Abstract
Background:
Premature hair graying (PHG) is often a matter of great concern for patients as it is viewed as a sign of increasing age, debility, decreasing vigor, and may lead to low self-esteem and psychological morbidity. Its etiopathogenesis is not completely understood but genetic, and various acquired factors have been implicated. The present study was undertaken to evaluate various epidemiological and biochemical variables associated with PHG.
Materials and Methods:
A cross-sectional case–control study was conducted at a tertiary care hospital in North India for 1 year which comprised 120 patients and equal number of controls. Various epidemiological variables were recorded and compared to controls. Serum ferritin, serum calcium, serum Vitamin D, serum Vitamin B12, lipid profile, thyroid profile, and fasting blood sugar were measured and compared among cases and controls.
Results:
Significantly higher proportion of cases had atopic diathesis, sedentary lifestyle, family history, history of smoking, and higher perceived stress values as compared to controls. Hair oiling seemed to protect against premature graying. Significantly, lower levels of serum calcium, ferritin, Vitamin B12, high-density lipoprotein-cholesterol, and high low-density lipoprotein-cholesterol were observed among cases.
Conclusion:
In the light of the present study, further studies with larger sample size are required to establish the definite etiological significance of these variables and formulate various preventive and therapeutic targets to prevent and treat PHG.
Key words: Atopic diathesis, oxidative stress, perceived stress scale, premature canities, premature graying
INTRODUCTION
Hair is an integral part of an individual's physical appearance and body image. Aging of hair follicle leads to reduced melanocyte function leading to graying. The term premature hair graying (PHG) or premature canities is used when graying occurs before the age of 20 in European, 25 in Asians, and 30 in Africans.[1] Graying of hair is a physiological phenomenon, and by the age of 50 years, 50% of the population have at least 50% gray hair known as the 50/50/50 rule of thumb.[2] However, a recent study reported that 6%–23% of people have 50% gray hair by 50 years of age.[3] As the age of the onset of PHG is dependent more on the genotype of the individual, it is subjected to racial variation.[4] On an average, Caucasians begin to gray in their mid30 s, Asians in their late 30s, and Africans in their mid40s.[5] Both sexes are equally affected.[6]
The current opinion about the etiology of PHG revolves around a robust genetic component with a speculated autosomal dominant pattern of inheritance, compounded with various acquired environmental factors, inflammation, and psychological stress.[5,7]
Various studies have also reported that environmental factors (e.g., UV light and climate), drugs, smoking, deficiency of trace elements, and nutritional deficiency, also play a role in PHG.[8] It has also been reported in patients with HIV, cystic fibrosis, and Hodgkin's lymphoma.[9,10,11] It may also occur in association with certain organ-specific autoimmune disorders such as pernicious anemia, vitiligo, hypothyroidism or hyperthyroidism, Addison's disease, premature hypogonadism, and as a part of various premature aging syndromes and atopic diathesis.[4] PHG has also been shown to be an important predictor of low mineral density and osteopenia and as a risk factor for coronary artery disease.[12,13,14]
There is a lack of epidemiological and clinical data on PHG from the Indian subcontinent. The present study is, therefore, undertaken to assess the risk factors associated with PHG, to reveal promising targets for future interventions.
MATERIALS AND METHODS
This was a case–control study conducted at the Postgraduate Department of Dermatology, Venereology, and Leprology at a tertiary care hospital in North India from November 2016 to October 2017 after obtaining approval from the Institutional Ethical Committee. In this study, PHG was defined as >5 gray hair in the scalp in patients <25 years of age. Control group comprised age- and sex-matched patients and attendants attending the Department for disease other than PHG.
Inclusion criteria
Clinically diagnosed patients of PHG
Age <25 years
Patients willing to participate in the study.
Exclusion criteria
Age >25 years
Patients with graying of hair as a part of other conditions such as vitiligo, cutaneous disease involving the scalp
Pregnant or lactating women
Patients who refused to give consent to undergo investigations.
A detailed history regarding the age of onset, origin, pattern, progression, family history, personal histories such as smoking and alcohol intake was taken and recorded on a predesigned pro forma. Biometric data such as height, weight, and body mass index were taken. Serum ferritin, serum calcium, serum Vitamin D, serum Vitamin B12, lipid profile, thyroid profile, and blood sugar (fasting) were measured in both cases and controls after taking informed consent.
