Abstract
Objectives:
To describe long-term mortality rate and to assess associations between mortality rate and antibiotic treatment of lower respiratory infection in patients with advanced dementia; antibiotic treatment allocation was independent of mortality risk—leaving less room for biased associations than in previous multicenter observational studies.
Design:
Prospective study (2004–2009). Multilevel Cox proportional hazard analyses with adjustment for mortality risk were used to assess associations between antibiotics and mortality using time-dependent covariates.
Setting:
A US Department of Veterans Affairs nursing home.
Participants:
Ninety-four residents with advanced dementia who developed 109 episodes. Measurements: Survival, treatment, mortality risk, illness severity, fluid intake, and several other patient characteristics.
Results:
Ten-day mortality was 48%, and 6-month mortality was 74%. Antibiotics were used in 77% of episodes. Overall, antibiotics were not associated with mortality rate (Hazard Ratio [HR] 0.70, Confidence Interval [CI] 0.38–1.30); however, antibiotics were associated with reduced 10-day mortality rate (HR 0.51, CI, 0.30–0.87; rate after 10 days: 1.5, CI 0.42–5.2). Benefit from antibiotics was less likely with inadequate fluid intake, and when experiencing the first episode.
Conclusion:
In our sample of male nursing home residents with advanced dementia and lower respiratory infection, mortality was substantial despite antibiotic treatment. Antibiotics prolonged life but in many cases only for several days. Treatment decisions should take into account that antibiotics may delay death but may also prolong the dying process, indicating a need for accurate prediction of mortality and study of characteristics that may alter effectiveness of antibiotics.
Keywords: Palliative care, treatment outcome, nursing homes, dementia, pneumonia, antibiotics
Lower respiratory infection (LRI), including pneumonia, is the cause of death in up to two thirds of patients with dementia in a variety of studies.1,2 Long-term mortality after the infection is substantial. Six-month mortality after curative treatment was between one third and one half of patients in previous studies.3–5
Comfort care may be appropriate especially in patients with later stages of dementia depending on prognosis, burden of treatment, patients’ previous wishes, and patients’ best interests.6–9 This may entail withholding of antibiotics or combining antibiotics with symptom relief.10,11 From a population point of view, appropriate antibiotic use is important because of concerns regarding increased antimicrobial resistance.7,12
Little is known about how antibiotic usage affects survival in this population. Previous reports have been observational; the first such study was performed in Bedford, MA, in the 1980s.13 Antibiotics prolonged survival in nursing home residents with fever, but not for those with more severe dementia. In a study in Missouri performed in the 1990s, antibiotic-untreated nursing home residents were not very sick and mortality was low.14 In contrast, in a Dutch study, antibiotic-untreated residents were much sicker than treated residents, because antibiotics were withheld in dying patients.11,15 Almost all antibiotic-untreated nursing home residents died within days of diagnosis, whereas in antibiotic-treated residents, mortality in the weeks and months after treatment was substantial.16 This raises the question if antibiotics can cause prolonged dying.
However, such large differences between treated and untreated patients complicate adjustment for confounding patient characteristics when examining associations between antibiotics and outcome, and complete adjustment remains highly uncertain. The objectives of our study were to describe long-term mortality and to assess associations between antibiotic treatment and short- as well as long-term mortality in nursing home residents with advanced dementia and LRI in a sample of residents with similar mortality risk in those treated and not treated with antibiotics. We hypothesized that antibiotics may be partially effective, possibly resulting in prolonged dying. Longer-term outcome could be either protective (eg, via a faster cure and less health damage) or result in excess mortality in the months after treatment if antibiotic-treated initial survivors were otherwise more vulnerable, eg, when at increased risk of developing functional impairment.17
Methods
A prospective observational study design was used, collecting data on residents with LRI of a 100-bed dementia special care unit. All residents had a physician’s diagnosis of dementia. The facility is the same US Department of Veterans Affairs nursing home in Bedford, MA, with a long-standing tradition of hospice care, where the first study of antibiotic usage and survival was performed in the 1980s.13 Preference for antibiotic treatment was generally determined in advance, with family input.8 Between February 2004 and November 2008,109 episodes of LRI developed in 94 residents with advanced dementia defined as being severely impaired in daily decision making (Cognitive Performance Scale18 [CPS] 5 or 6). Multiple episodes in the same resident were included from June 2004 onward. There was a gap in enrollment of new cases (episodes) between the summer of 2005 and the summer of 2006 because of staffing issues. Follow-up was until November 2009 by the time of which 91 of 94 residents had died.
