Most immune related studies on IL-18 have focused on its property to induce IFNγ. Indeed, Haruki Okamura and coworkers described in 1995 the release of a cytokine from Propionibacterium acnes-primed liver macrophages stimulated with LPS 1995 and gave the cytokine the name “IFNγ inducing factor” [1]. In this issue of the Journal, El-Darawish reports that recombinant IL-18 primes resting mouse spleen-derived NK cells to transduce effective signals, which allows IL-2 to trigger proliferation and survival of NK cells [2]. The main point of their study is that IL-18 is the driver of signal transduction via PI3/AKT, mTOR, ATG and LC3 in NK cells. IL-18 primed NK cells respond to IL-2 and increase CD25 expression. Using IL-18 deficient mouse NK cells, there is no significant expansion. There is considerable clinical importance to IL-18 in the context of NK cells survival. As described below in this commentary, IL-18 activation of NK cells is consistent with the pathogenesis of the hemophagocytic lymphohistiocytosis syndrome and the related macrophage activation syndrome. However, in their Significance Statement, the authors state “The concept that IL-18 is merely an IFNγ-inducer limited research of IL-18 roles in other cellular processes”. We disagree with this view that research on IL-18 was limited to the induction of IFNγ. On the contrary, research on IL-18 has been studied extensively during the last 20 years in models of human disease and cellular activation quite independent of IFNγ.
The early studies of IL-18 focused on its similarities with IL-1β because the precursor forms of both cytokines require caspase-1 cleavage to convert to active cytokines. However, IL-18 is hardly a recapitulation of IL-1β. The precursor of IL-18 is present constitutively in epithelial cells of the skin, liver, gastrointestinal tract and kidney of healthy humans and healthy mice, whereas IL-1β is not constitutive in health and is not found in epithelial cells [3]. Recent interest in IL-18 comes from missense mutations in NLRP3 where loss of control of caspase-1 cleavage results in a large spectrum of inflammatory diseases termed “autoinflammatory”. In general, manifestations of autoinflammatory diseases are independent of T-cells and IFNγ. Mice deficient in IL-18 spontaneously develop metabolic syndrome while eating a standard mouse diet. This study and several others reveal the anti-inflammatory properties of IL-18 as reviewed in [3]. IL-18 also plays a significant a role in myocardial suppression, heart failure and atherosclerosis [3]. In heart failure, it is difficult to envision a role for IFNγ [4]. Figure 1 lists properties of IL-18, which are independent as well as related to IFNγ [3].
Figure 1.
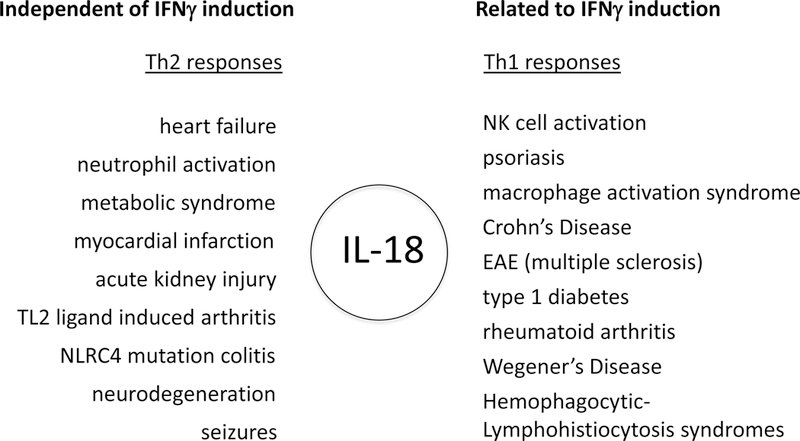
The role of IL-18 in disease related and unrelated to IFNγ. These data are derived from a review of IL-18 and IL-18BP [3].
The importance of IL-18 as an immunoregulatory cytokine is derived from inducing IFNγ from NK cells and T-cells, including CD8+ cytotoxic T-cells. Several human autoimmune diseases are associated with elevated production of IFNγ and IL-18 (Figure 1). Diseases such as systemic lupus erythematosus, rheumatoid arthritis, Type-1 diabetes, Crohn’s disease, psoriasis and graft versus host disease are mediated, in part, by IL-18 and IFNγ production. However, the best example for IL-18 causation in disease can be found in the hemophagocytic lymphohistiocytosis (HLH) syndromes. In HLH syndromes, one can observe the phagocytosis of cells by bone marrow macrophages and hence the adjective “hemophagocytic”. Primary HLH is a rare congenital disease, affecting children below the age of 1 year, due to inherited lymphocyte cytotoxicity defects. Secondary HLH occurs in adults complicating infections, lymphoma, or rheumatic diseases, especially systemic juvenile idiopathic arthritis and adult onset Still’s disease. Secondary HLH is called Macrophage Activation Syndrome, MAS.
IL-18 levels in the circulation of healthy subjects are low; the mean level in the plasma of 500 healthy Dutch men and women is 163 pg/mL [5]. The increases in circulating IL-18 during sepsis, Crohn’s Disease and active lupus are modest. The mean level of IL-18BP in 500 healthy subjects is 2,490 pg/mL [5], which is 10–20-fold molar excess of IL-18 in the circulation. With high affinity of IL-18BP for IL-18, levels of free IL-18 to engage the IL-18Rα are low. The default condition in health is that most if not all the IL-18 is bound to IL-18BP.
