Abstract
AIM:
This study aimed to evaluate the importance of IFN-γ in the diagnosis of pediatric TB and LTBI and to compare the IFN-γ levels.
METHODS:
We analysed 100 patients examined for possible M. tuberculosis infection or disease at the Institute of Respiratory Diseases in Children, Kozle, Skopje. Patients were divided into 2 groups: TB disease and LTBI. The following parameters were analyzed: demographic characteristics, history of previous exposure to active TB, BCG vaccination and presence of BCG scar, lung X-ray findings, tuberculin skin test by the Monteux method and the value of INF-γ according to the Quantiferon TB gold test, direct samples of acid-alcohol-resistant bacilli of sputum and Löwenstein Jensen cultures. Informed parental consent was obtained for each child included in the study.
RESULTS:
In the LTBI group 60.9% had a scar from the vaccination while in the TB group 50% had BCG scar. TST induration diameters in children with or without BCG scar were significantly larger in patients with active TB. Children with active TB had significantly higher IFN-γ levels than children with LTBI. The IFN-γ for the cut-off of 0.35 IU/ml, has 64% sensitivity for detection of LTBI, versus 80.6% sensitivity for active disease. Children with close TB contact had significantly higher IFN-γ levels. Correlation between TST induration diameter and IFN-γ levels was stronger in the TB group.
CONCLUSION:
IFN-γ levels are significantly higher in children with active TB, and children with close contact with TB patient. It has better sensitivity in active TB. Using both tests (IFN-γ and TST) can improve the diagnose of LTBI and TB in countries where vaccination with BCG is widespread.
Keywords: Children, Latent tuberculosis infection, Tuberculosis, Tuberculin skin test, IFN-γ
Introduction
World Health Organization defines tuberculosis (TB) as an infectious bacterial disease caused by Mycobacterium tuberculosis (M. tuberculosis). Patients with lung tuberculosis from whose sputum M. tuberculosis bacilli are isolated are the main source of the infection. M. tuberculosis, which was discovered in 1882 by Robert Koch, is anaerobic, facultative intracellular slow-growing acidophilic bacillus, naturally pathogenic only in humans [1] [2]. In children, TB usually develops as a result of close family contact with smear-positive TB patient.
According to WHO in 2016 there were 10.4 million new cases with TB, and 1.8 million deaths [3]. Tuberculosis is a contagious/infectious disease which is characterised by a high rate of morbidity and mortality in the world. Children account for 5 to 15% of the cases with tuberculosis worldwide; they are more often infected and have more severe forms of the disease [4]. In the R. Macedonia in 2012 the prevalence was 26/100,000. In 2014, 285 new cases of tuberculosis were discovered, the rate is 13.8 per 100.000 populations, which is 38 cases less in comparison with 2013 when the number of newly discovered cases amounted to 325 persons. In 2016 the incidence was 16 per 100000 population.
Diagnosis of tuberculosis in children is based on data about the history of the disease, epidemiologic data, clinical signs, laboratory analyses, x-ray examinations and immunologic examinations, tuberculin skin test (TST) and Interferon-Gamma Release Assays (IGRA) tests, while the unique secure proof for correct diagnosis is isolation of the causer from biologic material [5] [6]. Establishing the diagnosis in children may be difficult because the symptoms are often very discreet; there is not self-recognition of the disease; direct microscopic preparations are positive only in 10 to 15%; positive cultures are obtained in 30% of children, whereas in smaller age groups even less than in 20% [5] [6]. Latent tuberculosis infection (LTBI) is defined as a state of persistent immune response to stimulation by M. tuberculosis antigens with no evidence of clinically manifest active TB [7]. Common features for both TB and LTBI are positive TST and IGRA tests. LTBI is defined as an infection with tuberculous bacilli inside the granuloma where it remains in non-replicating condition but later it can be transformed into an active TB. However, recent experimental data support the dynamic model of LTBI presenting a continual endogenous reactivation and inflammatory response [8]. This has been supported by a Norwegian study from 2010 demonstrating that reactivation of tuberculosis is decreased over time [9]. The dynamic model offers explanation for the influence of isoniazid, a drug which influences the actively replicating bacilli only. As isoniazid prevents the episodes of reinfections with bacteria released from the resting phase, together with the delayed drainage and damaging of non-replicating bacteria in the stomach, latent infection weakens gradually [8]. The chance LTBI to develop into active tuberculosis during lifetime in infants amounts to 43%, in children at the age from 1 to 5 years it amounts to 29%, while in children aged from 11 to 15 years it is15%. In children with LTBI younger than five years the risk of TB development two years after the infection is 20-40% [10] [11]. The risk for active TB disease after infection depends on several factors, and the most important is the immunological status [7]. Prevention of active TB disease by treatment of LTBI is critical component of the WHO End TB Strategy [12]
The aim of this study was to evaluate the importance of the released IFN-γ from T lymphocytes IFN-γ in the diagnosis of pediatric TB and LTBI and to compare the IFN-γ levels between active TB and LTBI.