The diagnosis of PHG was made clinically and was graded into four groups as follows:
No gray hair
Mild: <10 gray hair
Moderate: 10–100 gray hair
Severe: >100 gray hair (Shin et al., 2015).[15]
Patients aged >15 years were evaluated for stress by perceived stress scale (PSS) questionnaire (Cohen et al., 1983).[16] Validity of this scale (PSS-10) has already been established.[17]
Scoring
It consists of ten items. Each item is rated on a five-point scale ranging from never (0) to almost always.[4] Positively worded items are reverse scored, and the ratings are summed, with higher scores indicating more perceived stress. PSS-10 scores are obtained by reversing the scores on the four positive items, for example, 0 = 4, 1 = 3, and 2 = 2. and then summing across all ten items. Items 4, 5, 7, and 8 are the positively stated items.
Interpretation
Total score perceived stress level as follows:
0–7 – much lower than average
8–11 – slightly lower than average
12–15 – average
16–20 – slightly higher than average
21 and over much higher than average.
Statistical analysis
The recorded data were compiled and entered in a spreadsheet (Microsoft Excel) and was analyzed using computer software Microsoft Excel and SPSS version 21.0 (IBM Corp. in Armonk, NY)for Windows. Data reported as mean ± standard deviation and proportions as deemed appropriate for quantitative and qualitative variables, respectively. The statistical difference in mean value between the two groups was tested using unpaired “t” test. The qualitative data were compared using Fisher's exact test. P < 0.05 was considered as statistically significant. All P values reported were two-tailed.
RESULTS
Epidemiological variables
A total of 120 cases with PHG and equal number of age- and sex-matched controls were enrolled in our study. The mean age of cases was 15.71 years and that of controls was 15.91 years. Majority of the cases were in 11–15 years age group (34.17%). Of 120 cases, 66 (55%) were male and 54 (45%) were female.
Majority of patients were students (79.17%), followed by employees (8.33%), housewives (6.67%), and businessmen (5.83%). About 22.50% of patients led sedentary lifestyle as compared to 7.50% in controls, the difference being statistically highly significant (P = 0.001). Nearly 11.67% of patients were smokers as compared to 0.83% in controls, the difference being statistically highly significant (P = 0.000). About 57.50% of patients were using hair oil as compared to 31.67% in cases. The difference between the two groups was statistically highly significant (P < 0.0001). A history of alcohol consumption was not present in any patient in any of the study participants.
The mean duration of PHG was 21.74 ± 13.86 months with a range of 2–60 months, while mean age at onset of graying of hair was 13.80 ± 4.68 years with a range of 2–22 years. In cases, the origin of premature graying was vertex in 47.50% of patients, frontal in 40%, temporal in 8.33%, and occipital in 4.17% patients.
In cases, the progression of premature graying was observed to be slow in 57.50% of patients, while it was rapid in 42.50% of patients. Diffuse pattern of graying was observed at presentation in the majority of patients (95%). However, there were 4.17% patients with only frontal involvement and 0.83% patients with only temporal involvement at presentation.
PSS was calculated for patients >15 years of age, which involved 67 cases and 61 controls.
In cases, PSS was significantly higher than average (>16) in 83.59% of patients as compared to 29.51% controls. Mean PSS score in cases was 21.20 versus 13.08 in controls, the difference being statistically highly significant (P < 0.0001) [Figure 1].
Figure 1.
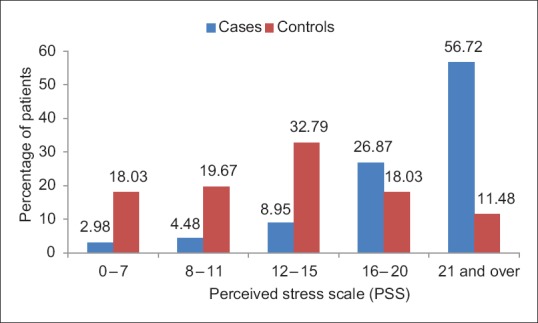
Bar chart showing the distribution of patients according to perceived stress scale in cases and controls
The family history of PHG was present in 65.83% of patients. Parental involvement was observed in 47.50% of patients and siblings in 0.83%, and both parental and sibling involvement was seen in 17.50% of patients. No family history was observed in 34.17% of patients.
