Abstract
BACKGROUND:
Retinopathy of Prematurity (ROP) is a potentially blinding vasoproliferative disease in premature babies. The presentation and course of ROP are determined by a complex interaction of a series of risk factors, including artificial reproductive technology (ART).
AIM:
To analyse and combine the information relating ART as an independent risk factor for retinopathy of prematurity.
METHODS AND MATERIAL:
The article is systematic review and meta-analysis using RevMan 5. Pubmed, Scopus and Medline were searched for articles from 1990 to 2018.
RESULTS:
Studies suggest that ROP is observed more frequently in ART children. They are more likely to be premature and of low birth weight than those conceived naturally. Results vary from just a tendency to a five-fold increase in risk to develop ROP in ART babies. At the same time, they might develop ROP later, and more mature newborns might be affected.
CONCLUSION:
The data relating ART as a risk factor for ROP is inconclusive, but most studies show at least a tendency. The ART newborns need to be considered as a risk group for ROP and observed with greater suspicion. Even more mature ART newborns might need to be screened in order not to miss any significant pathology.
Keywords: Premature, Assisted conception, Risk factor, Retinopathy of prematurity, Artificial reproductive technology
Introduction
Retinopathy of Prematurity (ROP) is a potentially blinding vasoproliferative disease in premature babies. Recent advances in neonatal care have improved the survival rates for premature infants, and this has been accompanied by an increase in the incidence of ROP [1] [2]. If it remains unrecognised and untreated, it can cause severe visual impairment and blindness in children. Nowadays ROP can be prevented by timely screening [3].
The presentation and course of ROP are determined by a complex interaction of several risk factors like gestational age, birth weight, as well as systemic risk factors like anaemia, sepsis, jaundice and multiple blood transfusions [4]. Other significant risk factors for developing ROP are: artificial ventilation (for more than 5 days), respiratory distress syndrome, intraventricular hemorrhage and periventricular leukomalacia, congenital heart disease, sepsis [5], low Apgar score in the first and fifth minutes, longer duration of oxygen therapy [3]; intrauterine hypotrophy, bronchopulmonary dysplasia [6]. Poor postnatal growth in the first weeks of life [7] [8] and early postnatal hyperglycemia [9] [10] [11] are also associated with later development of ROP, while phototherapy used to treat hyperbilirubinemia is considered a protective factor [6].
This study aims to analyse and combine the information relating ART as an independent risk factor for ROP.
Methods and Materials
Systematic review and meta-analysis (using RevMan 5) were performed. Online databases: Pubmed, Scopus and Medline were searched for articles on this topic from 1990 to 2018.
Results and Discussion
Some authors report they raised the frequency of ocular abnormalities in children born after in vitro fertilisation (IVF). In the study of Anteby major ocular malformations were observed in 12 (26%) of 47 children. These malformations included Coats disease, congenital cataract, congenital glaucoma, hypoplastic optic nerve head, idiopathic optic atrophy, coloboma with microphthalmos, and retinoblastoma [12]. A probability of fixation condition and visual deficiencies in these infants was also suggested [13]. These adverse outcomes of assisted conception are because the gametes are exposed to a variety of drugs, physically manipulated, nurtured in potentially hazardous conditions, and perhaps placed in an inappropriate uterine environment [1]. However, according to another study which included thirty-six IVF infants with ocular malformations, the risk, compared with non-IVF children, was not increased when adjusted for maternal age, parity, smoking, and body mass index (odds ratio, 1.05; 95% confidence interval, 0.75 to 1.47) [14].