Material and Methods
In this study, we have analysed 100 patients with possible M. tuberculosis infection or tuberculosis disease who were admitted at the Institute for Respiratory Diseases in Children, Kozle, Skopje in the period from September 2014 to August 2017. In that period the total number of registered patients with TB disease in our Institute was 61, and 215 with LTBI.
Inclusion criteria
Children, aged from 1 to 15 years, with no history of tuberculosis disease, BCG vaccinated, with a history of exposure to active TB.
In the patients included in the study the following parameters were analyzed: demographic characteristics, history of previous exposure to active TB and the type of contact (close, distant, unknown), BCG vaccination and presence of BCG scar, lung X-ray findings, tuberculin skin test (TST) by the Mantoux method and the value of INF- γ according to the commercial Quantiferon TB gold test, direct samples of acid-alcohol-resistant bacilli of sputum and Löwenstein Jensen cultures.
Informed parental consent was obtained for each child included in the study.
Patients were divided into 2 groups:
TB disease (n=36): children with symptoms suggestive of TB, with abnormal lung X-ray findings, with or without positive ARB smear microscopy
LTBI(n=64): asymptomatic patients, with a history of exposure to active TB, or tuberculin hyperreactors, with normal lung X-ray.
The TB group consists of 36 children, 19 males, 17 females, and the LTBI group consists of 64 children, 29 females and 35 males, aged from 1 to 15 years.
Exclusion criteria
One patient with immunodeficiency and malnutrition, one with congenital cardiopathy and one with osteoarticular tuberculosis were excluded from the study
Figure 1.
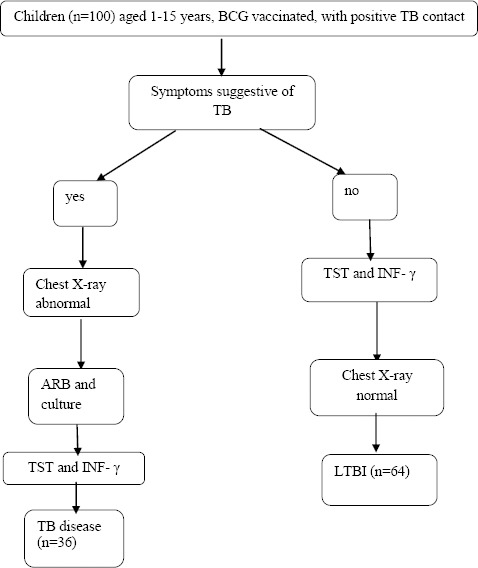
Included patients and study groups
Tuberculin skin test was performed on the volar aspect of the left forearm by injecting 0.1 ml purified protein derivative (PPD) of 5 tuberculin units (TU) intracutaneously. Measuring of induration diameter was made after 72 hours. According to the National tuberculosis programme, positive TST is considered a transversal induration diameter of more than 10 mm [13].
In our study, in patients who did not have a BCG vaccination scar, the value of TST diameter ≥ 6 mm was considered as borderline for a positive skin test. In patients who had a BCG scar, the value of TST diameter ≥ 15 mm was considered as borderline for a positive skin test. In patients who had a BCG scar, the value of TST test < 15 mm was considered as negative [14] [15].
Figure 2.
