Abstract
BACKGROUND:
Tuberculosis (TB) is one of the most common infectious diseases found in developing countries. One of the risk factors for TB is diabetes, a chronic metabolic disorder characterised by hyperglycemia. The altered in glucose metabolism will cause dysfunction of phagocyte and antibacterial that furthermore impaired activation of natural killer cells, dendritic cells. These together will alter the balance of T-cell immunity. Under hyperglycemic conditions, AGEs (advanced glycation end products) was increasingly formed and was believed to play a role in cell dysfunctions and diabetic complications. The CD4 deficiency will alter the immunity status in diabetes and TB with co-morbidity diabetes.
AIM:
This aim of this study was to evaluate CD4, and it’s relevant to Advanced Glycation End Products (AGEs) in TB with co-morbidity diabetes.
METHODS:
This is a case-control study with a total of 80 patients (40 diabetes and 40 TB with co-morbidity diabetes were recruited from Murni Teguh memorial Hospital Medan after ethical approval from Health Research Ethical Committee. The CD4, AGEs, Blood glucose and HbA1C were measured.
RESULTS:
There was no statistical difference of CD4, HbA1C and blood glucose within diabetes and TB with co-morbidity diabetes but BMI (p = 0.009) and AGEs (p = 0.001) did. The CD4 below 500 were seen in 15% diabetes and 25% in TB with co-morbidity diabetes but did not show statistical significance difference (p = 0.07). No correlation was found between CD4 and AGEs in TB with co-morbidity diabetes (p = 0.44).
CONCLUSION:
The CD4 was not correlated significantly with AGEs.
Keywords: Diabetes, TBs, CD4, AGEs
Introduction
Tuberculosis (TB) is one of the most common infectious diseases found in developing countries, in Indonesia the prevalence remains very high despite the decrease [1]. Study about TB including the risk factors, multidrug-resistant play an important part in the prevention and treatment of the disease. One of the risk factors of TB is diabetes, a chronic metabolic disorder characterised by hyperglycemia resulting from a defect in insulin secretion or resistance [2]. Diabetes Mellitus (DM) is considered one of the largest non-infectious health problems in the world; it is estimated there will be 380 million of people with diabetes in 2025 with the most in developing countries [3]. The chronic state of low-grade inflammation due to increased formation of advanced glycation end products, activation of proinflammatory mediators, and the increase of oxidative stress will impair the phagocytic and antibacterial activity of neutrophils and macrophages, that can further impaired activation of natural killer cells, dendritic cells, that can impair T Cell-mediated immune responses, to promote the establishment of acute intracellular bacterial infections in diabetic patients. The increased incidence of intracellular bacterial infections is one of many diabetic’s complications, one of the most common bacterial infection is TB [4] [5] [6] [7].
TBs is one of the most significant causes of death from intracellular bacterial infection especially in developing countries [8], data from a recent prospective study showed that diabetic patients have a threefold higher risk of developing TB, and at least 10-35% of patients with TB have co-morbidity diabetes [9].
The altered in host immune systems will increase the mortality of patients with TB and co-morbidity diabetes and are more likely to have cavitary lung lesions, increased relapsed rate and most likely to develop multidrug resistance TB [10] [11]. Also, the treatment of TB with Rifampicin, one of the major TB drug will increase the metabolism of oral antidiabetic drugs thus may complicate the glycemic control [12] [13] [14].
The altered in glucose metabolism will cause dysfunction of phagocyte and antibacterial that furthermore impaired activation of natural killer cells, dendritic cells. These together will alter the balance of T-cell immunity [15]. Under hyperglycemic conditions, AGEs (Advanced Glycation End Products) a group of heterogeneous compounds were increasingly formed and was believed to play a role in cell dysfunctions and diabetic complications [16]. Recent studies suggested that CD4 T cells are associated with control of chronic bacterial and viral infections [17] [18] [19], this also has been reported to play an important role in protective immunity against TB [20] [21]. The altered in the immune system and CD4 deficiency will alter the immunity status that leads to death in diabetes and TBs with co-morbidity diabetes [22].
This study aimed to evaluate CD4 and its relevant with AGEs in TB patients with co-morbidity diabetes.
