Abstract
Purpose:
CD133+ glioblastoma (GB) tumor stem-like cells (TSCs) have been defined as radioresistant. However, whereas previously classified relative to CD133− cells, the radiosensitivity of CD133+ TSCs with respect to the standard GB model, established glioma cell lines, has not been determined. Therefore, to better understand the radioresponse of this cancer stem cell, we have used established cell lines as a framework for defining their in vitro radioresponse.
Experimental Design:
The intrinsic radiosensitivity of CD133+ TSC cultures and established glioma cell lines was determined by clonogenic assay. The TSCs and established cell lines were also compared in terms of DNA double strand break (DSB) repair capacity and cell cycle checkpoint activation.
Results:
Based on clonogenic analysis, each of the six TSC cultures evaluated was more sensitive to radiation than the established glioma cell lines. Consistent with increased radiosensitivity, the DSB repair capacity as defined by neutral comet assay and γH2AX and Rad51 foci was significantly reduced in TSCs as compared to the cell lines. Whereas G2 checkpoint activation was intact, in contrast to the cell lines, DNA synthesis was not inhibited in TSCs after irradiation indicating the absence of the intra-S phase checkpoint.
Conclusions:
These data indicate that the mechanisms through which CD133+ TSCs respond to radiation are significantly different from those of the traditional GB in vitro model, established glioma cell lines. If TSCs play a critical role in GB treatment response, then such differences are likely to be of consequence in the development and testing of radiosensitizing agents.
Keywords: Glioblastoma stem cells, radiosensitivity, DNA damage response, DNA repair, cell cycle checkpoints
Introduction
Radiotherapy remains a primary treatment modality for glioblastomas (GBs) significantly contributing to the prolongation of patient survival. However, whereas many GBs initially respond, they essentially all recur; even in combination with surgery and chemotherapy, the median survival of patients with GB continues to be dismal with the vast majority succumbing to disease within 2 years of diagnosis (1). Towards improving the response of GB to radiotherapy, laboratory investigations aimed at defining the determinants of radiosensitivity and evaluating radiosensitizers have typically been performed using long established glioma cell lines. However, recent evidence, consistent with the cancer stem cell model, suggests that human glioblastomas (GBs) are driven and maintained by a subpopulation of clonogenic cells referred to as tumor stem-like cells (TSCs). Identification and isolation of GB TSCs has for the most part been based on the expression of stem cell associated protein CD133 (2–4). CD133+ cells isolated from GB surgical specimens have a number of properties in common with normal neural stem cells including continuous self renewal, expression of stem cell markers and at least partial differentiation along neuronal and glial pathways (2–4). Moreover, as few as 100 CD133+ cells have been reported to form invasive brain tumors in immuno-compromised mice that simulate the original primary tumor histology (5).
Data implicating TSCs as critical to GB growth and maintenance suggest that they should also have a major role in determining treatment response (6, 7). With respect to radiotherapy, Bao et al reported that CD133+ GB cells were more resistant to radiation than CD133- cells isolated from the same tumor (6). In this study, as compared to CD133-, irradiation of CD133+ cells resulted in a reduced level of apoptosis and an increased colony forming efficiency. The increased survival was attributed to a more efficient activation of the radiation-induced DNA damage response, both DNA repair and activation of cell cycle checkpoints, in CD133+ cells. Based on this comparison, it is now generally considered that TSCs contribute to GB radioresistance, which then implicates this subpopulation as a critical target for improving therapeutic outcome (8, 9). These results also imply that CD133+ cells provide an in vitro model system for studying mechanisms mediating GB radioresponse.
Whereas such an experimental model would be of considerable value with respect to fundamental studies of GB radioresponse as well as the identification of potential targets for radiosensitization, a number of critical questions remain. Quantitative clonogenic survival curves for CD133+ and CD133- cells were not provided in the initial analysis (6), which prevents the comparison of their radiosensitivity to other tumor cells. In addition, whereas the levels of specific proteins associated with cell cycle checkpoints were evaluated, the actual activation of the checkpoints after irradiation was not determined. Finally, whether the radioresponse of CD133+ TSCs differs from that of established glioma cell lines, which have long been the preclinical model used for developing potential GB therapies, was not addressed. The significance of such a comparison lies in the potential for identifying components/regulators “unique” to the radioresponse of TSCs and thus novel targets of GB radiosensitization and treatment, i.e. if the response of TSCs and established glioma cell lines differ. However, if TSCs respond in a manner similar to that of established cell lines, although of interest, it is unlikely that further study will generate new information relevant to GB radiosensitivity and its modification. Therefore, to generate additional insight into the radiosensitivity of GB TSCs in vitro, we compared CD133+ TSC lines to established glioma cell lines in terms of clonogenic survival and DNA damage response (DDR). As described here, CD133+ GB TSCs are more radiosensitive in vitro than established GB cell lines, with a reduced capacity to repair DNA double strand breaks (DSBs) and, although they have an intact G2 checkpoint, lack the intra-S phase checkpoint.
Materials and Methods
Neurosphere culture.
A total of six neurosphere cultures were used in this study. Three cultures NSC11, NSC20, and NSC23, isolated from three human GB surgical specimens, were kindly provided by Dr. Frederick Lang (M.D. Anderson Cancer Center, Houston, TX). Three additional neurosphere cultures (GBAM1, GBAM2, and GBMJ1) were generated at Moffitt Cancer Center from surgical specimens classified as glioblastoma according to World Health Organization criteria (10) and were obtained following informed consent in accordance with the local Institutional Review Board. The fresh GB specimens were dissociated mechanically, washed twice with ice-cold HBSS (Invitrogen), and then incubated at 37°C with trypsin (0.1%, Sigma) and DNAse (0.4%, Sigma) for 2–4 hours as described (4). For neurosphere formation single cell suspensions were added to standard tissue culture flasks containing DMEM/F-12 (Invitrogen, Carlsbad, CA), B27 supplement (Invitrogen) and human recombinant bFGF and EGF (50 ng/ml each, R&D Systems, Minneapolis, MN) as described (11). All cultures were maintained at 37°C in an atmosphere of 5% CO2/95% air.
