Abstract
BACKGROUND:
Purple sweet potato (Ipomoea Batatas L.) is one of the sources for anthocyanin, which promotes the health through antioxidant, anti-inflammatory, anti-cancer, neuroprotection, and anti-apoptosis activities. Oxidative stress has been shown to be the cause of apoptosis in ischemic stroke.
AIM:
The objective of this research was to delineate the pleiotropic effects of anthocyanin for neuroprotection during an acute stroke event.
METHODS:
Anthocyanin was extracted from Balinese cultivar of purple sweet potato and subsequently administered to rat models of induced ischemic stroke (labelled as treatment group), as well as a placebo (labelled as a control group). Several parameters were in turn evaluated, i.e. the activities of anti-apoptotic (Bcl-2) as well as pro-apoptotic (cytochrome c, caspase-3) molecules, and apoptosis rate. Bcl-2 levels were determined using the histochemical method, cytochrome c and caspase-3 via ELISA method, while apoptosis rate was measured by TdT-medicated Dutp-Nick End Labeling (TUNEL) assay.
RESULTS:
Bcl-2 expression demonstrated significantly higher Bcl-2 expression in the treatment compared with control group (median 31.2 vs. 1.1; p = 0.001). Accordingly, pro-apoptotic cytochrome c and caspase-3 levels were also found significantly lower in the treatment as opposed to control group (mean 4.17 vs. 8.06; p = 0.001; mean 3.81 vs. 8.02; p = 0.001). Ultimately, apoptosis rate was found markedly lower among treatment than control groups (mean 3.81 vs. control 21.97; p = 0.003).
CONCLUSION:
The results of this study indicated a significant neuroprotective effect of anthocyanin derived from Balinese cultivar of PSP. Anthocyanin was able to increase and reduce anti-apoptotic and pro-apoptotic protein levels, respectively, resulting in lesser cellular apoptotic rate when compared with placebo. The potential mechanism was thought mainly due to its anti-oxidant properties.
Keywords: Balinese cultivar of purple sweet potato extract, Bcl-2, Cytochrome c, Caspase 3, Apoptosis
Introduction
Purple sweet potato (Ipomoea Batatas L.), has different coloured tubers, comprising purple, white, red or yellow. Its roots and skin contain a lot of polyphenols, including anthocyanin and phenolic acids which are a source of vitamins A, B, C, Fe, Ca and phosphorus [1], [2]. Purple colour in the purple sweet potato (PSP) is due to high levels of anthocyanins. Higher anthocyanin in the PSP cause higher stability than other sources of anthocyanin [1], [3]. Anthocyanin possesses antioxidative properties [3], [4], maintains calcium ion homeostasis [5] [6], anti-apoptosis [7], [8], protective against cerebral ischemia [9], anti-inflammatory [10] and neuroprotective. Anthocyanin derived from Balinese cultivar have antioxidant effects by suppressing the production of malondialdehyde (MDA) in vivo and inducing endogenous antioxidants [11], [12].
Ischemic stroke happens because of blockage of blood flow to the brain, failing energy formation, cellular homeostatic disorders, acidosis and binding of calcium ions, excitotoxicity, reactive-oxygen species-mediated toxicity, glial cell activation, activation of complement, impaired blood brain barrier integrity and white blood cells infiltration [13]. Reduced cerebral blood circulation results in a decrease in ATP production, which is necessary for all brain cell activities. The failure of ATP formation will lead to depolarisation of cell membranes and excessive release of glutamate to extracellular space, thus activating NMDA and AMPA receptors, resulting in increased calcium ions in the cells, which in turn increases the formation of free radicals. Free radicals will cause cell death through necrosis and apoptosis mechanisms [13], [14].
