Abstract
BACKGROUND:
According to several authors, neutrophil extracellular traps (NETs) play an important role in the mechanisms of cancer development and metastatic processes, which allows them to be considered as a potential new target for the treatment of cancer.
AIM:
To investigate the presence of extracellular neutrophil traps in the blood of patients with cervical cancer on the background of the combined treatment.
MATERIALS AND METHODS:
The study was conducted in 28 patients with cervical cancer. Group 1 received only radiation therapy; Groups 2-radiation therapy with ftorafur; Group 3-radiation therapy with cisplatin. To determine the number of spontaneous extracellular neutrophilic traps in the blood of the examined individuals, we used a technique of I.I. Dolgushin and Yu.S. Andreeva.
RESULTS:
Peripheral blood neutrophils in 53.57% (33.87; 72.49) of cervical cancer patients showed the ability to generate NETs before treatment. The ability to form NETs was observed in neutrophils isolated from 66.67% (9.43; 99.16) patients of the Group 1. After radiation therapy with ftorafur, the ability of blood neutrophils to form NETs was observed in 50% (1.26; 98.74) of cervical cancer patients. After radiotherapy with cisplatin, 37.50% (15.20; 64.57) of patients were found to have NETs formation
CONCLUSION:
The ability to form NETs varied greatly after radiotherapy. The addition of chemotherapy drugs to radiation therapy did not increase the percentage of NETs in the blood of patients with cervical cancer but stimulated the appearance of basophil extracellular traps.
Keywords: Neutrophil Extracellular Traps, NETs, Cervical Cancer
Introduction
The issue of the role of neutrophil extracellular traps (NETs) in oncology is at the stage of the study. An in vitro study of the formation of NETs after stimulation of neutrophils obtained from patients with colorectal cancer and healthy donors of the same age group was carried out. It was found that neutrophils of patients’ blood formed spontaneous NETs. A significant increase in the number of NETs in response to the stimulus was recorded in patients with postoperative complications. There was no relationship between the number of NETs, the stage and the localisation of a tumour. According to the authors of the study, these results allow us to consider the formation of NETs as a potential target for therapy [1].
At the same time, according to other researchers, neutrophils isolated from the blood of patients with uterine cancer (stage Ia) had a reduced ability to form traps in response to stimulation by yeast cells [2].
It was suggested that the formation of NETs could be one of the mechanisms of tumour development. The basis for this assumption was the evidence that the granulocyte colony-stimulating factor, which is produced by many tumours and is determined in the blood of cancer patients, triggers the formation of NETs [3].
It has been shown by Garley M. et al., that tumour-associated neutrophil can form and release NETs, but their effects are controversial. There is evidence that NETs have anticarcinogenic properties, which associated with the direct destruction of tumour cells and stimulation of the immune system. In contrast, some authors point to the ability of traps to facilitate the migration of tumour cells or create a physical barrier between tumour cells and immunocompetent cells [4].
NETs are believed to be involved in the pathogenesis of the tumour process through the mechanism of tumour-associated thrombosis. Several models of triggering thrombosis with the participation of NETs have been proposed. Thus, it is shown that neutrophils can produce tissue factor (TF), which is released into the bloodstream during the formation of the trap. The tissue factor forms a complex with factor VII. Complex TF-VIIa can activate the coagulation cascade, leading to the formation of a thrombus. According to another model, factor XII can bind to DNA and histones in the composition of NETs and stimulates the formation of fibrin in the internal pathway [5], [6]. Another way of participation of NETs in the mechanism of thrombosis is the capture and activation of platelets. NETs threads bind platelets and promote their aggregation [7]. [8]. It was also suggested that adhesion molecules, including von Willebrand factor, fibronectin or fibrinogen, are involved in the interaction between NETs and platelets [9], [10].
According to several authors, NETs play an important role in the mechanisms of cancer development and metastatic processes, which allows them to be considered as a potential new target for the treatment of cancer [11], [12]. At the same time, they also express the opposite point of view on the role of NETs in oncopathology. Myeloperoxidase, proteinases and histones can be cytotoxic concerning tumour cells. Moreover, NETs itself is considered as a kind of skeleton for the capture of tumour cells, which prevents their further spread [13].