Physicians on staff diagnosed pneumonia by physical examination and clinical signs and symptoms compatible with pneumonia. In 4 cases, chest x-ray was performed and in these cases, pneumonia was confirmed. Because x-rays were not used in most of the residents, we further refer to LRI. We also assessed if surveillance diagnostic criteria for LRI19 were met. Briefly, more stringent LRI diagnostic criteria apply without chest x-ray, requiring at least 3 of cough, sputum, fever, pleuritic chest pain, physical findings, change in status, or breathing difficulty. The study protocol was approved by the local Institutional Review Board. A waiving of consent procedures applied because of the observational nature of the study.
Data Collection
We collected data on treatment, mortality risk, global illness severity, cognition, fluid intake, demographics, activities of daily living (ADLs), and symptoms and signs of LRI on a study form. Mortality risk was estimated according to an 8-item risk score that estimates 14-day mortality in nursing home residents with dementia and LRI when treated with antibiotics.2 It includes male gender, respiratory rate, respiratory difficulty, pulse rate, decreased alertness, fluid intake, eating dependency, and pressure sores. This score was developed in antibiotic-treated residents in the Netherlands, and validated well in antibiotic-treated nursing home residents with dementia in Missouri. When tested (unpublished data), discrimination was adequate also for untreated residents in 5 US and Dutch datasets. Global subjective illness severity by clinical judgment was estimated by the attending physician, nurse practitioner, or nurse, using Charlson’s illness severity rating, ranging from 1 (not ill) to 9 (moribund).20,21 Walking and eating dependency were measured with 4-level scales, referring to status at diagnosis.
Data Analyses and Statistics
Mortality rate was our main focus, as relevant to time to death, and hazard models were developed with antibiotic treatment as the independent variable. Adjustments included mortality risk as indicated by the validated risk score, illness severity, CPS, fulfillment of the diagnostic criteria, and episode. Models without adjustment for episode and with adjustment for morphine treatment, and for different time frames were explored. We further tested influence of fluid intake on the association of antibiotic treatment with mortality by adding it as an item separate from the score, because intake might affect antibiotic effectiveness. A priori, 10-day mortality rate (death rate until and including day 10 from diagnosis) was regarded as relevant in view of previous findings of a high death rate in the first week in antibiotic-untreated residents and possible prolonged dying because of antibiotics, and because antibiotic treatment was usually no longer than that.
Multilevel Cox proportional hazard analyses were performed using generalized estimating equations (GEE) regression accounting for clustering of episodes within residents, using an exchangeable working correlation matrix.22 Hazard ratios (HR) and 95% confidence intervals (CI) were calculated. We used time-dependent covariates for antibiotic treatment, fitting 2-period models with interaction terms for antibiotics. As in previous work, mortality rates soon after treatment differed for antibiotic-treated and untreated residents. Although the Cox proportional hazard assumption was met, the HR for antibiotics in the first 10 days differed significantly from the HR in the second period, justifying the 2-period approach (Likelihood ratio test, chi-square 5.83, 1 degree of freedom; P = .02). We tested interaction terms for 10-day mortality rate with antibiotic treatment to examine if associations with treatment differed by mortality risk, illness severity, cognitive status, fulfillment of diagnostic criteria, later episodes, or fluid intake.
Chi-square and independent t tests were used to compare treatment groups and episodes. Dependent and independent tests explored characteristics by episode. The data were complete except for a few missing data in the variables for diagnostic criteria and illness severity. Diagnostic variables were imputed using a conservative approach where missing data were coded as absent. We imputed the mean for illness severity in the multivariable models in 2 cases. The level of significance was .05. The GEE analyses were performed in Stata 10.1 (StataCorp, College Station, TX); other analyses were performed with SPSS 15.0.1.1 (SPSS, Chicago, IL).
Results
There were 109 episodes of LRI that developed in 94 residents; 81 residents had a single episode, 11 residents had 2 episodes, and 2 residents had 3 episodes. In all but 1 case (99%), there was abnormal chest auscultation. Mean respiratory rate was 27.4 per minute (SD 9.0), and mean pulse rate was 91 (SD 20). Decreased alertness was present in 94% of cases, and cough presented in 60%. Using the conservative approach, 80% met the LRI diagnostic criteria.
