Abstract
The activation of immune-defense mechanisms in response to a microbial attack must be robust and appropriately tailored to fight particular types of pathogens. Infection with intracellular microorganisms elicits a type 1 inflammatory response characterized by mobilization of T helper type 1 (TH1) cells to the site of infection, where they are responsible for the recruitment and activationof macrophages. At the center of the type 1 inflammatory response is the transcription factor T-bet, a critical regulator of the TH1 differentiation program. T-bet induces the production of interferon-γ (IFN-γ) and orchestrates the TH1 cell–migratory program by regulating the expression of chemokines and chemokine receptors. However, tight regulation of the type 1 inflammatory response is essential for the prevention of immunopathology and the development of organ-specific autoimmunity. In this review, we discuss how T-bet expression drives autoaggressive and inflammatory processes and how its function in vivo must be delicately balanced to avoid disease.
In 1986, Mosmann and Coffman made the landmark discovery that CD4+ T cells are not a homogenous cell population but can be categorized into the T helper type 1 (TH1) and TH2 subsets based on the cytokines that they secrete after being stimulated1. TH1 cells make interferon-γ (IFN-γ) as their hallmark cytokine, whereas the TH2 signature cytokines are interleukin 4 (IL-4), IL-5 and IL-13. After the introduction of the TH1-TH2 dichotomy to the immunology audience, considerable effort was made to discover the cytokine signaling pathways and transcription factors that initiate and stabilize commitment to the TH1 or TH2 lineage. This led to the identification of two major transcription factors, T-bet and GATA-3, as the master regulators of the TH1 and TH2 differentiation programs, respectively2,3. The field of helper T cell biology has gone through another wave of renaissance-like rejuvenation with identification of a third effector helper T cell subset, TH17 cells, characterized by the secretion of the following distinct panel of cytokines: IL-17A, IL-17F, IL-21 and IL-22 (refs. 4,5). T-bet has a unique role in the differentiation of all three subsets of helper T cells by promoting TH1 differentiation while simultaneously inhibiting the opposing TH2 and TH17 lineage-commitment programs2,6–8.
Naming T-bet ‘T-box expressed in T cells’ turned out to be a misnomer because subsequent studies showed that T-bet is expressed and has important roles in several immune-response cell types. As discussed below, the expression of T-bet in dendritic cells (DCs) is required for the priming of antigen-specific CD4+ T cells9,10. T-bet-deficient CD8+ T cells produce less IFN-γ and have diminished cytolytic activity11. In the absence of T-bet, B cells do not produce immunoglobulin G2a (IgG2a), and T-bet deficiency negatively affects the development and function of natural killer (NK) and NKT cells12,13. Because T-bet is expressed in many cell lineages of the immune system, it is not surprising that its expression affects immunoregulation at many stages of the immune response. We have chosen several examples to demonstrate the complex role of T-bet in the immune response. Although T-bet expression is required for protection against pathogens, exuberant T-bet-regulated immune responses can be a driving force in inflammatory diseases. In contrast, silencing T-bet can also be pathogenic, as shown by greater susceptibility of T-bet-deficient animals to asthma and allergies.
TH1 cells
In 2000, T-bet was isolated and shown to control the TH1 genetic program in naive CD4+ T cells (Fig. 1). T-bet directly activates Ifng (which encodes IFN-γ), and ectopic expression of T-bet in fully differentiated TH2 cells redirects them into the TH1 lineage2. During the initial polarization phase, signaling via the T cell antigen receptor (TCR) and IFN-γ–transcription factor STAT1 synergistically induces T-bet expression in helper T cell precursors14–16. Subsequent T-bet expression is driven by IL-12–STAT4 signaling in the absence of TCR stimulation14,16,17. In fact, cessation of TCR signaling is required for T-bet to induce expression of the IL-12 receptor β2 subunit and increase the responsiveness of TH1 cells to IL-12, thus leading to further amplification of T-bet expression17.
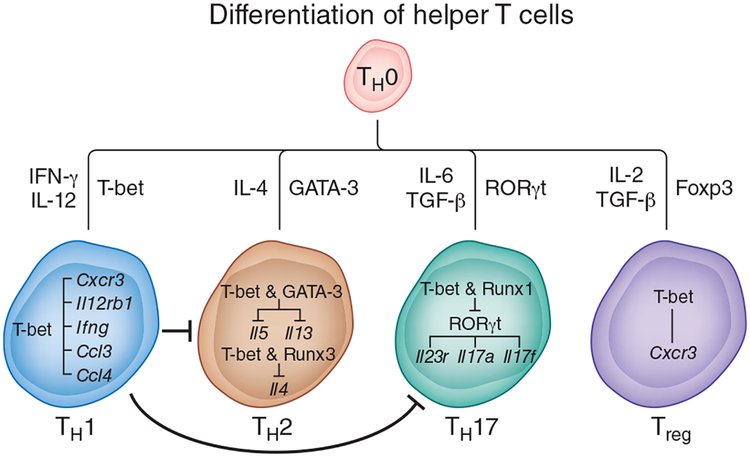
Figure 1.
Role of T-bet in the differentiation of helper T cells. When naive CD4+ T cells (TH0) are activated in the presence of IFN-γ and IL-12, they differentiate into the TH1 subset. The differentiation of TH1 cells is critically dependent on the transcription factor T-bet. The first wave of T-bet expression in CD4+ T cells is regulated by signaling via the TCR and IFN-γ. T-bet upregulates the gene encoding the IL-12 receptor β2 subunit (Il12rb1) and confers IL-12 responsiveness, which induces the second wave of sustained T-bet expression. T-bet promotes TH1 differentiation not onlyby upregulating Ifng but also by inducing the expression of genes encoding CXCR3 and chemokines responsible for the mobilization of leukocytes tothe site of inflammation. In addition to promoting the TH1 differentiation program, T-bet suppresses commitment to the TH2 or TH17 lineage. T-bet blocks TH2 differentiation by sequestering the TH2-specific transcription factor GATA-3 away from the Il5 and Il13 promoters. T-bet and Runx3 bind to the Il4 silencer and prevent Il4 expression. In developing TH17 cells, T-bet binds to Runx1 and blocks expression of the TH17 cell–specific transcription factor RORγt and consequently RORγt target genes (Il23r, Il17a and Il17f). In fully differentiated TH17 cells, T-bet expression is associated with the appearance of repressive epigenetic changes in the Rorc locus, which result in the repression of Rorc expression. In Treg cells, T-bet expression is required for upregulation of the gene encoding CXCR3 and for the recruitment of Treg cells to the site of inflammation. T-bet expression in Treg cells is also essential for their suppressive activity in the scurfy model of autoimmunity but not in most organ-specific inflammatory or autoimmune diseases.
Katie Vicari
By inducing IFN-γ production in CD4+ T cells, T-bet can control many aspects of inflammation and immunoregulation. For example, IFN-γ augments the antigen-processing and antigen-presenting ability of antigen-presenting cells, stimulates IgG2a production by B cells, induces the expression of cytokines and chemokines required for the recruitment of myeloid cells to the site of inflammation, and increases the expression of Toll-like receptors, nitric oxide synthase, and phagocyte oxidase by macrophages18. T-bet also orchestrates the TH1 cell–migratory program by directly controlling expression of the chemokine receptor CXCR3 and the chemokines CCL3 and CCL4 (refs. 19,20; Fig. 1). The aforementioned mechanisms are components of a very effective type 1 response designed to sequester, contain and destroy the invading pathogen (Fig. 2). Not surprisingly, deletion of the gene encoding T-bet (Tbx21) results in greater susceptibility to many intracellular pathogens, including Mycobacterium tuberculosis21, Leishmania major22, Staphylococcus aureus23 and Salmonella typhimurium24. However, it is easy to envision how these same mechanisms, when overactivated, could cause inflammation-associated tissue damage by autoreactive TH1 cells. Hence, T-bet-deficient mice show greater resistance to the development of several inflammatory and autoimmune diseases, including inflammatory bowel disease25, experimental autoimmune encephalomyelitis (EAE)26,27, arthritis10, systemic lupus erythematosus12 and type 1 diabetes9,28.
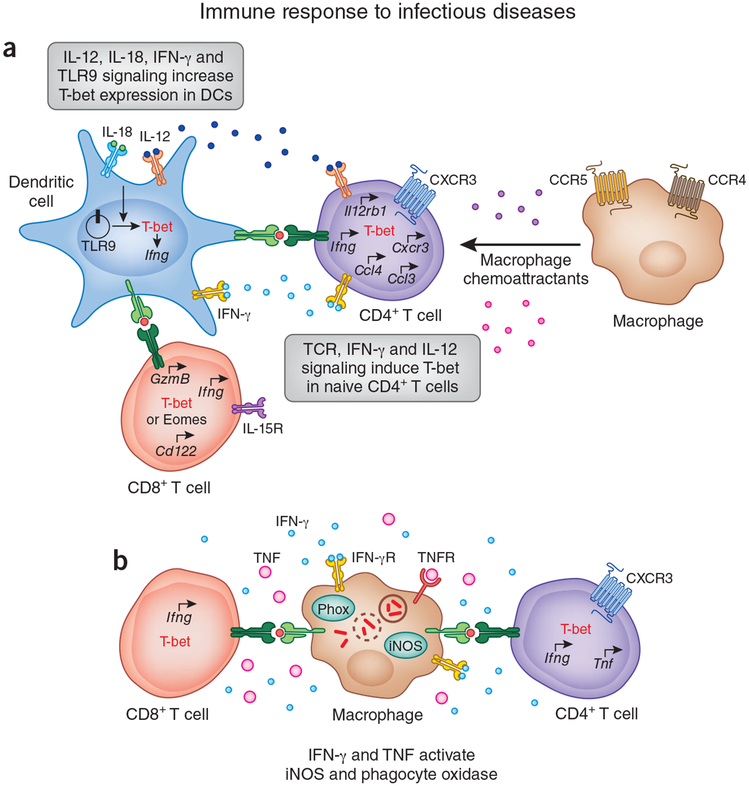
Figure 2.