Purpose of the study: to investigate the presence of extracellular neutrophil traps in the blood of patients with cervical cancer on the background of the combined treatment.
Material and Methods
The study was conducted in 28 patients with cervical cancer in the Karaganda Regional Oncology Centre in 2018. The average age of patients was 48.3 ± 1.9 years, of which 22 patients were diagnosed with stage IIb and 6 patients with stage III (FIGO 2009). The study was approved by the local Ethical Committee at the Medical University of Karaganda. Informed consent was obtained from the patients before they were recruited into the study.
All patients underwent radiation therapy (RT): external beam radiation (EBRT) was performed at the Clinac 600 C (3D CRT), brachytherapy was implemented on the “Agat VU” device. The patients were divided into 3 study groups. Patients of the 1st Group received only RT, 2nd group-RT with ftorafur oral at a dose of 1200 mg on the days of external beam radiation (800 mg in the morning and 400 mg in the evening), the total dose of 27,600 mg; 3 the group-RT with cisplatin at the rate of 40 mg/m2 7 days during the entire course of EBRT.
To determine the number of spontaneous extracellular neutrophilic traps in the blood of the examined individuals, we used a technique of I.I. Dolgushin and Yu.S. Andreeva [14]. The samples were stained with Hematoxylin-eosin and were examined microscopically under magnification of 1400. The results were expressed in the number of neutrophils that generated NETs in 100 neutrophils. Photos of NETs were obtained using the ToupView 3.7. We counted the percentage of patients whose smears contained NETs. Confidence intervals of percentages were determined by Clopper-Pearson.
Results
As a result of the study, it was shown that in practically healthy people, the ability of neutrophils to form NETs was not found, which corresponds with data from other researchers [15] [16]. Peripheral blood neutrophils in 53.57% (33.87; 72.49) of cervical cancer patients showed the ability to generate NETs before treatment. The number of NETs ranged from 1 to 23 in a smear (Figure 1).
Figure 1.
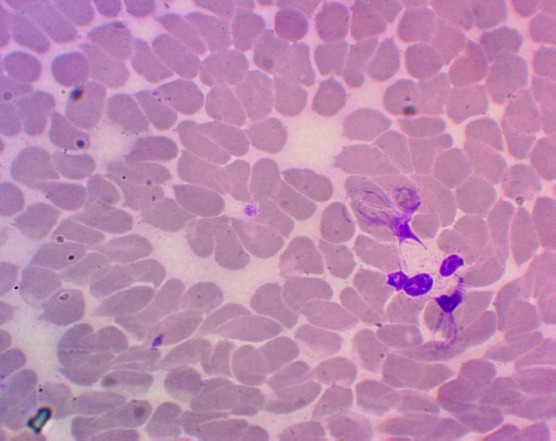
Neutrophil extracellular traps in the blood of a patient with cervical cancer (Hematoxylin-eosin staining, magnification 1400 x)
The dependence of NETs formation on the stage of cancer (IIb or III) was not found. NETs were not observed in 46.43% (27.51; 63.13) of the patients. In 3.57% (0.09; 18.35) of patients with cervical cancer, the formation of extracellular traps was detected not only by neutrophils but also by basophils.
The ability to form NETs was observed in neutrophils isolated from 66.67% (9.43; 99.16) patients of the 1st group. The number of NETs varied from 7 to 21 in a smear (Figure 2).
Figure 2.
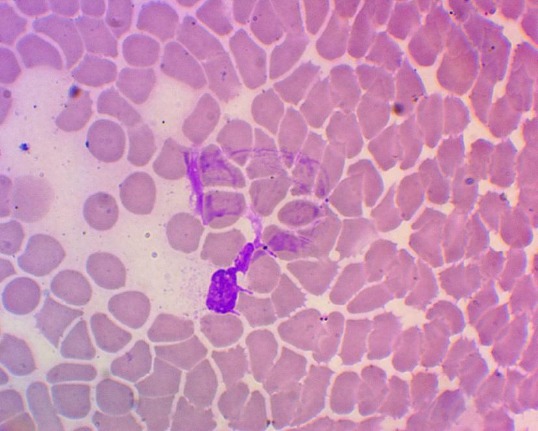
Neutrophil extracellular trap in the blood of a patient with cervical cancer after radiation therapy (hematoxylin-eosin staining, magnification 1400 x)
After radiation therapy with ftorafur, the ability of blood neutrophils to form NETs was observed in 50% (1.26; 98.74) of cervical cancer patients, but in a small amount - 1-2 in a smear (Figure 3). A feature of this group of patients should be considered the appearance in the blood of these patient’s basophil extracellular traps (from 1 to 7 in a smear).
