Abstract
The ability to dynamically allocate neural resources within the visual space is supported by a number of spectrally-specific oscillatory responses, and such visuospatial processing has been found to decline moderately with age and differ by sex. However, the direct effects of age and sex on these oscillatory dynamics remains poorly understood. Using magnetoencephalography (MEG), structural magnetic resonance imaging, and advanced source reconstruction and statistical methods, we investigated the impact of aging and sex on behavioral performance and the underlying neural dynamics during visuospatial processing. In a large sample spanning a broad age range, we find that a number of prototypical attention and perception network components, both spectrally- and spatially-defined, exhibit complex and uniquely informative relationships with age and sex. Specifically, neural responses in the theta range (4 – 10 Hz) were found to covary with chronological age in prefrontal and motor cortices, signifying a possible relationship between age and cognitive control. Further, we found that beta (18 – 24 Hz) activity covaried with age across a large swath of the somato-motor strip, supporting previous findings of motor planning and execution deficits with increasing age. Finally, gammafrequency (48–70 Hz) oscillations were found to exhibit robust covariance with age in superior parietal and temporo-parietal areas, indicating that the mapping of saliency in visual space is modulated by the normal aging process. Interestingly, behavioral performance and some of these oscillatory neural responses also exhibited interactions between age and sex, indicating sex differences in the evolution of the neural coding of visual perception as age increases. In particular, men were found to have stronger correlations between age and neural oscillatory responses during task performance than women in lateral occipital and superior temporal regions in the alpha band and in dorsolateral prefrontal cortex in the gamma band, while women exhibited more robust covariance between age and neural responses than men in inferior temporal and medial prefrontal cortex in the theta range.
Keywords: aging, sex differences, attention, neural oscillations
1. Introduction
The aging process affects every person and commonly brings with it modest declines in some cognitive functions. Included among these declines is a reduced ability to rapidly and preferentially allocate neural resources to salient environmental features (Chao and Knight, 1997; Plude and Doussard-Roosevelt, 1989; Stankov, 1988; Verhaeghen and Cerella, 2002), to perceive salient properties of these features in the visual space (Baccini et al., 2014; Bigelow et al., 2015; Jenkins et al., 2000; Moffat et al., 2006; Moffat et al., 2001), and to respond to these stimuli accordingly (Oliviero et al., 2006; Seidler et al., 2010; Smith et al., 1999). These gradual deteriorations in visuospatial perception have been documented for decades, however the macro-scale neuronal dynamics responsible for the declines are not fully understood. Although a number of functional MRI (fMRI) studies have strongly implicated increased BOLD activity in prefrontal, ventral temporal, and superior parietal regions in age-related attentional and perceptual decline (Alichniewicz et al., 2012; Geerligs et al., 2014; Iachini et al., 2009; Kim et al., 2008; Madden et al., 2007; Madden et al., 2004; Milham et al., 2002; Townsend et al., 2006), little knowledge exists regarding changes in the neural dynamics within these regions and beyond. For instance, differing patterns of rhythmic neural activity (i.e., neural oscillations) have been found to code distinct and dissociable components of visuoperceptual function (Busch et al., 2009; Doesburg et al., 2008; Edden et al., 2009; Handel et al., 2011; Landau and Fries, 2012; Landau et al., 2015; Spaak et al., 2014; Tallon-Baudry et al., 2005; Verbruggen et al., 2010; Vidal et al., 2006; Wiesman et al., 2017), but the effect of aging on these oscillatory neural codes during visuospatial processing is also an understudied topic. Identifying the oscillatory bases of visuospatial dysfunction in aging is of prime importance, as neural activity in different frequency ranges are thought to be produced by distinct neurophysiological mechanisms. In particular, the dynamics of gamma (>30 Hz) frequency oscillations have been repeatedly linked to local gamma-aminobutyric acid (GABA) concentration in the human brain (Edden et al., 2009; Gaetz et al., 2011; Muthukumaraswamy et al., 2009), and have also been found to covary reliably with the amplitude of fMRI responses in a number of studies (Lachaux et al., 2007; Logothetis et al., 2001; Niessing et al., 2005). Likewise, local GABA concentration has been shown to decline with age (Gao et al., 2013), and several metrics of gamma activity are also known to be modulated by age (Gaetz et al., 2011).
Like aging, the impact of biological sex on the oscillatory dynamics serving visuospatial processing is also poorly understood, and whether this relationship differs by age remains unknown. This is again surprising, as the structural connections between major attention and visual-network regions have been found to vary by sex (Ingalhalikar et al., 2014), and the strength of resting-state functional networks formed among these regions (i.e., fronto-parietal and visual networks) appear to be more strongly affected by the aging process in men relative to women (Scheinost et al., 2015). Taken together, these studies suggest sex differences in the oscillatory coding of visuospatial attention and perception, as well as a likely interaction between age and sex in the macro-scale neuronal dynamics serving visuospatial function.
In the current study, we utilize the unique combination of spatial (i.e., millimeter) and temporal (i.e., millisecond) precision offered by magnetoencephalography (MEG) to investigate the oscillatory dynamics and performance parameters during a visuospatial processing task that covary with age in a large cohort of healthy adult participants. More specifically, we used the millisecond sampling of neural data provided by MEG to examine the rapidly evolving neural dynamics underlying specific sub-processes of visuospatial processing, while still pinpointing the origin of these dynamics with high spatial accuracy. Additionally, this high temporal precision allowed us to examine oscillatory activity that fluctuates in amplitude within very short time intervals (e.g., gamma activity). Our research focus was three-fold. First, whole-brain covariance between age and source-imaged oscillatory responses was assessed, in order to determine which brain regions exhibited a relationship between aging and the local power in statistically-defined spectral windows. Next, sex differences were probed across the whole brain for each oscillatory neural response. Third, covariance maps for men and women were compared, in order to determine which oscillatory responses showed an interaction between age and sex. Our primary hypotheses were that age would covary with neural responses in multiple spectral windows, but most prominently in the theta (4–10 Hz) and gamma (>30 Hz) bands, as these have been strongly linked to visuospatial function (Busch et al., 2004; Jensen et al., 2014; Lisman and Jensen, 2013; Muthukumaraswamy and Singh, 2013; Vidal et al., 2006; Wiesman et al., 2017; Wiesman et al., 2018). Further, we expected that sex differences would appear in major visuospatial attention and perception regions, as such differences have been found in fMRI studies of working memory and executive function (Cosgrove et al., 2007; Gong et al., 2011; Sacher et al., 2013). Finally, we expected that age by sex interactions would be observed in key visuospatial attention and perception regions, and that these interactions would parallel the behavioral data and generally reflect more accelerated aging in men relative to women (Crimmins et al., 2011; Horvath et al., 2016; Simpkin et al., 2016).