Each of the neurosphere cultures were initially comprised of mixtures of CD133+ and CD133- cells. To allow for the specific evaluation of the CD133+ cells, FACS was used to sort CD133+ cells from each culture. Neurospheres were disaggregated into single cell suspensions by incubating in TryplE express (Sigma) at 37°C for five minutes followed by passaging through progressively smaller pipette tips. Single cell suspensions were then labeled with an anti-CD133 antibody conjugated to phycoerythrin (PE) (AC133, Miltenyi Biotec, Auburn, CA) under sterile conditions. Cells were analyzed on a FACSvantage cell sorter (BD, Franklin Lakes, NJ); cells positive for CD133 were collected, the purity of which was determined as >90% positive. For clonogenic analyses, the CD133− population was also collected from NSC11 and NSC20. The CD133+ cells isolated from each culture were then placed back into neurosphere forming culture conditions and used for all described experiments. Each of the six CD133+ cell cultures met the criteria for tumor stem-like cells (4): continuous self-renewal, differentiation along glial and neuronal pathways upon exposure to conditions in 10% serum as measured by GFAP and βIII tubulin expression, and tumor formation when injected intracerebrally into nu/nu mice ((12) and data not shown).
Established cell lines.
Three glioma cell lines were used in this study: U87 (American Type Culture Collection, Gaithersburg, MD), U251 and SF126 (DCTD Tumor Repository, NCI-Frederick, MD). The glioma cell lines were grown in monolayer in RPMI 1640 (Invitrogen) with 10% (U87, SF126) or 5% (U251) fetal bovine serum. The A-T fibroblast cell line GM02052 was purchased from Coriell Institute for Medical Research (Camden, NJ) and grown in MEM (Invitrogen) supplemented with 15% FBS, sodium pyruvate and non-essential amino acids (Invitrogen). Cultures were maintained at 370 in an atmosphere of 5% CO2/95% air.
Irradiation.
The established cell lines and the CD133+ TSCs were irradiated as monolayer cultures using a using an XRad 160 X-ray source (Precision XRay Inc., N. Branford, CT) at a dose rate of 2.5 Gy/min.
Clonogenic analysis.
Cell survival was defined using a colony forming efficiency assay. CD133+ neurospheres were disaggregated into single cell suspensions as described above. A specified number of cells were then seeded into poly-L-lysine coated 6-well plates, which allows for adherent colony formation, containing the serum-free growth media noted above. For established glioma lines, cultures were trypsinized to generate a single cell suspension and a specified number of cells were seeded into each well of 6-well tissue culture plates. After allowing cells time to attach without doubling (6 hours for glioma cell lines; 16 hours for TSCs and CD133- cells), cultures were irradiated. Ten to twelve days after seeding (established glioma lines), or fourteen to twenty-one days after seeding (CD133+ TSCs), colonies were stained with crystal violet, and the number of colonies containing at least 25 cells was determined and the surviving fractions were calculated. The same procedure was performed for CD133- cells isolated from the initial unsorted NSC11 and NSC20 neurosphere cultures, which were seeded into the same stem cell growth media as for CD133+ cells. Data presented are the mean ± SE of three independent experiments.
Immunofluorescent analyses of γH2AX and Rad51 foci.
CD133+ TSCs were seeded onto Lab-Tek CC2-treated tissue culture slides and glioma cell lines into Lab-Tek II standard tissue culture slides (Thermo Fisher, Rochester, NY) at least 24h before use in an experiment. At specified times after irradiation cultures were fixed with 4% paraformaldehyde, permeabilized with 0.2% NP40, and blocked with 1% bovine serum albumin (BSA) in PBS containing 5% goat serum. The slides were incubated with primary antibodies (1:500 dilution) to phospho-H2AX (Upstate Biotechnology, Charlottesville, VA) or RAD51 (Santa Cruz Biotechnology, ) for two hours at room temperature and with secondary antibodies (Alexa Fluor 488 goat anti-mouse IgG and Alexa Fluor 594 goat anti-rabbit IgG at 1:500, Molecular Probes, Eugene, OR) and mounted with anti-fade containing 4’,6-diamidino-2-phenylindole (Invitrogen). Cells were analyzed on a Zeiss upright fluorescent microscope. The number of foci was determined in 100 cells per condition and data presented as foci number per cell ± 95% confidence intervals.
Neutral comet assay.
As a measure of DNA double strand breaks (DSBs), the neutral comet assay was performed using a commercially available kit according to the recommendations from the manufacturer (Trevigen, Gaithersburg, MD) with slight modifications. Briefly, monolayer cultures were irradiated (10 Gy) and at specified times single cell suspensions were generated and washed with PBS. Cells were mixed with low melting agarose (1:10), and this mixture was pipetted onto the provided slides. Cell lysis was performed at 4°C for one hour. Cells were then subjected to electrophoresis for 20 minutes at room temperature, fixed with 70% ETOH and DNA was stained with SYBRgreen. Digital fluorescent images were obtained using the IP Labs software (Signal Analytics Corporation, VA). Data are expressed as % damage remaining, in which the Olive tail moment for the 0 time post-irradiation was set to 100% damage, with the remaining times post-irradiation normalized accordingly. Data represent the mean ± SE of three independent experiments.
Cell cycle phase analysis.
Evaluation of cell cycle phase distribution was performed using flow cytometry (FCM). The treatment protocols were essentially the same as in the clonogenic survival experiments, except that the cells were initially seeded into 10 cm dishes. All cultures were subconfluent at the time of collection. Cultures were collected for fixation, stained with propidium iodide, and analyzed by FCM with cell cycle distributions determined using MODFIT software (Verity Software, Topsham, ME).
G2 arrest.
To evaluate the activation of the G2 cell cycle checkpoint, mitotic cells were distinguished from G2 cells and the mitotic index was determined according to the expression of phosphorylated histone H3 (Upstate Biotechnology) as detected in the 4N population using the flow cytometric method of Xu et al. (13). Data are expressed as mean ± SE of three independent experiments.
Radioresistant DNA synthesis (intra-S-phase arrest).
