Abstract
Callosobruchus maculatus is an important predator of cowpeas. Due to infestation during storage, this insect affects the quality of seed and crop yield. This study aimed to investigate the effects of CrataBL, a multifunction protein isolated from Crataeva tapia bark, on C. maculatus larvae development. The protein, which is stable even in extreme pH conditions, showed toxic activity reducing the larval mass 45% and 70% at concentrations of 0.25% and 1.0% (w/w), respectively. Acting as inhibitor, CrataBL decreased by 39% the activity of cysteine proteinases from larval gut. Conversely, the activity of serine proteinases was increased about 8-fold. The toxic properties of CrataBL may also be attributed to its capacity of binding to glycoproteins or glycosaminoglycans. Such binding interferes with larval metabolism, since CrataBL-FITC was found in the fat body, Malpighian tubules, and in the feces of larvae. These results demonstrate the potential of this protein for controlling larvae development.
Keywords: bioinsecticide, C. maculatus, Crataeva tapia, glycosaminoglycan, inhibitor, lectin
Graphical Abstract
INTRODUCTION
The legumes (seeds of beans) are an important component of nutrition in the tropics. For example, the cowpea (Vigna unguiculata (L.) Walp) is an important source of carbohydrates and proteins in dietary needs of various countries. The bean beetle Callosobruchus maculatus (Fabricius) (Coleoptera: Bruchinae) is among the most important insect pests of cowpea, severely affecting the quality and storability of the produce, by attacking seeds during storage. Due to the extensive adaptability of these storage pests, the destruction of seeds is often extremely high, making the beans useless for human consumption or for replanting.1
Plants produce various metabolic products that may be responsible for pest resistance. These compounds can be classified as primary (proteinase inhibitors and lectins) and secondary (alkaloids and tannins) metabolites.2 Examples of toxic primary metabolites of plants are canatoxin isolated from Canavalia ensiformis,3 zeatoxin glycoprotein isolated from Zea mays,4 and the proteinase inhibitor isolated from Bauhinia rufa seeds.5
Lectins are proteins not belonging to the immune system that are able to reversibly bind to carbohydrates by recognizing specific sites on the target molecules, without altering the covalent structure of the glycosides.6–10 Thus lectins may bind to glyco components on the cell surface of viruses, bacteria, and on tissues from plant and animals source.9,11 Such interactions can subsequently trigger other responses, including antimicrobial, anti-tumor, mitogenic, and insecticidal activity.12–14 It has been reported that some plant lectins are toxic to insects belonging to the orders of Coleoptera, Diptera, Lepidoptera, and Isoptera.15-20
Crataeva tapia (Capparidaceae), also known as Crateva tapia, is a tree found in northeastern Brazil, where its wood is used in construction and for building canoes, because of its great resistance to putrefaction.21 CrataBL (Crataeva tapia Bark Lectin) is a bifunctional protein (lectin and protease inhibitor of trypsin and factor Xa) that was isolated from the Crataeva tapia bark. The primary and tertiary structure of this protein was characterized previously.22 CrataBL exhibits binding specificity to N-acetyl glucosamine, 23 glucose, and galactose.14 It was already demonstrated that this protein has several important biological functions including changes in the intrinsic pathway of the coagulation cascade proteins leading to the delay time of formation of the blood clot,24 as well as thrombus formation.25 In addition, CrataBL was shown to exhibit analgesic and anti-inflammatory,26–27 anti-tumoral,22,26 and insecticidal (against Nasutitermes corniger14) properties.
In this report, we present the results of monitoring the insecticidal effects of CrataBL on C. maculatus utilizing an insect bioassay.
MATERIALS AND METHODS
Purification of CrataBL.
The purification the CrataBL was accomplished following the previously described methodology.14,22 Briefly, protein from the fine powder of Crataeva tapia bark was extracted with saline (0.15 M NaCl) (10% w/v). The saline extract was subjected to protein fractionation with ammonium sulfate (0–30% and 30–60% (w/v)). The 30–60% fraction, containg lectin activity, was dialyzed against 0.01M phosphate citrate buffer pH 5.5 and applied to a CM-cellulose column that was previously equilibrated with the dialysis buffer. Elution of CrataBL was performed by adding the equilibration buffer plus 0.5 M NaCl. This eluate was applied to a Superdex 75 column attached to Äkta Purifier (GE Healthcare) and equilibrated with 0.15 M NaCl. The homogeneity of CrataBL preparation was followed by reverse phase chromatography in a C18 protein/peptide column (15 cm X 4.6 mm; Vydac) in a linear gradient of acetonitrile in trifluoroacetic acid (TFA) (0.1%, v/v) at a flow rate of 0.7 mL/min and monitored at 280 nm.
Circular Dichroism (CD) analysis.