Almost all residents were male (97%) (Table 1). The mean mortality risk score was 20.0, corresponding to “moderately high risk” of 2-week mortality with an estimated 40% 14-day mortality when treated with antibiotics.2 The mean illness severity score of 6.6 corresponds to “severely ill.”20 The 94 first episodes did not differ significantly from the 15 later episodes in mortality risk or in illness severity. Comparing first and last (second or third) episodes of the same 13 residents, mortality risk (16.4, SD 5.1, versus 20.7, SD 5.0; P = .01) and illness severity (5.3, SD 1.4, versus 6.4, SD 1.4; P = .047) were greater for the last episode.
Table 1.
Characteristics and Outcomes of Subjects with Dementia and LRI by Treatment
| Characteristics and Mortality in 94 Residents Developing 109 Episodes | Total (n = 109) | Antibiotics |
||
|---|---|---|---|---|
| yes (n = 84) | no (n = 25) | P Value | ||
| Age, mean (SD) [range] | 80.8 (5.8) [58–93] | 80.4 (5.9) | 82.1 (5.3) | .18 |
| Male gender, % | 97 | 98 | 96 | .66 |
| Risk score, mean (SD) [range] | 20.0 (5.3) [5–30] | 19.8 (5.1) | 20.6 (6.1) | .49 |
| Illness severity, mean (SD) [range] | 6.6 (1.6) [2–9] | 6.5 (1.5) | 7.0 (1.9) | .15 |
| Cognition, % CPS score | ||||
| 5 | 60 | 63 | 48 | .18 |
| 6 | 40 | 37 | 52 | |
| Full walking dependency, % | 72 | 69 | 80 | .29 |
| Fluid intake < 1.5 L/d, %* | 75 | 74 | 80 | .53 |
| (Unadjusted) mortality, % | ||||
| 10-day | 48 | 39 | 76 | .001 |
| 1-month | 61 | 56 | 76 | .07 |
| 6-month | 74 | 70 | 88 | .07 |
LRI, lower respiratory infection; CPS, Cognitive Performance Scale. Range: 0–6; 5 = severe (severely impaired decision making), and 6 = very severe cognitive impairment (total eating dependency over all shifts during the last 7 days); Illness severity range: 1 (not ill) – 9 (moribund) and values between 6 and 7 refer to “severely ill”; Risk score range: 0 – 31. Risk score 17–21 refers to “moderately high risk” of 14-day mortality (with antibiotics, 20 points: estimated mortality 40%; 21 points: 44%).
Oral fluid intake, average over preceding week. Intravenous fluids or hypodermoclysis were not administered.
Treatment
Antibiotic treatment was provided in 77%, and morphine in 62% of episodes. Morphine was more frequently provided when antibiotics were withheld than when antibiotics were used (80% versus 57%; P = .04; not in table). There were no significant differences (P ≥ .15) between the groups treated and untreated with antibiotics in mortality risk, illness severity, or other characteristics, although antibiotic-untreated residents tended to have less favorable characteristics (Table 1).
In 95% of cases, antibiotic treatment lasted at most 10 days. At the first day of treatment, antibiotics were intramuscular in 62% of cases, and oral in 38%. A single way of administration applied to 85% of those who received antibiotics; 15% had received both oral and parenteral antibiotics at some point during treatment. Antibiotics were mostly cephalosporins (65%; including combinations of 2 cephalosporins) or quinolones (24%), and in other cases, macrolides (5%), a combination of 2 different antibiotic types (5%), or trimethoprim-sulfamethoxazole (1%).
Mortality
Overall 10-day mortality was 48%, 1-month mortality was 61%, and 6-month mortality was 74% (Table 1). Only 10-day mortality was lower for residents treated with antibiotics (39% versus 76% in untreated residents; P = .001), although the difference at 1 and 6 months was marginally statistically significant (P = .07).
Antibiotics were associated with reduced mortality rate in bivariable multilevel analyses (HR 0.56, CI 0.34–0.92). This was because of a strong association with mortality rate in the first 10 days (HR 0.38, CI 0.23–0.64). The HR for mortality after 10 days was not smaller than 1: HR 1.2, CI 0.59–2.2.