Figure 3.
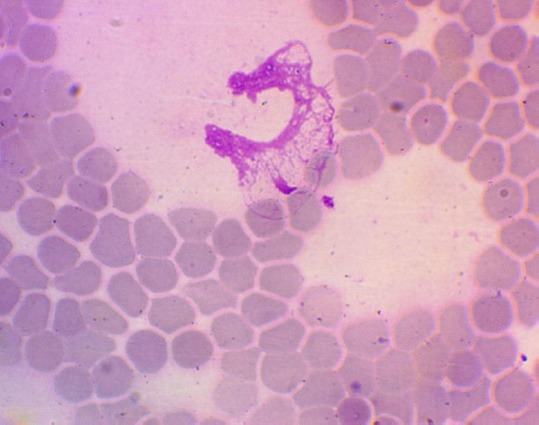
Neutrophil extracellular trap in the blood of a patient with cervical cancer after radiation therapy with ftorafur (Hematoxylin-eosin staining, 1400 x magnification)
After radiotherapy with cisplatin, 37.50% (15.20; 64.57) of patients were found to have NETs formation, and their number varied from 2 to 15 in a smear. In 12.50% (1.55; 38.35) of patients with cervical cancer, basophil extracellular traps were found in a number of 1 to 9 in a smear (Figure 4). It should be noted that neutrophil and basophil extracellular traps were found in the same patients.
Figure 4.
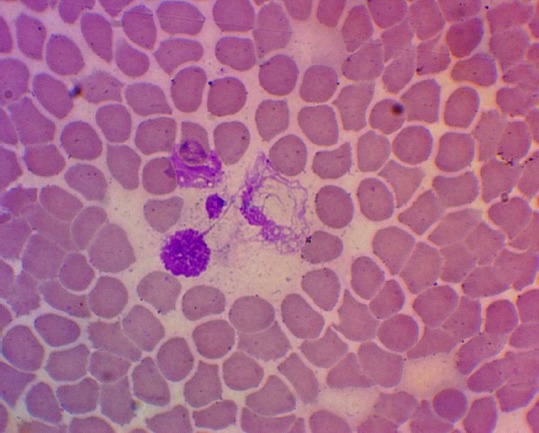
Neutrophil extracellular trap in the blood of a patient with cervical cancer after radiation therapy with cisplatin (Hematoxylin-eosin staining, 1400 x magnification)
Discussion
In all patients, the formation of NETs proceeds via suicidal NETosis which includes chromatin decondensation and releasing of network-like DNA after the rupture of the plasma membrane, i.e. according to the classical way [17]. It is noteworthy that not all neutrophils isolated from patients with cervical cancer have demonstrated the ability to form NETs. In our opinion, this is due to several reasons. First of all, neutrophils in the blood may be present in several subpopulations [18]. In some pathological states, including oncology, along with high-density neutrophils, the appearance of low-density neutrophils has been described [19]. It has been established that in cancer, low-density neutrophils exhibit a reduced ability to explode [20] oxidatively, but they are more susceptible to the formation of NETs [21]. According to other researchers, in the circulation, there are subpopulations of immature, mature and old neutrophils. The older the neutrophil, the easier it forms the NETs [22]. Trellakis S. et al. established that the neutrophils of the peripheral blood of patients with certain types of cancer had a prolonged period of life [23], which made it possible to assume that tumour cells probably produce factors that modulate the life of neutrophils [24]. The main cause of pre-activation of neutrophils is the excessive generation of reactive oxygen species as a result of ionising radiation. This assumption is based on literature data showing that hydrogen peroxide can be an inducer of NETs [25]. Addition of ftorafur and cisplatin to the radiotherapy did not lead to a significant increase in the number of NETs. In case of a combination of RT with cisplatin, the percentage of neutrophils capable of forming NETs was even lower than that before treatment. It is due to the decreased level of neutrophils under the influence of chemotherapy.