Description of performing and measuring induration in tuberculin skin test
Quantiferon TB Gold (QFT) analysis was performed at the Institute for Respiratory Diseases and TB in line with the guidelines of the manufacturer. The examination was performed in two phases: the incubation of the whole blood with antigens was made in the first phase, and in the second phase the measuring of IFN-γ was made by the ELISA method.
In 2-6 hours after blood taking, the test-tubes with blood were placed for incubation at 37ºC. 24 hours after incubation the test-tubes had been centrifuged, and the plasma was separated and frozen at -70ºC. The concentration of INF-γ was measured by the ELISA method (Enzyme-Linked Immunosorbent Assay) using the commercial test Quantiferon TB gold (Cellestis, QIAGEN Company). The values of INF-γ were expressed in international units on millimetre. As recommended by the manufacturer, the cut-off value for a positive test was INF- γ ≥ 0.35 IU/ml.
Results
A total of 100 children were analysed from whom 36 with TB disease and 64 with Latent tuberculosis infection. There was no significant difference between the sexes in both groups. Both sexes were equally represented. The age ranged from 1 to 15 years with an average age of 7.48 years.
In both groups, there was no significant difference between young children from 1-5 years old, and older than 5 years.
In the TB group, 55.6% were from the rural area, while in the LTBI group there was a significantly higher frequency of children from an urban area (70.3%).
The results obtained about BCG showed that all 100 children were BCG vaccinated at their birth. In the LTBI group, 60.9% had a scar from the vaccination while in 39.1% of the children the BCG scar could not be seen. In the TB group, there was an equal distribution of children with or without BCG scar (50%-50%).
According to the contact with a patient infected with active TB, our results showed that there is a significantly higher per cent of children with very close contact with active TB in the TB group. All of the children had a chest X-ray. In the LTBI group, all of them showed normal findings. In the TB group, chest X-rays were mostly with primary TB.
Direct specimens for acid-alcohol-resistant bacilli (ARB) from sputum as well as culture in the solid medium by Löwenstein Jensen were analysed in all children with active TB. Most of them, 88.9% were ARB negative, and only 11.1% ARB positive. Löwenstein Jensen cultures were positive in 22.2% of the TB cases and negative in the rest of them (77.8%). In the group of patients with LTBI most of the children, 89.1% were not analysed for ARB and Löwenstein Jensen culture. In only 10.9% of children, these analyses were performed and were all negative.
TST induration diameters in children with or without BCG scar were significantly larger in patients with active TB (Table 3 and 4).
Table 1.
Basic demographic characteristics of the patients
| Parameters | Children with LTBI (n = 64) | Children with TB (n = 36) | Significance |
|---|---|---|---|
| Gender | |||
| male | 54.7% | 52.8% | χ2 = 0.037 |
| female | 45.3% | 47.2% | p = 0.982 (n.s.) |
| Age | 6 (IQR = 7) (range 1 – 15) |
5.5 (IQR = 8) (range 1 – 15) |
F = 0.096 P = 0.953 (n.s.) |
| Age group | |||
| Under 5 years | 48.4% | 50.0% | χ2 = 0.453 |
| Older than 5 years | 51.6% | 50.0% | p = 0.797 (n.s.) |
| Area | |||
| Rural | 29.7% | 55.6% | χ2 = 6.821 |
| Urban | 70.3% | 44.4% | p = 0.033 (*) |
| BCG scar | |||
| Yes | 60.9% | 50.0% | χ2 = 1.587 |
| No | 39.1% | 50.0% | p = 0.452 (n.s.) |
Table 2.
Specific characteristics in children with TB and LTBI
| Parameters | Children with LTBI (n = 64) | Children with TB (n = 36) | Significance |
|---|---|---|---|
| Contact with active TB | χ2 = 7.523 p = 0.023 (*) |
||
| Close | 48.4% | 75.0% | |
| Distant | 46.9% | 19.4% | |
| Unknown | 4.7% | 5.6% | |
| Chest X-ray findings | χ2 = 100.000 p = 9.87·10-21(**) |
||
| Normal | 100.0% | 0.0% | |
| TB pleuritis | 0.0% | 8.3% | |
| Primary TB | 0.0% | 77.8% | |
| Cavities | 0.0% | 5.6% | |
| Hilar lymphadenitis | 0.0% | 8.3% | |
| ARB | χ2 = 75.071 p = 4.99·10-17(**) |
||
| positive | 0.0% | 11.1% | |
| negative | 10.9% | 88.9% | |
| Not analyzed | 89,1% | 0,0% | |
| Löwenstein–Jensen culture | χ2 = 75.694 p = 3.66·10-17(**) |
||
| Positive | 0.0% | 22.2% | |
| Negative | 10.9% | 77.8% | |
| Not analyzed | 89.1% | 0.0% |
Table 3.