Apoptosis in ischemic stroke can occur via extrinsic and intrinsic mechanisms. The intrinsic mechanism is induced by oxidative stress. Mitochondria are critical in the occurrence of intrinsic apoptosis due to oxidative stress. Increased ROS (reactive oxygen species) and intracellular calcium ions stimulate the release of pro-apoptotic family protein B cell lymphoma-2 protein (Bcl-2), such as Bcl-2 associated X protein (Bax), Bcl-2 antagonist killer (Bak), a Bcl-2 antagonist of death (Bad) and P53 from mitochondria. In mitochondria, there are also anti-apoptotic molecules such as Bcl-2 itself, Bcl-2 extra-long (Bcl-el), Bcl-2 homology of the ovary (Boo), protein kinase and extracellular signal-regulated kinase (ERK) that inhibit the activity of the pro-apoptotic protein. Under normal circumstances, there is a balance between anti- and pro-apoptotic proteins. When ischemic stroke occurs, this balance changes wherein there is an increase in the number of pro-apoptotic molecules, leaving open pores at the outer membrane of the mitochondria and outgoing pro-apoptotic proteins and will result in apoptotic cascades beginning with the cytochrome c secretion from mitochondria that will join apoptotic protease activating factor (APAF) and creating an apoptosome which triggers pro-caspase 9 into caspase 9 which further activates caspase-3 as the executor caspase, resulting in apoptosis [15], [16], [17].
Based on the mechanism of the occurrence of brain cell death (apoptosis) in ischemic stroke due to oxidative stress, and the antioxidant properties of Balinese cultivar PSP to neutralize oxidative stress, the researchers wanted to examine anthocyanin’s benefit obtained from PSP Balinese cultivar against oxidative stress in Wistar rats with ischemic stroke, by investigating bcl-2 anti-apoptotic molecules, pro-apoptotic molecules such as cytochrome c, caspase-3 and apoptosis rate.
Material and Methods
Three-month-old male Wistar rats 200-250 g in weight, obtained from Bio Farma Laboratory, Bandung, Indonesia was kept for a week in the cage at the place of research in Bioscience Laboratory Brawijaya University Malang for habituation. All animals used in this study were treated accordingly by adhering to National Institutes of Health guide for the care and use of laboratory animals. Food was provided according to a standardised protocol to ensure those rats were healthy. Rats were fed regularly, a night before the ischemic stroke induction protocol. In the morning, ischemic stroke was inducted according to previous methods [18]. A total of 20 rats were used for the study, each of which was 10 for the control and treatment group. The anthocyanin dosage used was 3 mL/day as previously used [19] intragastric only in the treatment group for 7 days, while all rats were decapitated under anaesthesia on the 8th day for further analyses.
PSP was extracted as follows: PSPs were rinsed under clear water before further processed. After peeled these sweet potatoes were cut transversely with 2-2.5 cm in thickness. Slices were subsequently added with water in 1:1 ratio, and then filtered with three layered-gauze. The liquid obtained from filtration was in turn heated until it boiled for 30 minutes for sterilisation purpose before filled into a bottle.
The tissue to be examined was reorganised with xylol, and 90%, 80%, and 70% of absolute alcohol, respectively were dropped for 5 minutes, then dropped with PBS (phosphate buffer saline) with pH of 7.4 for 10 minutes. It was subsequently blocked with BSA (bovine serum albumin), then mixed with primary antibody (monoclonal mouse anti-human Bcl-2). The preparation was then mixed with secondary IgG biotin antibody in PBS for one hour. The tissue then added with streptavidin HRP (horseradish peroxidase) at room temperature for 30 minutes. Afterwards, DAB was dropped for 20-40 minutes. Counterstain was subsequently performed using Meyer hematoxyline. The brownish colour is seen during histochemical analysis was derived from the bcl-2 protein cells. Cell counting was in turn performed by the axiovision ratio method, which can be downloaded at http://153.1.200:8080/immunoratio.
Wells of ELISA plate had been previously filled with cytochrome c antibody and caspase-3. The sample (serum) was filled into the well, then incubated at room temperature for 2 hours and washed three times. Then biotin was added and incubated for an hour. The phosphate buffered saline teonin (PBST) and HRP-avidin enzyme were added and incubated for an hour, then rinsed four times. TBM substrate was subsequently added and incubated for 5-10 minutes at room temperature. Lastly, 100 μL of stop solution was added, which turned the blue colored wells into yellow. The wells were then read with ELISA reader at 450 nm wavelength.
The rat’s cortical neuron cells were added with DNA fragmentation assay kit inserted into coplin jar, then washed with PBS for 5 minutes, and covered with protein kinase solution. After washed twice with PBS, samples were covered with the buffer derived from the kit then added with TdT incubation buffer. The samples were then placed in a dark room at 37°C in the incubator for an hour. It was soaked with anti-Brdu-biotin antibody for an hour at room temperature. It was in turn, washed with PBS then soaked with strevtavidin HRP conjugate. Cells which underwent apoptosis were visualized by adding the DAB subsrate and countered with HE before calculated by axiovision ratio.