A history of atopic diathesis was present in 40.83% of patients as compared to 10.83% in controls. The difference between the two groups was statistically highly significant (P < 0.0001). In cases, more patients had normal BMI (65%), followed by underweight (20%), overweight (12.50%), and obese (2.50%) which was statistically comparable with controls. In cases, PHG was severe in 38.34% of patients [Figure 2], moderate in 33.33% [Figure 3], and mild in 28.33% patients [Figure 4].
Figure 2.
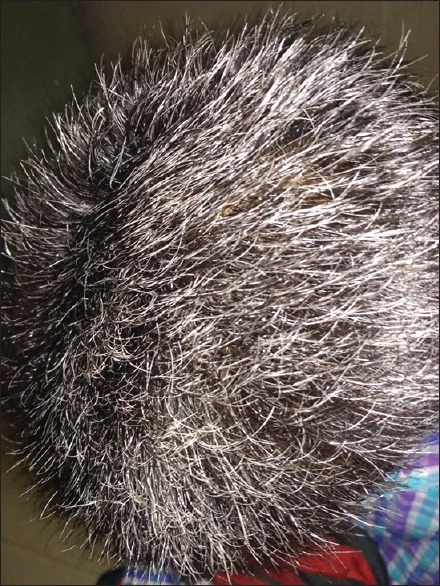
Severe grade of graying
Figure 3.
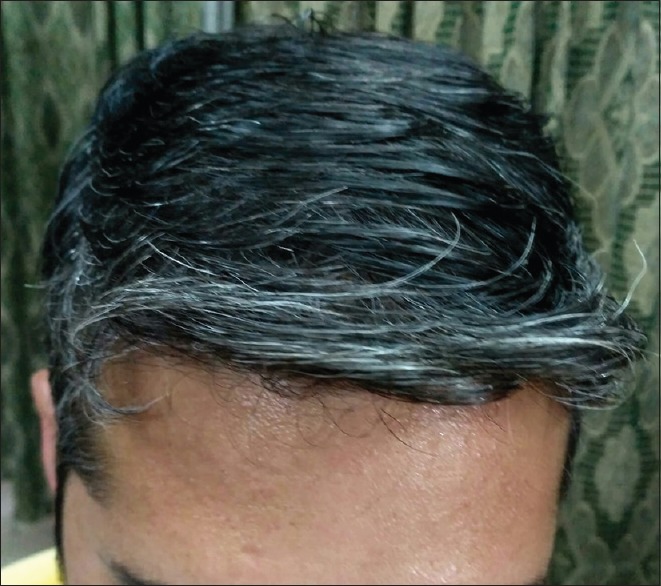
Moderate grade of graying
Figure 4.
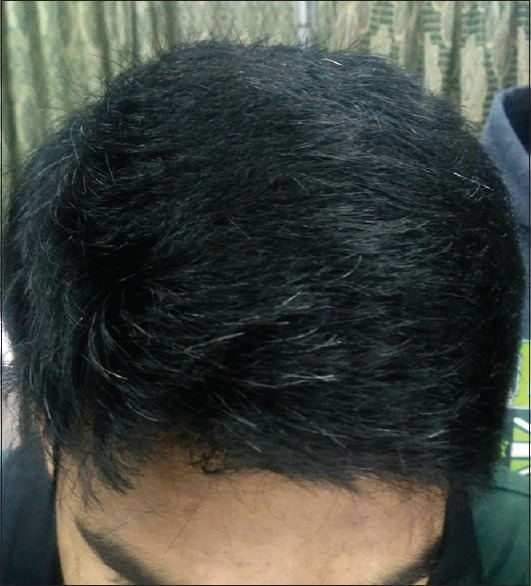
Mild grade of graying
Biochemical parameters
In cases, more patients had low serum calcium (48.33%) as compared to those in controls (25%). The difference between the two groups was statistically highly significant (P = 0.0003). However, the mean values of serum calcium in both the groups were statistically not significant (P = 0.30).
About 48.33% of patients had low serum ferritin as compared to 20.83% of controls. The difference between the two groups was statistically highly significant (P < 0.0001). Moreover, the mean value of serum ferritin in cases was less as compared to that of controls, the difference being statistically highly significant (P < 0.0001) [Figure 5].
Figure 5.