TST in children with BCG scar
| Parameter | Children with LTBI (n = 39) | Children with TB (n = 18) | Significance |
|---|---|---|---|
| Children with BCG scar | |||
| ТST induration diameter (mm) | 8 (IQR = 11, range 3 – 24) | 20 (IQR = 5, range 4 – 27) | Mann-Whitney test: U = 121.0 p = 0.00008(**) |
Table 4.
TST in children without BCG scar
| Parameter | Children with LTBI (n = 25) | Children with TB (n = 18) | Significance |
|---|---|---|---|
| Children without BCG scar | |||
| ТST induration diameter (mm) | 9 (IQR =13; range 2 – 34) | 15 (IQR = 7; range 3 – 24) | Mann-Whitney test:U = 135.5 p = 0.027(*) |
In all 100 children, IFN-γ was measured with commercial Quantiferon TB gold test.
In this study, the IFN-γ for the cutoff of 0.35 IU/ml, had 64% sensitivity for detection of LTBI, versus 80.6% sensitivity for active disease.
In our results, the Kruskal-Wallis test showed statistically highly significant differences in the levels of IFN-γ (IU/ml) among the two groups of patients. The Mann-Whitney tests confirmed that children with active TB have significantly higher IFN-γ levels than children with LTBI, (U = 649.5; **p = 0.0003).
According to that cutoff, there was the significantly higher frequency of patients with a positive test in the TB group (80.6%) compared with the LTBI group (64.1%; χ2 = 3.984, *p = 0.047)
Figure 3.
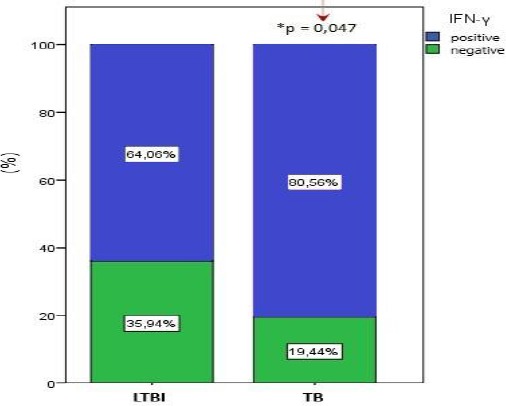
significantly higher frequency of patients with a positive test in the TB group (*p < 0.05)
In this study, we determined a new cut-off diagnostic value of IFN-γ level and its sensitivity and specificity for distinguishing LTBI from active TB in children in R. Macedonia. This was analysed with Receiver operating characteristic (ROC) curve.
Our analyses showed that IFN-γ level ≥ 0.822 IU/mL is optimal to discriminate children with LTBI and TB disease, with 74.3% sensitivity and 67.2% specificity (25.7% false negative and 32.8% false positive findings).
Figure 4.
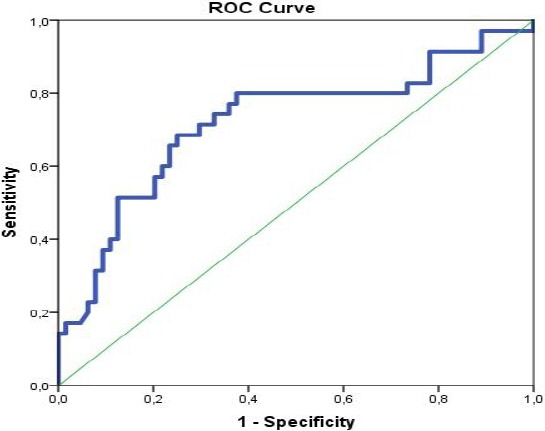
ROC analyses for determination of the cutoff diagnostic value of IFN-γ (IU/mL) for distinguishing children with TB disease from LTBI
About the influence of the age on IFN-γ levels, our results showed weak but statistically significant (*p = 0.013) and a positive correlation between the age and IFN-γ levels in both groups Correlation between TST induration diameter and IFN-γ levels was stronger in the group of children with active TB. We also compared the IFN-γ levels according to the contact with active TB patient.