Material and Methods
This is a case-control study to evaluate CD4 and its relevant with Advanced Glycation End Products (AGEs) in TBs patients with co-morbidity diabetes. The study received ethical approval from the Health Research Ethical Committee Medical Faculty Universitas Sumatera Utara/H.Adam Malik General Hospital Medan Indonesia (No.444/TGL/KEPK FK USU-RSUP HAM/2017). Only patients diagnosed with diabetes and who gave signed informed consent were admitted to the study. Patients were recruited between March 2017 and September 2017 from Murni Teguh Memorial Hospitals in Medan, Indonesia. A total of 80 patients (40 diabetes and 40 TB with co-morbidity diabetes were recruited and was admitted into the study. Their age ranged between 36 years and 86 years (mean 60.0 ± 10.0 years) with the Body mass index ranged between 16.4 and 32.1 (mean 23.8 ± 3.0). Their characteristics are shown in Table 1.
Table 1.
Characteristic of the samples
| Group | Mean Age (range) years | Mean BMI (range) | Mean Blood Glucose (range) mg/dl | Mean HbA1C (range)% | Mean CD4 (range) | Mean AGEs (range) μg/ml |
|---|---|---|---|---|---|---|
| Diabetes | 60 (44-73) | 24.6 (19.9-32.1) | 223 (85-568) | 7.8 (5-12) | 780 (198-1347) | 7.4 (1.24-53.32) |
| TB +diabetes | 60 (36-86) | 23 (16.4 -31.1) | 214 (90-351) | 8.8 (4-15) | 687 (142-1800) | 4.2 (1.28-21.5) |
Blood sampling was performed from a clean venepuncture using the Vacutainer system (Beckton Dickenson, New Jersey, USA). About 6 mL of blood was collected into EDTA and heparin tubes. Both tubes spun for 15 min at 2000g within an hour of blood collection. Plasma EDTA and heparin samples were aliquoted and kept at -70°C until assayed.
The following assays were performed: blood glucose, HbA1C, CD4, and AGEs was performed at the Integrated Laboratory Medical faculty University Sumatera Utara, Medan Indonesia. Blood glucose was measured with spectrophotometry, HbA1C with HPLC method, CD4 with flow cytometry, and AGEs with ELISA method.
Body mass index was determined by using the BMI calculator with body weight and height.
The Statistical Package for Social Sciences (SPSS22; SPSS Inc, Chicago, Il, USA) was used to perform the statistical analysis. Descriptive statistic and Non-parametric Mann Whitney U test were performed to evaluate the blood glucose, HbA1C, CD4, BMI and AGEs level in diabetes and TBs patients with co-morbidity diabetes. Spearman’s Rho correlation analysis was used to determine the correlation between CD4 against AGEs seen in diabetes and TB patients with co-morbidity diabetes. A P value of less than 0.05 was considered statistically significant.
Results
In total, 80 patients were studied, (40 diabetes patients, 40 TB with co-morbidity diabetes). Their characteristics and Laboratory assays are shown in table 1. The parameter statistic was shown in table 2. The Box plots of CD4 and AGEs levels between diabetes and TB with co-morbidity diabetes are shown in Figure 1 and 2.
Table 2.
Statistic of parameters studied in the diabetic group compared to those in TBs with comorbid diabetic
| Diabetic | TB +diabetic | p | |
|---|---|---|---|
| Blood glucose mean (SD) mg/dl | 223.4 ± 117.4 | 214.1 ± 78.6 | 0.75 |
| HbA1c mean(SD) % | 7.82 ± 1.75 | 8.78 ± 2.85 | 0.19 |
| CD4 mean(SD) | 779.8 ± 299.9 | 687.2 ± 380.5 | 0.074 |
| AGEs mean(SD) μg/ml | 7.4 ± 10.7 | 4.16 ± 5.9 | 0.001 |
| BMI mean(SD) | 24.6 ± 2.48 | 23.1 ± 3.33 | 0.009 |
Statistical significant p < 0.05.
Figure 1.
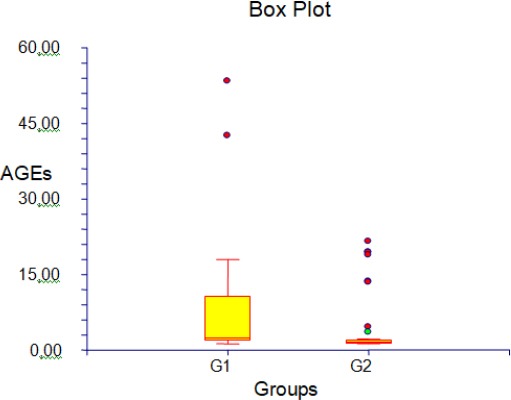
CD4 in diabetes and TB with co-morbidity diabetes; G1: diabetes; G2: TB with co-morbidity diabetes
Figure 2.