After adjustment for covariates, overall, antibiotics (with no time dependency) were not significantly associated with mortality (HR 0.70, CI 0.38–1.3). Subjective illness severity largely accounted for the difference between unadjusted and adjusted models; however, the association with lower 10-day mortality remained after adjustment (HR 0.51, CI, 0.30–0.87; for mortality after 10 days: 1.5, CI 0.42–5.2; Table 2). Mortality risk, illness severity, and not fulfilling the diagnostic criteria were independent risk factors of mortality (Table 2). Models with and without adjustment for episode were similar, as were models that included morphine treatment, and models for data collected before summer 2005 and later (not shown). Models in selected cases that met the diagnostic criteria were essentially the same; the antibiotics HR for mortality after 10 days tended to be larger but was not significant (2.6; CI, 0.83–7.8; Table 2).
Table 2.
Adjusted Multivariable Multilevel Associations of Treatment with Mortality Rate (n=109 Episodes)
| Independent Factor in the Single 2-Period Treatment Model | All Episodes (n = 109) Hazard Ratio (95% Confidence Interval) |
Selected Episodes That Met LRI Diagnostic Criteria (n = 87) Hazard Ratio (95% Confidence Interval) |
|---|---|---|
| Antibiotic treatment | ||
| mortality between diagnosis and 10 days (period 1) | 0.51(0.30–0.87) | 0.53 (0.28–1.0) |
| mortality after 10 days (period 2) | 1.5(0.42–5.2) | 2.6 (0.83–7.8) |
| Adjustments | ||
| mortality risk, per 5-point increment (0–31)* | 1.4(1.1–1.8) | 1.3 (0.92–1.7) |
| illness severity, per point increment (1–9) | 1.7(1.4–2.2) | 1.8 (1.3–2.4) |
| cognition (CPS 6 versus 5) | 0.71(0.46–1.1) | 0.80(0.48–1.3) |
| fulfillment of diagnostic criteria | 0.60(0.38–0.94) | Not applicable |
| later episode | 1.1(0.73–1.6) | 0.97 (0.65–1.4) |
LRI, lower respiratory infection; CPS, Cognitive Performance Scale. Range: 0–6; 5 = severe (severely impaired decision making), and 6 = very severe cognitive impairment (total eating dependency over all shifts during the last 7 days).
Discrimination and calibration of the validated risk score predicting 14-day mortality2 in the current sample was adequate for 10-day, 20-day, and even for 6-month outcome: c-statistic > 0.70; Hosmer-Lemeshow Goodness of Fit P > .05; observed mortality over score categories increased stepwise from 0% (“low risk”) to at least 50% (“high risk”), both in selected antibiotic-treated residents, and in the total samples.
When fluid intake was added to the (left) model of Table 2, associations were similar, and fluid intake was not an independent risk factor for mortality (HR 1.2; CI, 0.64–2.30). However, there was significant interaction with 10-day mortality rate between antibiotics and fluid intake (P = .012), and between later episodes and antibiotics (P = .001). The interactions indicated that inadequate fluid intake in the first 10 days decreased the protective effect of antibiotics, and that antibiotics had more favorable (adjusted) effect in later episodes compared with first episodes. There were no significant interactions between antibiotics and risk score, illness severity, cognition, or, as expected comparing models in Table 2, diagnostic criteria.