DNA synthesis was determined according to the incorporation of the thymidine analog 5-ethynyl 2′-deoxyuridine (EdU) into genomic DNA and was performed using the Click-IT EdU AlexaFluor 488 kit for flow cytometry (Invitrogen). Cells were irradiated with 12 Gy; 30 minutes later growth medium was replaced with medium containing EdU (10 µmol/L). After 30 minutes, the medium was replaced with EdU-free medium for 1 hour, and cells were fixed and stained according to manufacturer’s protocol. In addition, cells were stained with propidium iodide to determine DNA content and cell cycle phase distribution. Cells were analyzed by flow cytometry using CellQuest software (BD) and data were analyzed using FlowJo software by gating on the S-phase population, then determining the % EdU positive cells in that population. Data are expressed as mean ± SE of three independent experiments.
Results
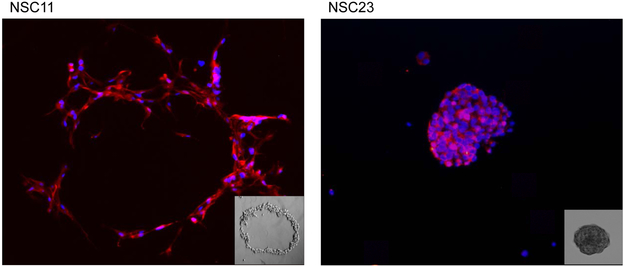
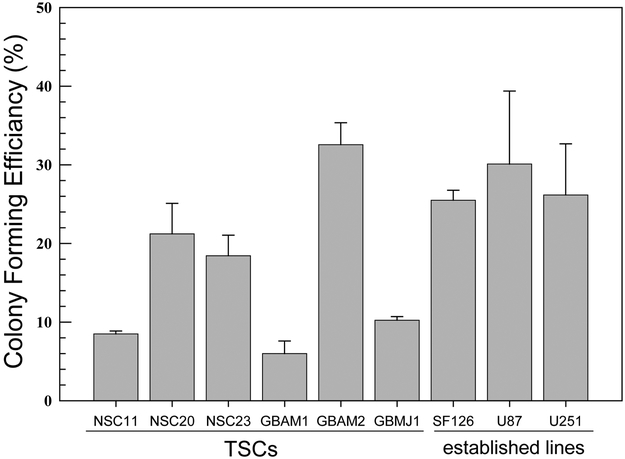
To compare the intrinsic radiosensitivities of the CD133+ TSCs and established glioma cell lines, it was necessary to perform clonogenic analyses under optimal growth conditions for each cell type. Towards this end, CD133+ neurosphere cultures were disaggregated into single cells and seeded at specified numbers into poly-L-lysine coated plates in neural basal media containing EGF and bFGF, i.e. stem cell growth media. Under these conditions the TSCs grow as adherent colonies, and in contrast to growth in media containing fetal calf serum, maintain their CD133 expression for at least 3 weeks after seeding (figure 1a), which is consistent with CD133 serving as a marker for in vitro clonogenicity (5) and the self-renewal characteristic of TSCs. Of note is the “crater” colony morphology of NSC11, which was the dominant morphology in half of the TSC cultures, and is similar to that reported for embryonic stem cells (14). The clonogenic analysis of the established glioma cell lines was also performed under conditions of optimal growth (i.e., 5–10% FBS). The colony forming efficiencies (CFEs) of untreated CD133+ TSC cultures and established glioma cell lines are shown in figure 1b.
Figure 1. Colony-forming efficiency.
(A) CD133+ TSCs were plated as single cells onto poly-L-lysine coated plates. Colonies were stained for CD133 (red) and counterstained with DAPI (blue) 21 days after seeding. An NSC11 colony is shown in the left panel (10X magnification) as an example of crater morphology; an NSC23 colony is shown in the right panel as an example of spheroid morphology. The insets are phase/contrast photomicrographs of representative colonies (10X magnification). (B) CD133+ TSCs and established glioma cell lines were seeded as single cells and colony-forming efficiency determined. Each value represents the mean ± SE of three independent experiments.
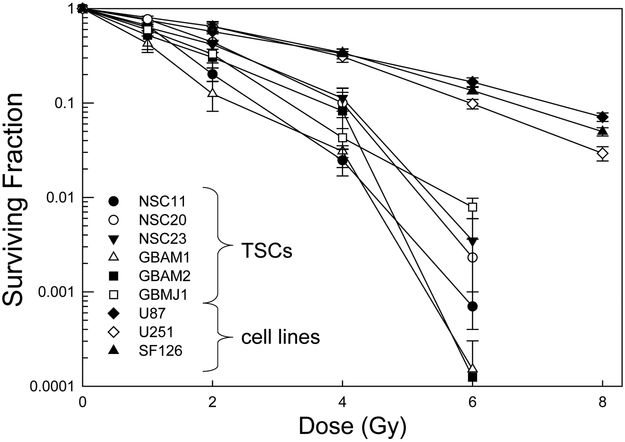
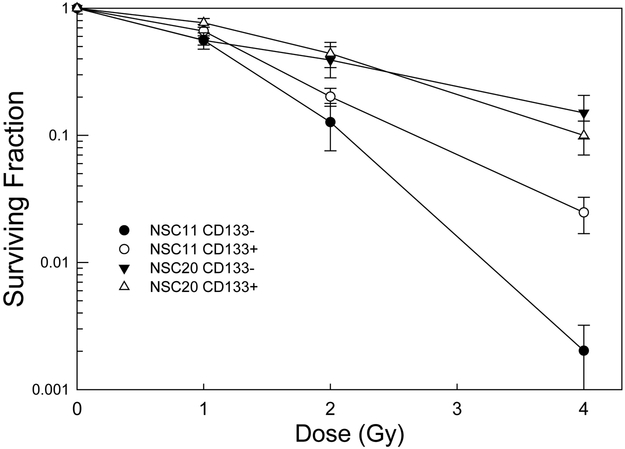
Radiation survival curves based on clonogenic analysis were generated for each of the CD133+ TSC cultures and established glioma cell lines. In these experiments TSCs and established cell lines were plated 16h or 6h before irradiation, respectively, time periods that allowed for cell attachment and yet no division. Following this protocol single cells and not micro-colonies are subjected to irradiation, which eliminates the confounding parameter of multiplicity and its effects on apparent radiosensitivity. As shown in figure 2A, whereas there was variability among the TSC cultures with respect to radiosensitivity, they were all considerably more radiosensitive than the traditional glioma cell lines. For example, after 4 Gy each of the stem cell lines had surviving fractions of less than 0.12; the surviving fractions for the established cell lines were approximately 0.33. These data thus indicate that CD133+ GB TSCs are more radiosensitive than the traditional glioma cell lines. We also compared the CD133+ cells to CD133- cells isolated from the initial unsorted NSC11 and NSC20 neurosphere cultures (figure 2B). CD133- cells were grown in stem cell growth media. It should be noted that CD133- cells isolated from the other neurosphere cultures did not proliferate sufficiently to form colonies when grown in stem cell growth media or serum containing media. For NSC11 neurospheres, the CD133+ TSCs were more resistant than CD133- cells, consistent with the previous results (6). However, there was essentially no difference in the radiation survival curves generated for the CD133+ and CD133- cells isolated from NSC20 neurosphere cultures. These results suggest that the radioresistance of CD133+ TSCs versus CD133- may be dependent on the tumor from which they were isolated.