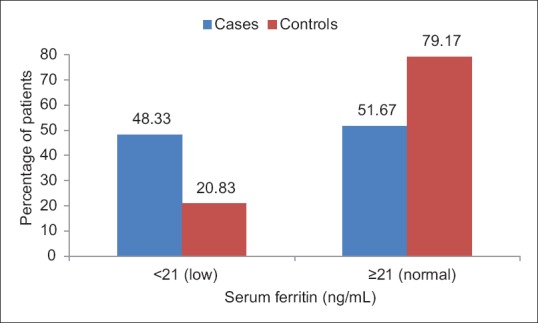
Bar chart showing the distribution of patients according to serum ferritin levels in cases and controls
Nearly 59.16% of patients had low serum Vitamin D as compared to 50% of controls, but the difference between the two groups was statistically not significant (P = 0.19) [Figure 6].
Figure 6.
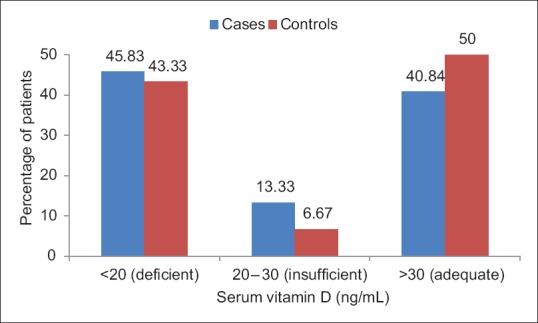
Bar chart showing the distribution of patients according to serum Vitamin D levels in cases and controls
Low serum Vitamin B12 was seen in 64.17% of cases as compared to 15% controls, and the difference between the two groups was statistically highly significant (P < 0.0001). Furthermore, the mean value of serum Vitamin B12 in cases was less as compared to that of controls, the difference being statistically highly significant (P < 0.0001).
Serum low-density lipoprotein (LDL) levels were significantly raised in cases (21.67%) as compared to controls (9.17%) (P = 0.01). However, mean serum LDL-cholesterol was comparable in both the groups (114.72 vs. 116.42 mg/dL), the difference being statistically not significant (P = 0.47).
Nearly 22.5% of patients had low serum high-density lipoprotein-cholesterol (HDL-C) as compared to those in controls (11.67%), the difference being statistically significant (P = 0.03). Furthermore, the mean value of serum HDL-C in cases was less as compared to that of controls, the difference being statistically highly significant (P < 0.0001) [Figure 7].
Figure 7.
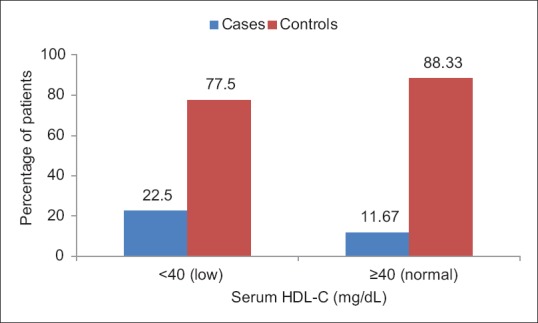
Bar chart showing the distribution of patients according to serum high-density lipoprotein cholesterol levels in cases and controls
Serum levels of total cholesterol, triglycerides, and very LDL were comparable between cases and controls.
Fasting blood sugar and thyroid profile were normal in cases as well as controls.
DISCUSSION
The mean age of cases in our study was 15.71 years; however, in a study conducted by Shin et al. (2015), the mean age of cases was 20.3 ± 1.5 years. The younger age seen in our study population can be because the age of inclusion in our study was <25 years, while in the study conducted by Shin et al. (2015), the criteria for inclusion were age <30 years.
About 55% of the studied patients were males, and this gender distribution is similar to a study conducted by Akin Belli et al. (2016)[18] where males outnumbered females. However, in a study conducted by Daulatabad et al. (2016)[19], female patients (51.9%) were seen more frequently than male patients (48.1%).
Majority of the cases and controls were students (79.17%). These results are in accordance with the study by Shin et al. (2015), in which majority of cases were students (82.4%). Similar results were seen in a study by Akin Belli A et al. (2016) with students comprising 91.7% of cases. The younger age of inclusion in the study explains the preponderance of students among the study group.
About 22.5% of cases led sedentary lifestyle as compared to 7.5% in controls, the difference being statistically highly significant (P = 0.001). Similar results were reported by Chakrabarty et al. (2016)[20], who observed significant number of patients with PHG had a sedentary lifestyle as compared to controls (P = 0.006).