Role of T-bet in immune response to pathogens. (a) DCs express T-bet in response to signaling via IL-12, IL-18, IFN-γ and Toll-like receptor 9 (TLR9). T-bet expression in DCs is required for activation of the TH1 differentiation program in naive CD4+ T cells. In concert with TCR signaling, IFN-γ and IL-12 derived from mature DCs induce T-bet expression in CD4+ T cells and initiate TH1 differentiation. T-bet regulates the expression of genes encoding CXCR3, CCL3 and CCL4 by TH1 cells. CXCR3 is required for the migration of TH1 cells, whereas CCL3 and CCL4 are responsible for the recruitment of myeloid cellsto the site of inflammation. T-bet and eomesodermin (Eomes) have redundant roles in regulating the effector transcriptional program in CD8+ T cells. Both T-bet and eomesodermin control IFN-γ production and expression of the genes encoding granzyme B (Gzmb) and CD122 (the IL-2 and IL-15 receptor (IL-15R) β-subunit; CD122) by CD8+ T cells. Hence, mice deficient in either T-bet or eomesodermin demonstrate partial loss of cytotoxicity or partial deficiency in cytokine production relative to that of mice lacking both genes. IFN-γ production and granzyme B expressionare essential in immunity to intracellular pathogens, whereas CD122 expression is required for IL-15 responsiveness and the maintenance of memory CD8+ T cell responses in vivo. (b) IFN-γ and TNF delivered by effector CD4+ and CD8+ T cells activate microbicidal mechanisms in infected macrophages by inducing expression of phagocyte oxidase (Phox) and inducible nitric oxide synthase (iNOS). Reactive oxygen and nitrogen species generated by these two enzymesare responsible for the destruction of intracellular microorganisms. IFN-γR, IFN-γ receptor; TNFR, TNF receptor.
Katie Vicari
T-bet, TH1 cells and immune responses to pathogens
The immune-response activities of TH1 cells are mediated largely by the hallmark cytokine IFN-γ (Fig. 2b). Not surprisingly, mice deficient in IFN-γ or its receptor are susceptible to an array of intracellular pathogens29–31. As T-bet has a pivotal role in the development of IFN-γ-producing cells, several groups have examined the role of T-bet in infectious disease models. T-bet-deficient mice cannot clear L. major infection, as shown by their greater parasite burden and larger lesions22. T-bet-deficient CD4+ T cells isolated from mice infected with L. major produce much less IFN-γ and have higher concentrations of the TH2 cytokines IL-4 and IL-5. The shift to a TH2 response accounts for the greater susceptibility of T-bet-deficient mice to L. major infection22. T-bet-deficient mice are also more susceptible to infection with M. tuberculosis or S. typhimurium than are wild-type control mice21,25. As expected, IFN-γ production by T-bet-deficient CD4+ T cells is much lower; however, in contrast to results obtained during L. major infection, in which there is a TH2 bias, T-bet-deficient CD4+ T cells do not produce more IL-4 and IL-5 in response to infection with M. tuberculosis or S. typhimurium. Instead, they have more production of the immunosuppressive cytokine IL-10. Thus, T-bet expression is essential for resistance to infection with M. tuberculosis or S. typhimurium, a function that is mediated through the regulation of TH1 differentiation and repression of IL-10 production21,24. Tbx21–/– mice are more susceptible to aerosol challenge with a Francisella tularensis live vaccine strain. Tbx21–/– NK cells do not traffic to the lungs in response to infection with the F. tularensis live vaccine strain, so the early source of NK cell–derived IFN- γ is absent and the very few IFN-γ-producing CD4+ T cells that infiltrated the lung are unable to control bacterial replication (V.L. and L.H.G., unpublished data). Interestingly, T-bet is not required for host resistance to infection with Listeria monocytogenes. During such infection, the early IFN-γ production by NK cells is not affected by the absence of T-bet32. Although IFN-γ production by antigen-specific CD4+ T cells is lower, there is no defect in the generation of IFN-γ-producing, antigen-specific CD8+ T cells. Hence, L. monocytogenes infection induces compensatory IFN-γ production by NK cells and CD8+ T cells through T-bet-independent pathways, which is sufficient to control bacterial replication32. Thus, the role of T-bet in immune responses to most intra-cellular bacterial pathogens ‘maps’ mainly to its role in regulating the generation of a TH1 response characterized by substantial induction of IFN-γ production. In addition, T-bet expression in CD4+ T cells suppresses the expression of anti-inflammatory cytokines (such as IL-10) and TH2 signature cytokines, which can skew the immune response to most pathogens from the protective TH1 response to the susceptible TH2 response.
T-bet, TH1 cells and inflammatory and autoimmune diseases
Inflammatory bowel disease is a chronic inflammatory disease of the gastrointestinal tract that can present in two different forms: Crohn’s disease and ulcerative colitis. Although the etiology of inflammatory bowel disease is not fully understood, it is suggested that deregulated cytokine production by cells of the immune system in response to gut microbiota has a pivotal role in driving the pathogenesis process. The following two main features distinguish Crohn’s disease from ulcerative colitis: the location of inflammatory lesions, and the types of cytokines produced by helper T cells. The lesions in Crohn’s disease are discontinuous, extend through multiple layers of the intestine and can affect both the small and large intestine. In contrast, ulcerative colitis is characterized by superficial mucosal ulcers restricted to the colon. Although Crohn’s disease is associated with more production of TH1 cytokines, such as IFN-γ and tumor necrosis factor (TNF), TH2 cytokines are thought to promote the immunopathology of ulcerative colitis33–35.
T-bet expression in TH1 cells contributes to the pathogenesis of Crohn’s disease. Several groups have detected more IFN-γ production and higher T-bet protein expression in lamina propria CD4+ T cells from patients with Crohn’s disease but not in those of patients with ulcerative colitis or healthy controls25,36. Higher T-bet expression is also detected in TH1-mediated mouse models of chronic intestinal inflammation but not in TH2-mediated models. Although overexpression of T-bet in naive CD4+ T cells causes severe TH1 cell–mediated chronic intestinal inflammation in immunocompromised mice, transfer of T-bet-deficient naive CD4+ T cells fails to induce this disease25. Interestingly, T-bet-deficient mice are more susceptible to oxazolone-induced, TH2 cell–mediated colitis due to enhanced IL-4 production by T-bet-deficient lamina propria CD4+ T cells. Genetic elimination of STAT6 (TH2-specific) signaling results in more production of IL-17A by Tbx21–/–Stat6–/– CD4+ T cells in the gut mucosa and induction of TH17 cell–dominant colitis37. These animal studies suggest that T-bet expression in CD4+ T cells delicately balances TH1, TH2 and TH17 responses in the gut mucosal immune system25.
T-bet expression in CD4+ T cells also has a role in the pathogenesis of type 1 diabetes (Fig. 3). This organ-specific autoimmune disease is caused by T cell–mediated destruction of the insulin-producing beta cells in the pancreas. Polymorphisms in TBX21 and TH1-related genes have been linked in humans to a greater risk of developing type 1 diabetes. The T-bet Gln33 polymorphism, which is present at a greater frequency in Japanese patients with type 1 diabetes, is responsible for more transcription from the IFNG promoter, which suggests that T-bet-mediated control of IFN-γ production is a contributing factor to the pathogenesis of this disease38. However, immunological analyses of mice deficient in IFN-γ or its receptor suggest that this may not be the only mechanism39,40. T-bet-deficient nonobese diabetic mice are fully protected from developing type 1 diabetes because of defects in both innate and adaptive immunity9. T-bet-deficient DCs are impaired in priming naive CD4+ T cells. In addition, T-bet-deficient CD4+ T cells do not proliferate efficiently in vivo to generate enough autoreactive cells to cause diabetes. Loss of T-bet in CD4+ T cells also impairs their ability to migrate and, as a result, T-bet-deficient CD4+ cells infiltrate the pancreas poorly and promote diabetes less effectively9. Thus, T-bet expression in DCs is required for the initiation of autoimmune diabetes, whereas the pathogenic role of T-bet in CD4+ T cells is important during later stages of pathogenesis9 (Fig. 3).
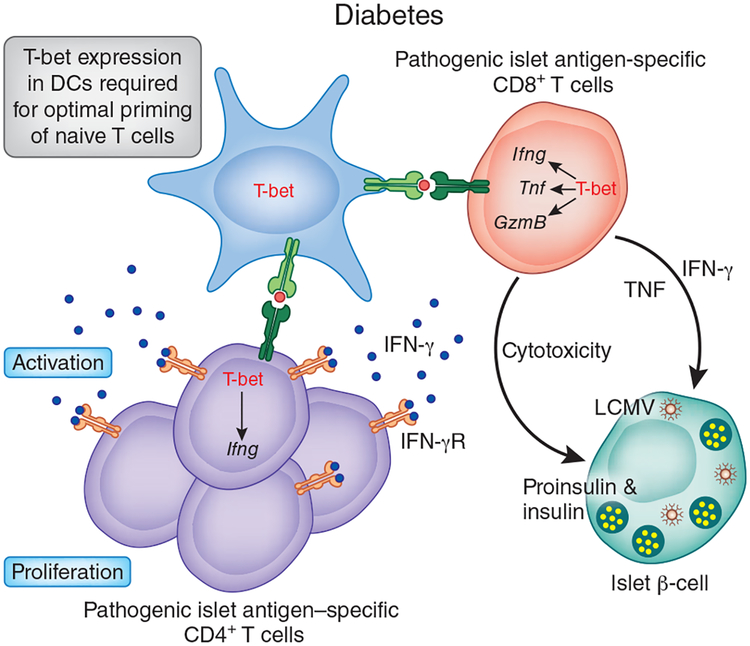
Figure 3.