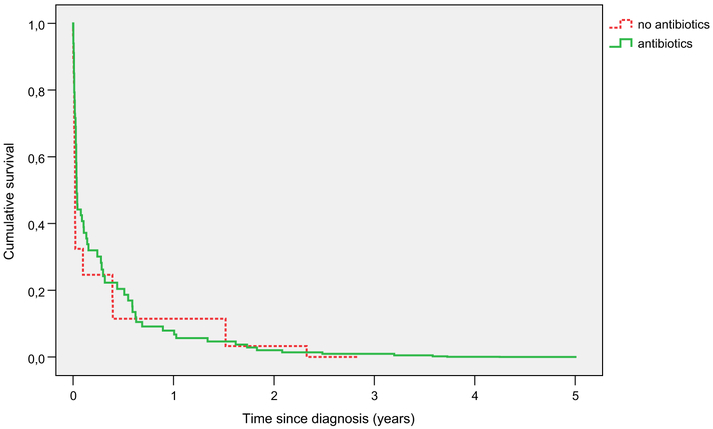
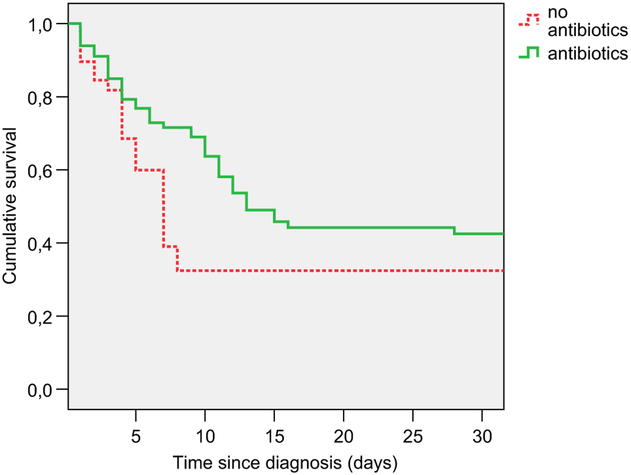
Adjusted survival curves for those treated and untreated with antibiotics over 5 years are shown in Figure 1 and detail over the first 30 days is provided in Figure 2. Figure 2 shows that about a quarter of antibiotic-treated residents had a survival benefit that developed between 5 and 10 days from diagnosis, and diminished roughly between 10 and 15 days. The parallel lines in Figures 1 and 2 show that for about 10% of residents the survival benefit persisted for at least a few months. Mean time to death in those who died in the first 30 days was 7.1 (SD 5.6) days for antibiotic-treated and 3.8 (SD 2.6) days for antibiotic-untreated residents.
Fig. 1.
Adjusted long-term survival after lower respiratory infection by antibiotic treatment. Solid line, survival after episodes treated with antibiotics; Dotted line, survival after episodes treated without antibiotics.
Fig. 2.
Adjusted 30-day survival after lower respiratory infection by antibiotic treatment. Solid line, survival after episodes treated with antibiotics; Dotted line, survival after episodes treated without antibiotics.
Discussion
There are 2 possible goals that are sought by administration of antibiotics to individuals with advanced dementia who develop LRI: extension of life and improvement of discomfort caused by this infection. Results of this study indicate that antibiotics are independently associated with decreased short-term mortality in a male nursing home population with advanced dementia. However, antibiotics provided a survival benefit that persisted over at least a few months for about 1 of 10 residents, whereas they may have prolonged the dying process with some days for as many or some more residents (roughly 15%), typically for residents who had insufficient fluid intake or those who may have experienced LRI for the first time. Most residents died within a month irrespective of antibiotics, and few residents were alive a year after the LRI.
Mortality after antibiotic treatment in this study was higher than in 4 previous studies,3–5 with three quarters (74%) dying within 6 months from diagnosis. This may be explained by higher mortality risk particularly in antibiotic-treated residents in the current study (mean risk score 19.8 versus 14.8 and 15.0 in previous Dutch studies, and 11.3 in a previous US study),4,5 which is not only attributed to predominantly male gender (+2 points). Earlier observational studies suffered from large differences between treated and untreated patients.11,14 The previous study performed in the same nursing home as the current study included patients with fever not distinguished by diagnosis, and had relatively low mortality rates.13
Effects of antibiotics on comfort at the end of life varies across studies and may depend on diagnoses, adequate adjustment for initial discomfort because physicians may treat for comfort,16 and other treatments provided. Decreased symptoms in antibiotic-treated dying patients was reported in an observational study with direct observation and adequate adjustment for discomfort at diagnosis,23 and antibiotics may therefore contribute to improved comfort. However, it is unclear if antibiotics still provide additional comfort with higher levels of treatment to relieve symptoms provided more recently.5 In an earlier study, discomfort was the same in residents with dementia treated with antibiotics or with palliative care, but this study included patients with fever caused by various infections,24 and LRI or pneumonia diagnosed by physicians specifically involves severe discomfort.23 In cancer patients receiving hospice care, antibiotics controlled symptoms in half of patients with a respiratory tract infection,25 and in the last week of life of cancer patients with variable infections, it was 9%.26 In other work, death with an infection (95% antibiotic-treated) in hospice patients was more frequently peaceful than death without infection (86% versus 52%; P < .001; the reported nonsignificant P value should be erroneous).27 Death from infection is therefore not necessarily related to suffering, and effects of antibiotics on comfort need further study.