Statistically significant number of patients were smokers as compared to controls in our study which is in accordance with a study conducted by Shin et al. (2015), in which the proportion of smokers was higher in patients as compared to controls (P = 0.013). Similarly, a study conducted by Zayed et al. (2013)[21] suggested that there is a significant relation (with an adjusted odds ratio [OR] of two and half) between the onset of PHG and cigarette smoking. The mechanisms by which smoking causes hair graying are incompletely understood. Smoking is well known to be a source of free radicals, which leads to increased oxidative stress[22] and damage to melanocytes. This theory is further supported by the observation that melanocytes in gray hair bulbs are frequently highly vacuolated, a common response to increased oxidative stress.[23]
Among cases, only 31.67% were using hair oil, while 57.50% controls had a history of the use of hair oil. Thus, the use of hair oil was seen to be protective against the development of PHG.
Family history of PHG was present in 65.83% of cases. Similarly, in a study conducted by Daulatabad et al. (2016), family history of PHG was obtained in 75% of the cases. In a study conducted by Shin et al. (2015), family history of PHG was paternal in 33.3% and maternal in 11.2%. Both parents were involved in 4.6% of patients.
A history of atopic diathesis was seen in 40.83% of cases as compared to 10.83% controls, and the difference was statistically highly significant. Our results are in accordance with the study conducted by Daulatabad et al. (2016), in which a history of atopic diathesis was seen in 36.5% of cases as compared to 9.6% controls (OR of 3.8).
The mean age at onset of graying was 13.8 ± 4.68 years with a range of 2–22 years. The earliest age of onset recorded was 2 years. In a study conducted by Daulatabad et al. (2016), the mean age at onset of graying was 11.6 ± 3.6 years with a range of 3–18 years, and the earliest age of onset seen was 3 years.
The mean duration of PHG was 21.74 ± 13.86 months with a range of 2–60 months. In a study conducted by Daulatabad et al. (2016), the mean duration of graying was 39.8 ± 37.3 months with a range of 4 months to 15 years.
The pattern of involvement at presentation was diffuse in the majority of the cases (95%). However, 4.17% of cases were having frontal involvement and only 0.83% with temporal involvement. The graying was severe in 38.34% of cases, moderate in 33.33% of cases, and mild in 28.33% cases. However, in a study conducted by Bhat et al. (2013), 11.5% patients had mild, 65.7% had moderate, and 23% had severe graying of hair. The origin of graying was vertex in 47.5% cases, followed by frontal (40%), temporal (8.33%), and occipital (4.17%). However, in a study conducted by Daulatabad et al. (2016), the origin of graying was most commonly seen in frontal region (48.1%), followed by vertex (34.6%), occipital (13.5%), and temporal (3.8%). Other hairy sites were not involved in any of the study participants.
The difference between mean BMI values of cases and controls in our study was not statistically significant. This is in contrast to the study done by Shin et al. (2015), in which the odds of having PHG were significantly higher in the overweight (P = 0.002) and obese (P < 0.001) groups than the normal weight group.
Cases and controls >15 years of age were evaluated for stress by PSS questionnaire (Cohen et al., 1983). A total of 67 cases and 61 controls were evaluated. The PSS score was significantly higher than average (≥16) in 83.59% cases as compared to 29.51% controls. Mean PSS score in cases was 21.2 compared to 13.08 in controls, the difference being statistically highly significant (P < 0.0001). Similarly, in a study conducted by Akin Belli et al. (2016), the mean PSS score in cases was 29.1 ± 6.6, while in controls, the mean PSS score was 27.9 ± 6.8 (P = 0.006). The process of hair graying includes a decrease in enzymes involved in melanogenesis, disruption of DNA repair, and loss of antioxidant mechanisms.[24] Repressed catalase protein expression and hydroxyl radical scavenging activities were recently found in gray hair follicles, and it was, therefore, noted that PHG is a result of oxidative damage in hair follicle melanocytes.[25] Arck et al. (2006)[26] also showed excess oxidative stress and melanocyte apoptosis in graying hair follicles. The relationship between oxidative stress and psychological disorders (emotional stress, anxiety, and depression) has been reported previously[27,28] In our study, higher PSS score, an indicator of higher emotional stress, which may be a source of oxidative stress, was significantly higher in cases as compared to controls.
