Abstract
‘Black Esophagus’ or acute esophageal syndrome (AEN) is extremely rare entity characterized by patchy or diffuse circumferential necrosis of esophagus. To date, there is minimal number of cases described with this phenomenon. The main objective of this case series is to discuss the diagnosis of extremely rare condition seen in two patients within 1 year and its effective management by adopting conservative measures in a tertiary hospital. The objective of our case series is to discuss the diagnosis of an extremely rare condition seen in two patients within a short span of 6 months and its effective management by adopting conservative measures in a tertiary care hospital. An overall conservative approach, serial endoscopic monitoring and effective follow-up resulted in zero mortality with successful outcomes.
INTRODUCTION
Acute esophageal syndrome (AEN) also called ‘Black Esophagus’ is rare clinical entity with 113 cases reported so far [1]. It is patchy or diffuse circumferential necrosis of intrathoracic esophagus of varying length [2]. The circumferential black/ischemic appearance gives the name ‘Black esophagus’. The reported incidence is 0.01–0.28% in various studies [3]. This clinical entity is classically more common in males with 4:1 ratio [4] and in sixth decade of life with an average age of 67 years [5].
Aim
The objective is to report two interesting patients diagnosed within a period of 6 months at a tertiary-care hospital. Their similar presentation, age group and management resulted in successful early and long-term outcomes.
CASE 1
A 78-year-old male presented with 3 days history of dull, intermittent chest pain associated with vomiting. He had no tachypnea, abdominal pain, hematemesis or melena. His past medical history included recent laparoscopic anterior resection and Hiatus hernia.
His hemodynamics was stable with no abnormal physical findings. Laboratory tests showed elevated white cell count. Biochemical studies, troponin levels and ECG were normal.
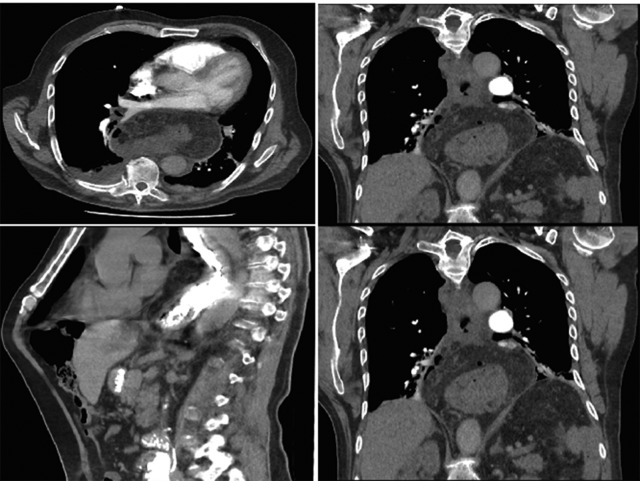
Computer tomography pulmonary angiogram (CTPA) done given recent surgery reported distal esophagus perforation with bibasal pleural effusion. No pulmonary embolism (Fig. 1).
Figure 1:
Hiatus hernia with associated mural thickening of esophagus and locules of gas showing localized perforation.
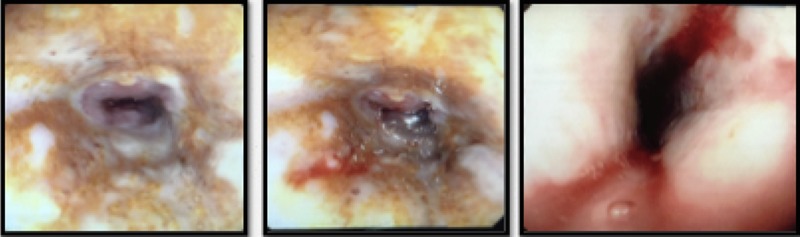
He was kept nil orally. Analgesia, antiemetics, IV proton pump inhibitor, and empirical IV antibiotics were commenced. Esophagastroscopy showed large hiatus hernia with reflux esophagitis. Middle-lower third esophagus appeared patchy, gangrenous from 27 cm to gastroesophageal-junction at 35 cm (Fig. 2). Multiple biopsies for viral culture and microbiology showed chronically inflamed cell infiltrates consistent with mucosal ischemia. No viral inclusions are seen. At the end of procedure, a PEG with jejunal extension tube was inserted to commence feeds.