CD spectra were obtained with a J-810 JASCO spectropolarimeter. Measurements were carried out at 25 °C at a CrataBL concentration of 10 μM in a 1 mm pathlength cuvette and were recorded in the 190–250 nm range as an average of eight scans. The results were expressed as the mean residue ellipticity, [θ], defined as [θ] = θobs/(10.C.l.n), where θobs is the CD in millidegrees, C is the protein concentration (M), l is the pathlength of the cuvette (cm), and n is the number of amino acid residues (165, as described by Ferreira et al. (2013)22). The CDPro software was used to estimate the fractions of the secondary structure28 and the Cluster program was used to determine the tertiary structure class.29
The effect of pH on the CrataBL conformation was determined by CD. CrataBL, at an initial concentration of 2.0 mg/mL, was diluted with 10 mM acetate/phosphate/borate (PBA buffer), pH 2.0; 4.0; 5.5; 6.0; 7.4; 8.0; 10.0; and 12.0 to a final concentration of 0.2 mg/mL. The protein was incubated for six hours at room temperature.
Insects.
C. maculatus colony was maintained at the Laboratório de Química e Função de Proteínas, Departamento de Bioquímica, Universidade Federal de São Paulo, São Paulo, SP, Brazil. The bruchids were reared on V. unguiculata host seeds (cv. Fradinho), purchased at supermarkets in the city, in glass bottles at 28 °C and 60–80% relative humidity inside a B.O.D. incubator.
The coats of V. unguiculata seeds were separated from cotyledons by manual peeling and a fine flour of V. unguiculata cotyledons was made. The flour was placed into a cylindrical brass mold containing variable concentrations (w/w) of CrataBL. These artificial seeds have a solid consistency, are 8 mm in diameter and 5 mm in height, with the final mass of 400 mg.30 After the removal of a seed from the mold it was exposed to three C. maculatus females (2 days old) over 24 h, under conditions described above. Subsequently the females were removed, and only four eggs were left on each seed, with the excess eggs removed. The control artificial seeds consisted of only V. unguiculata flour, without addition of CrataBL; they were maintained in the same conditions, including the number of eggs. Both the control and CrataBL-containing seeds were incubated for a period of 18 days (28 °C and relative humidity 60–80%). Upon completion of the incubation the seeds were opened and the larval mass, as well as the number of emerging larvae, were determined.
Conjugation of FITC (isothiocyanate fluoresceine) to CrataBL.
A solution of the compound FITC was prepared by dissolving 50 mg in 1 mL of anhydrous DMSO. In order to covalently couple it to CrataBL, this solution was immediately diluted in 0.75 M bicarbonate buffer pH 9.5 and then added to the solution of CrataBL, to yield the final concentration of 1 mg FITC for 1 mg of protein. The solution was kept in the dark and constantly rotated at room temperature for 1 h. After this period the solution was dialyzed through 14 kDa membrane against Milli-Q water to remove FITC which was not conjugated. The solution of the FITC-CrataBL complex was subsequently lyophilized and was used later for making artificial seeds.
Artificial seeds containing CrataBL coupled to FITC.
Artificial seeds which contained 1% CrataBL coupled to FITC (w/w) were made as described above. Artificial seeds used as controls included only V. unguiculata flour. After 18 days the larvae were removed and placed on glass plates for 30 min to collect feces. They were subsequently dissected in 0.15 M NaCl for collection of midgut, malpighian tubules, and fat body and were further analyzed through Leica confocal microscopy.
Enzyme activity assays.
Cysteine proteinase activity.
For extraction of enzymes from midgut, five larvae, after 18 days of maintenance on artificial seeds incorporating 1% (w/w) CrataBL, were dissected in 0.15 M NaCl and their midgut macerated with 250 µL of 0.1 M sodium phosphate buffer pH 6.3 with 0.01 M EDTA, 0.4 M NaCl and 0.005 M DTT. The extract was maintained in constant rotation at 5 °C for 1 h, centrifuged at 4000g for 5 min at 4 °C, and the supernatant was used for enzymatic assay.
Proteinase activity of the extract was measured using the 4.10−4 M peptide Z-Phe-Arg-pNan (Calbiochem Ltda, Drmstadt, Germany) as a substrate. Twenty microliters of the extract diluted 10x in 0.1 M sodium phosphate buffer pH 6.3 with 0.01 M EDTA, 0.4 M NaCl and 0.005 M DTT were incubated at 37 °C in microplate assay, in a 250 µL final volume of 0.1 M sodium phosphate buffer, pH 6.3 with 0.01 M EDTA, 0.4 M NaCl and 0.005 M DTT. The reaction was monitored for 30 min. Substrate hydrolysis was monitored by measuring the absorbance of the released p-nitroaniline at 405 nm in a spectrophotometer (Spectra max plus 384, Molecular Devices).
Serine proteinase activity.
The midgut enzymes were extracted as described above. However, in this case the maceration was performed in 150 µL of 0.05 M Tris/HCl buffer pH 8.0 with 0.02% CaCl2. The extract was maintained in constant rotation at 5 °C for 1 h and was subsequently centrifuged at 4000g for 5 min at 4 °C, with the supernatant used for enzymatic assays.