Possible effects of antibiotics on survival and comfort should be weighed against the burdens of antibiotic use. These burdens may affect both the individual and the whole society. For individuals, these include prolonging the dying phase if cure is not achieved and, sometimes, adverse effects of antibiotics. Considering the discomfort associated with LRI,23 possible length of suffering from LRI and risk of antibiotics prolonging this should be taken into account. Use of antibiotics can cause adverse drug reactions such as allergic reactions and diarrhea. Causes of diarrhea vary but nursing home residents are particularly vulnerable to Clostridium difficile.28 In general, prevalence of allergic reaction caused by antibiotics increases with age.28,29 Burdens of antibiotic use for the society include antibiotic costs and development of difficult-to-treat infections. Frequent use of antibiotics in nursing homes may result in epidemics of C difficile infection and development of highly resistant pathogens.12,28
We have not limited our study to radiographic-confirmed pneumonia, because effectiveness of antibiotics in a sample with a physician’s diagnosis of LRI is more clinically relevant. Further, our findings were essentially unaffected by including cases that did not meet the diagnostic LRI criteria in spite of a physician’s diagnosis of pneumonia. Adjustment for confounding factors is an issue in observational work. However, it is unlikely that our results are explained by unknown mortality risk factors because we adjusted with a carefully developed and externally validated risk score that also performed well in the current study, along with clinical judgment of illness severity; 2 potent determinants of prognosis that, combined, usually provide the best prediction.2,30–32 Importantly, these known risk factors were distributed almost equally between treatment groups. As a result, unadjusted results differed little from adjusted analyses, and further, our findings are robust in a sense that adjustment for clustering by episodes, or use of other possible, but less suitable, analyses for mortality rates (series of t tests or logistic regression), when explored, did not essentially change our findings. Therefore, we benefited from unique circumstances usually created for randomized trials.
Although our study enrolled fewer cases than previous studies, power was adequate to assess association of mortality rate with antibiotics. The smallest and power-determining group of untreated cases was relatively large in particular for a US study, and combined with short-term outcomes occurring in about half of residents, this involved favorable statistical efficiency. However, power was insufficient to determine which time-dependent model showed the best fit, because there were fewer deaths than in previous studies between 9 and 120 days from diagnosis. When exploring data-driven 3-period models in addition to the presented a priori—determined 2-period models, 9 and 120 days seemed the best time cut points, with a protective association in the first period (HR 0.47, CI, 0.27–0.80), a tendency for antibiotics to increase risk in the second period (HR 4.1, CI, 0.65–26), and no association in the third period (HR 0.90, CI, 0.21–3.9). The optimal 9-day cut point is obvious from Figure 2, because the slopes representing rates of mortality differ most by treatment in the first 9 days. Future studies may examine if antibiotics are indeed associated with overall excess mortality on midterm. Larger studies may also examine associations with different antibiotic types. Our study was limited to male residents with advanced dementia and severe acute illness, and further study should determine generalizability to other nursing home populations.
In the United States, there is a default of treatment with antibiotics.7 This does not apply to the US nursing home under study, with a long-standing tradition of hospice care and advance care planning.8,13 It also does not apply to the Netherlands, where antibiotics are withheld in about a quarter of residents with a physician’s diagnosis of pneumonia and dementis,5,11 especially in high-risk residents, who almost all die when not treated.11,14,15 by contrast, US treatment strategies are more driven by family wishes.33 Informing families and clinicians of poor prognosis in advanced dementia and modest effectiveness of antibiotics to prolong life and possible prolonged suffering may help balanced decision making to replace treatment with antibiotics by default.
Conclusion
For many dementia patients, whether or not to treat LRI with antibiotics remains a treatment dilemma. Our findings on probable effectiveness of antibiotics to extend life in some cases and prolong the dying process in others show the importance of accurate mortality prediction and of further study on characteristics that may alter antibiotic effectiveness, such as fluid intake. A combination of clinical judgment of risk and validated mortality risk scores for short-term and long-term outcome in this population, even if scores are most suitable to identify low-risk residents,2,34 warrants further study for help in decision making and improving outcome including survival and comfort for patients with dementia and LRI.