ART multiple birth babies make up a considerable proportion of the ROP screening burden, and their number is likely to increase as ART is increasingly available and utilised [15]. McKibbin et al. were one of the first to investigate the workload imposed by the treatment for infertility on a ROP screening programme. They reviewed the records of all babies born between August 1991 and December 1994 in the Assisted Conception Unit and of all babies screened for ROP over the same period. Of the babies born after ART, 20% fulfilled the ROP screening criteria. ROP of any stage was present in 23% of all the assisted conception babies screened [16]. As a follow up of McKibbin et al., C. Funnell and T. Dabbs made retrospective study utilising computerised databases of ROP screening, live births, assisted conception (AC) and multiple births between April 1st 2000 and August 31st 2003 at St James’s Hospital, Leeds. They concluded that the percentage of AC babies requiring ROP screening had fallen since McKibbin et al., This appeared to be at least partially due to the reduced multiple birth rates. This reduction in the multiple birth rates follows evidence that reducing the number of embryos transferred does not reduce the number of couples taking home a baby. Human Fertility and Embryology Association guidelines recommend transferring no more than two embryos in an IVF cycle. Changes in clinical practice at St James’s Hospital have significantly reduced the likelihood of AC babies requiring ROP screening and developing ROP [17]. However, even singleton births resulting from AC are more likely to be premature and of low birth weight than those conceived naturally [18]. This higher rate may be attributed to various infertility cofactors, such as uterine malformations, previous operative procedures that involved cervical dilatation, and a history of pelvic infection [19].
In twin births, both babies have the same gestational age and pre-natal conditions. However, twins may develop a varied ROP course depending on birth weight and other systemic factors. The profile of 56 pairs of twins with ROP was studied and analysed for differences in zone or need for treatment while studying possible causes for the varied outcome. In 45 pairs of twins (80%) the disease progressed identically in both eyes, while in 11 pairs (20%) the ROP showed differences in zone or need for treatment. Four of these pairs were discordant. In 3 of these 4 pairs, the heavier birth weight twin had a more severe ROP course. Twins can present with asymmetric ROP course, and it is, therefore, essential to examine both twins as per screening protocols [20]. Discordant twins (discordancy is defined as a difference of 15% or more in the birth weights of the twins) may be at increased risk for ROP because of factors related to their unequal growth. 38% (10) of the lower birthweight infants had higher grades of ROP than their bigger twin [10]. Ninety-nine infants from multiple gestation births and weighing ≤ 1500 g at birth were matched with infants from single births to clarify the relationship of multiple gestations to ROP. There was no significant difference in the incidence of ROP between the twins and the singletons (relative risk [RR] = 0.84, 95% confidence intervals [CI] = 0.61, 1.16). Logistic regression analysis confirmed that very low birth weight (VLBW), despite being single or multiple gestations, was the most significant predictor of ROP occurrence in either group. These results indicate that ROP screening in VLBW twins may be conducted according to the same standard protocols as for singletons [21]. Two other studies also showed no significant difference in stage of ROP between infants of single-gestation pregnancies vs those of multiple-gestation pregnancies [22] [6]. Surprisingly, V. Chernodrinska et al. even reported a lower risk for development of ROP in infants from multiple than from single birth [23].
There is some debate regarding whether ART constitutes an independent risk factor for ROP. Studies have reported conflicting results regarding this relationship. Some of them failed to demonstrate any association between the two. Friling et al. examined routinely a study group consisting of 363 infants with a birth weight (B, W) of ≤ 1500 gm, who were hospitalised in the neonatal unit of a single tertiary-care centre between 1998 and 2000. Data on gestational age (GA), BW, type of pregnancy (singleton/multiple), and type of conception (natural/assisted) were recorded, in addition to the ophthalmological results. In their sample, AC per se did not appear to be a risk factor for ROP. Singleton babies with a birth weight of ≤ 1500 g were more prone to develop ROP stages II and III than twins or triplets. GA and BW were the most significant factors associated with ROP [21]. An article of a Turkish team was published in 2016 analysing the medical records of consecutive premature triplets who had been screened for ROP in a single maternity hospital. The presence of ROP was not associated with the mode of conception (p = 0.674) [24].