In cases, more patients had low serum calcium (48.33%) as compared to those in the control group (25%). The difference between the two groups was statistically significant (P = 0.0003). However, the mean values of serum calcium in both the groups were statistically not significant (P = 0.30). These results are in accordance with a study conducted by Bhat et al. (2013) in which serum calcium levels were significantly lower in cases as compared to the control group (P = 0.018). Calcium has been involved in some steps of melanogenesis (Bhat et al., 2013). Furthermore, PHG has also been linked to decreased bone mineral density in previous studies.[29]
In cases, more patients had low serum ferritin (48.33%) as compared to controls (20.83%). The difference between the two groups was statistically highly significant (P < 0.0001). Moreover, the mean value of serum ferritin in cases was less as compared to that of controls, the difference being statistically highly significant (P < 0.0001). Our results are in accordance with a study conducted by Bhat et al. (2013) who found serum ferritin was significantly lower in cases as compared to controls (P = 0.03). Similarly, Chakrabarty et al. (2016) observed that mean serum ferritin was significantly lower in cases as compared to controls (P < 0.0001). Studies have postulated that iron affects melanogenesis. There is evidence provided by studies for the role of iron in the modulation of tyrosinase. It is reported that in a tautomerization reaction by dopachrome tautomerase, which is one of the later stages of melanin biosynthesis, the isomerization of dopachrome to dihydroxyindole-2-carboxylic acid occurs. This enzyme is a metalloenzyme with ferrous in at its active site (Chakraborty et al., 1992).
In cases, more patients had low serum Vitamin D (59.16%) as compared to those in controls (50%); however, the difference between the two groups was statistically not significant (P = 0.19). This is in accordance with study by Chakrabarty et al. (2016) in which there was no statistically significant difference between serum Vitamin D levels in cases and controls. However, Bhat et al. (2013) had reported significantly lower levels of serum Vitamin D in patients of PHG compared to controls.
In cases, more patients had low serum Vitamin B12 (64.17%) as compared to controls (15%). The difference between the two groups was statistically highly significant. These results are in accordance with a study conducted by Chakrabarty et al. (2016) in which serum Vitamin B12 levels were significantly lower in patients of PHG compared to controls (P < 0.001). The cells of hair follicles are considered to be rapidly dividing cells. Proliferation of these cells depends on synthesis of DNA, which is further dependent on sufficient supply of Vitamin B12.[30] Vitamin B12 also facilitates stabilization of the initial anagen phase of the hair follicle.[31]
Among cases, 21.67% had high serum LDL as compared to 9.17% in controls, the difference being statistically significant (P = 0.01). Furthermore, in cases, more patients had low serum HDL (22.50%) as compared to controls (11.67%), and the difference is statistically significant (P = 0.03). Similar results were seen in the study by Chakrabarty et al. (2016) which reported individuals with PHG had significantly lower levels of serum HDL as compared to the control group (P < 0.001). PHG has been linked with premature cardiovascular disease,[13] and this dyslipidemia could be a connecting factor in this linkage.
Dyslipidemia, a component of metabolic syndrome, is frequently seen in obese patients. The mean BMI between cases and controls in our study was statistically comparable, but dyslipidemia was statistically significantly present in cases as compared to controls. This can be explained by studies which have shown that even nonobese patients may have the presence of dyslipidemia and metabolic risk factors.[32] The upper body fat that was related to dyslipidemia in nonobese and anthropometric variables such as waist circumference and waist-to-hip ratio may be used for screening of dyslipidemia in these participants.[33]
CONCLUSION
The present study showed family history, atopic diathesis, sedentary lifestyle, smoking, and higher perceived stress to be associated with PHG. The use of hair oils was seem to be protective for premature graying. In the biochemical parameters, low serum calcium, ferritin, Vitamin B12, HDL, and high LDL were found to be associated with PHG. Further studies may throw more light in conclusively defining these putative associations.