Acknowledgments
The study was supported by the Geriatric Research Education and Clinical Center at the Bedford VA Medical Center, and in part by the National Institute on Aging-funded Boston University Alzheimer’s Disease Center (P30 AG013846). J.T.S. is supported by a career award of the Netherlands Organisation for Scientific Research (NWO, the Hague; Veni 916.66.073). NWO also provided 2 travel grants.
Footnotes
Preliminary results of the study were presented as a poster presentation at the IAGG (International Association of Gerontology and Geriatrics) World Congress of Gerontology and Geriatrics, Paris, July 2009.
References
- 1.Thomas BM, Starr JM, Whalley LJ. Death certification in treated cases of presenile Alzheimer’s disease and vascular dementia in Scotland. Age Ageing 1997;26:401–406. [DOI] [PubMed] [Google Scholar]
- 2.van der Steen JT, Mehr DR, Kruse RL, et al. Predictors of mortality for lower respiratory infections in nursing home residents with dementia were validated transnationally. J Clin Epidemiol 2006;59:970–979. [DOI] [PubMed] [Google Scholar]
- 3.Morrison RS, Siu AL Survival in end-stage dementia following acute illness. JAMA 2000;284:47–52. [DOI] [PubMed] [Google Scholar]
- 4.van der Steen JT, Mehr DR, Kruse RL, et al. Dementia, lower respiratory tract infection, and long-term mortality. J Am Med Dir Assoc 2007;8:396–403. [DOI] [PubMed] [Google Scholar]
- 5.van der Steen JT, Meuleman-Peperkamp I, Ribbe MW. Trends in treatment of pneumonia among Dutch nursing home patients with dementia. J Palliat Med 2009;129:789–795. [DOI] [PubMed] [Google Scholar]
- 6.Marcus EL, Clarfield AM, Moses AE. Ethical issues relating to the use of antimicrobial therapy in older adults. Clin Infect Dis 2001;33:1697–1705. [DOI] [PubMed] [Google Scholar]
- 7.Schwaber MJ, Carmeli Y. Antibiotic therapy in the demented elderly population: redefining the ethical dilemma. Arch Intern Med 2008;168: 349–350 [DOI] [PubMed] [Google Scholar]
- 8.Volicer L, Rheaume Y, Brown J, et al. Hospice approach to the treatment of patients with advanced dementia of the Alzheimer type. JAMA 1986;256: 2210–2213. [PubMed] [Google Scholar]
- 9.van der Steen JT, Muller MT, Ooms ME, et al. Decisions to treat or not to treat pneumonia in demented psychogeriatric nursing home patients: development of a guideline. J Med Ethics 2000;26:114–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Morrison RS, Siu AL. Mortality from pneumonia and hip fractures in patients with advanced dementia. JAMA 2000;284:2447–2448. [PubMed] [Google Scholar]
- 11.van der Steen JT, Ooms mE, Adèr HJ, et al. Withholding antibiotic treatment in pneumonia patients with dementia: A quantitative observational study. Arch Intern Med 2002;162:1753–1760. [DOI] [PubMed] [Google Scholar]
- 12.Yoshikawa TT. Antimicrobial resistance and aging: beginning of the end of the antibiotic era? J Am Geriatr Soc 2002;50:S226–S229. [DOI] [PubMed] [Google Scholar]
- 13.Fabiszewski KJ, Volicer B, Volicer L. Effect of antibiotic treatment on outcome of fevers in institutionalized Alzheimer patients. JAMA 1990;263: 3168–3172. [PubMed] [Google Scholar]
- 14.van der Steen JT, Kruse RL, Ooms ME, et al. Treatment of nursing home residents with dementia and lower respiratory tract infection in the United States and The Netherlands: An ocean apart. J Am Geriatr Soc 2004;52: 691–699. [DOI] [PubMed] [Google Scholar]
- 15.van der Steen JT, Helton MR, Ribbe MW. Prognosis is important in decision-making in Dutch nursing home patients with dementia and pneumonia. Int J Geriatr Psychiatry 2009;24:933–936. [DOI] [PubMed] [Google Scholar]
- 16.van der Steen JT, Ooms ME, Adèr HJ, et al. Medical treatment of acute illnesses in end-stage dementia. Arch Intern Med 2003;163:497–498. [DOI] [PubMed] [Google Scholar]