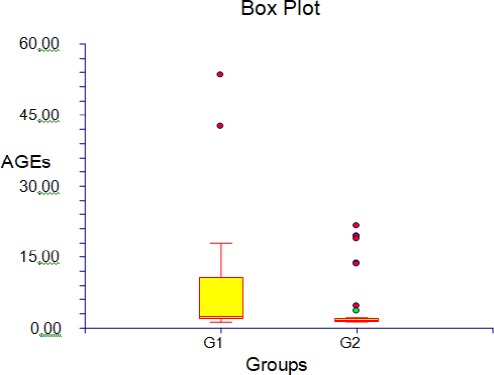
AGEs in diabetes and TB with co-morbidity diabetes; G1: diabetes; G2: TB with co-morbidity diabetes
Most of the patients were overweights in both groups, in the diabetes group there were 55% normoweight and 45% overweight and in TB with co-morbidity diabetes, there were 60% normoweight, 30% overweight and 10% underweight. The blood glucose levels were not well controlled either in diabetes or TBs with co-morbidity diabetes group. In the diabetes group there were 45% well controlled and 55% bad controlled. Meanwhile, in the TB with co-morbidity diabetes there were 35% well controlled and 65 % bad controlled. The immunity status in diabetes group was 85% good (CD4 > 500) and 15% bad (CD4 < 500). In the TB with co-morbidity diabetes 75% good and 25 % bad.
The mean level of the AGEs was 5.78 with the range 1.24-53.32 in diabetes group and 1.3-19.38 in the TB with co-morbidity diabetes group. No statistically significant differences were seen in blood glucose level (p = 0.75, HbA1C, p = 0.19 and CD4, p = 0.074) between the diabetes group and TB with co-morbidity diabetes, but BMI did with p = 0.009 and so did AGEs with p = 0.001.
In diabetes group there was no Statistical significant correlation found between CD4 and AGEs (p = 0.11), CD4 and blood glucose (p = 0.19), CD4 and HbA1C (p = 0.09), CD4 and BMI (p = 0.48). Statistical significant correlation was found between blood glucose and HbA1C (p = 0.013, r = 0.35), blood glucose and AGEs (p = 0.013, r = 0.35).
No statistical significant correlation was found between CD4 and AGEs (p = 0.44), CD4 and blood glucose (p = 0.08), CD4 and HbA1C (p = 0.45), CD4 and BMI (p = 0.44), but between blood glucose and HbA1C (p = 0.003, r = 0.43), HbA1C and AGEs (p = 0.003, r = 0.43).
Discussion
The chronic hyperglycemia will cause various complications; this was supported by the significant correlation between HbA1C and AGEs (p = 0.003) in the TB with co-morbidity diabetes group and significant correlation between blood glucose and AGEs (p = 0.013) in the diabetes group. This has been suggested by Helen et al. that AGEs play a rule in chronic inflammation [23].
The body mass index of the patients in the diabetes group was more with normoweight (22%) than overweight (18%), also in the TB with co-morbidity diabetes group (normoweight 24%, overweight 12%, underweight 4%). Obesity plays a role in metabolic syndrome that will worsen the chronic hyperglycemic effect. This was described by Ann Smith in The Epidemic of Obesity and Diabetes Trends and Treatments 2011 [24]. Underweight was found in the TB with co-morbidity diabetes group.
The CD4 ranged between 142 and 1800 with the mean 733. There were more cases (25%) with CD4 below 500 in the TB with co-morbidity diabetes group than in the diabetes group (15%), assumed that the immunity in the diabetes group is better than the TB with co-morbidity diabetes group, but the data did not show statistical significance difference (p = 0.07). Advanced glycation end products that play a role in cell dysfunctions and diabetic complications did not show the correlation with CD4 (p = 0.44) in the TB with co-morbidity diabetes group and p = 0.114 in the diabetes group. In conclusion, the CD4 below 500 were seen in 15% diabetes and 25% in TB with co-morbidity diabetes groups, but did not show statistical significance difference (p = 0.07). The AGEs was not correlated significantly with the CD4 in both groups (p = 0.44, p = 0.114). The HbA1C correlated significantly with AGEs (p = 0.003) in the TB with co-morbidity diabetes group and blood glucose with AGEs (p = 0.013) in the diabetes group. No statistically significant differences were seen in blood glucose level (p = 0.75, HbA1C, p = 0.19 and CD4, p = 0.074) between the diabetes group and TB with co-morbidity diabetes. But BMI did with p = 0.009 and so did AGEs with p = 0.001.