Limitations
Sample size of our study was small
The level of oxidative stress was not quantified due to lack of resources
Follow-up of patients after correcting the biochemical deficiencies was not done.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Chakrabarty S, Krishnappa PG, Gowda DG, Hiremath J. Factors associated with premature hair graying in a young Indian population. Int J Trichology. 2016;8:11–4. doi: 10.4103/0974-7753.179384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Keogh EV, Walsh RJ. Rate of greying of human hair. Nature. 1965;207:877–8. doi: 10.1038/207877a0. [DOI] [PubMed] [Google Scholar]
- 3.Panhard S, Lozano I, Loussouarn G. Greying of the human hair: A worldwide survey, revisiting the ‘50’ rule of thumb. Br J Dermatol. 2012;167:865–73. doi: 10.1111/j.1365-2133.2012.11095.x. [DOI] [PubMed] [Google Scholar]
- 4.Pandhi D, Khanna D. Premature graying of hair. Indian J Dermatol Venereol Leprol. 2013;79:641–53. doi: 10.4103/0378-6323.116733. [DOI] [PubMed] [Google Scholar]
- 5.Tobin DJ, Paus R. Graying: Gerontobiology of the hair follicle pigmentary unit. Exp Gerontol. 2001;36:29–54. doi: 10.1016/s0531-5565(00)00210-2. [DOI] [PubMed] [Google Scholar]
- 6.Westerhof W, Njoo D, Menke KE. Miscellaneous hypomelanoses: Disorders characterized by extracutaneous loss of pigmentation. In: Nordlund JJ, Biossy RE, Hearing VJ, King RA, Ortonne JP, editors. The Pigmentary System: Physiology and Pathophysiology. New York: Oxford University Press; 1998. pp. 690–705. [Google Scholar]
- 7.Sonthalia S, Sarkar R. Premature graying of hair. The voids and tiffs. Pigment Int. 2015;2:73–5. [Google Scholar]
- 8.Bhat RM, Sharma R, Pinto AC, Dandekeri S, Martis J. Epidemiological and investigative study of premature graying of hair in higher secondary and pre-university school children. Int J Trichology. 2013;5:17–21. doi: 10.4103/0974-7753.114706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wadhwa SL, Khopkar U, Nischal KC. Hair and scalp disorders. In: Valia RG, Valia RA, editors. IADVL Textbook of Dermatology. 3rd ed. Mumbai: Bhalani Publishing House; 2008. pp. 864–948. [Google Scholar]
- 10.Dalgic B, Egritas O. Gray hair and acrodermatitis enteropathica-like dermatitis: An unexpected presentation of cystic fibrosis. Eur J Pediatr. 2011;170:1305–8. doi: 10.1007/s00431-011-1447-0. [DOI] [PubMed] [Google Scholar]
- 11.Trakymiene SS, Abla O. Hodgkin lymphoma presenting with hair graying. J Pediatr Hematol Oncol. 2010;32:417–8. doi: 10.1097/MPH.0b013e3181df6172. [DOI] [PubMed] [Google Scholar]
- 12.Schnohr P, Lange P, Nyboe J, Appleyard M, Jensen G. Gray hair, baldness, and wrinkles in relation to myocardial infarction: The Copenhagen city heart study. Am Heart J. 1995;130:1003–10. doi: 10.1016/0002-8703(95)90201-5. [DOI] [PubMed] [Google Scholar]
- 13.Gould L, Reddy CV, Oh KC, Kim SG, Becker W. Premature hair graying: A probable coronary risk factor. Angiology. 1978;29:800–3. doi: 10.1177/000331977802901103. [DOI] [PubMed] [Google Scholar]
- 14.Dwivedi S, Jhamb R. Cutaneous markers of coronary artery disease. World J Cardiol. 2010;2:262–9. doi: 10.4330/wjc.v2.i9.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shin H, Ryu HH, Yoon J, Jo S, Jang S, Choi M, et al. Association of premature hair graying with family history, smoking, and obesity: A cross-sectional study. J Am Acad Dermatol. 2015;72:321–7. doi: 10.1016/j.jaad.2014.11.008. [DOI] [PubMed] [Google Scholar]
- 16.Cohen S, Kamarck T, Mermelstein R. Aglobal measure of perceived stress. J Health Soc Behav. 1983;24:385–96. [PubMed] [Google Scholar]