2. Materials and Methods
2.1. Participants
We enrolled 77 adult participants for this experiment (64 right-handed; 40 males), spanning a wide age range (Range = 22–72 years-old; Mean = 44 years; SD = 14.95 years). Exclusion criteria included any medical illness affecting CNS function, any neurological or psychiatric disorder, history of head trauma, and current substance abuse. The Institutional Review Board at the University of Nebraska Medical Center reviewed and approved this investigation. Written informed consent was obtained from each participant following detailed description of the study. All participants completed the same experimental protocol.
2.2. MEG Experimental Paradigm
We used a visuospatial discrimination task, termed Vis-Attend (Figure 1A), to engage visuospatial perception circuitry (Wiesman et al., 2017; Wiesman et al., 2018). During this task, participants were seated in a magnetically-shielded room and told to fixate on a crosshair presented centrally. After a variable ISI (range: 1900–2100 ms), an 8×8 grid was presented for 800 ms at one of four positions relative to the fixation: above right, below right, above left, or below and to the left. The left/right orientations were defined as a lateral offset of 75% of the grid from the center of fixation. Participants were instructed to respond via button press with their right hand as to whether the grid was positioned to the left (index finger) or right (middle finger) of the fixation point upon presentation of the grid. Each participant performed 240 trials (60 of each type) in a pseudo-randomized order concurrent with MEG recording.
Figure 1. Experimental paradigm and behavioral results.
(A) An illustration of the visuospatial task paradigm. (B) Relationship between reaction time (in milliseconds) on the visuospatial task, displayed on the x-axis, and age (in years) on the y-axis. A line of best-fit is overlaid on the graph.
2.3. MEG Data Acquisition
All recordings were conducted in a one-layer magnetically-shielded room with active shielding engaged for environmental noise compensation. Neuromagnetic responses were sampled continuously at 1 kHz with an acquisition bandwidth of 0.1– 330 Hz using a 306-sensor Elekta MEG system (Helsinki, Finland) equipped with 204 planar gradiometers and 102 magnetometers. Participants were monitored during data acquisition via real-time audio–video feeds from inside the shielded room. Each MEG dataset was individually corrected for head motion and subjected to noise reduction using the signal space separation method with a temporal extension (Taulu and Simola, 2006).
2.4. Structural MRI Processing and MEG Coregistration
Preceding MEG measurement, four coils were attached to the participant’s head and localized, together with the three fiducial points and scalp surface, using a 3-D digitizer (Fastrak 3SF0002, Polhemus Navigator Sciences, Colchester, VT, USA). Once the participant was positioned for MEG recording, an electric current with a unique frequency label (e.g., 322 Hz) was fed to each of the coils. This induced a measurable magnetic field and allowed each coil to be localized in reference to the sensors throughout the recording session. Since coil locations were also known in head coordinates, all MEG measurements could be transformed into a common coordinate system. With this coordinate system, each participant’s MEG data were coregistered with their individual structural T1-weighted MRI data prior to source space analyses using BESA MRI (Version 2.0). These neuroanatomic images were acquired with a Philips Achieva 3T X-series scanner using an eight-channel head coil and a 3D fast-field echo sequence with the following parameters: TR: 8.09 ms; TE: 3.70 ms; field of view: 24 cm; slice thickness: 1 mm with no gap; in-plane resolution: 1.0 × 1.0 mm. Structural MRI data were aligned parallel to the anterior and posterior commissures and transformed into standardized space. Following source analysis (i.e., beamforming), each participant’s 4.0 × 4.0 × 4.0 mm functional images were also transformed into standardized space using the transform that was previously applied to the structural MRI volume and spatially resampled to 1.0 × 1.0 × 1.0 mm.
2.5. MEG Preprocessing, Time-Frequency Transformation, and Sensor-Level Statistics
Cardiac and eyeblink artifacts were removed from the data using signal-space projection (SSP), which was subsequently accounted for during source reconstruction (Uusitalo and Ilmoniemi, 1997). The continuous magnetic time series was then divided into 2700 ms epochs, with the baseline extending from −400 to 0 ms prior to stimulus onset. Epochs containing artifacts were then rejected using a fixed threshold method, supplemented with visual inspection. Briefly, in MEG, the raw signal amplitude is strongly affected by the distance between the brain and the MEG sensor array, as the magnetic field strength falls off sharply as the distance from the current source increases. To account for this sort of variance across participants, as well as actual variance in neural response amplitude, we used an individually-determined threshold based on the distribution across all trials for both signal amplitude (mean = 1139.29 fT; SD = 239.91 fT) and signal gradient (mean = 171.00 fT/s; SD = 65.09 fT/s) to identify trials containing artifacts. In addition, all trials with incorrect behavioral responses were excluded from further analysis prior to artifact exclusion. In total, an average of 206.47 (SD = 8.55) trials per participant were used for further analysis.
The artifact-free epochs were next transformed into the time-frequency domain using complex demodulation, and the resulting spectral power estimations per sensor were averaged over trials to generate time-frequency plots of mean spectral density. The post-stimulus sensor-level data were then normalized per sensor and bin using the mean power during the −400 to 0 ms time period. The specific time-frequency windows used for subsequent source imaging were determined by statistical analysis of the sensor-level spectrograms across the entire array of gradiometers. Each data point in each spectrogram was initially evaluated using a mass univariate approach based on the general linear model. To reduce the risk of false positive results while maintaining reasonable sensitivity, a two stage procedure was followed to control for Type 1 error. In the first stage, one-sample t-tests were conducted on each data point and the output spectrograms (one per gradiometer) of t-values were thresholded at p < 0.01 to define time-frequency bins containing potentially significant oscillatory deviations across all participants. In stage two, the time-frequency bins that survived the threshold were clustered with temporally and/or spectrally neighboring bins that were also above the threshold (p < 0.01), and a cluster value was derived by summing all of the t-values of all data points in the cluster. Nonparametric permutation testing was then used to derive a distribution of cluster-values and the significance level of the observed clusters (from stage one) were tested directly using this distribution (Ernst, 2004; Maris and Oostenveld, 2007). For each comparison, at least 10,000 permutations were computed to build a distribution of cluster values. Based on these analyses, the time-frequency windows that contained significant oscillatory events across all participants and trials were subjected to a beamforming analysis. We did not consider time-frequency clusters that became significant after the mean RT across all participants, so as to focus on responses underlying visuospatial perception, rather than other processes inherent to the later portions of our task (i.e., motor initiation, response/errorchecking, etc.).