Baseline data of Bcl-2 expression, cytochrome c, caspase 3 and number of cells undergoing apoptosis were tested for normality using Shapiro Wilk. If the data was normally distributed, independent t-tests were conducted to find the difference in Bcl-2, cytochrome c, and caspase-3 expressions, as well as the number of cells undergoing apoptosis between the control and treatment group. In contrast, when the data was abnormally distributed, data transformation was performed. When the transformation results were normally distributed then the independent t-test was conducted, otherwise Mann-Whitney test was conducted to identify the difference of Bcl-2, cytochrome c, and caspase 3 expressions, as well as the number of cells undergoing apoptosis with 95% confidence interval (p < 0.05). All data were processed by using SPSS 20 for Windows.
Results
The Mann-Whitney test of Balinese cultivar PSP extract effect on Bcl-2 expression demonstrated significantly higher Bcl-2 expression in the treatment group (p < 0.05) compared with the control group (Table 1)
Table 1.
Analysis result of the Mann-Whitney test for Bcl-2 expression
| n | Median (minimum-maximum) | p* | |
|---|---|---|---|
| Control | 10 | 1.1 (0.9-4.1) | 0.001 |
| Treatment | 10 | 31.2 (7.6-65.9) |
signicant at p < 0.05.
To clarify Bcl-2 expression in the control and treatment groups, the histochemical features are presented in Figure 1.
Figure 1.
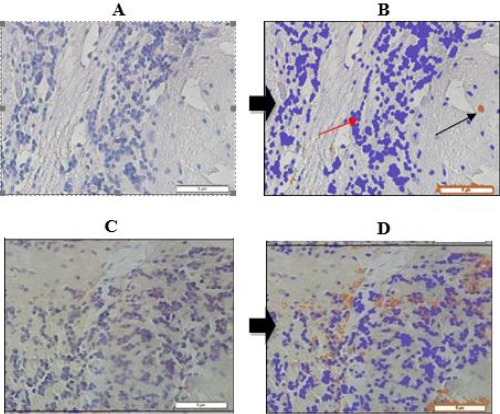
Bcl-2 expression in Wistar rats’ cerebral neuron. A) Bcl-2 expression before analyzed with Axio Vision Ratio (control); B) Bcl-2 expression after analyzed with Axio Vision Ratio (3.6%) (control); C) Bcl-2 expression before analyzed with Axio Vision Ratio (treatment); D) Bcl-2 expression after analyzed with Axio Vision Ratio (13.4%) (treatment); →Bcl-2 expression; →Pre-stained nucleus
Table 2 demonstrated cytochrome c levels among treatment group were markedly lower than control (p < 0.05).
Table 2.
Independent t-test of cytochrome c levels
| n | Mean ± SD | Mean difference (95% CI) | p* | |
|---|---|---|---|---|
| Control | 10 | 8.06 ± 0.07 | 3.89 (3.84-3.94) | 0.001 |
| Treatment | 10 | 4.17 ± 0.02 |
significant at p < 0.05.
Effect of purple sweet potato Balinese cultivar extract on caspase-3 level
To determine the difference in caspase-3, the Mann-Whitney test is presented in Table 3.
Table 3.
Mann-Whitney test result for caspase-3 level
| N | Median (minimum-maximum) | p* | |
|---|---|---|---|
| Control | 10 | 8.02 (7.99-8.16) | 0.001 |
| Treatment | 10 | 3.81 (3.75-3.84) |
significant at p < 0.05.
Mann-Whitney test showed that caspase-3 levels among treatment group were markedly lower than control (p = 0.001).
The effect of the purple sweet potato Balinese cultivar on apoptosis
TUNEL test results from Wistar rat on day 8 showed a lower number of apoptosis events in the treatment group when compared with control (p <0.05) as presented in Table 4.
Table 4.
Independent t-test of anthocyanin administration on apoptosis among Wistar rats with ischemic stroke between treatment and control group
| Group | N | Mean ± SD | Mean difference (95% CI) | p* |
|---|---|---|---|---|
| Control | 10 | 21.97 ± 13.92 | 18.16 (8.17-28.15) | 0.003 |
| Treatment | 10 | 3.81 ± 1.79 |
significant at p < 0.05.