- 17.Andreou E, Alexopoulos EC, Lionis C, Varvogli L, Gnardellis C, Chrousos GP, et al. Perceived stress scale: Reliability and validity study in Greece. Int J Environ Res Public Health. 2011;8:3287–98. doi: 10.3390/ijerph8083287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Akin Belli A, Etgu F, Ozbas Gok S, Kara B, Dogan G. Risk factors for premature hair graying in young Turkish adults. Pediatr Dermatol. 2016;33:438–42. doi: 10.1111/pde.12881. [DOI] [PubMed] [Google Scholar]
- 19.Daulatabad D, Singal A, Grover C, Chhillar N. Profile of Indian patients with premature canities. Indian J Dermatol Venereol Leprol. 2016;82:169–72. doi: 10.4103/0378-6323.168911. [DOI] [PubMed] [Google Scholar]
- 20.Chakraborty AK, Orlow SJ, Pawelek JM. Evidence that dopachrome tautomerase is a ferrous iron-binding glycoprotein. FEBS Lett. 1992;302:126–8. doi: 10.1016/0014-5793(92)80421-c. [DOI] [PubMed] [Google Scholar]
- 21.Zayed AA, Shahait AD, Ayoub MN, Yousef AM. Smokers' hair: Does smoking cause premature hair graying? Indian Dermatol Online J. 2013;4:90–2. doi: 10.4103/2229-5178.110586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Trüeb RM. Association between smoking and hair loss: Another opportunity for health education against smoking? Dermatology. 2003;206:189–91. doi: 10.1159/000068894. [DOI] [PubMed] [Google Scholar]
- 23.Trüeb RM. Pharmacologic interventions in aging hair. Clin Interv Aging. 2006;1:121–9. doi: 10.2147/ciia.2006.1.2.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Commo S, Gaillard O, Bernard BA. Human hair greying is linked to a specific depletion of hair follicle melanocytes affecting both the bulb and the outer root sheath. Br J Dermatol. 2004;150:435–43. doi: 10.1046/j.1365-2133.2004.05787.x. [DOI] [PubMed] [Google Scholar]
- 25.Shi Y, Luo LF, Liu XM, Zhou Q, Xu SZ, Lei TC, et al. Premature graying as a consequence of compromised antioxidant activity in hair bulb melanocytes and their precursors. PLoS One. 2014;9:e93589. doi: 10.1371/journal.pone.0093589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Arck PC, Overall R, Spatz K, Liezman C, Handjiski B, Klapp BF, et al. Towards a “free radical theory of graying”: Melanocyte apoptosis in the aging human hair follicle is an indicator of oxidative stress induced tissue damage. FASEB J. 2006;20:1567–9. doi: 10.1096/fj.05-4039fje. [DOI] [PubMed] [Google Scholar]
- 27.Srivastava R, Batra J. Oxidative stress and psychological functioning among medical students. Ind Psychiatry J. 2014;23:127–33. doi: 10.4103/0972-6748.151684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Matsushita M, Kumano-Go T, Suganuma N, Adachi H, Yamamura S, Morishima H, et al. Anxiety, neuroticism and oxidative stress: Cross-sectional study in non-smoking college students. Psychiatry Clin Neurosci. 2010;64:435–41. doi: 10.1111/j.1440-1819.2010.02109.x. [DOI] [PubMed] [Google Scholar]
- 29.Orr-Walker BJ, Evans MC, Ames RW, Clearwater JM, Reid IR. Premature hair graying and bone mineral density. J Clin Endocrinol Metab. 1997;82:3580–3. doi: 10.1210/jcem.82.11.4338. [DOI] [PubMed] [Google Scholar]
- 30.Volkov I, Press Y, Rudoy I. Vitamin B12 could be a “master key” in the regulation of multiple pathological processes. J Nippon Med Sch. 2006;73:65–9. doi: 10.1272/jnms.73.65. [DOI] [PubMed] [Google Scholar]
- 31.Krugluger W, Stiefsohn K, Laciak K. Vitamin B12 activates Wnt-pathway in human hair follicle by induction of beta catenin and inhibition of glycogen synthase kinase 3 transcription. J Cosmet Dermatol Sci Appl. 2011;1:25–9. [Google Scholar]
- 32.Shah SZ, Devrajani BR, Devrajani T, Bibi I. Frequency of dyslipidemia in obese versus non obese in relation to BMI, WHR, and WC. Pak J Sci. 2010;62:27–30. [Google Scholar]
- 33.Ito H, Nakasuga K, Ohshima A, Sakai Y, Maruyama T, Kaji Y, et al. Excess accumulation of body fat is related to dyslipidemia in normal-weight subjects. Int J Obes Relat Metab Disord. 2004;28:242–7. doi: 10.1038/sj.ijo.0802528. [DOI] [PubMed] [Google Scholar]