2.6. MEG Source Imaging and Statistics
Cortical networks were imaged through an extension of the linearly constrained minimum variance vector beamformer (Gross et al., 2001), which applies spatial filters to frequency domain sensor data in order to calculate voxel-wise source power for the entire brain volume. The single images are derived from the cross spectral densities of all combinations of MEG gradiometers averaged over the timefrequency range of interest, and the solution of the forward problem for each location on a grid specified by input voxel space. Following convention, we computed noise-normalized, source power per voxel in each participant using active (i.e., task) and passive (i.e., baseline) periods of equal duration and bandwidth. Such images are typically referred to as pseudo-t maps, with units (pseudo-t) that reflect noise-normalized power differences (i.e., active vs. passive) per voxel. MEG pre-processing and imaging used the Brain Electrical Source Analysis (BESA version 6.0) software.
To identify regions of activity that exhibited a significant relationship with age, whole-brain correlation maps were computed between the voxel-wise pseudo-t values across participants and their respective ages. Since reaction time was found to be significantly correlated with age, it was used as a covariate of no-interest, and the correlation maps were computed using partial correlation coefficients. The effect of participant sex on oscillatory neural responses was then explored by computing wholebrain unpaired t-test maps between the sexes. Next, to identify regions where a significant age-by-sex interaction was present, whole-brain correlation maps were computed individually for men and women using the method described above. From these maps, whole-brain bivariate correlation coefficient comparisons were computed using Fisher’s Z-transformation, which provided a voxel-wise map of zscores representing the normalized difference between men and women in the age/oscillatory power (pseudo-t) relationship. To account for multiple comparisons, a relatively strict significance threshold of p < .005 was used for the identification of significant clusters in all whole-brain statistical maps, accompanied with a cluster (k) threshold of at least 500 contiguous voxels. From these significant clusters, pseudo-t values per participant were extracted from the peak voxel (i.e., the voxel with the highest statistical value), which were then used in further hypothesis-driven analyses.
2.7. Peak Frequency Analysis
Peak frequency data were obtained by first extracting virtual sensor time-frequency data from the peak voxels identified in the whole-brain gamma correlation, and then finding the frequency within that band that had the highest amplitude response across the time window previously used in beamforming analysis of the same response. This resulted in one peak frequency value per participant per hemisphere, which were averaged within each person as no hemispheric effects were hypothesized.
2.8. Statistics
To evaluate the predictive capacity of the neurophysiological responses identified in the whole-brain analyses, reaction time and accuracy on the visuospatial task were individually regressed upon the extracted pseudo-t values described above. To meaningfully differentiate the predictive value of these responses, separate regressions were computed for those responses that covaried with age and those that exhibited an interaction between age and sex. Tables that comprehensively report the results of these regressions can be found in the supplement (Tables S1-S4). All correlations beyond those computed in the whole-brain statistical maps utilized the Pearson product-moment correlation coefficient, and any subsequent bivariate correlation coefficient comparisons were performed using the Fisher’s Z-transformation. For these tests a significance threshold of p < .05 was used. Whole-brain statistical maps were computed in MATLAB (Mathworks, Inc., Massachusetts, USA) using custom functions, and all other statistical analyses were performed in SPSS (Chicago, Illinois, USA).
3. Results
3.1. Demographics, Behavior, and Neuropsychology
To rule out any potential confounding factors in our participant group, correlations and unpaired t-tests were computed to determine whether age or sex, respectively, exhibited any statistically significant relationship with other demographic data. No significant relationships were found between age and highest level of education, handedness, or ethnicity. Further, no differences existed for age, education, handedness, or ethnicity between men and women. Each participant completed 240 trials of our visuospatial processing task, which has been found to elicit neural responses across a number of spectrally- and spatially-distinct visuospatial network components (Wiesman et al., 2017; Wiesman et al., 2018). In general, participants performed well on the task. The average reaction time was 610.18 milliseconds, and the average accuracy of responses was 97.84% correct. As was expected, reaction time on the task significantly correlated with age, such that as age increased so did reaction time (i.e., performance decreased; r = .43, p < .001; Figure 1B). Neither accuracy nor average reaction time on the task significantly differed between men and women.
3.2. Spectrally-specific Neural Oscillations
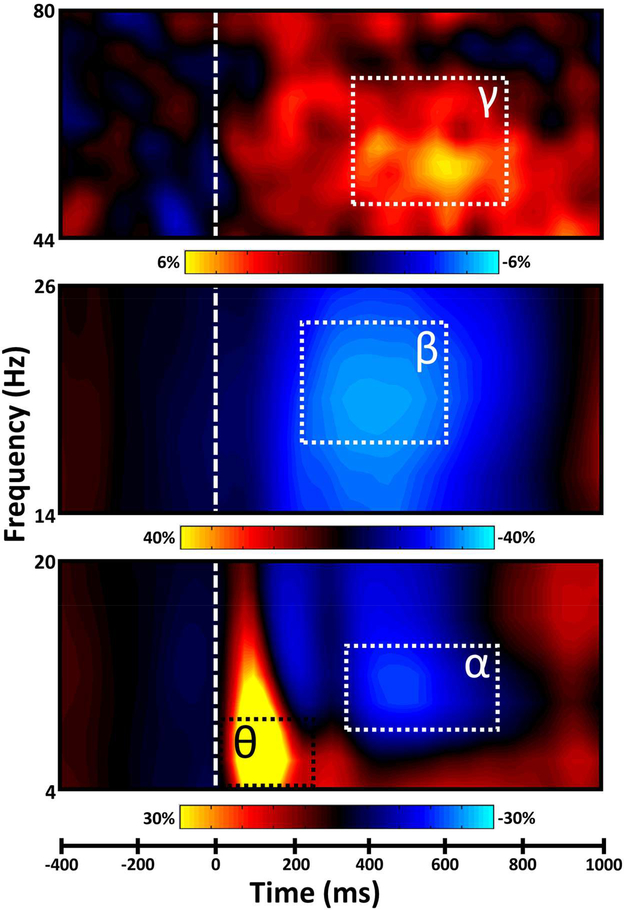
Prior to source reconstruction, significant oscillatory events were identified in the MEG sensor-level time-frequency data using stringent statistical criteria (see Materials and Methods). In agreement with previous investigations of visuospatial processing, significant neural responses were observed in four distinct time-frequency windows (Figure 2). These included a strong increase or synchronization in the theta (4–10 Hz) range over occipital and frontal cortices (bottom of Figure 2, sensor shown: M1743), extending temporally from stimulus onset to 250 ms post-stimulus onset. In a spatially-overlapping group of sensors (top of Figure 2, sensor M2513 is shown), there was also a broadband gamma (48–70 Hz) increase, which ranged temporally from 325–725 ms post-stimulus onset. In addition, there was a slightly more lateral decrease or desynchronization response in the alpha (8–14 Hz) band, which stretched from 350 to 750 ms post-stimulus onset. Finally, in medial parietal sensors (middle of Figure 2, sensor shown: M1622), a strong decrease in the beta (18–24 Hz) band was observed from 250 to 600 ms post-stimulus onset. To identify the anatomical basis of these four oscillatory responses, we performed source reconstruction on each response using a beamforming approach, which generated four whole-brain maps reflecting functional neural activity per participant.