To clarify the results of the apoptosis between control and treatment groups, the apoptosis was presented in Figure 2. Expression of apoptotic cells in the control group (A and B) and treatment group (C and D).
Figure 2.
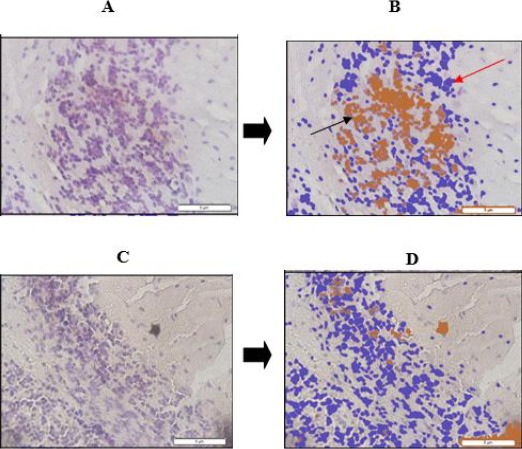
Expression of cerebral cortex neurons in Wistar rats undergoing apoptosis. A) Cellular expression undergoing apoptosis before analyzed with Axio Vision Ratio (control); B) Cellular expression undergoing apoptosis after analyzed with Axio Vision Ratio (28.8%) (control); C) Cellular expression undergoing apoptosis before analyzed with Axio Vision Ratio (treatment); D) Cellular expression undergoing apoptosis after analyzed with Axio Vision Ratio (6.8%) (treatment); →Pre-stained cells underwent DNA fragmentation; →Post-stained cells underwent DNA fragmentation
Discussion
The apoptotic cascade occurs due to an imbalance of anti-apoptotic protein (Bcl-2) with pro-apoptotic proteins (Bax and Bak). Oxidative stress has been demonstrated to cause apoptosis in ischemic strokes (apoptosis) [20]. Anthocyanin has been shown to exert antioxidant effects by increasing the production of endogenous antioxidants and as radical scavengers. Balinese cultivar of PSP also possesses antioxidant properties by increasing endogenous antioxidant production to counteract the effects of oxidative stress on ischemic stroke [12], [19], [21].
Consequently, apoptosis was inhibited. Inhibition of apoptotic cascade occurs when the amount of anti-apoptotic protein is in equilibrium or higher than pro-apoptotic protein, which in this study is evidenced by significantly higher Bcl-2 levels (p < 0.05) in the treatment group. A similar study was found in anthocyanins extracted from Ipomoea Batatas cultivar Ayamurasaki which has been shown to neutralise the effects of ROS and decrease p53 protein activity, so that apoptosis was lower by increasing Bcl-2 expression and decreasing Bak expression, thus increasing the Bcl2/Bak ratio (Han et al., [22]). The study by Yao et al., [23] also found that anthocyanin (quercetin) administered at a dosage of 10 and 20 mg/kg/day results in higher Bcl-2 expression (p < 0.05 and p < 0.01) in the treatment versus that in control group.
Cytochrome c is a protein consisting of 104 amino acids and has many functions such as electron transfer in the respiratory chain to form energy. Another function is to play a role in cardiolipin peroxide, ROS scavenger and apoptosis. Various cytochrome c functions are governed by their association with specific tissues. Under normal circumstances of bonding with Tyr97 and Tyr48, cytochrome c phosphorylation will form ATP. In a state of oxidative stress, bonding occurs with p66she and cytochrome c dephosphorylation occurs, which obviates its role from the respiratory chains of cytochrome c, so that binding with cardiolipin is released and cytochrome c exits from the mitochondria. The cytochrome c released from the mitochondria will bind to APAF forming an apoptosome, which will activate the subsequent apoptotic cascade (Gamdo et al., [24]). The PSP extract of Balinese cultivars has been shown to suppress oxidative stress and induce endogenous antioxidants [12], [19], [21], resulting in lower cytochrome c levels in the treatment than control (p < 0.05). These outcomes were similar to the research conducted by Lu et al., [7], which found that PSP colour (PSPC) can suppress cytochrome c elevation in the cytoplasm in older rats of Kunming strain induced by D-galactose. The suppression occurs because PSPC has the effect of increasing endogenous antioxidant expression such as Cu and Zn SOD and catalase. The study by Ullah et al., [26] also obtained the same result that on mouse-induced hippocampus neurons induced by kainic acid for 12 hours there was markedly elevated cytochrome c levels. In hippocampal neurons treated with anthocyanins before induction with kainic acid, there was markedly reduced cytochrome c levels (p < 0.05).