Acknowledgement
This study was supported by the Ministry of Research and Technology and Higher Education Republic Indonesia, under research grant TALENTA. There is no conflict of interest.
Footnotes
Funding: This study was supported by the Ministry of Research and Technology and Higher Education Republic Indonesia, under research grant TALENTA
Competing Interests: The authors have declared that no competing interests exist
Ethical approval
This study was done after the ethical approval under the Health Research Ethical Committee Medical Faculty of USU/HAM General Hospital Medan -Indonesia No. 44/TGL/KEPK FK-USU-RSUP HAM/2017.
References
- 1.Atkins RC, Zimmet P. Diabetic kidney disease: Act now or pay later. Saudi J Kidney Dus Transpl. 2012;21:217–21. [PubMed] [Google Scholar]
- 2.Mark A, Joshua A, Thomas FL, Francesco C. Diabetes and vascular disease: pathophysiology clinical consequences, and medical therapy: part I. Circulation. 2003 doi: 10.1161/01.CIR.0000091257.27563.32. PMid:14517147. [DOI] [PubMed] [Google Scholar]
- 3.Alberti KG, Zimmet PF. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus. Provisional report of a WHO consultation. Diabetic medicine. 1998;15(7):539–53. doi: 10.1002/(SICI)1096-9136(199807)15:7<539::AID-DIA668>3.0.CO;2-S. https://doi.org/10.1002/(SICI)1096-9136(199807)15:7<539:: AID-DIA668>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 4.Dobler CC, Flack JR, Marks GB. Risk of Tuberculosis among people with diabetes mellitus: an Australian nationwide cohort study. BMJ Open. 2012;2:e000666. doi: 10.1136/bmjopen-2011-000666. https://doi.org/10.1136/bmjopen-2011-000666 PMid:22331390 PMCid: PMC3282295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Young F, Wotton CJ, Critchley JA, et al. Increased risk of Tuberculosis disease in people with diabetes mellitus: record-linkage study in a UK population. J Epidemiol Community Health. 2012;66:519–23. doi: 10.1136/jech.2010.114595. https://doi.org/10.1136/jech.2010.114595 PMid:21109542. [DOI] [PubMed] [Google Scholar]
- 6.Leung CC, Lam TH, Chan WM, et al. Diabetic control and risk of Tuberculosis: a cohort study. Am J Epidemiol. 2008;167:1486–94. doi: 10.1093/aje/kwn075. https://doi.org/10.1093/aje/kwn075 PMid:18400769. [DOI] [PubMed] [Google Scholar]
- 7.Perez A, Brown HS, III, Restrepo BI. Association between Tuberculosis and diabetes in the Mexican border and non-border regions of Texas. Am J Trop Med Hyg. 2006;74:604–11. https://doi.org/10.4269/ajtmh.2006.74.604 PMid:16606993 PMCid: PMC1464139. [PMC free article] [PubMed] [Google Scholar]
- 8.WHO. Global TBs Report 2013. Geneva: World Health Organisation; 2013. URL http://apps.who.int/iris/bitstream/10665/91355/1/9789241564656_eng.pdf . [Google Scholar]
- 9.Restrepo BI, Camerlin AJ, Rahbar MH, et al. Cross-sectional assessment reveals high diabetes prevalence among newly-diagnosed Tuberculosis cases. Bull World Health Organ. 2011;89:352–9. doi: 10.2471/BLT.10.085738. https://doi.org/10.2471/BLT.10.085738 PMid:21556303 PMCid: PMC3089389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Peleg AY, Weerarathna T, McCarthy JS, Davis TM. Common infections in diabetes: Pathogenesis, management, and relationship to glycaemic control. Diabetes Metab Res Rev. 2007;23:3–13. doi: 10.1002/dmrr.682. https://doi.org/10.1002/dmrr.682 PMid:16960917. [DOI] [PubMed] [Google Scholar]
- 11.Dooley KE, Chaisson RE. Tuberculosis and diabetes mellitus: Convergence of two epidemics. Lancet Infect Dis. 2009;9:737–46. doi: 10.1016/S1473-3099(09)70282-8. https://doi.org/10.1016/S1473-3099(09)70282-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ruslami R, Aarniutse RE, Alisjahbana B, van der Ven AJ, van Crevel R. Implications of the global increase of diabetes for Tuberculosis control and patient care. Trop Med Int Health. 2010;15:1289–99. doi: 10.1111/j.1365-3156.2010.02625.x. https://doi.org/10.1111/j.1365-3156.2010.02625.x PMid:20955495. [DOI] [PubMed] [Google Scholar]