As we can see from Table 6, patients with close contact have significantly higher IFN-γ levels (*p = 0.002).
Table 5.
IFN-γ levels (IU/ml)
| Parameter | Children with LTBI (n = 64) | Children with TB | Significance |
|---|---|---|---|
| Levels of IFN-γ (IU/mL) | 0.475 (IQR = 1.082) (range 0.030 – 3.042) | 2.037 (IQR = 2.246) (range 0.024 – 4.764) | Kruskal-Wallis test: F = 46.042 p = 1.00·10-10(**) |
The test was considered positive with titer ≥ 0.35 IU/ml.
Table 6.
Comparison of the IFN-γ levels according to the contact with active TB patient
| Parameter | Close contact (n = 58) | Distant contact (n = 37) | Significance |
|---|---|---|---|
| IFN-γ (IU/mL) | 1.54 (IQR = 2.44) (range 0.04 – 4.76) | 0.41 (IQR = 0.70) (range 0.02 – 4.47) | Mann-Whitney test: U = 666.0 p = 0.002 (*) |
Discussion
When we speak about the eradication of TB, it is not enough only to treat patients with active tuberculosis but to diagnose and to treat adequately those with LTBI.
Until recently, tuberculin skin test by the Mantoux method has been the uniquely available immunologic test for diagnosis of LTBI. It is an in vivo test that is based on measurement of the reaction of postponed hypersensitivity after injecting a mixture of mycobacterial antigens, PPD subcutaneously on the forearm [16]. PPD of 5 tuberculin units (TU) is used in our country. The size of induration on the site of injection is proportional to the strength of the immunologic response to competent cells. Induration diameter is read after 72 hours [17].
A positive outcome can be expected if two to eight weeks have passed after the infection with M. tuberculosis. Since the solution of PPD contains more than 200 protein components that are common for most of the mycobacteria, tuberculin test may give false-positive results in persons vaccinated with BCG or who were in contact with nontuberculous mycobacteria [18].
False-negative results may be found in persons with damaged or immature cell immunity such as patients infected with HIV, patients with iatrogenic-caused immunosuppression, children in younger age due to weak reactivity on the skin or if the result is falsely read as negative [19].
According to the above said it is clear that TST is not a secure test for detecting LTBI especially in countries where there is BCG vaccination such as in our country. In our study, TST induration diameters in children with or without BCG scar were significantly larger in patients with active TB.
Since 2004 the Quantiferon TB gold test has been used, and it has largely contributed to the diagnosis of LTBI and TB. The specific antigens that are used in this test are early secreted antigenic target-6KD (ESAT-6), culture filtrate protein-10KD (CFR-10) and TB 7.7. These antigens do not exist in BCG (Bacillus Calmette-Guerin) and most of the nontuberculous mycobacteria, except in M. Kansai, M. szulgai, M. marinum, M. flavescens and M. gastric [20]. Therefore, the chance of having false-positive results of IGRA tests is very small since T lymphocytes in healthy BCG-vaccinated uninfected persons as well as in those infected by nontuberculous mycobacteria do not secrete gamma interferon after stimulation with the mixture of antigens ESAT-6, CFP-10 and TB 7.7 [21].
The positive features of IGRA tests are their high diagnostic sensitivity and specificity, reproducibility and possible standardisation. The research of Pai et al., (2008) showed 99% specificity of Quantiferon TB gold test in persons who were not BCG-vaccinated and 96% in BCG-vaccinated persons, while the sensitivity reached 78% [22]. Sun et al., found a sensitivity for all TB disease in children of 70% for ELISA (range: 57%–96%), 62% for ELISPOT (range: 40%–100%), and 71% for the TST (range: 43%–100%) [23]. In our study, the IFN-γ for the cutoff of 0,35 IU/ml, had 64% sensitivity for detection of LTBI, versus 80,6% sensitivity for active disease.