Caspase is an amino-terminal prodomain carboxy-terminal protease domain. Based on its role, caspase is classified as the initiator and executor. Caspase is generally in an inactive state called pro-caspase and will become active when there is a death signal which will then be followed by cell death (apoptosis). Caspase 3, 6 and 7 act as the executor’s caspases and are inactive in the form of a procaspase dimer. This procaspase will become active when there is a split between large and small subunits, resulting in a conformational change so that it becomes mature caspase. Activation of caspase 3 occurs by a chain reaction through the release of cytochrome c which binds to APAF to form apoptosome. Apoptosome then activates procaspase 9 into caspase 9. Caspase 9 then activates caspase 3 as the executor’s caspase. Caspase 3 will destroy and degrade cell components such as structural proteins in the cytoskeleton, cell nucleus proteins and enzymes involved in cellular repair and activate DNAase enzymes, thus detaching from their association with the caspase DNAse (ICAD) inhibitor [26], [27].
The effect of Balinese cultivar of PSP on Bcl-2 in this study was markedly different between those two groups, wherein the treatment group was higher in the treatment group, resulting in the inhibition of proapoptotic protein release such as cytochrome c, so that its level was lower in the treatment group which leads to a lower apoptotic activity. Consequently, apoptosis cascade will be reduced so that the level of caspase-3 in the treatment group in this study was also lower (p < 0.05) than in the control group (Table 3). A similar study was obtained by Yao et al., [23] in which caspase-3 expression on the cerebral cortex cells of rats ligated in the medial cerebral arteries was found to be lower in the anthocyanin-treated group (quecertin). A study conducted by Shah et al., [28], obtained similar results, i.e. prenatal rat neurons induced with ethanol demonstrated significantly higher levels of caspase-3 in the sham group as opposed to those treated with anthocyanin with a dose of 0.1 mg/mL (p < 0.05). Similar results were obtained by Badshah et al., [29], which examined the effect of anthocyanin against the toxic beta-amyloid effects injected intraventricularly in the hippocampus, in which caspase-9 and -3 were significantly higher among those rats without anthocyanin treatment (p < 0.05) as opposed to shame.
Apoptosis or programmed cell death is a normal life process. Disorders in apoptosis will trigger the onset of various diseases such as cancer, Parkinson’s, Alzheimer’s and stroke. Apoptosis occurs due to the formation of pores in the outer membrane of the mitochondria. The outer membranes of the mitochondria are formed as a response to the increased permeability of small molecule solution in the intermembrane of the mitochondria. Antiapoptotic proteins such as Bcl-2, Bcl-XL and Mcl inhibit the cascade of proapoptotic signals such as Bax. It migrates to the outer layer of the mitochondrial membrane to form pores, which induces cytochrome c release into the cytoplasm to stimulate the subsequent apoptotic cascade [17], [29], [30], [31].
In this study, we found that Bcl-2 expression was higher in the treatment than in the control group. The cytochrome c and caspase-3 levels were found lower in the treatment group thus resulting in lower apoptosis rate (p < 0.05).
Similar results have also been found in other investigators such as Shin et al., [32] who examined the anthocyanin’s neuroprotective effect against rat’s middle cerebral artery occlusion and reperfusion. In anthocyanin-treated rats, infarct volume and the number of apoptotic cells were significantly lower than placebo (p < 0.05). Anthocyanin exerts its neuroprotective effect by significantly inhibiting JNK and p53 activities. In mice treated with anthocyanin, JNK and p53 levels were significantly lower (p < 0.05). A study by Ye et al., [6] investigated the effect of PSPC on pheochromocytoma cells induced by beta-amyloid. Cells induced with beta-amyloid previously treated with PSPC have shown decreased calcium ion levels, improved mitochondrial membrane potential, and reduced caspase-3 expression and DNA fragmentation, all of which ameliorate apoptosis. Lu et al., [7] investigated the PSPC effect of Korean black soybean on apoptosis among older rats induced with D galactose. D-galactose administration induced a higher apoptosis rate in cerebral cortex neurons and hippocampus as opposed to the control group (p < 0.01). Conversely, simultaneous administration of PSPC and D-galactose decreased the apoptosis rate of cerebral cortex and hippocampus neurons. The effect of PSPC here is by increasing the concentration of antioxidants such as Cu, and ZN-SOD and catalase.