Figure 2:
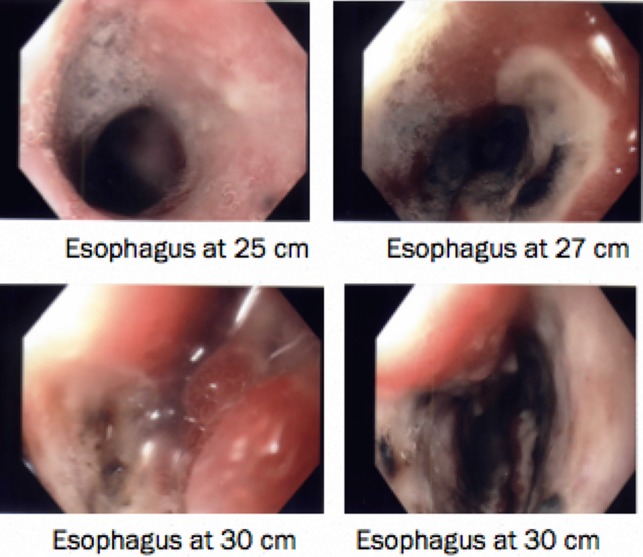
Patchy gangrenous esophagus from 24–30 cm.
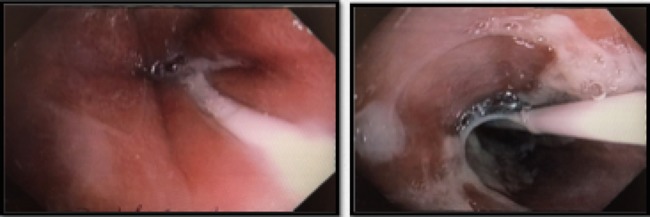
Interval gastroscopy in 2 weeks showed healing mucosal ischemia with slough from 27–30 cm (Fig. 3). Clear fluids were commenced and gradually upgraded to normal diet. Follow-up CT showed no contrast extravasation within posterior mediastinum (Fig. 4) and gastroscopy in 8 weeks showed healed esophagus.
Figure 3:

Healing mucosal ischemia from upper, mid and distal esophagus.
Figure 4:
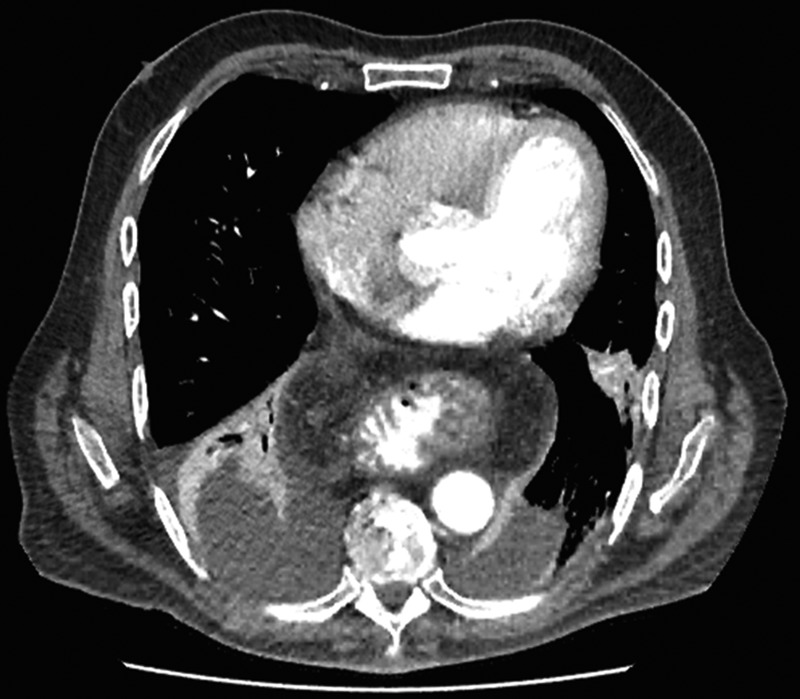
CT scan at 2 weeks showing no contrast extravasation in posterior mediastinum.
CASE 2
An 82-year-old male presented with intermittent epigastric pain, nausea, anorexia and vomiting for 2 days. He last opened bowels or passed flatus 2 days ago. No History of shortness of breath, coffee-ground vomit or melena. His past history included ischemic heart disease and diverticular disease.