Figure 2. Spectral time course of task-related neural responses.
Each representative plot displays the significant oscillatory responses identified in the sensor-level analyses. Time (in milliseconds) is denoted on the x-axis at bottom, and frequency (in Hz) is denoted on the y-axis of each respective plot. The dashed white line at 0 ms indicates the onset of the visual stimulus, and the color scale bar for percent change from baseline is displayed below each respective plot. All spectrograms represent groupaveraged data from a gradiometer sensor that was representative of the significant neural response in that part of the MEG array. The same representative sensors were selected in all participants.
3.3. Relationships between Age and Oscillatory Neural Responses
To examine the relationship between age and the oscillatory responses described above, the participant-level functional maps were used to compute whole-brain correlation maps, with age as a covariate of interest and reaction time as a covariate of no-interest. This revealed significant correlationclusters between age and neural activity in seven distinct brain regions, spread across three different oscillatory responses (Figure 3A). Specifically, clusters of theta activity in the left frontal eye fields (FEF; k = 7903, p < .005, corrected), right dorsolateral prefrontal cortex (dlPFC; k = 575; p < .005, corrected), and right postcentral gyrus (k = 1467; p < .005, corrected) correlated with age, such that as age increased, theta responses in these regions decreased. In contrast to the focal theta correlations, beta activity was negatively correlated with age across the entirety of the somato-motor strip (k = 311,376; p < .005, corrected). It is important to note that, although the directionality of this correlation was negative, the beta response itself is a desynchronization (i.e., a decrease from baseline), and thus this relationship should be interpreted as stronger neural oscillations being associated with increasing age. Three regions of activity in the gamma band also significantly correlated with age, including the bilateral superior parietal cortices (SPC; right-SPC: k = 38,787, p < .005, corrected; left-SPC: k = 12,542, p < .005, corrected). Of note, the strength of gamma oscillations in the right, but not the left SPC also significantly predicted accuracy on the task (p = .001). Within the right-SPC cluster, a significant sub-cluster was detected in the right temporo-parietal junction (TPJ), which also exhibited a relationship with age (k = 3,039, p < .005, corrected). Note that the functional map of alpha oscillatory activity (8–14 Hz, 350–750 ms) did not correlate with age in any region.
Figure 3. Spectrally-specific relationships between age and visuospatial neural responses.
(A) Whole brain visualizations of the relationship between age and neural oscillatory responses within each frequency band, including theta responses the left FEF, right dlPFC, and right postcentral gyrus, beta activity in the somato-motor strip, and gamma activity in the bilateral SPC and right TPJ. Greek letters in the upper left of each map indicate the frequency band, and the color scale bar representing the statistical significance of all presented relationships is displayed in the upper middle. (B) Relationship between peak gamma frequency (in Hz; averaged across the bilateral SPC clusters) is displayed on the xaxis, and age (in years) on the y-axis.
Converging lines of evidence suggest a likely role for GABA in the peak frequency of gamma oscillations in parietal, motor, and visual cortices (Edden et al., 2009; Gaetz et al., 2011; Muthukumaraswamy et al., 2009), as well as a decline in GABAergic neurotransmission with increasing age (Gao et al., 2013; He et al., 2016; Hua et al., 2008; Leventhal et al., 2003). To evaluate the impact of aging on gamma peak frequency, we extracted the peak voxel time series from the significant clusters and identified the peak frequency (see Section 2.7), which indicated a significant relationship (r = −.29, p = .012), such that as age increased, gamma peak frequency in the SPC decreased (Figure 3B).
3.4. Relationships between Sex and Oscillatory Neural Responses
Next, we investigated the effect of biological sex on the same neural oscillatory responses, and found that gamma activity in two right-lateralized regions significantly varied by sex. One was located near the right inferior cerebellum (k = 7,989, p < .005, corrected; Mwomen = 0.56, Mmen = 0.08), and the other in the right SPC (k = 1,769, p < .005, corrected; Mwomen = 1.20, Mmen = 0.29); both were significantly stronger in women relative to men.
3.5. Age by Sex Interactions in Behavior and Neural Oscillations
An interaction between age and sex was observed in the behavioral reaction time data. Specifically, the relationship between age and reaction time was significantly more positive for men than for women (z = 2.00, p = .045), such that as men aged their performance on the task decreased more rapidly than women (Figure 4).
Figure 4. Behavioral interaction between age and sex.
Group-wise relationships between reaction time (in milliseconds; x-axis) on the visuospatial task and age (in years; y-axis) for men (red) and women (blue). The respective Pearson correlation coefficient for each group is indicated in the upper left corner, and a line of best-fit for each group is overlaid on the graph.
To investigate which neural oscillations exhibited an interaction between age and sex, the participant-level maps were used to derive whole-brain interaction maps per oscillatory response (i.e., theta, alpha, beta and gamma). Five significant age by sex interaction clusters were identified from these maps, distributed across three distinct frequency bands (Figure 5). Specifically, a significant theta cluster was identified in the left mPFC (k = 821, p < .005, corrected), and follow-up testing revealed that women exhibited a positive relationship between theta oscillatory power and age, while men exhibited a negative relationship (rwomen = .39; rmen = −.34). A similar theta cluster was identified in the right middle temporal cortices (k = 9,526, p < .005, corrected; rwomen = .36; rmen = −.41). Two interaction clusters were also present in the alpha band; one near left occipito-temporal cortices (k = 29,962, p < .005, corrected; rwomen = .52; rmen = −.30), and the other in the right anterior superior temporal cortices (k = 10,354, p < .005, corrected; rwomen = .46; rmen = −.34). Since the alpha response was actually a decrease in power, these results indicate that alpha oscillatory activity in these regions is getting stronger as men get older, and that the opposite is true in women. Finally, a significant age-by-sex interaction was found for gamma oscillations in the right dorsolateral prefrontal cortex (dlPFC; k = 1,803, p < .005, corrected; rwomen = −.42; rmen = .37). Interestingly, oscillations in this gamma cluster became stronger as men aged, while the opposite was true in women, and the strength of gamma oscillations in this region was the only neurophysiological marker to significantly predict both reaction time (p = .005) and accuracy (p = .008) on the task. Note that no age-by-sex interactions were found for beta activity.
Figure 5. Spectrally-specific interactions between age and sex in oscillatory neural responses.