In conclusion, this study proved that the PSP Balinese cultivar extract exerted neuroprotection on Wistar rats with ischemic stroke reflected by higher Bcl-2, cytochrome c, and caspase-3 levels, while lowering the apoptosis rate.
Acknowledgement
This paper is part of a doctoral dissertation submitted to the Post Graduate Programme Faculty of Medicine, Udayana University in 2017.
Footnotes
Funding: This research did not receive any financial support
Competing Interests: The authors have declared that no competing interests exist
References
- 1.Suprapta DN, Antara M, Arya N, Sudana M, Duniaji AS, Sudarma M. Review of cultivation and consumption of various yam types as an alternative food source. Report of Research Results BAPEDA Bali and Faculty of Agriculture Udayana University. 2004 [Google Scholar]
- 2.Pasca-Panda V, Sonkambe M. Phytochemical constituents and pharmacological activities of Ipomoea batatas L. Int J Res Phytochem Pharmacol. 2012;2(1):25–34. [Google Scholar]
- 3.Montilla EC, Hillebrand S, Winterhalter P. Anthocyanins in purple sweet potato (Ipomoea batatas L.) varieties. Fruit, Vegetable and Cereal Science and Biotechnology. 2011;5(2):19–23. [Google Scholar]
- 4.de Pascual-Teresa S. Molecular mechanisms involved in the cardiovascular and neuroprotective effects of anthocyanins. Archives of biochemistry and biophysics. 2014;559:68–74. doi: 10.1016/j.abb.2014.04.012. https://doi.org/10.1016/j.abb.2014.04.012 PMid:24791600. [DOI] [PubMed] [Google Scholar]
- 5.Shih PH, Wu CH, Yeh CT, Yen GC. Protective effects of anthocyanins against amyloid β-peptide-induced damage in Neuro-2A cells. Journal of agricultural and food chemistry. 2011;59(5):1683–9. doi: 10.1021/jf103822h. https://doi.org/10.1021/jf103822h PMid:21302893. [DOI] [PubMed] [Google Scholar]
- 6.Ye J, Meng X, Yan C, Wang C. Effect of purple sweet potato anthocyanins on β-amyloid-mediated PC-12 cells death by inhibition of oxidative stress. Neurochemical research. 2010;35(3):357–65. doi: 10.1007/s11064-009-0063-0. https://doi.org/10.1007/s11064-009-0063-0 PMid:19771514. [DOI] [PubMed] [Google Scholar]
- 7.Lu J, Wu DM, Zheng YL, Hu B, Zhang ZF. Purple sweet potato colour alleviates d-galactose-induced brain aging in old mice by promoting survival of neurons via PI3K pathway and inhibiting cytochrome c-mediated apoptosis. Brain Pathology. 2010;20(3):598–612. doi: 10.1111/j.1750-3639.2009.00339.x. https://doi.org/10.1111/j.1750-3639.2009.00339.x PMid:198635;44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim HG, Ju MS, Shim JS, Kim MC, Lee SH, Huh Y, Kim SY, Oh MS. Mulberry fruit protects dopaminergic neurons in toxin-induced Parkinson's disease models. British Journal of Nutrition. 2010;104(1):8–16. doi: 10.1017/S0007114510000218. https://doi.org/10.1017/S0007114510000218 PMid:20187987. [DOI] [PubMed] [Google Scholar]
- 9.Bhuiyan MI, Kim JY, Ha TJ, Kim SY, Cho KO. Anthocyanins extracted from black soybean seed coat protect primary cortical neurons against in vitro ischemia. Biological and Pharmaceutical Bulletin. 2012;35(7):999–1008. doi: 10.1248/bpb.b110628. https://doi.org/10.1248/bpb.b110628 PMid:22791144. [DOI] [PubMed] [Google Scholar]