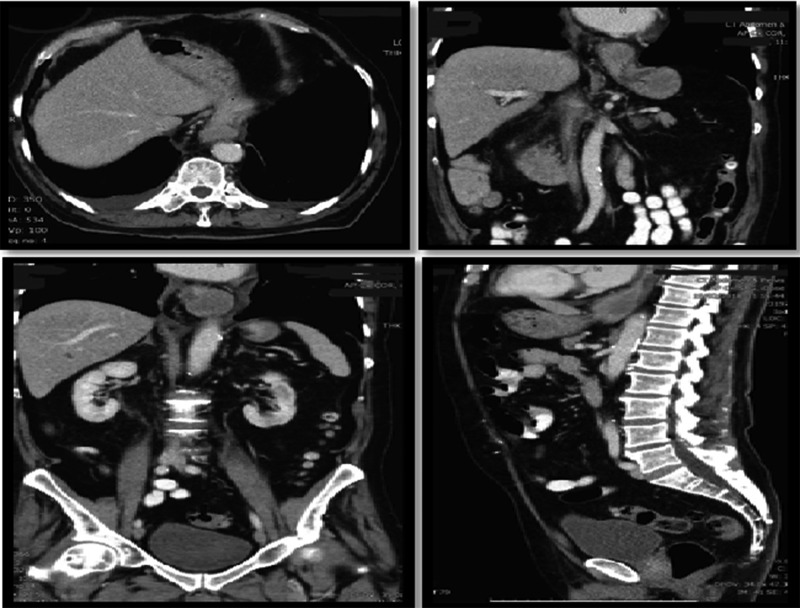
On assessment, he was hypertensive, normal heart rate and temperatures. Abdominal exam was unremarkable. Laboratory tests were all within normal range. CT Abdomen showed distal esophageal mural thickening suggestive of esophagitis in small hiatus hernia with bilateral pleural effusions (Fig. 5).
Figure 5:
Hiatus hernia with associated mural thickening of esophagus.
He was commenced on high-dose PPI, IV Antibiotics and analgesia. Emergency gastroscopy showed middle third of esophagus consistent with esophagitis and circumferential ischemia/necrosis in distal esophagus 27–34 cm (Fig. 6). Mucosal biopsies taken revealed ulcerated squamocolumnar mucosa with necrosis and inflammation. No CMV inclusions were identified.
Figure 6:
Circumferential ischemia/necrosis in distal esophagus.
Nasojejunostomy tube was inserted and feeds were commenced. Interval gastroscopy in 2weeks revealed superficial sloughing of esophagus from 27 to 34 cm consistent with partial thickness esophageal necrosis (Fig. 7). Clear fluids were commenced and gradually upgraded to full diet over the course of 6 weeks. Follow-up gastroscopy at 9 weeks showed completely healed mucosa (Fig. 8).
FIGURE 7:
Interval endoscopy at 2 weeks showing signs of healing (Superficial slough at the area of partial necrosis).
Figure 8:
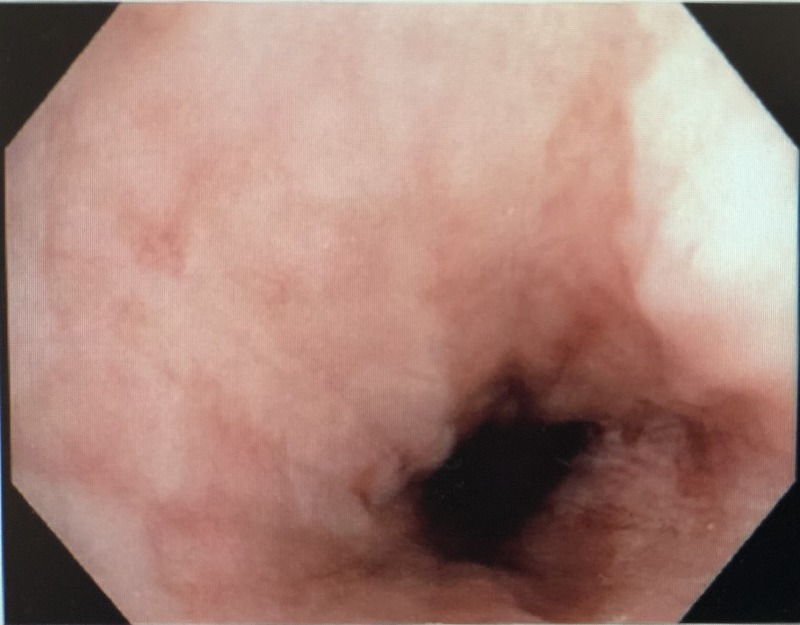
Normal esophageal mucosa at 8 weeks.