Whole brain visualizations of the interaction between age and sex in the visuospatial-related oscillatory neural responses within different bands, including significant theta clusters in the left mPFC and right middle temporal cortices, alpha clusters in the left occipito-temporal cortices and right anterior temporal cortices, and gamma clusters in the right dlPFC. Greek letters to the left indicate the frequency band pertaining to each map, and the color scale bar representing the statistical significance of all presented relationships is displayed at the bottom. As the legend in the upper right suggests, warm colors indicate clusters where men exhibited a more positive relationship between age and neural responses at that frequency, while cool colors indicate clusters where women exhibited a more positive relationship. It is important to note that the oscillatory response of interest in the alpha band was a decrease from baseline, and so a negative relationship between age and activity at this frequency actually suggests an increasing neural response with increasing age.
4. Discussion
In this study, we evaluated the effects of age and sex on dynamic oscillatory neural responses during a visuospatial processing task. We performed source reconstruction on several attention and perceptionrelated neural responses and found that behavioral performance and some of these neural responses covaried with age and differed by sex, including a parietal gamma response that is known to be a functionally-significant marker of visuospatial processing. Further, we showed that some of these oscillatory neural responses exhibit an interaction between age and sex, signifying differences between men and women in regard to the effects of aging on neural and cognitive function.
4.1. Spectrally-specific Relationships between Age and Perception-related Oscillatory Neural Responses
Our analyses revealed that multiple prototypical attention and perception-related oscillatory responses covary with age, and that the anatomical location of these responses include major visuospatial-network hubs. For example, theta oscillations correlated with age in three regions, including the right dlPFC and left FEF. Oscillatory theta activity has been strongly tied to the temporal parcellation and long-distance transmission of stimulus information (Başar et al., 2001; Fellrath et al., 2016; Jensen and Tesche, 2002; Landau et al., 2015; Wiesman et al., 2017), particularly in attention networks that commonly include the prefrontal cortices and frontal eye fields. Taken together, the negative correlation observed between activity in these frontal network components and age suggests a decline in the executive control system with the progression of age.
In addition to theta, gamma oscillations in three brain regions correlated with age, and all three of these regions are integral components of well-established visuospatial attention networks. The right TPJ is commonly associated with the detection of novel or salient stimuli (Corbetta and Shulman, 2002), while the bilateral SPC has been robustly tied to the mapping of this saliency in visual space during cognitively-demanding tasks (Bisley and Goldberg, 2010). The relationship between these regions and advancing age is of particular interest due to their spectral definition. Specifically, gamma band oscillations in posterior brain regions have been linked to the encoding and binding of task-relevant stimulus features, and are enhanced by increased attentional load (Jensen et al., 2007; Uhlhaas et al., 2011). Thus, our finding of increasing gamma oscillatory activity in these regions with age, coupled with decreasing performance on the task, likely signifies an insufficient compensatory effort by the aging brain to enhance processing within these visuospatial networks. In addition, the observed negative relationship between the peak gamma frequency of the SPC response and age lends further support to the notion of age-related aberrations in neurotransmitter levels in these brain regions. GABAergic function has been found to decrease with age in numerous studies (Gao et al., 2013; He et al., 2016; Hua et al., 2008; Leventhal et al., 2003), and exhibits a positive relationship with gamma-band peak frequency in motor, parietal, and visual cortices (Edden et al., 2009; Gaetz et al., 2011; Muthukumaraswamy et al., 2009). Thus, the negative relationship observed in the current study between peak gamma frequency and age likely signifies an age-related deficiency in local GABAergic activity within these brain regions.
Finally, consistent with previous studies (Heinrichs-Graham et al., 2018; Heinrichs-Graham and Wilson, 2016; Rossiter et al., 2014), the beta desynchronization response in the somato-motor cortices correlated negatively with age. Although this correlation was negative, it is important to remember that the response of interest was a desynchronization (i.e., a decrease from baseline levels), and thus should be interpreted as increasing in magnitude with age. Of note, we observed a similar age-related relationship with beta in a previous study of movement sequences, but the sample in that study consisted only of older and younger groups (i.e., no ~35–60 year-old middle range). Since there was no overlap between the sample in the previous study (Heinrichs-Graham and Wilson, 2016) and the current study, our beta findings can be interpreted as strongly confirmatory, and a partial replication in an independent sample.
4.2. Spectrally-specific Sex Differences in Visuospatial Neural Responses
In contrast to the relationships with age, sex differences were found in only one oscillatory response. Compared to men, women exhibited significantly increased levels of gamma activity in the right inferior cerebellum and the right SPC. The difference in the SPC may reflect a differential ability to map salient information to the visual space between the sexes. However, both groups performed equally well on the task, and so it may represent a compensatory mechanism that enables better task performance when cognitive load is high. Further studies are definitely needed to better understand this effect. A sex difference in the same direction was also observed in the cerebellum, however this finding was less expected and also deserves further investigation. Interestingly, the global functional connectivity of the cerebellum with all other brain regions was recently found to be decreased in women compared to men (Ingalhalikar et al., 2014), which could signify enhanced intra-regional processing.
4.3. Age by Sex Interactions in Perception-related Oscillatory Responses
Aging affects the sexes differently, and our observed interactions between age and sex in the neural coding of visuospatial processing reveal new insights into fundamental aspects of human brain function. For example, we found an interaction between age and sex in the strength of theta oscillations in the left mPFC, such that women exhibited a positive relationship between theta mPFC activity and age, while men exhibited a negative relationship. This region has been found to be a critical cortical generator of the frontal midline theta signal (Asada et al., 1999; Ishii et al., 1999), which is thought to be central to response choice during cognitive tasks, as well as in error monitoring and learning (Cavanagh and Shackman, 2015; Luu et al., 2004). In addition, men’s performance decreased more rapidly with age than women’s, and thus this interaction might represent more efficient error monitoring along the aging spectrum for women relative to men. An interaction between age and sex was also observed in the strength of theta and alpha oscillations in the right middle and superior temporal cortices, respectively, which was more surprising. Interestingly, gray matter thinning with age has been found to be exacerbated in these regions (Allen et al., 2005; Fjell et al., 2009), as well as an accompanying reduction in glucose metabolism (Kalpouzos et al., 2009). It is possible that this thinning affects cognitive function for men and women differentially across the aging spectrum, and further investigation into this possibility is warranted. Another interaction cluster observed in the alpha range likely represented a very different cognitive phenomenon. Briefly, alpha desynchronizations in posterior cortices are widely accepted as being indicative of stimulus-related cortical dis-inhibition, and are strongly tied to visual perception (Başar et al., 2001; Handel et al., 2011; van Dijk et al., 2008). Once again, it is important to note that the oscillatory response of interest in the alpha band was a desynchronization, and so a negative relationship actually implies an increasing neural response with age. Therefore, the age-by-sex interaction we found in the occipito-temporal cortex likely represents an enhanced dis-inhibition in “bottom-up” visuospatial pathways in men, compared to women, as age increases. Future studies should evaluate whether there are clear sex differences in the brain’s method for compensating across the aging spectrum, as our current data suggests that these distinct oscillatory changes are probably both beneficial (i.e., not maladaptive). Finally, an interaction between age and sex was found for gamma oscillations in the right dlPFC. Gamma activity in the right dlPFC is associated with higher-level cognitive control and stimulus selection (Benchenane et al., 2011), and so the interaction found over this region implies a differential relationship between age and executive function for men compared to women. Like the theta findings, this enhanced gamma with age is probably beneficial and could be a compensatory mechanism for supplementing age-related performance declines in other brain regions. Supporting this interpretation, the strength of gamma oscillations in this region predicted both higher accuracy and lower reaction time (both indicating better performance) on the task.