Some studies show only a tendency of ART being an independent risk factor for ROP, without proving statistically significant results, so their data do not rule out a possible association. One of the first teams to study these problems [16] reviewed the records of all babies born between August 1991 and December 1994 as a result of treatment in the Assisted Conception Unit, and all babies screened for ROP over the same period. Of the babies born after AC treatment, 20% fulfilled the ROP screening criteria. ROP of any stage was present in 23% of all the assisted conception babies screened. This group also accounted for a large proportion of those reaching stage 3 disease and of those requiring treatment [16]. Watts and Adams found an association between the development of threshold severe ROP and ART (specifically IVF). They carried out a retrospective study between Dec. 1995 and Dec. 1998 of infants in a single neonatal unit serving the Brent and Harrow area of North West Thames, requiring screening and treatment of ROP. In this study, 11.7% of the group requiring screening was conceived by AC. Of all babies requiring treatment for ROP, 28.6% were born after AC. Of the AC group, 83.3% were conceived by IVF. AC using IVF rather than other techniques appeared to be the major risk factor for the development of threshold ROP. The authors advise increased vigilance when screening babies conceived by the IVF methods of AC [25]. Barker et al. performed a retrospective audit of all multiple birth babies admitted to a tertiary neonatal unit, who met the UK ROP screening criteria. A total of 205 babies met the criteria, of whom 87.3% were twins. They found no significant difference between the numbers of babies developing ROP in the ART vs non-ART groups. However, the estimated odds of developing ROP were slightly higher in the ART babies [15].
The first team to demonstrate a statistically significant association between ART and severe ROP requiring treatment in infants in the US performed their studies in Weill Cornell Medical Center Neonatal Intensive Care Unit at the New York-Presbyterian Hospital from 2002 to 2008. According to their studies AC placed infants at greater risk for treatment-requiring ROP [OR 4.5; 95% CI 1.3-15.5; p = 0.0150] [26]. Using multifactor analysis, the key finding was that, regardless of birth weight, ART was associated with a nearly five-fold increased risk of severe ROP requiring treatment, after controlling for potential confounders (OR 4.70, CI 1.52–14.57; P = 0.007). Gestational age was also a significant risk factor, and both gestational age and ART appear to be independent risk factors associated with risk of severe ROP requiring laser [27]. A few years later Yau et al., using univariate analysis showed IVF was significant independent risk factors for Type 1 ROP. A retrospective review of medical records was performed for all neonates of multiple gestations (n = 153) screened for ROP between January 2007 and December 2012 in 2 neonatal intensive care units in Hong Kong [28]. The same team a year later published an article which included all infants (n = 513) that were screened from this same period. It did not show IVF as an independent risk factor for ROP but it still showed a tendency/ odd ratio = 2.07/CI = 0.47- 6.54/ [29].
Minasion and Fielder reported that severe ROP could affect larger and more mature babies conceived through IVF, compared with those who are not. They presented three babies conceived IVF who were close to the limits of the current screening criteria [30]. Chan et al., also observed that although the mean gestational age at birth was similar between the ART and the natural conception groups, the mean time of treatment for severe ROP was approximately one week later in the ART group (36 2/7 weeks) [27]. Watts and Adams also noted that for infants developing Stage 3 severe ROP, those conceived through IVF were born at larger gestational ages on average and with heavier birth weights than infants who received other forms of ART [25]. These differences in weight and gestational age between babies requiring treatment for severe ROP in the ART and natural conception groups may also have implications for screening criteria.
We included all the retrospective studies with newborns screened for ROP investigating the association between ART and ROP as an independent risk factor (Table 1).
Table 1.
ART as an independent risk factor for ROP
| Author/year | Country | Follow-up | Sample size | Natural conception | ART | Odds ratio | Significance |
|---|---|---|---|---|---|---|---|
| McKibbin, 1996 | England | 3 years | 267 | 233 | 44 | - | p>0,05 only tendency |
| Watts, 2000 | England | 3 years | 179 | 152 | 27 | - | p>0.05 only tendency ROP 3 |
| Friling, 2007 | Israel | 2 years | 363 | 204 | 159 | - | P>0,05, no significance |
| Chan P.,2010 | USA | 5 years | 399 | 253 | 146 | 4.70; [CI], 1.52–4.57 | 0,007 ROP 3 |
| Şekeroğlu M., 2016 | Turkey | 2 years | 54 | 18 | 36 | - | 0,674 |
| Barker L, 2017 | USA | 5 years | 205 | 125 | 80 | - | 0,837 |
| Yau GS, 2016 | China | 5 years | 513 | --- | ---- | 2.07/CI= 0.47- 6.54 | 0,3 ROP 1 |
Of all the included studies only four had sufficient data and homogeneity and were included in the meta-analysis. We used random effect forest plot to pool the results of those studies answering if ART is an independent risk factor for ROP stage 3.