Figure 2. Radiosensitivity of CD133+ TSCs and established glioma lines.
Cells were plated, allowed to attach and exposed to graded doses of X rays. A). Colony-forming efficiency was determined 10–14 (glioma cell lines) or 14–21 (CD133+ TSCs) days later, and survival curves generated. B) The survival curves corresponding to CD133+ TSCs isolated from NSC11 and NSC20 neurosphere cultures were re-plotted with those generated for CD133- cells isolated from the same initial neurosphere cultures. Colonies of CD133- cells were determined at 18 days after irradiation. Each value represents the mean ± SE of three independent experiments.
Given the consistently greater radiosensitivity of the 6 CD133+ TSCs as compared to the established glioma cell lines, subsequent studies addressed the fundamental processes responsible. For these experiments, we focused on 2 TSC cultures (NSC11; NSC20) and 2 established lines (U87; SF126). In these studies CD133+ TSCs were seeded onto poly-L-lysine coated tissue culture plates and established glioma cell lines onto standard tissue culture plastic at least 24h before analysis and thus evaluated under the same monolayer growth conditions used to generate the cell survival curves (figure 2A).
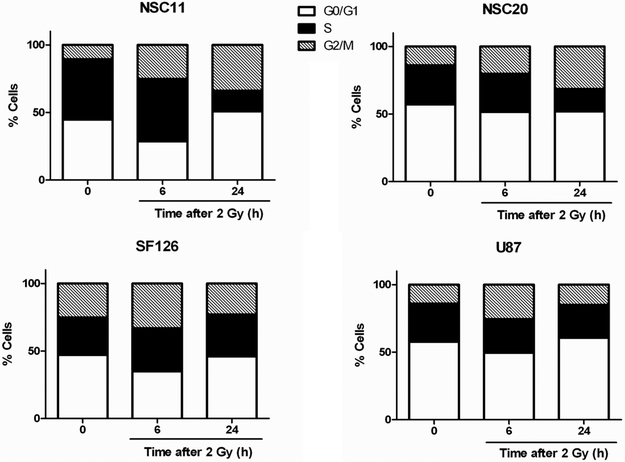
Distribution through the cell cycle could account for differences in radiosensitivity with cells in S-phase considered to be more radioresistant. However, as shown in figure 3, the percentage of S-phase cells in U87, SF126 and NSC20 cultures were essentially the same; NSC11 cultures had a higher S-phase percentage. These results indicate that the differences between the radiosensitivities of the TSCs and established glioma cell lines cannot be simply attributed to cell cycle phase distribution. The redistribution of cells after exposure to 2 Gy is also shown in figure 3. At 24h after irradiation, the TSC cultures had a higher percentage of cells in G2/M as compared to the established cell lines. In other cell types such an accumulation in G2/M has been attributed to cells that were irradiated in S phase, which then failed to undergo S-phase arrest (15). It should be noted that in these cell cycle analyses, radiation was not found to induce a significant sub-G1 population in any of the cultures or cell lines evaluated, including when the dose was increased to 6 Gy (data not shown). Thus, consistent with solid tumor cell lines in general, these data indicate that apoptotic death is an infrequent event after irradiation of TSCs and moreover, does not account for the increased radiosensitivity of TSCs.
Figure 3. Cell cycle phase distribution.
Cells were seeded 24h before irradiation (2 Gy) and collected at 6 and 24 h for analysis of cell cycle phase distribution. Results are representative of three independent experiments.
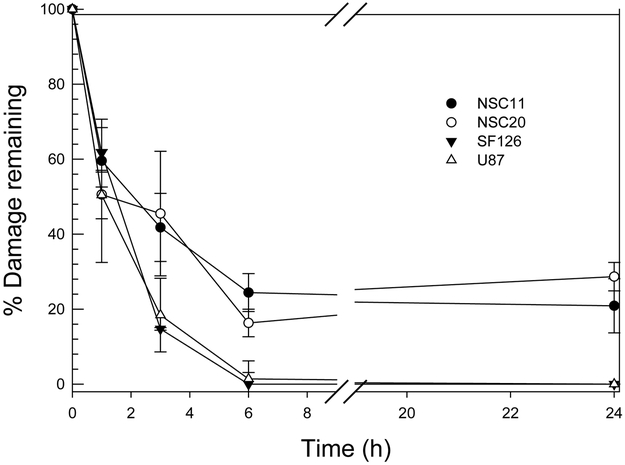
A major determinant of cellular radiosensitivity is the repair of DNA double strand breaks (DSBs), the DNA lesion primarily responsible for radiation-induced cell death. As an initial approach to defining the DSB repair capacity we applied the neutral comet assay. In these experiments, cells grown in monolayer were irradiated with 10 Gy and collected for analysis immediately after irradiation or at times out to 24h. As expected, there was no difference among the culture types in the initial level of radiation-induced DSBs as measured by the Olive tail moment (data not shown). Typical of established cell lines, within 6h after 10 Gy, the percentage of DNA damage remaining in the SF126 and U87 cell lines returned to control levels (Fig. 4a). In contrast, there was a significant percentage of DNA damage remaining in the TSCs at 6h, which remained at essentially the same levels out to at least 24h post-irradiation. These data suggest that TSCs have a reduced capacity to repair radiation-induced DSBs as compared to the established glioma cell lines. Of note, in their analysis of DNA repair in CD133+ TSCs, Bao et al used the alkaline comet assay performed after 3 Gy (6), a method that measures the induction and repair of DNA single strand breaks (16), which are of little significance in radiation-induced cell death.