Role of T-bet in the pathogenesis of autoimmune diabetes. T-bet-deficient nonobese diabetic mice are protected from developing type 1 diabetes because of defects in their innate and adaptive immune systems. The priming ability of T-bet-deficient DCs is diminished, which results in the activation of fewer autoreactive TH1 cells. Less cytokine production by T-bet-deficient TH1 cells, which also have defective migration to the pancreas, causes the overall low-grade inflammatory response in the target organwith minimal damage. T-bet expression in CD8+ T cells is required for their pathogenicity in the RIP-LCMV transgenic model of virus-induced type 1 diabetes. T-bet-deficient mice have many fewer CD8+ effector-memory cells that produce IFN-γ and TNF and also have poor migratory potential.
Katie Vicari
T-bet, TH2 cells and asthma
In addition to promoting the TH1 developmental program, T-bet inhibits the differentiation of TH2 cells6. The generation of TH2 cells is dependent on IL-4–STAT6 signaling and upregulation of the TH2 master regulator GATA-3 (refs. 3,41–43). T-bet blocks TH2 differentiation in the following two ways: it directly inhibits expression of the TH2-driving cytokine IL-4 (refs. 44,45), and it prevents GATA-3 from activating the Il5 and Il13 promoters6 (Fig. 1). The Il4 promoter and enhancer are hyper-acetylated in Tbx21–/– CD4+ TH1 cells, which reflects the suppressive effect of T-bet on the Il4 locus45. The mechanism of Il4 suppression also involves cooperative binding of the transcription factors Runx3 and T-bet at the Il4 silencer44. Phosphorylation of T-bet Tyr525 mediated by the kinase Itk is required for the interaction of T-bet with GATA-3 and sequestration of GATA-3 from the Il5 and Il13 promoters. Consequently, mice with targeted deletion of T-bet have a greater frequency of TH2 cells and spontaneously develop an airway hyper-responsiveness associated with overproduction of TH2 cytokines (IL-4, IL-5 and IL-13) and more peribronchial and perivenular infiltration by eosinophils and lymphocytes46. In this context, T-bet deficiency induces a pathological state in the lungs characterized by goblet cell hyperplasia, more collagen deposition and myofibroblast proliferation, which are indicative of the chronic airway remodeling often seen in patients with chronic asthma46. This remodeling is driven by the absence of T-bet-mediated repression of the pro-fibrotic cytokines IL-13 and transforming growth factor-β (TGF-β)47. In patients with asthma, T-bet expression by lung CD4+ T cells is much lower, and several different T-bet polymorphisms have been associated with allergic asthma48,49. T-bet overexpression shifts the cytokine balance to the TH1 response and attenuates goblet cell hyperplasia and mucus hyperproduction in mice after chronic allergen exposure50. Hence, similar to its role in the gut, T-bet controls the airway inflammatory response to environmental antigens by regulating the TH1-TH2 balance.
T-bet and TH17 cell–mediated immunopathology
The differentiation of TH17 cells is induced by TGF-β and IL-6 or IL-21, which upregulate expression of the TH17 cell–specific transcription factor RORγt51–55. Several transcription factors are involved in promoting TH17 differentiation by increasing RORγt expression, including STAT3 (ref. 56,57), IRF4 (ref. 58), Batf59 and Runx1 (ref. 60). It has been demonstrated that T-bet expression exerts a negative effect on commitment to the TH17 lineage7,8. T-bet binds to Runx1 and interferes with its transcriptional activity in uncommitted helper T cells, thus preventing induction of RORγt even under TH17-polarizing conditions7 (Fig. 1). Other studies have shown that T-bet is induced in fully differentiated TH17 cells in response to IL-12 and type I–type II interferon signaling and is responsible for introducing repressive epigenetic changes in the locus encoding RORγ (Rorc) that shut down RORγt expression61,62. Consequently, T-bet-deficient mice have a higher frequency of TH17 cells in several disease models63–66.
T-bet-deficient recipients develop accelerated rejection of cardiac allografts and vasculopathy despite their profound deficiency in IFN-γ-producing CD4+ T cells65. Interestingly, the accelerated allograft rejection is associated with severe vascular inflammation characterized by infiltration of neutrophils and IL-17A-producing CD4+ cells. Neutralization of IL-17A prevents vascular inflammation and suppresses accelerated rejection of cardiac allografts in Tbx21–/– mice. These results suggest that T-bet expression prevents the generation of CD4+ TH17 cells able to mediate strong alloimmune responses, which cause allograft rejection65.
Tbx21–/– mice are more susceptible to challenge with Trypanosome cruzi despite having a normal frequency of antigen-specific IFN-γ TH1 cells. The pathology is caused by the induction of a robust TH17 response and chronic neutrophilia, which increases the morbidity and mortality of T. cruzi–infected mice. Similarly, T-bet-dependent signaling prevents TH17 cell–mediated immunopathology in response to infection with Schistosoma mansoni64. Functional characterization of Tbx21–/– CD4+ T cell responses in S. mansoni–infected mice after immunization with soluble egg antigen and complete Freund’s adjuvant has shown much higher expression of the TH17-specific cytokines IL-23, IL-17A, IL-21, IL-22 and TNF and of the neutrophil-specific chemoattractants CXCL1 and CXCL2. This augmented TH17 response is accompanied by lower expression of the TH2-associated genes Il4, Il5 and Il10 (ref. 64). Thus, schistosome egg–induced immunopathology in Tbx21–/– mice could be explained by a strong TH17 response with concurrently lower expression of immunomodulatory cytokines.
In an antigen-induced allergic airway inflammation model, Tbx21–/– mice show more severe airway hyper-responsiveness that does not correlate with more production of TH2 cytokines. Tbx21–/– mice have higher concentrations of IL-17A than do control mice, which are associated with substantial influx of eosinophils and neutrophils into the lung67,68. Neutralization of IL-17A results in less infiltration by neutrophils and less airway inflammation. Thus, T-bet prevents the development of allergies and asthma not only by inhibiting the differentiation of TH2 cells but also by regulating the TH1-TH17 balance in the airways.
T-bet, TH17 cells and autoimmune diseases
Rheumatoid arthritis is a chronic, inflammatory autoimmune disease that affects mainly the synovium of peripheral joints, although tissue damage can also encompass the lungs, pericardium and sclera. The inflammation of synovial tissue often leads to the destruction of articular cartilage, bone erosion and joint deformities. It was thought that TH1 cells cause damage in the joints mainly through IFN-γ-driven inflammatory mechanisms; however, conflicting data have been reported about the role of IFN-γ in various animal models of rheumatoid arthritis. In some studies, IFN-γ is actually protective. In the microbial S. aureus–induced sepsis and arthritis model, expression of T-bet and IFN-γ results in lower incidence of the disease23. In collagen-induced arthritis, mice deficient in the IFN-γ receptor have an accelerated disease onset characterized by more infiltration of neutrophils and macrophages and severe destruction of tissues and bones69,70. The disease is mild but not completely eliminated in IFN-γ-deficient mice after immunization with proteoglycan. Eventually, IFN-γ-deficient and T-bet-deficient mice succumb to arthritis because of much greater TH17 responses that convert proteoglycan-induced arthritis, which is normally an IL-17A-independent model, into IL-17A-dependent arthritis63. The results from collagen-induced arthritis and proteoglycan-induced arthritis models would suggest that expression of IFN-γ and T-bet has an immunomodulatory effect on the development of arthritis by constraining the magnitude of TH17 responses (Fig. 4).
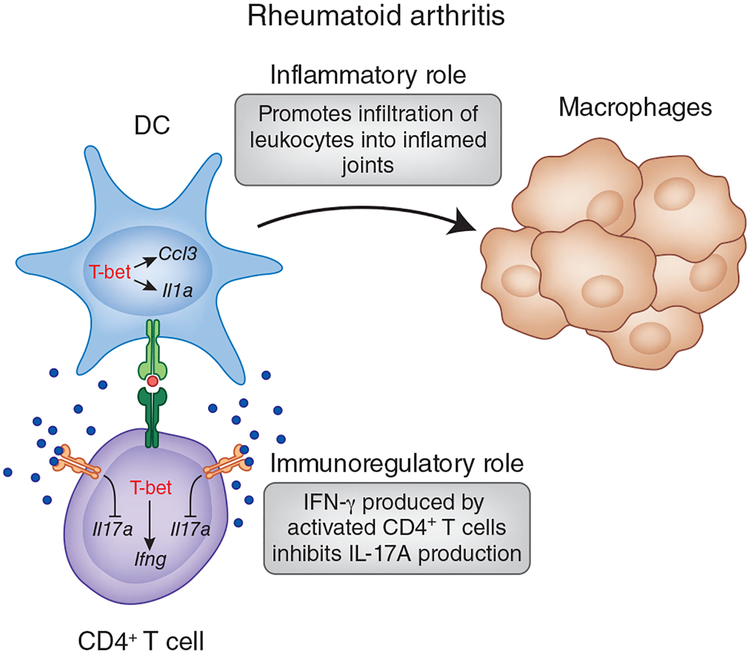
Figure 4.
Role of T-bet in the pathogenesis of rheumatoid arthritis. T-bet-deficient mice are protected from developing passive collagen antibody-induced arthritis. In this model, T-bet expression in DCs is required for disease pathogenesis. T-bet-deficient DCs are not efficient antigen-presenting cells and activate TH1 cells poorly. In the absence of T-bet, DCs produce much less IL-1α and CCL3 and recruit fewer leukocytes to the joints. In contrast, the role of T-bet in CD4+ T cells is less straightforward. In certain models, such as collagen- or proteoglycan-induced arthritis, expression of T-bet and IFN-γ may have an immunomodulatory effect on the development of arthritis by constraining the magnitude of TH17 responses.