Regarding IFN-γ levels differences in children with LTBI and active TB, Whittaker et al., [24] described a lower amount of IFN-γ in LTBI patients compared to active TB, whereas Latorre et al. described no significant difference between these two groups [25]. Our results showed statistically highly significant differences in the levels of IFN-γ (IU/ml) among the two groups of patients. Children with active TB had significantly higher IFN-γ levels than children with LTBI (U = 649.5; **p = 0.0003). Connell et al., in 2 subsequent studies, showed a good agreement between positive IGRAs and TB disease, whereas a poor correlation between IGRAs and TST for the diagnosis of LTBI in children was found [26] [27].
About the influence of the age on IFN-γ levels, our results showed weak but statistically significant (*p = 0.013) and a positive correlation between the age and IFN-γ levels in both groups. Nakaoka et al. reported a decreased response to QFT in children < 5 years of age [28].
Correlation between TST induration diameter and IFN-γ levels was stronger in the group of children with active TB. Onur at al. reported that QFT positivity differed significantly according to TST induration diameter and the positivity rate was significantly higher in patients with an induration diameter of 10–14 and ≥ 15 mm compared to other diameters [29]. In our study, we also compared the IFN-γ levels in patients with close or distant contact with active TB. The results showed that patients with close contact have significantly higher IFN-γ levels (*p = 0.002). Kang et al., found the positive QFT rates 4%, 10%, 44% and 81% in low risk, casual contact, close contact, and active TB patients, respectively [30].
The limitations of this study are that we hadn’t investigated children without risk of TB infection, so we couldn’t make a correlation between IFN-γ levels in healthy children and children with LTBI and determine a new cut-off value of IFN-γ that can distinguish children with LTBI from healthy controls. The specificity of IFN-γ couldn’t be analysed too.
In conclusion, the present study indicates that IFN-γ levels are significantly higher in children with active TB, and children with close contact with TB patient. It has better sensitivity in active TB, and the correlation between TST induration diameter and IFN-γ levels is stronger in children with active TB versus children with LTBI. Using both tests (IFN-γ and TST) can improve the diagnose of LTBI in children in countries like R. Macedonia where vaccination with BCG is widespread. According to our results that IFN-γ has better sensitivity and stronger correlation with TST inactive TB, these tests can contribute to better and certain diagnose of TB disease, because of a lack of microbiological proof for M. tuberculosis in children.
Footnotes
Funding: This research did not receive any financial support
Competing Interests: The authors have declared that no competing interests exist
References
- 1.Report WHO. 2010, Global tuberculosis control-surveillance, planning, financing. Geneva, Switzerland: 2010. [Google Scholar]
- 2.World Health Organization. Global Tuberculosis Control: Surveillance, Planning, Financing. Geneva, Switzerland: World Health Organization; 2002. [Google Scholar]
- 3.World Health Organization. Global Tuberculosis Report 2016. WHO; 2016. [Google Scholar]
- 4.Perez-Porcuna TM, Ascaso C, Malheiro A, Abellana R, Martins M, et al. Mycobacterium tuberculosis Infection in Young Children: Analyzing the Performance of the Diagnostic Tests. PLoS ONE. 2014;9(5):e97992. doi: 10.1371/journal.pone.0097992. https://doi.org/10.1371/journal.pone.0097992 PMid:24879374 PMCid: PMC4039466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Starke JR. Tuberculosis in children. Semin Respir Crit Care Med. 2004;25:353–64. doi: 10.1055/s-2004-829507. https://doi.org/10.1055/s-2004-829507 PMid:16088476. [DOI] [PubMed] [Google Scholar]