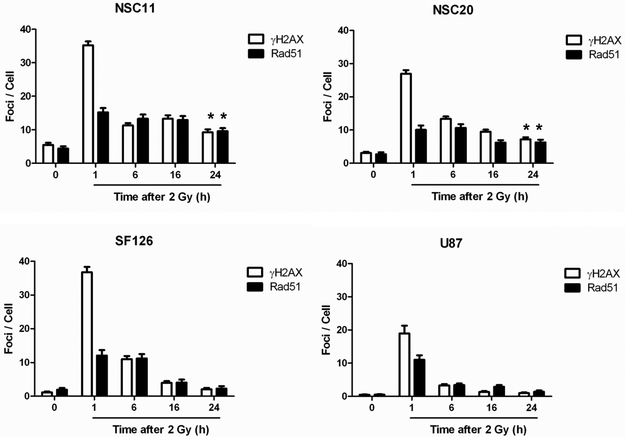
Figure 4. DNA DSB Repair.
(A) Neutral comet assay. Cells were irradiated (10 Gy) and analyzed at times out to 24h. Data are expressed as percent damage remaining in which the tail moment immediately after radiation corresponds to 100% damage. Data represent the mean ± SE of three independent experiments. (B) Induction and dispersal of γH2AX foci and Rad51 foci. Cells were irradiated (2 Gy) and analyzed at times out to 24h. Data are expressed as mean ± 95% confidence intervals. *p<0.01 for control cells versus 24 hours post-irradiation.
As an additional measure of DSB repair, the dispersal of phosphorylated histone H2AX (γH2AX) nuclear foci, which has been established as a sensitive indicator of DSBs, was determined. In these experiments, cells were exposed to 2 Gy and the number γH2AX foci/cell determined out to 24h (Fig. 4B). The number of foci/cell in each line decreased between 1 and 6h. However, whereas foci numbers returned to control levels by 24h in the established glioma cell lines, a significant number of residual γH2AX foci remained in the TSCs. Thus, the analysis of γH2AX, which is a chromatin level manifestation of DSBs, is consistent with the neutral comet data indicating that the DSB repair capacity of TSCs is significantly reduced as compared to established glioma cell lines.
DSBs are repaired in mammalian cells via non-homologous end-joining (NHEJ) and homologous recombination repair (HRR). Whereas γH2AX is a marker of DSB in general (17), Rad51 foci are considered an indicator of those DSBs subject to HRR (18). As shown in figure 4b, exposure of each of the cell types to 2 Gy resulted in detectable Rad51 foci by 1h. In the established cell lines, as for γH2AX, the number of Rad51 foci began to decrease after 1h, returning to control levels by 24h post-irradiation. In contrast, Rad51 foci in the TSCs changed little after 1h and remained significantly above control levels out to at least 24h. Because γH2AX levels in the TSCs decreased dramatically between 1 and 6h post-irradiation whereas RAD51 foci remained at essentially the same levels from 1–24h, these results suggest that the DSB repair defect in the TSC lines may involve some aspect of HRR.
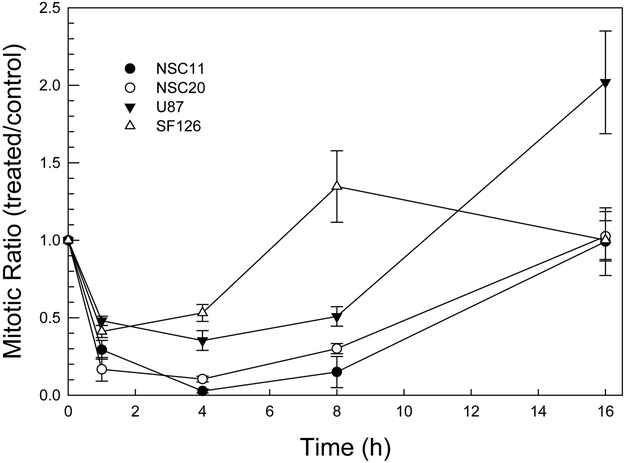
In addition to DSB repair, the DNA damage response includes the activation of cell cycle checkpoints. To compare the radiation-induced activation of the G2 checkpoint in TSCs and established glioma cell lines the method of Xu et al was used, which distinguishes between G2 and mitotic cells (15). This assay determines the percentage of mitotic cells in the 4N population (mitotic index) according to the flow cytometric analysis of phosphorylated histone H3, which is specifically expressed in mitotic cells. As shown in Figure 5, irradiation of both cell types resulted in the decrease in mitotic cells by 1h reaching a maximum reduction by approximately 4h, consistent with the rapid onset of G2 arrest and with previously published results (15); (19). These results suggest that TSCs effectively activate the G2 checkpoint in response to radiation. However, whereas the mitotic index of the TSC cultures returned to control levels by 16h, the mitotic index of established lines was increased relative to control cells by 8h (SF126) or 16h (U87) after irradiation. Such an overshoot has been observed previously by Xu et al and attributed to radiation-induced cell cycle synchronization (15).
Figure 5. G2 checkpoint activation.
Cells were irradiated (2 Gy) and phospho-H3 expression was analyzed in the 4N population at times out to 24h. Data are expressed as mitotic ratio, the percentage of phospho-H3 positive cells in irradiated/unirradiated levels. Values represent the mean ± SE of three independent experiments.