Katie Vicari
Although the animal studies discussed above indicate TH17 cells are the main culprit in disease initiation and progression, CD4+ T cells isolated from the joints of children with inflammatory arthritis have high expression of both RORγt and T-bet and produce both IL-17A and IFN-γ71. The relationship between IL-17A and IFN-γ is complex, and although animal studies suggest that IFN-γ could be potentially used as a negative regulator of TH17 cells, trials have not shown a substantial clinical benefit for recombinant human IFN-γ in rheumatoid arthritis72. In the setting of rheumatoid arthritis, targeting the upstream mediators of TH17 differentiation, such as IL-1, IL-6, IL-23 and TNF, or downstream effector functions of TH17 cells involved in osteoclastogenesis and bone erosion may represent more effective therapeutic options.
In most of the disease models discussed above, T-bet deficiency is associated with more pathology due to deregulated TH17 responses and heightened infiltration of neutrophils into target tissues. The exception to this rule is EAE, the most commonly used animal model of multiple sclerosis, in which more TH17 cells in the central nervous system (CNS) are not sufficient to elicit and sustain neuroinflammation in T-bet-deficient mice. These results suggest that the function of T-bet in the pathology of EAE and multiple sclerosis may not be straightforward7.
Multiple sclerosis is a chronic inflammatory disease of the CNS characterized by multifocal areas of leukocyte infiltration, demyelination and axonal damage that often result in paralysis. Components of cell-mediated and humorally mediated immunity were long thought to be involved in inducing and propagating neuroinflammation. Both TH1 cells and TH17 cells have been linked to multiple sclerosis, mainly through the production of their signature cytokines, IFN-γ and IL-17A. However, there is some discrepancy between human multiple sclerosis and EAE in the role of IFN-γ in disease pathogenesis. Patients with multiple sclerosis who have received recombinant IFN-γ have a greater frequency of relapses and exacerbation of symptoms, which suggests that IFN-γ contributes to the pathology of multiple sclerosis lesions73. That hypothesis has been supported by a clinical trial in which administration of neutralizing antibody to IFN-γ has shown clinical benefits74. In contrast, mice deficient in IFN-γ or its receptor develop exacerbated disease that has been attributed to less apoptosis of effector CD4+ T cells and a change in the composition of lymphocytic infiltration in the CNS75,76. Interestingly, T-bet-deficient mice are protected from developing EAE, which indicates the existence of T-bet-dependent but IFN-γ-independent mechanisms that contribute to disease development. Many studies have addressed the role T-bet in the pathology of EAE and multiple sclerosis, but the question of how T-bet expression drives autoreactive responses in the CNS still remains unanswered.
T-bet is upregulated substantially in circulating CD4+ T cells and CD8+ T cells from the peripheral blood of patients with relapsing multiple sclerosis77. Brief treatment with a high dose of glucocorticoid has beneficial effects on the functional recovery of such patients78. Activated glucocorticoid receptors physically interact with T-bet and diminish its DNA-binding activity79. Glucocorticoid treatment results in much lower T-bet expression in CD4+ T cells and CD8+ T cells in patients with relapsing multiple sclerosis that correlates with the lower IFN-γ production and improved clinical response80. Similarly, IFN-β treatment results in lower expression of IFN-γ and T-bet in patients with relapsing multiple sclerosis; however, much lower T-bet expression is observed only in patients responsive to IFN-β treatment81. Thus, targeting T-bet expression in patients with multiple sclerosis could be therapeutically beneficial and could lead to amelioration of the disease by many mechanisms that may not be exclusively limited to IFN-γ production.
Much attention has been directed to TH17 cells and their role in multiple sclerosis because neutralization of IL-17A through genetic targeting or the administration of neutralizing antibodies results in lower disease severity82–84. However, T-bet-deficient mice, which have stronger TH17 responses in the CNS than do wild-type mice, do not develop paralysis; this suggests that TH17 cells and IL-17A are not sufficient to cause EAE in T-bet-deficient hosts7,85. Some scientists argue that both TH1 cells and TH17 cells are needed to cause substantial damage in the CNS. For example, TH17 cells are the first to infiltrate the CNS, and they are required for orchestration of the infiltration of TH1 cells, which then initiate and sustain neuroinflammation86. Alternatively, polyfunctional CD4+ T cells that simultaneously produce IL-17A and IFN-γ may be more pathogenic than CD4+ T cells that produce either IL-17A or IFN-γ. There is some evidence to suggest that cells producing both IL-17A and IFN-γ may be better equipped for transmigration across the blood-brain barrier than are cells producing either IL-17A or IFN-γ. Upregulation of the integrin ligand ICAM-1 in response to IFN-γ, combined with the barrier-disrupting effects of IL-17A, could contribute to the greater migratory ability of IFN-γ-producing TH17 cells87
IFN-γ-producing TH17 cells with dual expression of T-bet and RORγt have also been detected in the CNS of mice with EAE88,89. Although animal studies have shown that TH17 cells are a distinct lineage in their own right, these cells have a great deal of flexibility and plasticity and acquire a TH1-like phenotype quickly after exposure to IL-12 or IL-23. This acquisition of a TH1-like phenotype is dependent on T-bet and STAT4 and involves the introduction of repressive epigenetic changes in the Rorc locus of TH17 cells61. Although T-bet limits the magnitude of TH17 cell responses, T-bet-deficient TH17 cells are not pathogenic85. These observations suggest that T-bet may have complex regulatory roles in TH17 biology; it has a negative role because it restrains the amount of IL-17A produced by TH17 cells and has a positive role by controlling a gene or a subset of genes required for the encephalitogenic potential of TH17 cells. As IFN-γ and IL-17A–IL-17F are dispensable for the propagation of neuroinflammation, the ‘genetic fingerprint’ that renders a cell pathogenic remains unknown. The following three factors have been shown to be important for the encephalitogenic potential of CD4+ T cells: IL-23, STAT4 and T-bet26,90,91. We propose here that T-bet has a central role along with IL-23 and STAT4, converging on the same signaling pathway that ultimately results in the induction of T-bet. In support of our hypothesis is an elegant study of fate mapping of IL-17-producing T cells showing that IL-23 is key for the induction of T-bet, expression of both IL-17A and IFN- γ and subsequent deviation to a TH1-like phenotype92. These IL-23-induced T-bet-expressing TH17 cells have greater encephalitogenic potential than do ‘regular’ TH17 cells89. It remains to be demonstrated whether STAT4 is phosphorylated in TH17 cells in response to signaling through the IL-23 receptor and whether STAT4 induces T-bet expression in a similar way in TH17 cells and TH1 cells.
So, how can the knowledge of transcription factors, cytokines and T cells be used to come up with better treatment strategies for multiple sclerosis? Mouse studies have shown that IFN-γ is not required for development of EAE; however, data from studies of patients with multiple sclerosis should not be ignored. Although IFN-γ is not the sole culprit in driving disease progression, targeting IFN-γ can disrupt the T-bet+IFN-γ+ self-amplification loop and indirectly decrease T-bet expression in circulating lymphocytes. Targeting of T-bet directly, by inhibiting either its expression or its activity, has always been an attractive possibility, although transcription factors have proven to be a difficult class of proteins to target. However, given the widespread expression of T-bet in cells of the immune system, inhibition of T-bet may render patients immunocompromised. In that case, the options left are silencing T-bet in a cell-specific manner or targeting T-bet-regulated genes that contribute to the pathology of multiple sclerosis without substantially altering T-bet expression. Although much attention has been focused on CD4+ T cells, multiple sclerosis is not solely mediated by these cells. Conditional deletion of T-bet in various cell lineages will identify the importance of T-bet in pathogenic functions of other cells of the immune system and may lead to new potential therapeutic targets.
T-bet and other helper T cells
T-bet is also expressed in a subset of regulatory T cells (Treg cells)93. The development of Treg cells is regulated by lineage-specific transcription factor Foxp3 (ref. 94); however, T-bet controls the expression of a subset of genes encoding molecules that influence the migration and homeostasis of Treg cells during TH1 cell–mediated immune responses93. T-bet-deficient Treg cells cannot control TH1 responses in the scurfy mouse model of autoimmunity93. However, the in vitro and in vivo suppressive functions of Treg cells are unaffected by the loss of T-bet25,26,47,95. Tbx21–/– Treg cells show a stronger protective effect in TH1 cell–mediated colitis than do wild-type Treg cells, and the enhanced protective effect is associated with augmented TGF-β production25. Tbx21–/– Treg cells are as potent as wild-type Treg cells in preventing innate immunity–driven ulcerative colitis and autoimmune diabetes8,95. The observed discrepancies in the role of T-bet in Treg cells could be explained by the different inflammatory disease models used to test Treg cell function. Foxp3-deficient scurfy mice succumb to severe, systemic multiorgan autoimmunity, and under these overwhelming inflammatory conditions, the suppressive function of T-bet-deficient Treg cells may be compromised. In contrast, in other models focused on testing the function of Treg cells in organ-restricted inflammatory or autoimmune diseases, T-bet deficiency in Treg cells has no effect on their suppressive functions. Future studies will clarify the role of T-bet in the biology of Treg cells under different inflammatory conditions.
The activation of naive CD4+ T cells in the presence of IL-4 and TGF-β results in development of CD4+ helper T cells producing both IL-9 and IL-10 (TH9 cells) that are inflammatory despite having more IL-10 production96,97. TH9 cells are able to induce EAE in an adoptive-transfer model, and when transferred along with CD45RBhi CD4+ T cells, TH9 cells induce colitis and peripheral neuritis in recipients deficient in recombination-activating gene 1 (Rag1)96,98. Because IL-9 is very important in intestinal responses to helminthes, TH9 cells are important in protective immunity to Trichuris muris97. T-bet is not expressed in TH9 cells differentiated in vitro96,97. However, in a passive EAE model and in a T cell–transfer model of colitis, these TH9 cells regain the ability to produce IFN-γ, analogous to the plasticity of TH17 cells96,98. Hence, it would be interesting to see how T-bet deficiency or T-bet expression influences the development and effector functions of these cells.