- 13.Baker MA, Harries AD, Jeon CY, et al. The impact of diabetes on Tuberculosis treatment outcomes: a systematic review. BMC Med. 2011;9:81. doi: 10.1186/1741-7015-9-81. https://doi.org/10.1186/1741-7015-9-81 PMid:21722362 PMCid: PMC3155828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Magee MJ, Foote M, Maggio DM, et al. Diabetes mellitus and risk of all-cause mortality among patients with Tuberculosis in the state of Georgia, 2009–2012. Ann Epidemiol. 2014;24:369–75. doi: 10.1016/j.annepidem.2014.01.012. https://doi.org/10.1016/j.annepidem.2014.01.012 PMid:24613196 PMCid: PMC4011933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kelly H, Jodie M, Tahnee Br, Brenda G, Rush C, Natkunam K. Immunological mechanisms contributing to the double burden of diabetes and intracellular bacterial infections. Immunology. 2014;144:171–185. doi: 10.1111/imm.12394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kerstin Nowotny, Tobias Jung, Annika Höhn, Daniela Weber, Tilman Grune. Advanced Glycation End Products and Oxidative Stress in Type 2 Diabetes Mellitus. Biomolecules. 2015;5:194–222. doi: 10.3390/biom5010194. https://doi.org/10.3390/biom5010194 PMid:2578610;7 PMCid: PMC4384119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Darrah PA, Patel DT, De Luca PM, Lindsay RW, Davey DF, Flynn BJ, Hoff ST, Andersen P, Reed SG, Morris SL, Roederer M. Multifunctional T H 1 cells define a correlate of vaccine-mediated protection against Leishmania major. Nature medicine. 2007;13(7):843. doi: 10.1038/nm1592. https://doi.org/10.1038/nm1592 PMid:17558415. [DOI] [PubMed] [Google Scholar]
- 18.Ciuffreda D, et al. Polyfunctional HCV-specific T-cell responses are associated with effective control of HCV replication. Eur J Immunol. 2008;38:2665–77. doi: 10.1002/eji.200838336. https://doi.org/10.1002/eji.200838336 PMid:18958874. [DOI] [PubMed] [Google Scholar]
- 19.Kannanganat S, Kapogiannis BG, Ibegbu C, Chennareddi L, Goepfert P, Robinson HL, Lennox J, Amara RR. Human immunodeficiency virus type 1 controllers but not noncontrollers maintain CD4 T cells coexpressing three cytokines. Journal of virology. 2007;81(21):12071–6. doi: 10.1128/JVI.01261-07. https://doi.org/10.1128/JVI.01261-07 PMid:17728221 PMCid: PMC2168799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kalsdorf B, Scriba TJ, Wood K, Day CL, Dheda K, Dawson R, Hanekom WA, Lange C, Wilkinson RJ. HIV-1 infection impairs the bronchoalveolar T-cell response to mycobacteria. American journal of respiratory and critical care medicine. 2009;180(12):1262–70. doi: 10.1164/rccm.200907-1011OC. https://doi.org/10.1164/rccm.200907-1011OC PMid:19797156 PMCid: PMC2796736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Day CL, Abrahams DA, Lerumo L, van Rensburg EJ, Stone L, O'rie T, Pienaar B, de Kock M, Kaplan G, Mahomed H, Dheda K. Functional capacity of Mycobacterium tuberculosis-specific T cell responses in humans is associated with mycobacterial load. The Journal of Immunology. 2011:1101122. doi: 10.4049/jimmunol.1101122. https://doi.org/10.4049/jimmunol.1101122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hull MW, Phillips P, Montaner JS. Changing global epidemiology of pulmonary manifestations of HIV/AIDS. Chest. 2008;134(6):1287–98. doi: 10.1378/chest.08-0364. https://doi.org/10.1378/chest.08-0364 PMid:19059959. [DOI] [PubMed] [Google Scholar]
- 23.Helen V. Uribarri Advanced Glycation End Products (AGE) and Diabetes: Cause, Effect, or Both? Curr Diab Rep. 2014;14(1):453. doi: 10.1007/s11892-013-0453-1. https://doi.org/10.1007/s11892-013-0453-1 PMid:24292971 PMCid: PMC3903318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ann SB. The Epidemic of Obesity and Diabetes Trends and Treatments. Tex Heart Inst J. 2011;38(2):142–144. [PMC free article] [PubMed] [Google Scholar]