- 6.Marais BJ, Gie RP, Hesseling AC, et al. A refined symptom-based approach to diagnose pulmonary tuberculosis in children. Pediatrics. 2006;118:1350–9. doi: 10.1542/peds.2006-0519. https://doi.org/10.1542/peds.2006-0519 PMid:17079536. [DOI] [PubMed] [Google Scholar]
- 7.Gatahun H, Mateelli A, Chaisson RE, Raviglione M. Latent Mycobacterium tuberculosis infection. Engl J Med. 2015;372(22):2127–35. doi: 10.1056/NEJMra1405427. https://doi.org/10.1056/NEJMra1405427 PMid:26017823. [DOI] [PubMed] [Google Scholar]
- 8.Cardona PJ. A dynamic reinfection hypothesis of latent tuberculosis infection. Infection. 2009;37:80–6. doi: 10.1007/s15010-008-8087-y. https://doi.org/10.1007/s15010-008-8087-y PMid:19308318. [DOI] [PubMed] [Google Scholar]
- 9.Wiker HG, Mustafa T, Bjune GA, Harboe M. Evidence for waning of latency in a cohort study of tuberculosis. BMC Infect Dis. 2010;10:1–10. doi: 10.1186/1471-2334-10-37. https://doi.org/10.1186/1471-2334-10-37 PMid:20178619;PMCid: PMC2843612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shingadia D, Novelli V. Diagnosis and treatment of tuberculosis in children. Lancet Infect Dis. 2003;3:624–32. doi: 10.1016/s1473-3099(03)00771-0. https://doi.org/10.1016/S1473-3099(03)00771-0. [DOI] [PubMed] [Google Scholar]
- 11.Marais BJ, Pai M. Recent advances in the diagnosis of childhood tuberculosis. Arch Dis Child. 2007;92:446–52. doi: 10.1136/adc.2006.104976. https://doi.org/10.1136/adc.2006.104976 PMid:17449528 PMCid: PMC2083717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Uplekar M, Weil D, Lonnroth K, Jaramillo E, Lienhardt C, Dias HM, et al. WHO's new end TB strategy. Lancet. 2015;385(9979):1799–801. doi: 10.1016/S0140-6736(15)60570-0. https://doi.org/10.1016/S0140-6736(15)60570-0. [DOI] [PubMed] [Google Scholar]
- 13.Review of the national tuberculosis programme on former Yugoslav Republic of Macedonia. 2016:24–25. [Google Scholar]
- 14.Beyers N, Gie RP, Schaaf HS, Van Zyl S, Talent JM, Nel ED, Donald PR. A prospective evaluation of children under the age of 5 years living in the same household as adults with recently diagnosed pulmonary tuberculosis. The International Journal of Tuberculosis and Lung Disease. 1997;1(1):38–43. PMid:9441057. [PubMed] [Google Scholar]
- 15.Wang L, Turner MO, Elwood RK, et al. A meta-analysis of the effect of Bacille Calmette Guerin vaccination on tuberculin skin test measurements. Thorax. 2002;57:804–809. doi: 10.1136/thorax.57.9.804. https://doi.org/10.1136/thorax.57.9.804 PMid:12200526 PMCid: PMC1746436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.American Thoracic Society. Diagnostic standards and classification of tuberculosis in adults and children. Am J Respir Crit Care Med. 2000;161:1376–95. doi: 10.1164/ajrccm.161.4.16141. https://doi.org/10.1164/ajrccm.161.4.16141 PMid:10764337. [DOI] [PubMed] [Google Scholar]
- 17.Jakjovski Lj. Belodrobna tuberkuloza vo detska vozrast. Skopje: Nova Makedoniaj; 1996. [Google Scholar]
- 18.Diel R, Loddenkemper R, Meywald-Walter K, Gottschalk R, Nienhaus A. Comparative performance of tuberculin skin test, QuantiFERON-TB-Gold InTube assay, and T-Spot. TB test in contact investigations for tuberculosis. Chest. 2009;135:1010–8. doi: 10.1378/chest.08-2048. https://doi.org/10.1378/chest.08-2048 PMid:19017873. [DOI] [PubMed] [Google Scholar]
- 19.Lalvani A, Thillai M. Diagnosis of tuberculosis: principles and practice of using interferon-γrelease assays (IGRAs) Breathe. 2009;5:303–9. https://doi.org/10.1183/18106838.0504.302. [Google Scholar]