En masse, these findings provide new insight into the differential mechanisms by which the brain processes visuospatial information as a function of age and sex. Many of the spatially- and spectrally-specific neural responses identified in these analyses are well-supported components of visual attention and perception networks, and our findings suggest that the integrity of these responses is dynamically modulated by chronological age, biological sex, and interactions between the two. By better understanding differences in the apparatus of visuospatial coding in the human brain due to aging and sex, we can better grasp the nuances of brain and cognitive aging, and human brain function in general.
Supplementary Material
5. Acknowledgements
This research was supported by grants R01-MH103220 and R01-MH116782 (TWW) and F31-AG055332 (AIW) from the National Institutes of Health, grant #1539067 from the National Science Foundation (TWW), and by a Research Support Fund grant from the Nebraska Health System and the University of Nebraska Medical Center. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
6. References:
- Alichniewicz KK, Brunner F, Klünemann HH, Greenlee MW, 2012. Structural and functional neural correlates of visuospatial information processing in normal aging and amnestic mild cognitive impairment. Neurobiol Aging 33, 2782–2797. [DOI] [PubMed] [Google Scholar]
- Allen JS, Bruss J, Brown CK, Damasio H, 2005. Normal neuroanatomical variation due to age: the major lobes and a parcellation of the temporal region. Neurobiol Aging 26, 1245–1260; discussion 12791282. [DOI] [PubMed] [Google Scholar]
- Asada H, Fukuda Y, Tsunoda S, Yamaguchi M, Tonoike M, 1999. Frontal midline theta rhythms reflect alternative activation of prefrontal cortex and anterior cingulate cortex in humans. Neurosci Lett 274, 29–32. [DOI] [PubMed] [Google Scholar]
- Baccini M, Paci M, Del Colletto M, Ravenni M, Baldassi S, 2014. The assessment of subjective visual vertical: comparison of two psychophysical paradigms and age-related performance. Atten Percept Psychophys 76, 112–122. [DOI] [PubMed] [Google Scholar]
- Başar E, Basar-Eroglu C, Karakas S, Schurmann M, 2001. Gamma, alpha, delta, and theta oscillations govern cognitive processes. Int J Psychophysiol 39, 241–248. [DOI] [PubMed] [Google Scholar]
- Benchenane K, Tiesinga PH, Battaglia FP, 2011. Oscillations in the prefrontal cortex: a gateway to memory and attention. Curr Opin Neurobiol 21, 475–485. [DOI] [PubMed] [Google Scholar]
- Bigelow RT, Semenov YR, Trevino C, Ferrucci L, Resnick SM, Simonsick EM, Xue QL, Agrawal Y, 2015. Association Between Visuospatial Ability and Vestibular Function in the Baltimore Longitudinal Study of Aging. J Am Geriatr Soc 63, 1837–1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisley JW, Goldberg ME, 2010. Attention, intention, and priority in the parietal lobe. Annu Rev Neurosci 33, 1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busch NA, Debener S, Kranczioch C, Engel AK, Herrmann CS, 2004. Size matters: effects of stimulus size, duration and eccentricity on the visual gamma-band response. Clin Neurophysiol 115, 1810–1820. [DOI] [PubMed] [Google Scholar]
- Busch NA, Dubois J, VanRullen R, 2009. The phase of ongoing EEG oscillations predicts visual perception. J Neurosci 29, 7869–7876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanagh JF, Shackman AJ, 2015. Frontal midline theta reflects anxiety and cognitive control: metaanalytic evidence. J Physiol Paris 109, 3–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao LL, Knight RT, 1997. Prefrontal deficits in attention and inhibitory control with aging. Cereb Cortex 7, 63–69. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL, 2002. Control of goal-directed and stimulus-driven attention in the brain. Nat Rev Neurosci 3, 201–215. [DOI] [PubMed] [Google Scholar]
- Cosgrove KP, Mazure CM, Staley JK, 2007. Evolving knowledge of sex differences in brain structure, function, and chemistry. Biol Psychiatry 62, 847–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crimmins EM, Kim JK, Langa KM, Weir DR, 2011. Assessment of cognition using surveys and neuropsychological assessment: the Health and Retirement Study and the Aging, Demographics, and Memory Study. J Gerontol B Psychol Sci Soc Sci 66 Suppl 1, i162–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doesburg SM, Roggeveen AB, Kitajo K, Ward LM, 2008. Large-scale gamma-band phase synchronization and selective attention. Cereb Cortex 18, 386–396. [DOI] [PubMed] [Google Scholar]
- Edden RA, Muthukumaraswamy SD, Freeman TC, Singh KD, 2009. Orientation discrimination performance is predicted by GABA concentration and gamma oscillation frequency in human primary visual cortex. J Neurosci 29, 15721–15726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst MD, 2004. Permutation methods: a basis for exact inference. Statistical Science 19, 676–685. [Google Scholar]
- Fellrath J, Mottaz A, Schnider A, Guggisberg AG, Ptak R, 2016. Theta-band functional connectivity in the dorsal fronto-parietal network predicts goal-directed attention. Neuropsychologia 92, 20–30. [DOI] [PubMed] [Google Scholar]
- Fjell AM, Westlye LT, Amlien I, Espeseth T, Reinvang I, Raz N, Agartz I, Salat DH, Greve DN, Fischl B, Dale AM, Walhovd KB, 2009. High consistency of regional cortical thinning in aging across multiple samples. Cereb Cortex 19, 2001–2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaetz W, Edgar JC, Wang DJ, Roberts TP, 2011. Relating MEG measured motor cortical oscillations to resting γ-aminobutyric acid (GABA) concentration. Neuroimage 55, 616–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao F, Edden RA, Li M, Puts NA, Wang G, Liu C, Zhao B, Wang H, Bai X, Zhao C, Wang X, Barker PB, 2013. Edited magnetic resonance spectroscopy detects an age-related decline in brain GABA levels. Neuroimage 78, 75–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geerligs L, Saliasi E, Maurits NM, Renken RJ, Lorist MM, 2014. Brain mechanisms underlying the effects of aging on different aspects of selective attention. Neuroimage 91, 52–62. [DOI] [PubMed] [Google Scholar]