Initially it was proposed that the main function of CD4+ helper T cells is to provide ‘help’ to B cells by regulating their proliferation and immunoglobulin class switching. A particular subset of CD4+ T cells, follicular helper T cells (TFH cells), is found in the germinal centers whose specialized functions promote B cell–mediated humoral immunity. The development of TFH cells is critically dependent on IL-6, IL-21 and STAT3, which induce expression of the TFH cell–specific transcription factor Bcl-6 (refs. 99,100). Although TFH cells do not express T-bet, it is interesting that T-bet-deficient mice have poor IgG2a responses. This observation warrants more complete investigation of a potential role for T-bet in the development of TFH cells and the outcome of TFH cell–B cell interactions.
T-bet expression promotes the development of proinflammatory diseases mediated by TH1 cells; however, T-bet deficiency can also cause pathological changes characterized by overexuberant TH2 and TH17 responses due to the loss of T-bet-mediated negative regulatory mechanisms. Hence, precise calibration of T-bet in CD4+ T cells is essential for maintenance of immunological homeostasis.
T-bet in other cells of the immune system
CD8+ T cells have a critical role in antiviral and antitumor immunity. T-bet and eomesodermin regulate the transcriptional program of effector cytolytic T lymphocytes101,102 (Fig. 2a). Overexpression of eomesodermin or T-bet is sufficient to induce the expression of IFN-γ, perforin and granzyme B in CD8+ T cells and, conversely, expression of dominant negative mutants of eomesodermin or T-bet results in less IFN-γ production and granzyme B expression102. Mice with the combined loss of Tbx21 and the gene encoding eomesodermin (Eomes) have additive defects in the expression of cytolytic T lymphocyte–associated genes and also lack memory CD8+ T cells103,104. The expression of T-bet and eomesodermin is required for high expression of CD122 (the IL-2 and IL-15 receptor β-subunit) and for specifying IL-15 responsiveness103. These results suggest that T-bet and eomesodermin have cooperative and partially redundant functions in regulating both IL-15-mediated homeostasis and the transcriptional program of cytolytic T lymphocytes. Furthermore, a gradient of T-bet expression specifies a short-lived effector cell fate or memory precursor effector cell fate. Under inflammatory conditions, high T-bet expression promotes the generation of terminally differentiated short-lived effector cells with a KLRGhiIL-7Rlo phenotype. In contrast, CD8+ T cells with small amounts of T-bet will develop into long-lived, self-renewing memory CD8+ T cells105. It has been shown that IL-12-augmented activity of the kinase mTOR is essential for sustained T-bet expression and the generation of effector CD8+ T cells106. The treatment of IL-12-conditioned CD8+ T cells with rapamycin, a chemical inhibitor of mTOR activity, blocks T-bet expression and promotes sustained eomesodermin expression. Higher eomesodermin expression in CD8+ T cells enhances the generation of long-lived, memory CD8+ T cells. Thus, balance between the transcription factors T-bet and eomesodermin, as ‘instructed’ by mTOR activity, can determine the CD8+ effector cell fate versus memory cell fate106.
T-bet expression in CD8+ T cells is required for the generation of CD8+ T cell–dependent autoimmune diabetes28. In the RIP-LCMV mouse model, lymphocytic choriomeningitis virus (LCMV) antigens are transgenically expressed in pancreatic beta cells under control of the rat insulin promoter107,108. CD8+ T cell–dependent autoimmune diabetes is rapidly induced in these mice after infection with LCMV. Notably, the virus is cleared from these mice before the onset of autoimmune diabetes. Islet destruction is dependent on concomitant production of TNF and IFN-γ and the cytotoxic function of CD8+ T cells (Fig. 3). In this model, primary antiviral CD8+ T cell responses develop normally in T-bet-deficient mice. However, there is a profound and intrinsic defect in the generation of effector-memory CD8+ T cell responses specific for LCMV-derived antigens28. As a result, T-bet-deficient mice are very well protected from developing type 1 diabetes. This observation indicates T-bet as a potential therapeutic target for the treatment of autoimmune diabetes28.
The functional importance of T-bet expression in CD8+ T cells is also highlighted by the greater susceptibility of T-bet-deficient mice to LCMV, whose clearance is largely dependent on CD8+ T cells11. T-bet-deficient CD8+ T cells have compromised cytolytic activity and many fewer IFN-γ-producing CD8+ T cells. These impaired effector functions of Tbx21–/– CD8+ T cells provide inadequate protection against LCMV infection and result in greater susceptibility of Tbx21–/– mice to LCMV11. Interestingly, mice with double deficiency in T-bet and eomesodermin (Tbx21–/–Eomes–/– mice) develop a progressive inflammatory and wasting syndrome characterized by multiorgan infiltration of neutrophils after infection with LCMV. Considerable infiltration by neutrophils is more characteristic of bacterial and fungal infections, not viral infection104. Functional analysis of CD8+ T cells has shown substantial induction of Rorc and TH17 signature genes (those encoding the IL-23 receptor, IL-17A, IL-21 and IL-22)104. Eomes–/– CD8+ T cells do not express IL-17A; however, there is evidence of deregulated expression of signature genes of IL-17-producing cytotoxic T cells (TC17 cells) in Tbx21–/– mice, although it is less severe than that seen in Tbx21−/−Eomes−/− mice104. Depletion of CD8+ T cells prevents the virus-induced immunopathology and neutrophilia in Tbx21–/–Eomes–/– mice, but depletion of CD4+ T cells does not. These results suggest that expression of T-bet and eomesodermin is essential for activation of the CD8+ T cell effector program, which is effective against viral pathogens, and for simultaneous suppression of the TC17 genetic program, which is involved in defense against extracellular pathogens104. More production of IL-17A by Tbx21–/– CD8+ T cells with accompanying neutrophilia is also observed in autoimmune myocarditis and in the rejection of cardiac allografts109,110. Reminiscent of the role of T-bet in suppressing the TH17 differentiation program in CD4+ T cells, T-bet expression is also needed to suppress the TC17 response in CD8+ T cells and prevent the development of TC17 response–mediated immunopathology.
Analyses of T-bet-deficient mice has shown that T-bet regulates immunoglobulin (IgG) class switching in B cells. T-bet expression in B cells is induced in response to signaling by IFN-γ, Toll-like receptor 9 and the costimulatory molecule CD40 and is linked to the induction of IgG2a, IgG2b and IgG3 and repression of IgG1 and IgE12,111,112. T-bet expression in B cells can be beneficial or detrimental depending on the pathological state. For example, induction of T-bet in B cells can be an efficacious treatment strategy for ameliorating IgE-mediated allergic responses and asthma because T-bet acts as a negative regulator of IgE production112. In contrast, higher expression of T-bet in B cells initiates IgG2a class switching. The ability of T-bet to stimulate IgG2a production by B cells is essential for the pathogenesis of mouse lupus12. The progeny of T-bet-deficient mice bred onto the lupus-susceptible background are protected from immune-complex glomerulonephritis. These mice have much less glomerular, interstitial and perivascular inflammation and less deposition immune complexes in the kidneys, associated with impaired IgG2a production by Tbx21–/– B cells. Thus, T-bet expression in B cells can contribute to the generation of pathological autoantibodies on an autoimmunity-susceptible background.
In addition to its role in cells of adaptive immunity, T-bet has critical developmental and functional roles in the innate immune system. T-bet is important for the normal development and survival of NK and NKT cells. Tbx21–/– NKT cells do not produce IFN-γ after stimulation with α-galactosylceramide, and they fail to undergo homeostatic proliferation in response to IL-15 because of their lack of expression of CD122 (the IL-15 receptor β-subunit)13. NK cells from T-bet-deficient mice are functionally immature and hyperactivated and undergo more apoptosis13. T-bet expression is required for the cytotoxic activity and sustained IFN-γ production of NK cells in response to infection with murine cytomegalovirus13. T-bet deficiency in NK cells severely compromises antitumor responses and results in greater susceptibility to metastatic melanoma113. The transfer of wild-type NK cells protects Tbx21–/– mice from the metastasis of melanoma to the lungs. The lungs of Tbx21–/– mice have a considerable deficiency in NK cells in after melanoma challenge that is not associated with trafficking defects but instead reflects less survival of T-bet-deficient NK cells. The very few remaining Tbx21–/– NK cells have impaired IFN-γ production and cytotoxic activity113. These results suggest that augmented T-bet expression in NK cells may enhance antitumor responses and affect tumor metastasis.
DCs rapidly upregulate T-bet in response to IFN-γ and Toll-like receptor 9 signaling114 (Fig. 2a). T-bet is essential for optimal production of IFN-γ by DCs and for the priming of TH1 cells114,115. DCs genetically engineered to have high expression of T-bet promote the priming of TH1 and cytotoxic T cell type 1 responses independently of IL-12 (ref. 116). Naive CD4+ T cells and CD8+ T cells have higher cell surface expression of CXCR3 and IL-12 receptor β2, express more granzyme B and produce higher concentrations of IFN-γ when primed with T-bet-overexpressing DCs versus than when primed with control DCs116. Hence, DCs modified to overexpress T-bet could be potentially used to boost antitumor immunity.
T-bet in DCs has a pivotal role in modulating host-commensal relationships in the gastrointestinal tract through its regulation of TNF production by colonic DCs95. T-bet binds to the Tnf promoter and inhibits Tnf promoter activity driven by the transcription factor NF-kB in a dose-dependent manner. Loss of T-bet in immunocompromised mice (Tbx21–/–Rag2–/– mice) causes deregulated TNF production by colonic DCs and more apoptosis of gut epithelial cells. These events lead to the breakdown of the intestinal epithelial barrier and continuous influx of gut bacteria into the underlying mucosal tissue, which fuels more TNF production and inflammation. The cytokine-driven inflammation in the gut of Tbx21–/–Rag2–/– mice represents a unique environment conducive for the generation and expansion of colitogenic bacteria, which can then infect immunocompetent hosts and initiate and perpetuate chronic gut inflammation independently of T-bet95. These studies raise several important questions. What is the role of the immune system in shaping the composition of microbial flora in the gut? Can the host microbial community instigate and drive inflammatory diseases in humans? Can chronic intestinal inflammation be stopped with immunotherapy or by the colonization of a host with beneficial bacteria? Studies have shown that the inclusion of probiotic bacteria in the diet alleviates colonic inflammation117. Furthermore, restoring T-bet expression specifically in DCs efficiently lowers TNF production and prevents the development of colitis and colitis-associated colorectal cancer in Tbx21–/–Rag2–/– mice118. Better understanding of how T-bet regulates biological processes in cells of adaptive and innate immune systems in the context of intestinal inflammation is required for the identification of new ways of interfering with T-bet-mediated inflammatory pathways in cells of the adaptive immune response while preserving the immunoregulatory role of T-bet in DCs.