Primary HLH and MAS are a life-threatening conditions due to a “cytokine storm” characterized by high levels of free IL-18, soluble CD25, IFNγ and TNFα, generalized cytopenia with marked inflammatory lesions in the liver, and hemophagocytosis in the bone marrow. In animal models of the disease, IFNγ plays a central pathogenic role [6]. In humans, unusually high concentrations of IL-18 have been reported [7]; however, an imbalance between IL-18 and IL-18BP results in an excess of free IL-18 [7]. Although levels of free IL-18 in the circulation are below 1 ng/mL, in inflammatory diseases such as severe sepsis, in the active phase of MAS as well as in systemic juvenile idiopathic arthritis and adult onset Still’s disease, free IL-18 is usually in the range of 5–7 ng/mL. IL-18 concentrations significantly correlate with clinical status and the biologic markers of MAS such as anemia, hypertriglyceridemia, and hyperferritinemia and also with markers of Th1 lymphocyte or macrophage activation [8].
Recently, children with a mutation in NLRC4 experience a life-threatening hyperinflammation state that is MAS, mostly manifested by intractable colitis. Free IL-18 is markedly elevated (10 ng/mL) in these patients and treatment with IL-18BP provides resolution of the inflammatory state, confirming the important role of IL-18 in this disease and the pathogenesis of MAS [9]. It is intriguing to view the excessively high circulating free IL-18 concentrations in MAS and the well-known stimulatory effects of IL-18 on NK cytotoxicity and IFNγ production. Membrane IL-18 may account for a significant effect of IL-18 on NK cells [10]. Since IL-18 stimulates CD25 expression on NK cells and induces autophagy, there is IL-2-induced NK cell proliferation as shown in El-Darawish’s report [2]. That report therefore makes an important contribution to IL-18 and IFNγ-mediated disease. Thus, neutralization of IL-18 with IL-18BP is likely to be effective in congenital HLH syndromes and acquired MAS as well as to prevent progression to MAS in systemic juvenile idiopathic arthritis and adult onset Still’s disease. Although these diseases relate to IFNγ and NK cells, neutralization of IL-18 may be effective in heart and renal failure, diseases that have minor if any role for IFNγ.
Acknowledgments
These studies are supported by NIH Grant AI-15614 and the Interleukin Foundation (to CAD). GK is supported by grants from Agence Nationale de Recherche-Maladies Rares (ANR-07-MRAR-019) and Projet National de Recherche Clinique (A0-1109-44).
References
- 1.Okamura H, Nagata K, Komatsu T, Tanimoto T, Nukata Y, Tanabe F, Akita K, Torigoe K, Okura T, Fukuda S, Kurimoto M (1995) A novel costimulatory factor for gamma interferon induction found in the livers of mice causes endotoxic shock. Infect Immun 63, 3966–3972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.El-Darawish Y, Li W, K, Y., Pencheva M, Oka N, Yamanishi H, Matsuyama T, Tanaka Y, N., M., Okamura H (2017) IL-18 primes murine NK cells 1 for proliferation by promoting protein synthesis, survival and autophagy. J Leuko Biol in press. [DOI] [PubMed]
- 3.Dinarello CA, Novick D, Kim S, Kaplanski G (2013) Interleukin-18 and IL-18 Binding Protein. Front Immunol 4, 289–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Toldo S, Mezzaroma E, O’Brien L, Marchetti C, Seropian IM, Voelkel NF, Van Tassell BW, Dinarello CA, Abbate A (2014) Interleukin-18 mediates interleukin-1-induced cardiac dysfunction. Am J Physiol Heart Circ Physiol 306, H1025–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ter Horst R, Jaeger M, Smeekens SP, Oosting M, Swertz MA, Li Y, Kumar V, Diavatopoulos DA, Jansen AF, Lemmers H, Toenhake-Dijkstra H, van Herwaarden AE, Janssen M, van der Molen RG, Joosten I, Sweep FC, Smit JW, Netea-Maier RT, Koenders MM, Xavier RJ, van der Meer JW, Dinarello CA, Pavelka N, Wijmenga C, Notebaart RA, Joosten LA, Netea MG (2016) Host and environmental factors influencing individual human cytokine responses. Cell 167, 1111–1124 e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chiossone L, Audonnet S, Chetaille B, Chasson L, Farnarier C, Berda-Haddad Y, Jordan S, Koszinowski UH, Dalod M, Mazodier K, Novick D, Dinarello CA, Vivier E, Kaplanski G (2012) Protection from inflammatory organ damage in a murine model of hemophagocytic lymphohistiocytosis using treatment with IL-18 binding protein. Front Immunol 3, 239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mazodier K, Marin V, Novick D, Farnarier C, Robitail S, Schleinitz N, Veit V, Paul P, Rubinstein M, Dinarello CA, Harle JR, Kaplanski G (2005) Severe imbalance of IL-18/IL-18BP in patients with secondary hemophagocytic syndrome. Blood 106, 3483–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kaplanski G (2018) Interleukin-18: Biological properties and role in disease pathogenesis. Immunol Rev 281, 138–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Canna SW, Girard C, Malle L, de Jesus A, Romberg N, Kelsen J, Surrey LF, Russo P, Sleight A, Schiffrin E, Gabay C, Goldbach-Mansky R, Behrens EM (2017) Life-threatening NLRC4-associated hyperinflammation successfully treated with IL-18 inhibition. J Allergy Clin Immunol 139, 1698–1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bellora F, Castriconi R, Doni A, Cantoni C, Moretta L, Mantovani A, Moretta A, Bottino C (2012) M-CSF induces the expression of a membrane-bound form of IL-18 in a subset of human monocytes differentiating in vitro toward macrophages. Eur J Immunol 42, 1618–26. [DOI] [PubMed] [Google Scholar]