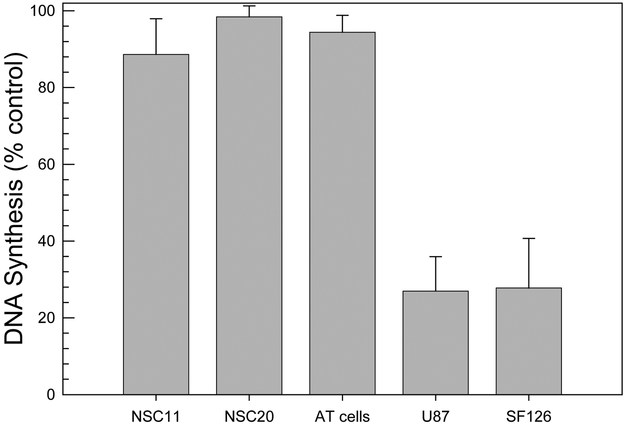
To define the activation of the intra-S phase checkpoint, DNA synthesis was determined according to EdU (a thymidine analog) incorporation (figure 6). As a control for cells that do not activate the intra-S phase checkpoint after irradiation, an AT fibroblast cell line was evaluated; these cells contain mutated ATM and are characterized by radioresistant DNA synthesis (20). Irradiation (12 Gy) of the established cell lines resulted in a decrease in DNA synthesis of approximately 70%, which corresponds to the activation of the intra-S phase checkpoint and is consistent with previous results generated from other tumor and normal cell lines (21, 22). However, irradiated TSC cultures maintained the same level of DNA synthesis as in control cells, a response similar to that of the AT cell line. These data indicate that in contrast to established glioma and other human tumor cell lines, CD133+ TSCs are unable to activate the intra-S checkpoint in response to radiation.
Figure 6. Intra-S-phase checkpoint activation.
Thirty minutes after irradiation (12 Gy), cells received a 30 minute pulse of EdU (10 µM) followed by incubation for 1h in EdU-free growth medium before analysis. DNA synthesis was defined according to EdU incorporation. Data are expressed as percent untreated control. Values represent the mean ± SE of three independent experiments.
Discussion
It was initially reported that CD133+ GB TSCs are radioresistant as compared to CD133- tumor cells (6), a finding that has received considerable attention. More recently, a cancer stem cell population isolated from a mouse breast tumor model was also found to be radioresistant as compared to tumor cells without a stem-like phenotype (23). The data presented here, however, indicate that not all CD133+ TSCs are radioresistant as compared to CD133- cells. Ropolo et al (24) recently reported that, in contrast to the initial study (6), the DNA DSB repair capacity of CD133+ GB cells was not significantly different from CD133- cells. Thus, it appears that there is at least some tumor type dependence regarding the relative radiosensitivities of GB TSCs and CD133- cells. However, regardless of whether there is a difference in the in vitro radiosensitivity of CD133+ cells and non-stem like tumor cells, their putative role in GB growth and maintenance implicates TSCs as a determinant of treatment response. Thus, whereas defining the radiosensitivity of CD133- cells is of interest in terms of the radiobiology of tumor subpopulations, with respect to the development of strategies that enhance GB response to radiotherapy, of potentially greater relevance is a detailed understanding of the specific processes mediating TSC radioresponse. Towards this end, the studies presented here focused on CD133+ GB TSCs.
In that radiosensitivity/resistance is a relative term, the radioresponse of CD133+ TSCs was compared to that of established glioma lines, the traditional in vitro GB model. Clonogenic survival curves revealed that each of the 6 CD133+ TSC cultures was more radiosensitive than the established glioma lines. Whereas providing a reference point for characterizing the radioresponse of the TSCs, the significance of their relatively greater in vitro radiosensitivity in terms of GB resistance in situ is unclear. Results generated from a variety of established tumor cell lines (23–25) as well as primary cultures (26) have shown that in vitro radiosensitivity does not predict in vivo tumor radioresponse. Specifically, established glioma cell lines do not simulate GB radioresistance; their radiosensitivities are not different from that of cell lines initiated from other histologies that typically respond to radiotherapy (24). However, the consistent difference between TSCs and established glioma cell lines in terms of their radiation survival curves does imply that the mechanisms mediating their respective radiosensitivities are different. Established cell lines have long served as the experimental model for investigating GB radiosensitivity and its modification, i.e., radiosensitization. The current targeted approach to radiosensitizer development depends on an understanding of the molecular determinants of radiosensitivity, which then provides a source of potential targets. The significance of the survival curves shown in figure 2A is the implication that the molecular determinants of TSC radiosensitivity, and thus potential targets for radiosensitization, differ from those in established cell lines.
Using established glioma cell lines as a benchmark for expected results, we have begun to define the components of the radiation-induced DNA damage response in CD133+ TSC cultures. Because the repair of DNA DSBs is essential to the survival of an irradiated cell, the DSB repair capacity of CD133+ TSCs was analyzed according to two parameters – γH2AX foci and neutral comet formation. At sites of radiation-induced DSBs the histone H2AX is rapidly phosphorylated (γH2AX) forming readily visible nuclear foci (17, 25), which then disperse as a function of time. The number of γH2AX foci remaining at 24h after irradiation has been shown to correlate with cellular radiosensitivity (26, 27). Whereas γH2AX reflects a chromatin level response to DSBs, the neutral comet assay provides a more direct measure of DNA strand breaks and in contrast to the alkaline comet assay, selectively detects DSBs over single strand breaks (16). Thus, as illustrated by the different time courses and radiation dose requirements, γH2AX and neutral comet analyses quantify different manifestations of DSBs and thus different aspects of the repair process. Compared to the established glioma cell lines, both analyses indicated that CD133+ TSCs have a reduced capacity to repair radiation-induced DSBs, which is likely to be a major contributor to their relatively greater degree of radiosensitivity. Moreover, based on the comparison to Rad51 foci formation and dispersal, the DSB repair defect in the TSCs appears to involve homologous recombination repair (HRR). Although Rad51 foci provide an indication of HRR activity, functional analysis of this repair pathway in TSCs is required to confirm a defect. However, these results suggest that targeting HRR may be an effective therapy against CD133+ TSCs. For example, PARP inhibitors have been shown to be extremely toxic to HRR deficient cells (28, 29).