- 17.Binder EF, Kruse RL, Sherman AK, et al. Predictors of short-term functional decline in survivors of nursing home-acquired lower respiratory tract infection. J Gerontol A Biol Sci Med Sci 2003;58:60–67. [DOI] [PubMed] [Google Scholar]
- 18.Morris JN, Fries BE, Mehr DR, et al. MDS Cognitive Performance Scale. J Gerontol 1994;49:M174–M182. [DOI] [PubMed] [Google Scholar]
- 19.McGeer A, Campbell B, Emori TG, et al. Definitions of infection for surveillance in long-term care facilities. Am J Infect Control 1991;19:1–7. [DOI] [PubMed] [Google Scholar]
- 20.Charlson ME, Sax FL, MacKenzie CR, et al. Assessing illness severity: Does clinical judgment work? J Chronic Dis 1986;39:439–452. [DOI] [PubMed] [Google Scholar]
- 21.Charlson ME, Hollenberg JP, Hou J, et al. Realizing the potential of clinical judgment: A real-time strategy for predicting outcomes and cost for medical inpatients. Am J Med 2000;109:189–195. [DOI] [PubMed] [Google Scholar]
- 22.Agresti A Categorical Data Analysis. 2nd ed. New York, NY: Wiley; 2002. [Google Scholar]
- 23.van der Steen JT, Pasman HR, Ribbe MW, et al. Discomfort in dementia patients dying from pneumonia and its relief by antibiotics. Scand J Infect Dis 2009;41: 143–151. [DOI] [PubMed] [Google Scholar]
- 24.Hurley AC, Volicer BJ, Volicer L. Effect of fever-management strategy on the progression of dementia of the Alzheimer type. Alzheimer Dis Assoc Disord 1996;10:5–10. [PubMed] [Google Scholar]
- 25.White PH, Kuhlenschmidt HL, Vancura BG, et al. Antimicrobial use in patients with advanced cancer receiving hospice care. J Pain Symptom Manage 2003; 25:438–443. [DOI] [PubMed] [Google Scholar]
- 26.Nakagawa S, Toya Y, Okamoto Y, et al. Can anti-infective drugs improve the infection-related symptoms of patients with cancer during the terminal stages of their lives? J Palliat Med 2010;13:535–540. [DOI] [PubMed] [Google Scholar]
- 27.Vitetta L, Kenner D, Sali A. Bacterial infections in terminally ill hospice patients. J Pain Symptom Manage 2000;20:326–334. [DOI] [PubMed] [Google Scholar]
- 28.Deneve C, Janoir C, Poilane I, et al. New trends in Clostridium difficile virulence and pathogenesis. Int J Antimicrob Agents 2009;33:S24–S28. [DOI] [PubMed] [Google Scholar]
- 29.Macy E, Poon K-Y T. Self-reported antibiotic allergy incidence and prevalence: Age and sex effects. Am J Med 2009;122:778.e1–778.e7. [DOI] [PubMed] [Google Scholar]
- 30.van der Steen JT, Ooms ME, van der Wal G, et al. Withholding or starting antibiotic treatment in patients with dementia and pneumonia: Prediction of mortality with physicians’ judgment of illness severity and with specific prognostic models. Med Decis Making 2005;25:210–221. [DOI] [PubMed] [Google Scholar]
- 31.Chow E, Harth T, Hruby G, et al. How accurate are physicians’ clinical predictions of survival and the available prognostic tools in estimating survival times in terminally ill cancer patients? A systematic review. Clin Oncol (R Coll Radiol) 2001;13:209–218. [DOI] [PubMed] [Google Scholar]
- 32.Glare PA, Sinclair CT. Palliative medicine review: Prognostication. J Palliat Med 2008;11:84–103. [DOI] [PubMed] [Google Scholar]
- 33.Helton MR, van der Steen JT, Daaleman TP. A cross-cultural study of physician treatment decisions for demented nursing home patients who develop pneumonia. Ann Fam Med 2006;4:221−227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.van der Steen JT, Albers G, Licht-Strunk E, et al. A validated risk score to estimate mortality risk in patients with dementia and pneumonia: Barriers to clinical impact. Int Psychogeriatr; 2010. In press. [DOI] [PubMed] [Google Scholar]