The results show that the studies with the biggest weight are Chan, 2010 and Wastts, 2000. All the studies are homogenous (heterogeneity Chi2 = 1.46, I = 0%). There is a positive association between ART and ROP stage 3 – Odds ratio = 1.64/0.96-2.83/ even though the test for overall effect does not show statistical significance /p = 0.07/ (Figure 1). The results of the risk ratio are also positive 1.56 [0.96, 2.52] (Figure 2).
Figure 1.
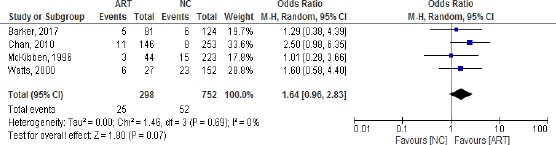
Odds Ratios
Figure 2.
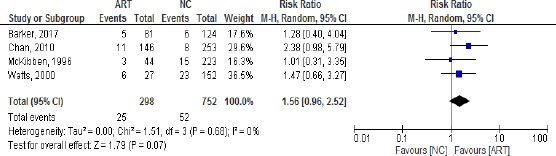
Risk Ratio
Conclusion
ART babies represent a large percentage of babies screened for ROP, and their number is likely to increase. Research on ART as a risk factor for ROP is present, though the subject became a popular study topic in recent years. Although the data relating artificial reproduction as an independent risk factor ROP is inconclusive, it still raises several questions which need further examination:
- Multiple gestations are rejected as an independent risk factor, though it indirectly leads to earlier birth and lower weight. Another thing to consider is discordancy between twins, which increases the risk of ROP.
- Even though results from the literature review are inconsistent, meta-analysis shows at least an increased tendency to develop ROP stage 3. Combined with the fact that ROP in ART infants develops later and affects heavier newborns, a change of screening criteria might be required in the future.
Further studies on this topic are needed, to fully recognise the potential differences in the ocular development of ART babies compared to naturally conceived ones.
Footnotes
Funding: This research did not receive any financial support
Competing Interests: The authors have declared that no competing interests exist
References
- 1.Gibson DL, Sheps SB, Uh SH, Schechter MT, McCormick AQ. Retinopathy of prematurity-induced blindness: birth weight-specific survival and the new epidemic. Pediatrics. 1990;6:405–12. [PubMed] [Google Scholar]
- 2.Valentine PH, Jackson JC, Kalina RE, Woodrum DE. Increased survival of low birth weight infants: impact on the incidence of retinopathy of prematurity. Pediatrics. 1989;84(3):442–5. PMid:2788864. [PubMed] [Google Scholar]
- 3.Alajbegovic-Halimic J, Zvizdic D, Alimanovic-Halilovic E, Dodik I, Duvnjak S. Risk factors for retinopathy of prematurity in prematurely born children. Medical Archives. 2015;69(6):409. doi: 10.5455/medarh.2015.69.409-413. https://doi.org/10.5455/medarh.2015.69.409-413 PMid:26843736 PMCid: PMC4720470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dutta S, Narang S, Narang A, Dogra M, Gupta A. Risk factors of threshold retinopathy of prematurity. Indian Pediatr. 2004;41(7):665–71. PMid:15297681. [PubMed] [Google Scholar]
- 5.Yang CS, Chen SJ, Lee FL, Hsu WM, Liu JH. Retinopathy of Prematurity: Screening, Incidence and Risk Factors Analysis. :201. [PubMed] [Google Scholar]
- 6.Mladenov O, Chernodrinska V, Petkova I, Dimitrova G, Kemilev P, et al. Retinopathy of Prematurity –Incidence and Risk Factors in Bulgaria. International Journal of Pharmaceutical Science Invention. 2017;6(7):18–23. [Google Scholar]
- 7.Hellstrom A, Hard AL, Engstrom E, Niklasson A, Andersson E, Smith L, et al. Early weight gain predicts retinopathy in preterm infants: new, simple, efficient approach to screening. Pediatrics. 2009;123:e638–45. doi: 10.1542/peds.2008-2697. https://doi.org/10.1542/peds.2008-2697 PMid:19289449. [DOI] [PubMed] [Google Scholar]