- 20.Andersen P, Munk ME, Doherty TM, et al. Specific immune-based diagnosis of tuberculosis. Lancet. 2000;356:1099–104. doi: 10.1016/s0140-6736(00)02742-2. https://doi.org/10.1016/S0140-6736(00)02742-2. [DOI] [PubMed] [Google Scholar]
- 21.Chun JK, Kim CK, Kim HS. i sur The role of whole blood interferon gamma assay for the detection of latent tuberculosis infection in bacillus Calmette-Guerin vaccinated children. Diagn Microbiol Infect Dis. 2008;62:389–94. doi: 10.1016/j.diagmicrobio.2008.08.022. https://doi.org/10.1016/j.diagmicrobio.2008.08.022 PMid:18990532. [DOI] [PubMed] [Google Scholar]
- 22.Pai M, Zwerlig A, Menzies D. Systematic review: T-cell based assays for the diagnosis of latent tuberculosis infection –an update. Ann Intern Med. 2008;149:177–84. doi: 10.7326/0003-4819-149-3-200808050-00241. https://doi.org/10.7326/0003-4819-149-3-200808050-00241 PMid:18593687 PMCid: PMC2951987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sun L, Xiao J, Miao Q, et al. Interferon gamma release assay in diagnosis of pediatric tuberculosis: a meta-analysis. FEMS Immunol Med Microbiol. 2011;63(2):165–173. doi: 10.1111/j.1574-695X.2011.00838.x. https://doi.org/10.1111/j.1574-695X.2011.00838.x PMid:22077219. [DOI] [PubMed] [Google Scholar]
- 24.Whittaker E, Gordon A, Kampmann B. Is IP-10 a better biomarker for active and latent tuberculosis in children than IFN gamma? PLoS One. 2008;3:e3901. doi: 10.1371/journal.pone.0003901. https://doi.org/10.1371/journal.pone.0003901 PMid:19065267 PMCid: PMC2588495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Latorre I, De Souza-Galvao M, Ruiz-Manzano J, et al. Quantitative evaluation of T-cell response after specific antigen stimulation in active and latent tuberculosis infection in adults and children. Diagn Microbiol Infect Dis. 2009;65:236–46. doi: 10.1016/j.diagmicrobio.2009.07.015. https://doi.org/10.1016/j.diagmicrobio.2009.07.015 PMid:19822269. [DOI] [PubMed] [Google Scholar]
- 26.Connell TG, Curtis N, Ranganathan SC, et al. Performance of a whole blood interferon gamma assay for detecting latent infection with Mycobacterium tuberculosis in children. Thorax. 2006;61:616–620. doi: 10.1136/thx.2005.048033. https://doi.org/10.1136/thx.2005.048033 PMid:16601088 PMCid: PMC2104654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Connel TG, Ritz N, Paxton GA, et al. A three-way comparison of tuberculin skin testing, quantiFERON-TB gold and T-SPOT. TB in children. PLoS ONE. 2008;3:e2624. doi: 10.1371/journal.pone.0002624. https://doi.org/10.1371/journal.pone.0002624 PMid:18612425 PMCid: PMC2440545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nakaoka H, Lawson L, Squire SB, Coulter B, Ravn P, Brock I, Hart CA, Cuevas LE. Risk for tuberculosis among children. Emerging infectious diseases. 2006;12(9):1383. doi: 10.3201/eid1209.051606. https://doi.org/10.3201/eid1209.051606 PMid:17073087 PMCid: PMC3294731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Onur H, Hatipoğlu S, Arıca V, Hatipoğlu N, Arıca SG. Comparison of quantiferon test with tuberculin skin test for the detection of tuberculosis infection in children. Inflammation. 2012;35(4):1518–24. doi: 10.1007/s10753-012-9466-1. https://doi.org/10.1007/s10753-012-9466-1 PMid:22535495 PMCid: PMC3397234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kang YA, Lee HW, Yoon HI, Cho B, Han SK, Shim YS, Yim JJ. Discrepancy between the tuberculin skin test and the whole-blood interferon γassay for the diagnosis of latent tuberculosis infection in an intermediate tuberculosis-burden country. Jama. 2005;293(22):2756–61. doi: 10.1001/jama.293.22.2756. https://doi.org/10.1001/jama.293.22.2756 PMid:15941805. [DOI] [PubMed] [Google Scholar]