- 10.Wang D, Wei X, Yan X, Jin T, Ling W. Protecatechuic acid, a metabolite of anthocyanins, inhibits monocyte adhesion and reduces atherosclerosis in apolipoprotein E-deficient mice. J Agric Food Chem. 2010;58:12722–8. doi: 10.1021/jf103427j. https://doi.org/10.1021/jf103427j PMid:21090717. [DOI] [PubMed] [Google Scholar]
- 11.Jawi IM, Suprapta DN, Dwi SU, Wiwiek I. Purple Sweet Potato reduced Mice'Blood and Liver MDA Levels after Maximum Physical Exertion. Jurnal Veteriner Jurnal Kedokteran Hewan Indonesia. 2008;9(2):65–72. [Google Scholar]
- 12.Jawi IM, Wita IW, Suprapta DN. Aqueous Extract of Purple Sweet Potato Tuber Increases SOd-2 and decrease VCAM-1 Expression by increasing Nrf2 Expression in The Aortic Endothelia of Hypercholetrolemic rabbits. Journal of Biology, Agriculture and Healthcare. 2014;4(10):76–84. [Google Scholar]
- 13.Woodruff TM, Thundyil J, Tang SC, Sobey CG, Taylor SM, Arumugam TV. Pathophysiology, treatment, and animal and cellular models of human ischemic stroke. Molecular neurodegeneration. 2011;6(1):11. doi: 10.1186/1750-1326-6-11. https://doi.org/10.1186/1750-1326-6-11 PMid:21266064 PMCid: PMC3037909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jordan J, Moreno-Parrado L, Anton-Martinez DA, Jellinger Kf, Galindo M. Modulation of apoptosis in acute ischemic stroke as treatment challenges. Current Immunology Reviews. 2012;8(1):39–49. https://doi.org/10.2174/157339512798991209. [Google Scholar]
- 15.Youle RJ, Strasser A. The BCL-2 protein family: opposing activities that mediate cell death. Nature reviews Molecular cell biology. 2008;9(1):47. doi: 10.1038/nrm2308. https://doi.org/10.1038/nrm2308 PMid:18097445. [DOI] [PubMed] [Google Scholar]
- 16.Niizuma K, Yoshioka H, Chen H, Kim GS, Jung JE, Katsu M, Okami N, Chan PH. Mitochondrial and apoptotic neuronal death signaling pathways in cerebral ischemia. Biochimica et Biophysica Acta (BBA)-Molecular Basis of Disease. 2010;1802(1):92–9. doi: 10.1016/j.bbadis.2009.09.002. https://doi.org/10.1016/j.bbadis.2009.09.002 PMid:19751828 PMCid: PMC2790539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vaux LD. Apoptogenic factor released from mitochondria. Biochim Biophys Acta. 2011;1813:546–550. doi: 10.1016/j.bbamcr.2010.08.002. https://doi.org/10.1016/j.bbamcr.2010.08.002 PMid:20713095. [DOI] [PubMed] [Google Scholar]
- 18.Adnyana IM, Sudewi AA, Samatra DP, Suprapta DN. A simple method to stimulate ischemic stroke in Wistar rat for animal testing. BALI MEDICAL JOURNAL. 2017;6(1):156–60. https://doi.org/10.15562/bmj.v6i1.430. [Google Scholar]
- 19.Jawi IM, Arijana IG, Subawa AA, Wirasuta IM. The Pharmacological Mechanisms of Anthocyanin in Aqueous Extract of Purple Sweet Potato as Antihyperglycemic Herbal Remedy. Global Journal of Medical Research. 2016 [Google Scholar]
- 20.Zhang H, Li Q, Li Z, Mei Y, Guo Y. The protection of Bcl-2 overexpression on rat cortical neuronal injury caused by analogous ischemia/reperfusion in vitro. Neuroscience research. 2008;62(2):140–6. doi: 10.1016/j.neures.2008.07.002. https://doi.org/10.1016/j.neures.2008.07.002 PMid:18723055. [DOI] [PubMed] [Google Scholar]
- 21.Suwarba IGN. Adjuvant Therapy of Purple Sweet Potato (Ipomoea batatas L.). Reduced 8-hydroxy-2 deoxyguanosine, Interleukin 6, Increased superoxide dismutase, Improved EEG, and reduced Refractory Focal Epileptic Seizures in Children. Dissertation. Doctoral Program Udayana University, Denpasar. 2016 [Google Scholar]