- 8.Lofqvist C, Andersson E, Sigurdsson J, Engstrom E, Hard AL, Niklasson A, et al. Longitudinal postnatal weight and insulin-like growth factor I measurements in the prediction of retinopathy of prematurity. Arch Ophthalmol. 2006;124:1711–8. doi: 10.1001/archopht.124.12.1711. https://doi.org/10.1001/archopht.124.12.1711 PMid:17159030. [DOI] [PubMed] [Google Scholar]
- 9.Blanco CL, Baillargeon JG, Morrison RL, Gong AK. Hyperglycemia in extremely low birth weight infants in a predominantly Hispanic population and related morbidities. J Perinatol. 2006;26:737–41. doi: 10.1038/sj.jp.7211594. https://doi.org/10.1038/sj.jp.7211594 PMid:16929343. [DOI] [PubMed] [Google Scholar]
- 10.Fellows RR1, McGregor ML, Bremer DL, Rogers GL, Miller D. Retinopathy of prematurity in discordant twins. J Pediatr Ophthalmol Strabismus. 1995;32(2):86–8. doi: 10.3928/0191-3913-19950301-06. PMid:7629675. [DOI] [PubMed] [Google Scholar]
- 11.Garg R, Agthe AG, Donohue PK, Lehmann CU. Hyperglycemia and retinopathy of prematurity in very low birth weight infants. J Perinatol. 2003;23:186–94. doi: 10.1038/sj.jp.7210879. https://doi.org/10.1038/sj.jp.7210879 PMid:12732854. [DOI] [PubMed] [Google Scholar]
- 12.Anteby I, Cohen E, Anteby E, Ben Ezra D. Ocular manifestations in children born after in vitro fertilisation. Arch Ophthalmol. 2001;119(10):1525–9. doi: 10.1001/archopht.119.10.1525. https://doi.org/10.1001/archopht.119.10.1525 PMid:11594955. [DOI] [PubMed] [Google Scholar]
- 13.Jafarzadehpur E, Kermani RM, Mohhamadi AR, Nateghi MR, Fazeli AS, Kashi KM. Ocular Manifestations in Infants Resulted from Assisted Reproductive Technology (ART) Journal of family & reproductive health. 2013;7(4):181. PMid:24971123 PMCid: PMC4064753. [PMC free article] [PubMed] [Google Scholar]
- 14.Tornqvist K, Finnström O, Källén B, Lindam A, Nilsson E, Nygren KG, Olausson PO. Ocular malformations or poor visual acuity in children born after in vitro fertilisation in Sweden. American journal of ophthalmology. 2010;150(1):23–6. doi: 10.1016/j.ajo.2010.01.035. https://doi.org/10.1016/j.ajo.2010.01.035 PMid:20447615. [DOI] [PubMed] [Google Scholar]
- 15.Barker L, Bunce C, Husain S, Adams GG. Is artificial reproductive technology a risk factor for retinopathy of prematurity independent of the generation of multiple births? Eur J Ophthalmol. 2017;27(2):174–178. doi: 10.5301/ejo.5000832. https://doi.org/10.5301/ejo.5000832 PMid:27445066. [DOI] [PubMed] [Google Scholar]
- 16.McKibbin M, Dabbs TR. Assisted conception and retinopathy of prematurity. Eye. 1996;10(4):476. doi: 10.1038/eye.1996.105. https://doi.org/10.1038/eye.1996.105 PMid:8944102. [DOI] [PubMed] [Google Scholar]
- 17.Funnell CL, Dabbs TR. Assisted conception and retinopathy of prematurity:8-year follow-up study. Eye. 2007;21(3):383. doi: 10.1038/sj.eye.6702215. https://doi.org/10.1038/sj.eye.6702215 PMid:16410811. [DOI] [PubMed] [Google Scholar]
- 18.McFaul PB, Patel N, Mills J. An audit of the obstetric outcome of 148 consecutive pregnancies from assisted conception: implications for neonatal services. Br J Obstet Gynaecol. 1993;100(9):820–5. doi: 10.1111/j.1471-0528.1993.tb14306.x. https://doi.org/10.1111/j.1471-0528.1993.tb14306.x PMid:8218001. [DOI] [PubMed] [Google Scholar]