T-bet expression in DCs is also required for the initiation and progression of rheumatoid arthritis (Fig. 4). Several aspects of DC biology can contribute to the pathogenesis of rheumatoid arthritis, including the priming of autoreactive TH1 cells and TH17 cells and the production of chemokines and proinflammatory cytokines such as IL-1, IL-6 and TNF. T-bet-deficient mice are protected in the passive collagen antibody–induced arthritis model, in which the importance of T-bet expression has been ‘mapped’ to DC function10. Adoptive transfer of T-bet expressing DCs is sufficient to induce the pathology of collagen antibody–induced arthritis in T-bet-deficient mice and in Tbx21–/–Rag2–/– mice. The effect of T-bet on DC function is dual. In addition to being required for the proper activation of helper T cells, T-bet regulates production of the inflammatory cytokine IL-1α and the chemokine CCL3 (MIP-1α). Thus, less production of IL-1α and CCL3 by DCs contributes to the diminished infiltration of CD4+ T cells and inflammation in T-bet-deficient mice10. These results suggest that targeting T-bet in DCs could be therapeutically beneficial in the treatment of rheumatoid arthritis.
Concluding remarks and future directions
In the past 25 years, remarkable progress has been made in the understanding of helper T cells. Four unique CD4+ T cell lineages have been identified—TH1, TH2, TH17 and Treg—whose differentiation and functions are regulated by the lineage-specific transcription factors T-bet, GATA-3, RORγt and Foxp3, respectively2,3,52,94. Each helper T cell subset is equipped with the ability to produce a distinct panel of effector cytokines. Such specialization ensures that the right types of cells are mobilized to the site of infection and that the appropriate effector mechanisms are activated in response to a specific challenge. However, differentiated T cells remain flexible and, in response to the external milieu, can dynamically change their cytokine profile to that of an opposing lineage; hence, in an affected organ, TH1-like TH17 cells or TH1-like TH2 cells are often present119,120. In contrast, TH1 cells seem to be terminally differentiated and, to our knowledge, there have been no reported cases in which fully committed TH1 cells have been converted into one of the other helper T cell subsets in vivo. The rigidity of the TH1 phenotype could be attributed to the dominant nature of T-bet, whose re-expression in TH17 and TH2 cells in response to IL-12 and/or type I–type II interferon signaling contributes to epigenetic changes in major cytokine loci and acquisition of the T 1-like phenotype119,120. T cell flexibility is beneficial to the host, as the immune system can quickly respond to a changing environment. However, the fine line between protective immunity and destructive inflammation-associated pathology must always be balanced. T-bet expression is associated with both. T-bet expression in the immune system is required for protection against infection and anti-tumor immunity; however, high T-bet expression can lead to TH1-mediated autoimmunity. Conversely, T-bet deficiency can lead to greater susceptibility to TH2 cell–or TH17 cell–mediated immunopathology. Many animal models have demonstrated that loss of T-bet expression protects the host from developing several different autoimmune diseases, which suggests that silencing Tbx21 could be therapeutic. However, transcription factors are notoriously difficult to target. In addition, the precise ‘titration’ of T-bet activity to therapeutic but not pathogenic amounts will be technically challenging. Hence, an alternative approach is to develop small-molecule inhibitors that partially block T-bet activity and/or function by identifying and modulating key T-bet-regulated genes that can be targeted pharmacologically. Studies of T-bet in autoimmune diseases have largely focused on its most famous target, IFN-γ. However, there is increasing evidence that there is an IFN-γ-independent but T-bet-dependent component to the pathogenesis of autoimmune diseases. One key unanswered question about the role of T-bet in autoimmunity is the nature of these other ‘pathogenic’ downstream effectors.
It is clear that T-bet has a prominent regulatory role in helper T cells. However, T-bet is expressed in several different cell lineages of the hematopoietic system, including DCs, NK cells, NKT cells, B cells and CD8+ T cells. How T-bet expression in these cells contributes to the pathogenesis of autoimmune disease is still poorly understood. In this review, we have attempted to highlight the importance of T-bet expression in other cells of the adaptive and innate immune systems in initiating and driving disease pathogenesis. Better understanding of how T-bet functions in T cells and other cells of the immune system may allow the development of effective treatments for autoimmune disease without compromising host immune defenses.
ACKNOWLEDGMENTS
Supported by the US National Institutes of Health (P01 NS038037 and CA112663 to L.H.G.), the Danone Group and the Cancer Research Institute (V.L.).
Footnotes
COMPETING FINANCIAL INTERESTS
The authors declare competing financial interests: details accompany the full-text HTML version of the paper at http://www.nature.com/natureimmunology/.
References
- 1.Mosmann TR, Cherwinski H, Bond MW, Giedlin MA & Coffman RL Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J. Immunol 136, 2348–2357 (1986). [PubMed] [Google Scholar]
- 2.Szabo SJ et al. A novel transcription factor, T-bet, directs Th1 lineage commitment. Cell 100, 655–669 (2000). [DOI] [PubMed] [Google Scholar]
- 3.Zheng W & Flavell RA The transcription factor GATA-3 is necessary and sufficient for Th2 cytokine gene expression in CD4 T cells. Cell 89, 587–596 (1997). [DOI] [PubMed] [Google Scholar]
- 4.Harrington LE et al. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat. Immunol 6, 1123–1132 (2005). [DOI] [PubMed] [Google Scholar]
- 5.Park H et al. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat. Immunol 6, 1133–1141 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hwang ES, Szabo SJ, Schwartzberg PL & Glimcher LH T helper cell fate specified by kinase-mediated interaction of T-bet with GATA-3. Science 307, 430–433 (2005). [DOI] [PubMed] [Google Scholar]
- 7.Lazarevic V et al. T-bet represses TH17 differentiation by preventing Runx1-mediated activation of the gene encoding RORγt. Nat. Immunol 12, 96–104 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Villarino AV, Gallo E & Abbas AK STAT1-activating cytokines limit Th17 responses through both T-bet-dependent and -independent mechanisms. J. Immunol 185, 6461–6471 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Esensten JH, Lee MR, Glimcher LH & Bluestone JA T-bet-deficient NOD mice are protected from diabetes due to defects in both T cell and innate immune system function. J. Immunol 183, 75–82 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang J et al. Transcription factor T-bet regulates inflammatory arthritis through its function in dendritic cells. J. Clin. Invest 116, 414–421 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sullivan BM, Juedes A, Szabo SJ, von Herrath M & Glimcher LH Antigen-driven effector CD8 T cell function regulated by T-bet. Proc. Natl. Acad. Sci. USA 100, 15818–15823 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Peng SL, Szabo SJ & Glimcher LH T-bet regulates IgG class switching and pathogenic autoantibody production. Proc. Natl. Acad. Sci. USA 99, 5545–5550 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Townsend MJ et al. T-bet regulates the terminal maturation and homeostasis of NK and Vα14i NKT cells. Immunity 20, 477–494 (2004). [DOI] [PubMed] [Google Scholar]
- 14.Afkarian M et al. T-bet is a STAT1-induced regulator of IL-12R expression in naive CD4+ T cells. Nat. Immunol 3, 549–557 (2002). [DOI] [PubMed] [Google Scholar]
- 15.Lighvani AA et al. T-bet is rapidly induced by interferon-γ in lymphoid and myeloid cells. Proc. Natl. Acad. Sci. USA 98, 15137–15142 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mullen AC et al. Role of T-bet in commitment of TH1 cells before IL-12-dependent selection. Science 292, 1907–1910 (2001). [DOI] [PubMed] [Google Scholar]
- 17.Schulz EG, Mariani L, Radbruch A & Hofer T Sequential polarization and imprinting of type 1 T helper lymphocytes by interferon-γ and interleukin-12. Immunity 30, 673–683 (2009). [DOI] [PubMed] [Google Scholar]
- 18.Hu X & Ivashkiv LB Cross-regulation of signaling pathways by interferon-γ: implications for immune responses and autoimmune diseases. Immunity 31, 539–550 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jenner RG et al. The transcription factors T-bet and GATA-3 control alternative pathways of T-cell differentiation through a shared set of target genes. Proc. Natl. Acad. Sci. USA 106, 17876–17881 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lord GM et al. T-bet is required for optimal proinflammatory CD4+ T-cell trafficking. Blood 106, 3432–3439 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sullivan BM et al. Increased susceptibility of mice lacking T-bet to infection with Mycobacterium tuberculosis correlates with increased IL-10 and decreased IFN-γ production. J. Immunol 175, 4593–4602 (2005). [DOI] [PubMed] [Google Scholar]
- 22.Szabo SJ et al. Distinct effects of T-bet in TH1 lineage commitment and IFN-γ production in CD4 and CD8 T cells. Science 295, 338–342 (2002). [DOI] [PubMed] [Google Scholar]
- 23.Hultgren OH, Verdrengh M & Tarkowski A T-box transcription-factor-deficient mice display increased joint pathology and failure of infection control during staphylococcal arthritis. Microbes Infect 6, 529–535 (2004). [DOI] [PubMed] [Google Scholar]