In addition to DSB repair, a critical component of the radiation-induced DNA damage response is cell cycle checkpoint activation. In their investigation, Bao et al reported that critical checkpoint regulatory proteins (ATM, Chk1, Chk2 and Rad17) were phosphorylated in CD133+ GB cells after irradiation (6). However, whether the CD133+ cells actually underwent arrest at the specified locations in the cell cycle after irradiation (i.e., checkpoint activation) was not determined. As shown here, although displaying an intact G2 checkpoint, the CD133+ TSC cultures were deficient in activating intra-S phase arrest. Whether the abrogated S-phase checkpoint contributes to the relative radiosensitivity of TSCs versus established cell lines is unclear. Xu et al. reported that the lack of an S-phase checkpoint alone does not enhance radiosensitivity (13). However, the S-phase checkpoint does play a critical role in genome maintenance (30) Thus, an abrogated S-phase arrest in the CD133+ TSC is consistent with the proposed role of genomic instability of cancer stem cells as a driving force of tumor development and heterogeneity (31).
The defective DNA damage response of the CD133+ TSCs may seem counter-intuitive for cells supposedly critical to the GB initiation and maintenance. However, this defect is consistent with the recently proposed concept that the activation of the DNA damage response provides a critical barrier to cancer development (32, 33). Data now suggest that oncogene-induced DNA damage response inhibits cell proliferation in the early stages of tumorigenesis; in this situation only tumor cells with mutated or epigenetically silenced repair and/or checkpoint genes continue to divide (34). The consequence of this selection procedure is that the cells that ultimately form a malignant tumor have a defective DNA damage response and exhibit a high degree of genomic instability (34). The role of the DNA damage response in preventing tumor development has for the most part been defined using human tissue specimens and in vivo model systems (32–34). Glioma cell lines, as well as the vast majority of established tumor cell lines, have an apparently intact DDR and thus, as noted by Halazonetis et al (35), may not accurately represent human tumor cells in situ. In contrast, the compromised DSB repair and checkpoint activation of CD133+ TSCs suggests that they may better simulate those tumor cells that have escaped the DNA damage response barrier and may thus provide a more relevant in vitro model of human tumors.
Although the compromised DNA damage response of CD133+ TSCs in vitro may be consistent with human tumor cells in vivo, in light of the radioresistance of GB, the enhanced radiosensitivity that accompanies such a defect seems paradoxical. This may suggest a critical role for the microenvironment as a determinant of radiosensitivity or, not mutually exclusive, that the putative genomic instability of the TSCs facilitates the generation and selection of resistant cells. Alternatively, the significance of in vitro radiosensitivity based solely on long established tumor cell lines may require further analysis. Clearly, the implication of the in vitro radioresponse of CD133+ TSCs and the mechanisms involved require additional investigation. However, although a number of questions remain, the data presented here indicate that the processes regulating the radioresponse of the long studied established glioma cell lines are substantially different from those operative in CD133+ TSCs. If these TSCs play a critical role in GB treatment response, then such differences are likely to be of consequence in translational studies aimed at the development and testing of potential radiosensitizing agents.
Translational Relevance.
CD133+ glioblastoma tumor stem-like cells (TSCs) are assumed to be radioresistant and thus likely to contribute to the resistance of glioblastoma in situ. However, this conclusion is based only on the comparison of these TSCs to CD133- cells. As shown here, as compared to the traditional glioblastoma model of established cell lines, the TSCs were more radiosensitive with a defective DNA damage response. Because in vitro measures of radiosensitivity do not predict tumor response in vivo, the significance of the relative radiosensitivity of the TSCs to glioblastoma response in situ is unclear. However, these data indicate that the mechanisms mediating TSC radioresponse differ from those in the traditional model. Because glioma cell lines have long been used to define molecular determinants of radiosensitivity, if TSCs play a role in glioblastoma treatment response, then exploiting such differences may aid in the development of effective radiosensitizing agents.
Contributor Information
Amy M. McCord, Drug Discovery Department, Moffitt Cancer Center, Tampa, FL
Muhammad Jamal, Drug Discovery Department, Moffitt Cancer Center, Tampa, FL.
Eli S. Williams, Drug Discovery Department, Moffitt Cancer Center, Tampa, FL
Kevin Camphausen, Radiation Oncology Branch, National Cancer Institute, Bethesda, MD.
Philip J. Tofilon, Drug Discovery Department, Moffitt Cancer Center, Tampa, FL
References
- 1.Laperriere N, Zuraw L, Cairncross G. Radiotherapy for newly diagnosed malignant glioma in adults: a systematic review. Radiother Oncol 2002;64: 259–73. [DOI] [PubMed] [Google Scholar]
- 2.Galli R, Binda E, Orfanelli U, et al. Isolation and characterization of tumorigenic, stem-like neural precursors from human glioblastoma. Cancer Res 2004;64: 7011–21. [DOI] [PubMed] [Google Scholar]
- 3.Hemmati HD, Nakano I, Lazareff JA, et al. Cancerous stem cells can arise from pediatric brain tumors. Proc Natl Acad Sci U S A 2003;100: 15178–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Singh SK, Hawkins C, Clarke ID, et al. Identification of human brain tumour initiating cells. Nature 2004;432: 396–401. [DOI] [PubMed] [Google Scholar]