- 19.Perri T, Chen R, Yoeli R, Merlob P, Orvieto R, Shalev Y, Ben-Rafael Z, Bar-Hava I. Clinical Assisted Reproduction: Are Singleton Assisted Reproductive Technology Pregnancies at Risk of Prematurity? Journal of assisted reproduction and genetics. 2001;18(5):245–9. doi: 10.1023/A:1016614217411. https://doi.org/10.1023/A:1016614217411 PMid:11464574 PMCid: PMC3455328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Azad R, Chandra P, Patwardhan SD, Gupta A. Profile of asymmetrical retinopathy of prematurity in twins. Indian journal of ophthalmology. 2010;58(3):209. doi: 10.4103/0301-4738.62645. https://doi.org/10.4103/0301-4738.62645 PMid:20413923 PMCid: PMC2886251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Friling R, Axer-Siegel R, Hersocovici Z, Weinberger D, Sirota L, Snir M. Retinopathy of prematurity in assisted versus natural conception and singleton versus multiple births. Ophthalmology. 2007;114(2):321–4. doi: 10.1016/j.ophtha.2006.11.010. https://doi.org/10.1016/j.ophtha.2006.11.010 PMid:17270680. [DOI] [PubMed] [Google Scholar]
- 22.Blumenfeld LC, Siatkowski RM, Johnson RA, Feuer WJ, Flynn JT. Retinopathy of prematurity in multiple-gestation pregnancies. American journal of ophthalmology. 1998;125(2):197–203. doi: 10.1016/s0002-9394(99)80092-0. https://doi.org/10.1016/S0002-9394(99)80092-0. [DOI] [PubMed] [Google Scholar]
- 23.Chernodrinska V, Oscar A, Cherninkova S. RetCam screening of prematurely born children and evaluation of the risk factors for the development of retinopathy. Pediatriya. 2011;51(4):21–23. [Google Scholar]
- 24.Şekeroğlu MA, Hekimoğlu E, Çelik Ü, Kale Y, Baş AY. Retinopathy of prematurity in triplets. Turkish journal of ophthalmology. 2016;46(3):114. doi: 10.4274/tjo.94815. https://doi.org/10.4274/tjo.94815 PMid:27800273; PMCid: PMC5076293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Watts P, Adams GG. In vitro fertilisation and stage 3 retinopathy of prematurity. Eye. 2000;14:330–3. doi: 10.1038/eye.2000.82. https://doi.org/10.1038/eye.2000.82 PMid:11026994. [DOI] [PubMed] [Google Scholar]
- 26.Wong R, Yonekawa Y, Sun G, DeAngelis MM, Morrison M, et al. Update: Assisted Conception and Progression of Retinopathy of Prematurity. Investigative Ophthalmology & Visual Science. 2009;50:3143. [Google Scholar]
- 27.Chan P, Yonekawa Y, Morrison M, Sun G, Wong R, et al. Association between assisted reproductive technology and advanced retinopathy of prematurity. Clin Ophthalmol. 2010;4:1385–1390. doi: 10.2147/OPTH.S15587. PMid:21179223 PMCid: PMC2999553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yau GS, Lee JW, Tam VT, Yip S, Cheng E, Liu CC, Chu BC, Wong IY. Incidence and risk factors for retinopathy of prematurity in multiple gestations: a Chinese population study. Medicine. 2015;94:18. doi: 10.1097/MD.0000000000000867. https://doi.org/10.1097/MD.0000000000000867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yau GS, Lee JW, Tam VT, Liu CC, Yip S, Cheng E, Chu BC, Yuen CY. Incidence and risk factors of retinopathy of prematurity from 2 neonatal intensive care units in a Hong Kong Chinese population. The Asia-Pacific Journal of Ophthalmology. 2016;5(3):185–91. doi: 10.1097/APO.0000000000000167. https://doi.org/10.1097/APO.0000000000000167 PMid:27183289. [DOI] [PubMed] [Google Scholar]
- 30.Minasian M, Fielder A. IVF babies with ROP at higher gestational age and birth weight: implications of changing screening criteria. Br J Ophthalmol. 2005;89(8):1066. doi: 10.1136/bjo.2004.062935. https://doi.org/10.1136/bjo.2004.062935 PMid:16024870 PMCid: PMC1772773. [DOI] [PMC free article] [PubMed] [Google Scholar]