- 24.Ravindran R, Foley J, Stoklasek T, Glimcher LH & McSorley SJ Expression of T-bet by CD4 T cells is essential for resistance to Salmonella infection. J. Immunol 175, 4603–4610 (2005). [DOI] [PubMed] [Google Scholar]
- 25.Neurath MF et al. The transcription factor T-bet regulates mucosal T cell activation in experimental colitis and Crohn’s disease. J. Exp. Med 195, 1129–1143 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bettelli E et al. Loss of T-bet, but not STAT1, prevents the development of experimental autoimmune encephalomyelitis. J. Exp. Med 200, 79–87 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lovett-Racke AE et al. Silencing T-bet defines a critical role in the differentiation of autoreactive T lymphocytes. Immunity 21, 719–731 (2004). [DOI] [PubMed] [Google Scholar]
- 28.Juedes AE, Rodrigo E, Togher L, Glimcher LH & von Herrath MG T-bet controls autoaggressive CD8 lymphocyte responses in type 1 diabetes. J. Exp. Med 199, 1153–1162 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Flynn JL et al. An essential role for interferon-γ in resistance to Mycobacterium tuberculosis infection. J. Exp. Med 178, 2249–2254 (1993). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huang S et al. Immune response in mice that lack the interferon-γ receptor. Science 259, 1742–1745 (1993). [DOI] [PubMed] [Google Scholar]
- 31.Harty JT & Bevan MJ Specific immunity to Listeria monocytogenes in the absence of IFN γ. Immunity 3, 109–117 (1995). [DOI] [PubMed] [Google Scholar]
- 32.Way SS & Wilson CB Cutting edge: immunity and IFN-γ production during Listeria monocytogenes infection in the absence of T-bet. J. Immunol 173, 5918–5922 (2004). [DOI] [PubMed] [Google Scholar]
- 33.Fuss IJ et al. Disparate CD4+ lamina propria (LP) lymphokine secretion profiles in inflammatory bowel disease. Crohn’s disease LP cells manifest increased secretion of IFN-γ, whereas ulcerative colitis LP cells manifest increased secretion of IL-5. J. Immunol 157, 1261–1270 (1996). [PubMed] [Google Scholar]
- 34.Plevy SE et al. A role for TNF-α and mucosal T helper-1 cytokines in the pathogenesis of Crohn’s disease. J. Immunol 159, 6276–6282 (1997). [PubMed] [Google Scholar]
- 35.Targan SR, Deem RL, Liu M, Wang S & Nel A Definition of a lamina propria T cell responsive state. Enhanced cytokine responsiveness of T cells stimulated through the CD2 pathway. J. Immunol 154, 664–675 (1995). [PubMed] [Google Scholar]
- 36.Matsuoka K et al. T-bet upregulation and subsequent interleukin 12 stimulation are essential for induction of Th1 mediated immunopathology in Crohn’s disease. Gut 53, 1303–1308 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yang Y, Xu J, Niu Y, Bromberg JS & Ding Y T-bet and eomesodermin play critical roles in directing T cell differentiation to Th1 versus Th17. J. Immunol 181, 8700–8710 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sasaki Y et al. Identification of a novel type 1 diabetes susceptibility gene, T-bet. Hum. Genet 115, 177–184 (2004). [DOI] [PubMed] [Google Scholar]
- 39.Hultgren B, Huang X, Dybdal N & Stewart TA Genetic absence of γ-interferon delays but does not prevent diabetes in NOD mice. Diabetes 45, 812–817 (1996). [DOI] [PubMed] [Google Scholar]
- 40.Serreze DV et al. Interferon-gamma receptor signaling is dispensable in the development of autoimmune type 1 diabetes in NOD mice. Diabetes 49, 2007–2011 (2000). [DOI] [PubMed] [Google Scholar]
- 41.Kaplan MH, Schindler U, Smiley ST & Grusby MJ Stat6 is required for mediating responses to IL-4 and for development of Th2 cells. Immunity 4, 313–319 (1996). [DOI] [PubMed] [Google Scholar]
- 42.Kurata H, Lee HJ, O’Garra A & Arai N Ectopic expression of activated Stat6 induces the expression of Th2-specific cytokines and transcription factors in developing Th1 cells. Immunity 11, 677–688 (1999). [DOI] [PubMed] [Google Scholar]
- 43.Zhu J, Guo L, Watson CJ, Hu-Li J & Paul WE Stat6 is necessary and sufficient for IL-4’s role in Th2 differentiation and cell expansion. J. Immunol 166, 7276–7281 (2001). [DOI] [PubMed] [Google Scholar]
- 44.Djuretic IM et al. Transcription factors T-bet and Runx3 cooperate to activate Ifng and silence Il4 in T helper type 1 cells. Nat. Immunol 8, 145–153 (2007). [DOI] [PubMed] [Google Scholar]
- 45.Avni O et al. TH cell differentiation is accompanied by dynamic changes in histone acetylation of cytokine genes. Nat. Immunol 3, 643–651 (2002). [DOI] [PubMed] [Google Scholar]
- 46.Finotto S et al. Development of spontaneous airway changes consistent with human asthma in mice lacking T-bet. Science 295, 336–338 (2002). [DOI] [PubMed] [Google Scholar]
- 47.Finotto S et al. Asthmatic changes in mice lacking T-bet are mediated by IL-13. Int. Immunol 17, 993–1007 (2005). [DOI] [PubMed] [Google Scholar]
- 48.Munthe-Kaas MC et al. T cell-specific T-box transcription factor haplotype is associated with allergic asthma in children. J. Allergy Clin. Immunol 121, 51–56 (2008). [DOI] [PubMed] [Google Scholar]
- 49.Raby BA et al. T-bet polymorphisms are associated with asthma and airway hyper-responsiveness. Am. J. Respir. Crit. Care Med 173, 64–70 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kiwamoto T et al. Transcription factors T-bet and GATA-3 regulate development of airway remodeling. Am. J. Respir. Crit. Care Med 174, 142–151 (2006). [DOI] [PubMed] [Google Scholar]
- 51.Bettelli E et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature 441, 235–238 (2006). [DOI] [PubMed] [Google Scholar]
- 52.Ivanov II et al. The orphan nuclear receptor RORγt directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell 126, 1121–1133 (2006). [DOI] [PubMed] [Google Scholar]
- 53.Mangan PR et al. Transforming growth factor-beta induces development of the TH17 lineage. Nature 441, 231–234 (2006). [DOI] [PubMed] [Google Scholar]
- 54.Veldhoen M, Hocking RJ, Atkins CJ, Locksley RM & Stockinger B TGFβ in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity 24, 179–189 (2006). [DOI] [PubMed] [Google Scholar]
- 55.Korn T et al. IL-21 initiates an alternative pathway to induce proinflammatory TH17 cells. Nature 448, 484–487 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhou L et al. IL-6 programs TH-17 cell differentiation by promoting sequential engagement of the IL-21 and IL-23 pathways. Nat. Immunol 8, 967–974 (2007). [DOI] [PubMed] [Google Scholar]
- 57.Nurieva R et al. Essential autocrine regulation by IL-21 in the generation of inflammatory T cells. Nature 448, 480–483 (2007). [DOI] [PubMed] [Google Scholar]
- 58.Brustle A et al. The development of inflammatory TH-17 cells requires interferon-regulatory factor 4. Nat. Immunol 8, 958–966 (2007). [DOI] [PubMed] [Google Scholar]
- 59.Schraml BU et al. The AP-1 transcription factor Batf controls TH17 differentiation. Nature 460, 405–409 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhang F, Meng G & Strober W Interactions among the transcription factors Runx1, RORγt and Foxp3 regulate the differentiation of interleukin 17-producing T cells. Nat. Immunol 9, 1297–1306 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mukasa R et al. Epigenetic instability of cytokine and transcription factor gene loci underlies plasticity of the T helper 17 cell lineage. Immunity 32, 616–627 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bending D et al. Epigenetic changes at il12rb2 and tbx21 in relation to plasticity behavior of th17 cells. J. Immunol 186, 3373–3382 (2011). [DOI] [PubMed] [Google Scholar]
- 63.Doodes PD et al. IFN-γ regulates the requirement for IL-17 in proteoglycan-induced arthritis. J. Immunol 184, 1552–1559 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rutitzky LI, Smith PM & Stadecker MJ T-bet protects against exacerbation of schistosome egg-induced immunopathology by regulating Th17-mediated inflammation. Eur. J. Immunol 39, 2470–2481 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yuan X et al. A novel role of CD4 Th17 cells in mediating cardiac allograft rejection and vasculopathy. J. Exp. Med 205, 3133–3144 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Guo S, Cobb D & Smeltz RB T-bet inhibits the in vivo differentiation of parasite-specific CD4+ Th17 cells in a T cell-intrinsic manner. J. Immunol 182, 6179–6186 (2009). [DOI] [PubMed] [Google Scholar]
- 67.Durrant DM, Gaffen SL, Riesenfeld EP, Irvin CG & Metzger DW Development of allergen-induced airway inflammation in the absence of T-bet regulation is dependent on IL-17. J. Immunol 183, 5293–5300 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Furuta S et al. Overlapping and distinct roles of STAT4 and T-bet in the regulation of T cell differentiation and allergic airway inflammation. J. Immunol 180, 6656–6662 (2008). [DOI] [PubMed] [Google Scholar]
- 69.Manoury-Schwartz B et al. High susceptibility to collagen-induced arthritis in mice lacking IFN-γ receptors. J. Immunol 158, 5501–5506 (1997). [PubMed] [Google Scholar]
- 70.Vermeire K et al. Accelerated collagen-induced arthritis in IFN-γ receptor-deficient mice. J. Immunol 158, 5507–5513 (1997). [PubMed] [Google Scholar]
- 71.Nistala K et al. Th17 plasticity in human autoimmune arthritis is driven by the inflammatory environment. Proc. Natl. Acad. Sci. USA 107, 14751–14756 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cannon GW et al. Double-blind trial of recombinant γ-interferon versus placebo in the treatment of rheumatoid arthritis. Arthritis Rheum 32, 964–973 (1989). [DOI] [PubMed] [Google Scholar]
- 73.Panitch HS, Hirsch RL, Schindler J & Johnson KP Treatment of multiple sclerosis with γ interferon: exacerbations associated with activation of the immune system. Neurology 37, 1097–1102 (1987). [DOI] [PubMed] [Google Scholar]