- 22.Han YT, Chen XH, Xie J, Zhan SM, Wang CB, Wang LX. Purple sweet potato pigments scavenge ROS, reduce p53 and modulate Bcl-2/Bax to inhibit irradiation-induced apoptosis in murine thymocytes. Cellular Physiology and Biochemistry. 2011;28(5):865–72. doi: 10.1159/000335801. https://doi.org/10.1159/000335801 PMid:22178939. [DOI] [PubMed] [Google Scholar]
- 23.Yao RQ, Qi DS, Yu HL, Liu J, Yang LH, Wu XX. Quercetin attenuates cell apoptosis in focal cerebral ischemia rat brain via activation of BDNF–TrkB–PI3K/Akt signaling pathway. Neurochemical research. 2012;37(12):2777–86. doi: 10.1007/s11064-012-0871-5. https://doi.org/10.1007/s11064-012-0871-5 PMid:22936120. [DOI] [PubMed] [Google Scholar]
- 24.Hüttemann M, Pecina P, Rainbolt M, Sanderson TH, Kagan VE, Samavati L, Doan JW, Lee I. The multiple functions of cytochrome c and their regulation in life and death decisions of the mammalian cell: From respiration to apoptosis. Mitochondrion. 2011;11(3):369–81. doi: 10.1016/j.mito.2011.01.010. https://doi.org/10.1016/j.mito.2011.01.010 PMid:21296189 PMCid: PMC3075374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ullah I, Park HY, Kim MO. Anthocyanins Protect against Kainic Acid-induced Excitotoxicity and Apoptosis via ROS-activated AMPK Pathway in Hippocampal Neurons. CNS neuroscience & therapeutics. 2014;20(4):327–38. doi: 10.1111/cns.12218. https://doi.org/10.1111/cns.12218 PMid:24393263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li X, Wen W, Liu K, Zhu F, Malakhova M, Peng C, Li T, Kim HG, Ma W, Cho YY, Bode AM. Phosphorylation of caspase-7 by p21-activated protein kinase (PAK) 2 inhibits chemotherapeutic drugs-induced apoptosis of breast cancer cell lines. Journal of Biological Chemistry. 2011:jbc–M111. doi: 10.1074/jbc.M111.236596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.ali Shah S, Ullah I, Lee HY, Kim MO. Anthocyanins protect against ethanol-induced neuronal apoptosis via GABAB1 receptors intracellular signaling in prenatal rat hippocampal neurons. Molecular neurobiology. 2013;48(1):257–69. doi: 10.1007/s12035-013-8458-y. https://doi.org/10.1007/s12035-013-8458-y PMid:23645118. [DOI] [PubMed] [Google Scholar]
- 28.Badshah H, Kim TY, Kim MO. Protective effect of anthocyanin against amyloid beta-induced neurotoxicity in vivo and in vitro. Neurochemistry International. 2015;80:51–59. doi: 10.1016/j.neuint.2014.10.009. https://doi.org/10.1016/j.neuint.2014.10.009 PMid:25451757. [DOI] [PubMed] [Google Scholar]
- 29.Tait SW, Green DR. Mitochondria and cell death: outer membrane permeabilization and beyond. Nature reviews Molecular cell biology. 2010;11(9):621. doi: 10.1038/nrm2952. https://doi.org/10.1038/nrm2952 PMid:20683470. [DOI] [PubMed] [Google Scholar]
- 30.Azad N, Iyer AKV. Reactive Oxygen Species and Apoptosis. In: Laher I, editor. System Biology of Free Radical and Antioxidants. Berlin: Springer-Verlag Berlin Heidelberg; 2014. pp. 113–127. https://doi.org/10.1007/978-3-642-30018-9_15. [Google Scholar]
- 31.Shin WH, Park SJ, Kim EJ. Protective effect of anthocyanins in middle cerebral artery occlusion and reperfusion model of cerebral ischemia in rats. Life sciences. 2006;79(2):130–7. doi: 10.1016/j.lfs.2005.12.033. https://doi.org/10.1016/j.lfs.2005.12.033 PMid:16442129. [DOI] [PubMed] [Google Scholar]