- 5.Singh SK, Clarke ID, Terasaki M, et al. Identification of a cancer stem cell in human brain tumors. Cancer Res 2003;63: 5821–8. [PubMed] [Google Scholar]
- 6.Bao S, Wu Q, McLendon RE, et al. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature 2006;444: 756–60. [DOI] [PubMed] [Google Scholar]
- 7.Baumann M, Krause M, Hill R. Exploring the role of cancer stem cells in radioresistance. Nat Rev Cancer 2008;8: 545–54. [DOI] [PubMed] [Google Scholar]
- 8.Hambardzumyan D, Squatrito M, Holland EC. Radiation resistance and stem-like cells in brain tumors. Cancer Cell 2006;10: 454–6. [DOI] [PubMed] [Google Scholar]
- 9.Strojnik T, Rosland GV, Sakariassen PO, Kavalar R, Lah T. Neural stem cell markers, nestin and musashi proteins, in the progression of human glioma: correlation of nestin with prognosis of patient survival. Surg Neurol 2007;68: 133–43; discussion 43–4. [DOI] [PubMed] [Google Scholar]
- 10.Louis DN, Ohgaki H, Wiestler OD, et al. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol 2007;114: 97–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee J, Kotliarova S, Kotliarov Y, et al. Tumor stem cells derived from glioblastomas cultured in bFGF and EGF more closely mirror the phenotype and genotype of primary tumors than do serum-cultured cell lines. Cancer Cell 2006;9: 391–403. [DOI] [PubMed] [Google Scholar]
- 12.Jiang H, Gomez-Manzano C, Aoki H, et al. Examination of the therapeutic potential of Delta-24-RGD in brain tumor stem cells: role of autophagic cell death. J Natl Cancer Inst 2007;99: 1410–4. [DOI] [PubMed] [Google Scholar]
- 13.Xu B, Kim S, Kastan MB. Involvement of Brca1 in S-phase and G(2)-phase checkpoints after ionizing irradiation. Mol Cell Biol 2001;21: 3445–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schulz TC, Palmarini GM, Noggle SA, Weiler DA, Mitalipova MM, Condie BG. Directed neuronal differentiation of human embryonic stem cells. BMC Neurosci 2003;4: 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xu B, Kim ST, Lim DS, Kastan MB. Two molecularly distinct G(2)/M checkpoints are induced by ionizing irradiation. Mol Cell Biol 2002;22: 1049–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Olive PL. DNA damage and repair in individual cells: applications of the comet assay in radiobiology. Int J Radiat Biol 1999;75: 395–405. [DOI] [PubMed] [Google Scholar]
- 17.Rogakou EP, Pilch DR, Orr AH, Ivanova VS, Bonner WM. DNA double-stranded breaks induce histone H2AX phosphorylation on serine 139. J Biol Chem 1998;273: 5858–68. [DOI] [PubMed] [Google Scholar]
- 18.Tashiro S, Walter J, Shinohara A, Kamada N, Cremer T. Rad51 Accumulation at Sites of DNA Damage and in Postreplicative Chromatin. J Cell Biol 2000;150: 283–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Iliakis G, Wang Y, Guan J, Wang H. DNA damage checkpoint control in cells exposed to ionizing radiation. Oncogene 2003;22: 5834–47. [DOI] [PubMed] [Google Scholar]
- 20.Lavin MF. Ataxia-telangiectasia: from a rare disorder to a paradigm for cell signalling and cancer. Nat Rev Mol Cell Biol 2008;9: 759–69. [DOI] [PubMed] [Google Scholar]
- 21.Falck J, Petrini JH, Williams BR, Lukas J, Bartek J. The DNA damage-dependent intra-S phase checkpoint is regulated by parallel pathways. Nat Genet 2002;30: 290–4. [DOI] [PubMed] [Google Scholar]
- 22.Larner JM, Lee H, Little RD, Dijkwel PA, Schildkraut CL, Hamlin JL. Radiation down-regulates replication origin activity throughout the S phase in mammalian cells. Nucleic Acids Res 1999;27: 803–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Diehn M, Cho RW, Lobo NA, et al. Association of reactive oxygen species levels and radioresistance in cancer stem cells. Nature 2009;458: 780–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ropolo M, Daga A, Griffero F, et al. Comparative analysis of DNA repair in stem and nonstem glioma cell cultures. Mol Cancer Res 2009;7: 383–92. [DOI] [PubMed] [Google Scholar]
- 25.Sedelnikova OA, Rogakou EP, Panyutin IG, Bonner WM. Quantitative detection of (125)IdU-induced DNA double-strand breaks with gamma-H2AX antibody. Radiat Res 2002;158: 486–92. [DOI] [PubMed] [Google Scholar]
- 26.Banath JP, Macphail SH, Olive PL. Radiation sensitivity, H2AX phosphorylation, and kinetics of repair of DNA strand breaks in irradiated cervical cancer cell lines. Cancer Res 2004;64: 7144–9. [DOI] [PubMed] [Google Scholar]
- 27.Olive PL, Banath JP. Phosphorylation of histone H2AX as a measure of radiosensitivity. Int J Radiat Oncol Biol Phys 2004;58: 331–5. [DOI] [PubMed] [Google Scholar]
- 28.Bryant HE, Schultz N, Thomas HD, et al. Specific killing of BRCA2-deficient tumours with inhibitors of poly(ADP-ribose) polymerase. Nature 2005;434: 913–7. [DOI] [PubMed] [Google Scholar]
- 29.Farmer H, McCabe N, Lord CJ, et al. Targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy. Nature 2005;434: 917–21. [DOI] [PubMed] [Google Scholar]
- 30.Aguilera A, Gomez-Gonzalez B. Genome instability: a mechanistic view of its causes and consequences. Nat Rev Genet 2008;9: 204–17. [DOI] [PubMed] [Google Scholar]
- 31.Lagasse E Cancer stem cells with genetic instability: the best vehicle with the best engine for cancer. Gene Ther 2008;15: 136–42. [DOI] [PubMed] [Google Scholar]
- 32.Bartkova J, Bakkenist CJ, Rajpert-De Meyts E, et al. ATM activation in normal human tissues and testicular cancer. Cell Cycle 2005;4: 838–45. [DOI] [PubMed] [Google Scholar]
- 33.Gorgoulis VG, Vassiliou LV, Karakaidos P, et al. Activation of the DNA damage checkpoint and genomic instability in human precancerous lesions. Nature 2005;434: 907–13. [DOI] [PubMed] [Google Scholar]
- 34.Bartek J, Lukas J, Bartkova J. DNA damage response as an anti-cancer barrier: damage threshold and the concept of ‘conditional haploinsufficiency’. Cell Cycle 2007;6: 2344–7. [DOI] [PubMed] [Google Scholar]
- 35.Halazonetis TD, Gorgoulis VG, Bartek J. An Oncogene-Induced DNA Damage Model for Cancer Development. Science 2008;319: 1352–5. [DOI] [PubMed] [Google Scholar]