- 74.Skurkovich S et al. Randomized study of antibodies to IFN-γ and TNF-α in secondary progressive multiple sclerosis. Mult. Scler 7, 277–284 (2001). [DOI] [PubMed] [Google Scholar]
- 75.Ferber IA et al. Mice with a disrupted IFN-γ gene are susceptible to the induction of experimental autoimmune encephalomyelitis (EAE). J. Immunol 156, 5–7 (1996). [PubMed] [Google Scholar]
- 76.Willenborg DO, Fordham S, Bernard CC, Cowden WB & Ramshaw IA IFN-γ plays a critical down-regulatory role in the induction and effector phase of myelin oligodendrocyte glycoprotein-induced autoimmune encephalomyelitis. J. Immunol 157, 3223–3227 (1996). [PubMed] [Google Scholar]
- 77.Frisullo G et al. pSTAT1, pSTAT3, and T-bet expression in peripheral blood mono-nuclear cells from relapsing-remitting multiple sclerosis patients correlates with disease activity. J. Neurosci. Res 84, 1027–1036 (2006). [DOI] [PubMed] [Google Scholar]
- 78.Nos C et al. Clinical impact of intravenous methylprednisolone in attacks of multiple sclerosis. Mult. Scler 10, 413–416 (2004). [DOI] [PubMed] [Google Scholar]
- 79.Liberman AC et al. The activated glucocorticoid receptor inhibits the transcription factor T-bet by direct protein-protein interaction. FASEB J 21, 1177–1188 (2007). [DOI] [PubMed] [Google Scholar]
- 80.Frisullo G et al. Glucocorticoid treatment reduces T-bet and pSTAT1 expression in mononuclear cells from relapsing remitting multiple sclerosis patients. Clin. Immunol 124, 284–293 (2007). [DOI] [PubMed] [Google Scholar]
- 81.Drulovic J et al. Expression of Th1 and Th17 cytokines and transcription factors in multiple sclerosis patients: does baseline T-bet mRNA predict the response to interferon-beta treatment? J. Neuroimmunol 215, 90–95 (2009). [DOI] [PubMed] [Google Scholar]
- 82.Chen Y et al. Anti-IL-23 therapy inhibits multiple inflammatory pathways and ameliorates autoimmune encephalomyelitis. J. Clin. Invest 116, 1317–1326 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yang XO et al. Regulation of inflammatory responses by IL-17F. J. Exp. Med 205, 1063–1075 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hu Y et al. IL-17RC is required for IL-17A- and IL-17F-dependent signaling and the pathogenesis of experimental autoimmune encephalomyelitis. J. Immunol 184, 4307–4316 (2010). [DOI] [PubMed] [Google Scholar]
- 85.Yang Y et al. T-bet is essential for encephalitogenicity of both Th1 and Th17 cells. J. Exp. Med 206, 1549–1564 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Reboldi A et al. C–C chemokine receptor 6-regulated entry of TH-17 cells into the CNS through the choroid plexus is required for the initiation of EAE. Nat. Immunol 10, 514–523 (2009). [DOI] [PubMed] [Google Scholar]
- 87.Kebir H et al. Preferential recruitment of interferon-γ-expressing TH17 cells in multiple sclerosis. Ann. Neurol 66, 390–402 (2009). [DOI] [PubMed] [Google Scholar]
- 88.Abromson-Leeman S, Bronson RT & Dorf ME Encephalitogenic T cells that stably express both T-bet and RORγt consistently produce IFNγ but have a spectrum of IL-17 profiles. J. Neuroimmunol 215, 10–24 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ghoreschi K et al. Generation of pathogenic TH17 cells in the absence of TGF-β signalling. Nature 467, 967–971 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Chitnis T et al. Effect of targeted disruption of STAT4 and STAT6 on the induction of experimental autoimmune encephalomyelitis. J. Clin. Invest 108, 739–747 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Cua DJ et al. Interleukin-23 rather than interleukin-12 is the critical cytokine for autoimmune inflammation of the brain. Nature 421, 744–748 (2003). [DOI] [PubMed] [Google Scholar]
- 92.Hirota K et al. Fate mapping of IL-17-producing T cells in inflammatory responses. Nat. Immunol 12, 255–263 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Koch MA et al. The transcription factor T-bet controls regulatory T cell homeostasis and function during type 1 inflammation. Nat. Immunol 10, 595–602 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Fontenot JD, Gavin MA & Rudensky AY Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat. Immunol 4, 330–336 (2003). [DOI] [PubMed] [Google Scholar]
- 95.Garrett WS et al. Communicable ulcerative colitis induced by T-bet deficiency in the innate immune system. Cell 131, 33–45 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Dardalhon V et al. IL-4 inhibits TGF-beta-induced Foxp3+ T cells and, together with TGF-β, generates IL-9+IL-10+Foxp3–effector T cells. Nat. Immunol 9, 1347–1355 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Veldhoen M et al. Transforming growth factor-β ‘reprograms’ the differentiation of T helper 2 cells and promotes an interleukin 9–producing subset. Nat. Immunol 9, 1341–1346 (2008). [DOI] [PubMed] [Google Scholar]
- 98.Jager A, Dardalhon V, Sobel RA, Bettelli E & Kuchroo VK Th1, Th17, and Th9 effector cells induce experimental autoimmune encephalomyelitis with different pathological phenotypes. J. Immunol 183, 7169–7177 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Nurieva RI et al. Generation of T follicular helper cells is mediated by interleukin-21 but independent of T helper 1, 2, or 17 cell lineages. Immunity 29, 138–149 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Nurieva RI et al. Bcl6 mediates the development of T follicular helper cells. Science 325, 1001–1005 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Cruz-Guilloty F et al. Runx3 and T-box proteins cooperate to establish the transcriptional program of effector CTLs. J. Exp. Med 206, 51–59 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Pearce EL et al. Control of effector CD8+ T cell function by the transcription factor Eomesodermin. Science 302, 1041–1043 (2003). [DOI] [PubMed] [Google Scholar]
- 103.Intlekofer AM et al. Effector and memory CD8+ T cell fate coupled by T-bet and eomesodermin. Nat. Immunol 6, 1236–1244 (2005). [DOI] [PubMed] [Google Scholar]
- 104.Intlekofer AM et al. Anomalous type 17 response to viral infection by CD8+ T cells lacking T-bet and eomesodermin. Science 321, 408–411 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Joshi NS et al. Inflammation directs memory precursor and short-lived effector CD8+ T cell fates via the graded expression of T-bet transcription factor. Immunity 27, 281–295 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Rao RR, Li Q, Odunsi K & Shrikant PA The mTOR kinase determines effector versus memory CD8+ T cell fate by regulating the expression of transcription factors T-bet and Eomesodermin. Immunity 32, 67–78 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Oldstone MB, Nerenberg M, Southern P, Price J & Lewicki H Virus infection triggers insulin-dependent diabetes mellitus in a transgenic model: role of anti-self (virus) immune response. Cell 65, 319–331 (1991). [DOI] [PubMed] [Google Scholar]
- 108.Ohashi PS et al. Ablation of “tolerance” and induction of diabetes by virus infection in viral antigen transgenic mice. Cell 65, 305–317 (1991). [DOI] [PubMed] [Google Scholar]
- 109.Burrell BE, Csencsits K, Lu G, Grabauskiene S & Bishop DK CD8+ Th17 mediate costimulation blockade-resistant allograft rejection in T-bet-deficient mice. J. Immunol 181, 3906–3914 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Rangachari M et al. T-bet negatively regulates autoimmune myocarditis by suppressing local production of interleukin 17. J. Exp. Med 203, 2009–2019 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Gerth AJ, Lin L & Peng SL T-bet regulates T-independent IgG2a class switching. Int. Immunol 15, 937–944 (2003). [DOI] [PubMed] [Google Scholar]
- 112.Liu N, Ohnishi N, Ni L, Akira S & Bacon KB CpG directly induces T-bet expression and inhibits IgG1 and IgE switching in B cells. Nat. Immunol 4, 687–693 (2003). [DOI] [PubMed] [Google Scholar]
- 113.Werneck MB, Lugo-Villarino G, Hwang ES, Cantor H & Glimcher LH T-bet plays a key role in NK-mediated control of melanoma metastatic disease. J. Immunol 180, 8004–8010 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Lugo-Villarino G, Ito S, Klinman DM & Glimcher LH The adjuvant activity of CpG DNA requires T-bet expression in dendritic cells. Proc. Natl. Acad. Sci. USA 102, 13248–13253 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Lugo-Villarino G, Maldonado-Lopez R, Possemato R, Penaranda C & Glimcher LH T-bet is required for optimal production of IFN-γ and antigen-specific T cell activation by dendritic cells. Proc. Natl. Acad. Sci. USA 100, 7749–7754 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Lipscomb MW et al. Ectopic T-bet expression licenses dendritic cells for IL-12-independent priming of type 1 T cells in vitro. J. Immunol 183, 7250–7258 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Veiga P et al. Bifidobacterium animalis subsp. lactis fermented milk product reduces inflammation by altering a niche for colitogenic microbes. Proc. Natl. Acad. Sci. USA 107, 18132–18137 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Garrett WS et al. Colitis-associated colorectal cancer driven by T-bet deficiency in dendritic cells. Cancer Cell 16, 208–219 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Hegazy AN et al. Interferons direct Th2 cell reprogramming to generate a stable GATA-3+T-bet+ cell subset with combined Th2 and Th1 cell functions. Immunity 32, 116–128 (2010). [DOI] [PubMed] [Google Scholar]
- 120.Lee YK et al. Late developmental plasticity in the T helper 17 lineage. Immunity 30, 92–107 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]