In conclusion, peripheral blood neutrophils generate NETs in 53.57% (33.87; 72.49) of cervical cancer patients before treatment. There are no NETs in healthy people.
There was no dependence of the formation of NETs on the stage of cervical cancer (IIb or III).
The ability to form NETs varied greatly after Radiotherapy. The addition of chemotherapy drugs to RT did not increase the percentage of NETs in the blood of patients with cervical cancer but stimulated the appearance of basophil extracellular traps.
Footnotes
Funding: This research did not receive any financial support
Competing Interests: The authors have declared that no competing interests exist
References
- 1.Richardson JJR, Hendrickse C, Gao-Smith F, Thickett DR. Neutrophil Extracellular Trap Production in Patients with Colorectal Cancer In Vitro. Int. J. Inflam. 2017;2017:4915062. doi: 10.1155/2017/4915062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abakumova TV, Antoneeva II, Gening TP, Dolgova DR, Gening SO. Phenotype of peripheral blood neutrophils in the initial stage of endometrial. cancer. 2016;58(1):23–29. [PubMed] [Google Scholar]
- 3.Demers M, Krause DS, Schatzberg D, et al. Cancers predispose neutrophils to release extracellular DNA traps that contribute to cancer-associated thrombosis. Proc Natl Acad Sci USA. 2012;109(32):13076–13081. doi: 10.1073/pnas.1200419109. https://doi.org/10.1073/pnas.1200419109 PMid:22826226 PMCid: PMC3420209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Garley M, Jabłońska E, Dąbrowska D. NETs in cancer. Tumour Biol. 2016;37(11):14355–14361. doi: 10.1007/s13277-016-5328-z. https://doi.org/10.1007/s13277-016-5328-z PMid:27614687. [DOI] [PubMed] [Google Scholar]
- 5.Von Brühl ML, Stark K, Steinhart A, et al. Monocytes neutrophils and platelets cooperate to initiate and propagate venous thrombosis in mice in vivo. J Exp Med. 2012;209(4):819–35. doi: 10.1084/jem.20112322. https://doi.org/10.1084/jem.20112322 PMid:22451716 PMCid: PMC3328366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wolberg AS, Aleman MM, Leiderman K, Machlus KR. Procoagulant activity in hemostasis and thrombosis: Virchow's triad revisited. Anesth Analg. 2012;114(2):275–85. doi: 10.1213/ANE.0b013e31823a088c. https://doi.org/10.1213/ANE.0b013e31823a088c PMid:22104070 PMCid: PMC3264782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ma AC, Kubes P. Platelets neutrophils and neutrophil extracellular traps (NETs) in sepsis. J Thromb Haemost. 2008;6(3):415–20. doi: 10.1111/j.1538-7836.2007.02865.x. https://doi.org/10.1111/j.1538-7836.2007.02865.x PMid:18088344. [DOI] [PubMed] [Google Scholar]
- 8.Fuchs TA, Bhandari AA, Wagner DD. Histones induce rapid and profound thrombocytopenia in mice. Blood. 2011;118(13):3708–14. doi: 10.1182/blood-2011-01-332676. https://doi.org/10.1182/blood-2011-01-332676 PMid:21700775 PMCid: PMC3186342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fuchs TA, Brill A, Duerschmied D, et al. Extracellular DNA traps promote thrombosis. Proc Natl Acad Sci USA. 2010;107(36):15880–5. doi: 10.1073/pnas.1005743107. https://doi.org/10.1073/pnas.1005743107 PMid:20798043 PMCid: PMC2936604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fuchs TA, Brill A, Wagner DD. Neutrophil extracellular trap (NET) impact on deep vein thrombosis. Arterioscler Thromb Vasc Biol. 2012;32(8):1777–83. doi: 10.1161/ATVBAHA.111.242859. https://doi.org/10.1161/ATVBAHA.111.242859 PMid:22652600 PMCid: PMC3495595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cools-Lartigue J, Spicer J, Najmeh S, Ferri L. Neutrophil extracellular traps in cancer progression. Cell Mol Life Sci. 2014;71(21):4179–94. doi: 10.1007/s00018-014-1683-3. https://doi.org/10.1007/s00018-014-1683-3 PMid:25070012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Erpenbeck L, Schön MP. Neutrophil extracellular traps: protagonists of cancer progression? Oncogene. 2017;36(18):2483–90. doi: 10.1038/onc.2016.406. https://doi.org/10.1038/onc.2016.406 PMid:27941879. [DOI] [PubMed] [Google Scholar]