DISCUSSION
First described by Goldenburg et al. in 1990 [6], Gurvits classified AEN as a distinct syndrome in 2007. Disease pathophysiology is unclear but it involves ischemic insult to the esophagus causing circumferential necrotizing oesophagitis or stroke.
AEN has multifactorial etiology resulting from impaired defense barriers and tissue hypoperfusion resulting in gastric content’s influx causing mucosal ischemia [7]. Predisposing factors such as diabetes, atherosclerosis, volvulus, shock, irradiation, trauma, and infections such as cytomegalovirus, Candida-albicans and Klebsiella-pneumoniae [8] have also been linked.
Seventy percent of the presentations include hematemesis, coffee-ground emesis and melena. Radiological modalities partially aid in demonstrating pathology but diagnosis is made endoscopically [9] and later confirmed histologically.
Treatment is restoring hemodynamics, gastric acid suppression, restricting oral intake, and intravenous antibiotics. Surgical esophagectomy has been described in cases of AEN with full thickness perforation with a hiatus hernia [10].
Interestingly, both our patients were older than 75 with hiatus hernia. Diagnosis was clinically challenging as none of them had classical presentation of hematemesis/melena.
Our success story is paramount as we had excellent outcomes with conservative measures. Patient’s clinical and hemodynamic stability and close surveillance with serial endoscopies reassured that esophageal resection, stent or mediastinal drainage was not required. Fifty percent of the cases in literature observing signs of mucosal healing provide reassurance that oral intake can be resumed.
Prognosis of AEN is poor with one in three patients dying (38%). Patients that survive AEN can have stenosis or strictures seen in >10% of patients. Both our patients have had 3 and 6 monthly follow-ups and to date neither of them exhibit any complications.
CONFLICT OF INTEREST STATEMENT
None declared.
REFERENCES
- 1. Day A, Sayegh M. Acute oesophageal necrosis: a case report and review of the literature. Int J Surg 2010;8:6–14. [DOI] [PubMed] [Google Scholar]
- 2. Worrell SG, Oh DS, Greene CL, DeMeester SR, Hagen JA. Acute esophageal necrosis: a case series and long-term follow-up. Ann Thorac Surg 2014;98:341–2. [DOI] [PubMed] [Google Scholar]
- 3. Lacy BE, Toor A, Bensen SP, Rothstein RI, Maheshwari Y. Acute esophageal necrosis: report of two cases and a review of the literature. Gastrointest Endosc 1999;49:527–32. [DOI] [PubMed] [Google Scholar]
- 4. Worrell SG, Oh DS, Greene CL, DeMeester SR, Hagen JA. Acute esophageal necrosis: a case series and long-term follow-up. Ann Thorac Surg 2014;98:341–2. [PubMed: 24996722]. [DOI] [PubMed] [Google Scholar]
- 5. Gurvits GE. Black esophagus: acute esophageal necrosis syndrome. World J Gastroenterol 2010;16:3219–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Goldenberg SP, Wain SL, Marignani P. Acute necrotizing esophagitis. Gastroenterology 1990;98:493–6. [DOI] [PubMed] [Google Scholar]
- 7. Gurvits GE, Shapsis A, Lau N, Gualtieri N, Robilotti JG. Acute esophageal necrosis: rare syndrome. J Gastroenterol 2007;42:29–38. [DOI] [PubMed] [Google Scholar]
- 8. Liu YH, Lin YS, Chen HJ, Tu CY, Chen W. Klebsiella pneumoniae deep neck infection with acute necrotizing esophagitis. South Med J 2009;102:219. [DOI] [PubMed] [Google Scholar]
- 9. Gurvits GE. Black esophagus: new insights and multicenter international experience in 2014. Dig Dis Sci 2015;60:444–53. [DOI] [PubMed] [Google Scholar]
- 10. Kram M, Gorenstein L, Eisen D, Cohen D. Acute esophageal necrosis associated with gastric volvulus. Gastrointest Endosc 2000;51:610–2. [DOI] [PubMed] [Google Scholar]