- Gong G, He Y, Evans AC, 2011. Brain connectivity: gender makes a difference. Neuroscientist 17, 575–591. [DOI] [PubMed] [Google Scholar]
- Gross J, Kujala J, Hamalainen M, Timmermann L, Schnitzler A, Salmelin R, 2001. Dynamic imaging of coherent sources: Studying neural interactions in the human brain. Proc Natl Acad Sci U S A 98, 694699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handel BF, Haarmeier T, Jensen O, 2011. Alpha oscillations correlate with the successful inhibition of unattended stimuli. J Cogn Neurosci 23, 2494–2502. [DOI] [PubMed] [Google Scholar]
- He X, Koo BB, Killiany RJ, 2016. Edited Magnetic Resonance Spectroscopy Detects an Age-Related Decline in Nonhuman Primate Brain GABA Levels. Biomed Res Int 2016, 6523909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrichs-Graham E, McDermott TJ, Mills MS, Wiesman AI, Wang YP, Stephen JM, Calhoun VD, Wilson TW, 2018. The lifespan trajectory of neural oscillatory activity in the motor system. Dev Cogn Neurosci 30, 159–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrichs-Graham E, Wilson TW, 2016. Is an absolute level of cortical beta suppression required for proper movement? Magnetoencephalographic evidence from healthy aging. Neuroimage 134, 514–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvath S, Gurven M, Levine ME, Trumble BC, Kaplan H, Allayee H, Ritz BR, Chen B, Lu AT, Rickabaugh TM, Jamieson BD, Sun D, Li S, Chen W, Quintana-Murci L, Fagny M, Kobor MS, Tsao PS, Reiner AP, Edlefsen KL, Absher D, Assimes TL, 2016. An epigenetic clock analysis of race/ethnicity, sex, and coronary heart disease. Genome Biol 17, 171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua T, Kao C, Sun Q, Li X, Zhou Y, 2008. Decreased proportion of GABA neurons accompanies agerelated degradation of neuronal function in cat striate cortex. Brain Res Bull 75, 119–125. [DOI] [PubMed] [Google Scholar]
- Iachini I, Iavarone A, Senese VP, Ruotolo F, Ruggiero G, 2009. Visuospatial memory in healthy elderly, AD and MCI: a review. Curr Aging Sci 2, 43–59. [DOI] [PubMed] [Google Scholar]
- Ingalhalikar M, Smith A, Parker D, Satterthwaite TD, Elliott MA, Ruparel K, Hakonarson H, Gur RE, Gur RC, Verma R, 2014. Sex differences in the structural connectome of the human brain. Proc Natl Acad Sci U S A 111, 823–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii R, Shinosaki K, Ukai S, Inouye T, Ishihara T, Yoshimine T, Hirabuki N, Asada H, Kihara T, Robinson SE, Takeda M, 1999. Medial prefrontal cortex generates frontal midline theta rhythm. Neuroreport 10, 675–679. [DOI] [PubMed] [Google Scholar]
- Jenkins L, Myerson J, Joerding JA, Hale S, 2000. Converging evidence that visuospatial cognition is more age-sensitive than verbal cognition. Psychol Aging 15, 157–175. [DOI] [PubMed] [Google Scholar]
- Jensen O, Gips B, Bergmann TO, Bonnefond M, 2014. Temporal coding organized by coupled alpha and gamma oscillations prioritize visual processing. Trends Neurosci 37, 357–369. [DOI] [PubMed] [Google Scholar]
- Jensen O, Kaiser J, Lachaux JP, 2007. Human gamma-frequency oscillations associated with attention and memory. Trends Neurosci 30, 317–324. [DOI] [PubMed] [Google Scholar]
- Jensen O, Tesche CD, 2002. Frontal theta activity in humans increases with memory load in a working memory task. Eur J Neurosci 15, 1395–1399. [DOI] [PubMed] [Google Scholar]
- Kalpouzos G, Chételat G, Baron JC, Landeau B, Mevel K, Godeau C, Barré L, Constans JM, Viader F, Eustache F, Desgranges B, 2009. Voxel-based mapping of brain gray matter volume and glucose metabolism profiles in normal aging. Neurobiol Aging 30, 112–124. [DOI] [PubMed] [Google Scholar]
- Kim HJ, Park HK, Park JR, Choi MH, Lee HW, Chung SC, 2008. Effects of aging on visuospatial performance and cerebral activation and lateralization: an FMRI study. Int J Neurosci 118, 781–791. [DOI] [PubMed] [Google Scholar]
- Lachaux JP, Fonlupt P, Kahane P, Minotti L, Hoffmann D, Bertrand O, Baciu M, 2007. Relationship between task-related gamma oscillations and BOLD signal: new insights from combined fMRI and intracranial EEG. Hum Brain Mapp 28, 1368–1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landau AN, Fries P, 2012. Attention samples stimuli rhythmically. Curr Biol 22, 1000–1004. [DOI] [PubMed] [Google Scholar]
- Landau AN, Schreyer HM, van Pelt S, Fries P, 2015. Distributed Attention Is Implemented through Theta-Rhythmic Gamma Modulation. Curr Biol 25, 2332–2337. [DOI] [PubMed] [Google Scholar]
- Leventhal AG, Wang Y, Pu M, Zhou Y, Ma Y, 2003. GABA and its agonists improved visual cortical function in senescent monkeys. Science 300, 812–815. [DOI] [PubMed] [Google Scholar]
- Lisman JE, Jensen O, 2013. The θ-γ neural code. Neuron 77, 1002–1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logothetis NK, Pauls J, Augath M, Trinath T, Oeltermann A, 2001. Neurophysiological investigation of the basis of the fMRI signal. Nature 412, 150–157. [DOI] [PubMed] [Google Scholar]
- Luu P, Tucker DM, Makeig S, 2004. Frontal midline theta and the error-related negativity: neurophysiological mechanisms of action regulation. Clin Neurophysiol 115, 1821–1835. [DOI] [PubMed] [Google Scholar]
- Madden DJ, Spaniol J, Whiting WL, Bucur B, Provenzale JM, Cabeza R, White LE, Huettel SA, 2007. Adult age differences in the functional neuroanatomy of visual attention: a combined fMRI and DTI study. Neurobiol Aging 28, 459–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madden DJ, Whiting WL, Provenzale JM, Huettel SA, 2004. Age-related changes in neural activity during visual target detection measured by fMRI. Cereb Cortex 14, 143–155. [DOI] [PubMed] [Google Scholar]
- Maris E, Oostenveld R, 2007. Nonparametric statistical testing of EEG- and MEG-data. J Neurosci Methods 164, 177–190. [DOI] [PubMed] [Google Scholar]