- 13.Berger-Achituv S, Brinkmann V, Abed UA, et al. A proposed role for neutrophil extracellular traps in cancer immunoediting. Frontiers Immunol. 2013;4:48. doi: 10.3389/fimmu.2013.00048. https://doi.org/10.3389/fimmu.2013.00048 PMid:23508552 PMCid: PMC3589747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dolgushin II, Andreeva YuS. Method for detection of extracellular neutrophilic traps: Russian Federation patent for invention 84702384844; publ. 04.01.2008. [In Russian] [Google Scholar]
- 15.Korotina OL, Generalov II. Neutrophil extracellular traps: mechanisms of formation, functions. Immunopathology, allergology, infectology. 2012;4:23–32. [Google Scholar]
- 16.Brinkmann V, Zychlinsky A. Beneficial suicide: why neutrophils die to, make NETs. Nature Rev. 2007;5:577–82. doi: 10.1038/nrmicro1710. https://doi.org/10.1038/nrmicro1710. [DOI] [PubMed] [Google Scholar]
- 17.Phillipson M, Kubes P. The neutrophil in vascular inflammation. Nature medicine. 2011;17(11):1381–90. doi: 10.1038/nm.2514. https://doi.org/10.1038/nm.2514 PMid:22064428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gerasimov IG. Neutrophilic functional heterogeneity. Russian clinical laboratory diagnostics. 2006;2:34–6. [Google Scholar]
- 19.Avila J, Adrover JM, Hidalgo A. Neutrophils in Homeostasis, Immunity, and Cancer. Immunity. 2017;46(17):15–28. doi: 10.1016/j.immuni.2016.12.012. https://doi.org/10.1016/j.immuni.2016.12.012 PMid:28099862. [DOI] [PubMed] [Google Scholar]
- 20.Sagiv JY, Michaeli J, Assi S, et al. Phenotypic diversity and plasticity in circulating neutrophil subpopulations in cancer. Cell Rep. 2015;10:562–573. doi: 10.1016/j.celrep.2014.12.039. https://doi.org/10.1016/j.celrep.2014.12.039 PMid:25620698. [DOI] [PubMed] [Google Scholar]
- 21.Villanueva E, Yalavarthi S, Berthier CC, et al. Netting neutrophils induce endothelial damage, infiltrate tissues, and expose immunostimulatory molecules in systemic lupus erythematosus. J Immunol. 2011;187:538–52. doi: 10.4049/jimmunol.1100450. https://doi.org/10.4049/jimmunol.1100450 PMid:21613614 PMCid: PMC3119769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ortmann W, Kolaczkowska E. Age is the work of art?Impact of neutrophil and organism age on neutrophil extracellular trap formation. Cell Tissue Res. 2018;371(3):473–88. doi: 10.1007/s00441-017-2751-4. https://doi.org/10.1007/s00441-017-2751-4 PMid:29250748 PMCid: PMC5820386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Trellakis S, Bruderek K, Dumitru CA, et al. Polymorphonuclear granulocytes in human head and neck cancer: enhanced inflammatory activity, modulation by cancer cells and expansion in advanced disease. Int J Cancer. 2011;129:2183–93. doi: 10.1002/ijc.25892. https://doi.org/10.1002/ijc.25892 PMid:21190185. [DOI] [PubMed] [Google Scholar]
- 24.Danilova AB, Baldueva IA. Neutrophils as tumor microenviroment member. Problems of oncology. 2016;62(1):35–44. [PubMed] [Google Scholar]
- 25.Savochkina AYu. Neutrophil extracellular traps: mechanisms of formation, detection methods, biological role: dissertation abstract. Dr. Med. Sciences. Chelyabinsk. 2012:48. [Google Scholar]