- Milham MP, Erickson KI, Banich MT, Kramer AF, Webb A, Wszalek T, Cohen NJ, 2002. Attentional control in the aging brain: insights from an fMRI study of the stroop task. Brain Cogn 49, 277296. [DOI] [PubMed] [Google Scholar]
- Moffat SD, Elkins W, Resnick SM, 2006. Age differences in the neural systems supporting human allocentric spatial navigation. Neurobiol Aging 27, 965–972. [DOI] [PubMed] [Google Scholar]
- Moffat SD, Zonderman AB, Resnick SM, 2001. Age differences in spatial memory in a virtual environment navigation task. Neurobiol Aging 22, 787–796. [DOI] [PubMed] [Google Scholar]
- Muthukumaraswamy SD, Edden RA, Jones DK, Swettenham JB, Singh KD, 2009. Resting GABA concentration predicts peak gamma frequency and fMRI amplitude in response to visual stimulation in humans. Proc Natl Acad Sci U S A 106, 8356–8361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthukumaraswamy SD, Singh KD, 2013. Visual gamma oscillations: the effects of stimulus type, visual field coverage and stimulus motion on MEG and EEG recordings. Neuroimage 69, 223–230. [DOI] [PubMed] [Google Scholar]
- Niessing J, Ebisch B, Schmidt KE, Niessing M, Singer W, Galuske RA, 2005. Hemodynamic signals correlate tightly with synchronized gamma oscillations. Science 309, 948–951. [DOI] [PubMed] [Google Scholar]
- Oliviero A, Profice P, Tonali PA, Pilato F, Saturno E, Dileone M, Ranieri F, Di Lazzaro V, 2006Effects of aging on motor cortex excitability. Neurosci Res 55, 74–77. [DOI] [PubMed] [Google Scholar]
- Plude DJ, Doussard-Roosevelt JA, 1989. Aging, selective attention, and feature integration. Psychol Aging 4, 98–105. [DOI] [PubMed] [Google Scholar]
- Rossiter HE, Davis EM, Clark EV, Boudrias MH, Ward NS, 2014. Beta oscillations reflect changes in motor cortex inhibition in healthy ageing. Neuroimage 91, 360–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacher J, Neumann J, Okon-Singer H, Gotowiec S, Villringer A, 2013. Sexual dimorphism in the human brain: evidence from neuroimaging. Magn Reson Imaging 31, 366–375. [DOI] [PubMed] [Google Scholar]
- Scheinost D, Finn ES, Tokoglu F, Shen X, Papademetris X, Hampson M, Constable RT, 2015. Sex differences in normal age trajectories of functional brain networks. Hum Brain Mapp 36, 1524–1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidler RD, Bernard JA, Burutolu TB, Fling BW, Gordon MT, Gwin JT, Kwak Y, Lipps DB, 2010. Motor control and aging: links to age-related brain structural, functional, and biochemical effects. Neurosci Biobehav Rev 34, 721–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpkin AJ, Hemani G, Suderman M, Gaunt TR, Lyttleton O, Mcardle WL, Ring SM, Sharp GC, Tilling K, Horvath S, Kunze S, Peters A, Waldenberger M, Ward-Caviness C, Nohr EA, Sørensen TI, Relton CL, Smith GD, 2016. Prenatal and early life influences on epigenetic age in children: a study of mother-offspring pairs from two cohort studies. Hum Mol Genet 25, 191–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith CD, Umberger GH, Manning EL, Slevin JT, Wekstein DR, Schmitt FA, Markesbery WR, Zhang Z, Gerhardt GA, Kryscio RJ, Gash DM, 1999. Critical decline in fine motor hand movements in human aging. Neurology 53, 1458–1461. [DOI] [PubMed] [Google Scholar]
- Spaak E, de Lange FP, Jensen O, 2014. Local entrainment of alpha oscillations by visual stimuli causes cyclic modulation of perception. J Neurosci 34, 3536–3544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stankov L, 1988. Aging, attention, and intelligence. Psychol Aging 3, 59–74. [DOI] [PubMed] [Google Scholar]
- Tallon-Baudry C, Bertrand O, Henaff MA, Isnard J, Fischer C, 2005. Attention modulates gammaband oscillations differently in the human lateral occipital cortex and fusiform gyrus. Cereb Cortex 15, 654–662. [DOI] [PubMed] [Google Scholar]
- Taulu S, Simola J, 2006. Spatiotemporal signal space separation method for rejecting nearby interference in MEG measurements. Phys Med Biol 51, 1759–1768. [DOI] [PubMed] [Google Scholar]
- Townsend J, Adamo M, Haist F, 2006. Changing channels: an fMRI study of aging and cross-modal attention shifts. Neuroimage 31, 1682–1692. [DOI] [PubMed] [Google Scholar]
- Uhlhaas PJ, Pipa G, Neuenschwander S, Wibral M, Singer W, 2011. A new look at gamma? High- (>60 Hz) γ-band activity in cortical networks: function, mechanisms and impairment. Prog Biophys Mol Biol 105, 14–28. [DOI] [PubMed] [Google Scholar]
- Uusitalo MA, Ilmoniemi RJ, 1997. Signal-space projection method for separating MEG or EEG into components. Med Biol Eng Comput 35, 135–140. [DOI] [PubMed] [Google Scholar]
- van Dijk H, Schoffelen JM, Oostenveld R, Jensen O, 2008. Prestimulus oscillatory activity in the alpha band predicts visual discrimination ability. J Neurosci 28, 1816–1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verbruggen F, Aron AR, Stevens MA, Chambers CD, 2010. Theta burst stimulation dissociates attention and action updating in human inferior frontal cortex. Proc Natl Acad Sci U S A 107, 1396613971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhaeghen P, Cerella J, 2002. Aging, executive control, and attention: a review of meta-analyses. Neurosci Biobehav Rev 26, 849–857. [DOI] [PubMed] [Google Scholar]
- Vidal JR, Chaumon M, O’Regan JK, Tallon-Baudry C, 2006. Visual grouping and the focusing of attention induce gamma-band oscillations at different frequencies in human magnetoencephalogram signals. J Cogn Neurosci 18, 1850–1862. [DOI] [PubMed] [Google Scholar]
- Wiesman AI, Heinrichs-Graham E, Proskovec AL, McDermott TJ, Wilson TW, 2017. Oscillations during observations: Dynamic oscillatory networks serving visuospatial attention. Hum Brain Mapp 38, 5128–5140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiesman AI, O’Neill J, Mills MS, Robertson KR, Fox HS, Swindells S, Wilson TW, 2018. Aberrant occipital dynamics differentiate HIV-infected patients with and without cognitive impairment. Brain. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.