Proteinase activity of the extract was measured using α-benzoyl-DL-arginine p-nitroanilide (BAPA) (Bachem, Bubendorf, Switzerland) as a substrate. Forty microliter of the extract were incubated with 20 µL 0.01 M BAPA for in microplate assay in a 250 µL final volume at 37 °C for 120 min. Substrate hydrolysis was monitored by measuring the absorbance of released p-nitroaniline at 405 nm in a spectrophotometer (Spectra max plus 384, Molecular Devices).
RESULTS AND DISCUSSION
Circular Dichroism (CD) analysis.
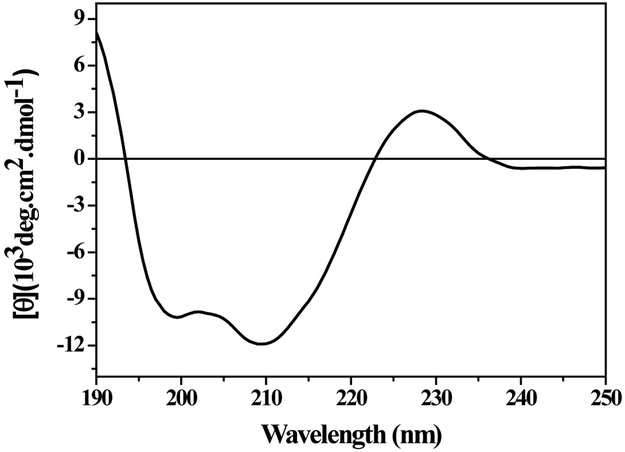
The CD spectrum of CrataBL in pH 7.4 is characterized by two negative bands, one at around 198 nm and the other one around 210 nm (Figure 1). This indicates that this protein possesses a large fraction of β-sheet structure and a low content (or complete lack) of α-helix structure, because no peaks were observed around 208 nm and 222 nm.31 Additionally, the CD spectrum of CrataBL shows a positive contribution below 190 nm and a positive band around 228 nm that are due to contribution of aromatic residues and disulfide bridges.32 (CrataBL contains four tyrosine residues, three tryptophan residues, seven phenylalanine residues and two disulfide bridges.22) The contents of secondary structure estimated using the CDPro software were 2% α-helix, 46% β-sheet, 21% β-turn, 31% irregular structures and an RMSD (root mean square deviation) was lower than 0.1%. Cluster analysis showed CrataBL to be a β-class protein (proteins containing mainly β structure), and this result was corroborated by CDPro analysis (46% all-β structures) and by crystallographic data which showed that this lectin belongs to the β-trefoil superfamily.22 Proteinase inhibitors from the Kunitz family33,34 have secondary structure content similar to that of CrataBL. CD spectra of other Kunitz family inhibitors are generally similar, although, for example, the soybean trypsin inhibitor (STI), a model protein of this family, displays only one negative band around 200 nm in his CD spectrum. However, the papaya Kunitz-type trypsin inhibitor33 has a very similar CD spectrum that includes positive and negative CD bands, as observed in the CD spectrum of the CrataBL.
Figure 1.
Measurements of the circular dichroism of CrataBL. A CD spectrum of CrataBL was obtained in 0.01 M PBA pH 7.4, at 25 °C. Measurements are the averages of eight scans using a solution containing 10 μM of protein. CD spectrum deconvolution using CDPro software calculated 2% α-helix, 46% β-sheet, 21% β-turn, 31% irregular structures.
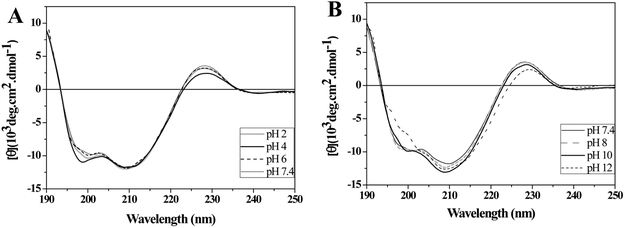
The secondary structure of CrataBL was not affected by the changes in pH ranging from 2.0 to 10.0, as shown by the CD spectra (Figure 2). Furthermore, an increase in pH to 12.0 resulted in the loss of CD band intensity centered at 199.5 nm and the reduction of the light intensity of the band with a maximum at 228.5 (Figure 2B). However, the profile of protein CD spectrum at this highly basic pH is not characteristic of disordered structure, suggesting that the molecule still retains considerable native-like order. These results demonstrate the stability of the secondary structure of the protein at both the high and low extremes of pH. The β-trefoil structure of CrataBL, also common in many other lectins and protease inhibitors,33,35 leads to increased stability of these proteins required in the performance of their biological functions.
Figure 2.
Circular dichroism spectra of CrataBL at different pH values. CD spectra of CrataBL (0.2 mg/ml) were obtained after incubation for 6 hours in phosphate-borate-acetate buffer at pH (A) 2.0; 4.0; 6.0 and 7.4, (B) 7.4; 8.0; 10.0 and 12.0.
The conformational stability of the CrataBL at different pH values makes this protein a good target for use in a bioassay with C. maculatus larvae, considering that the midgut of such insects has pH value around 5.6 to 6.2.36
Insect assay.
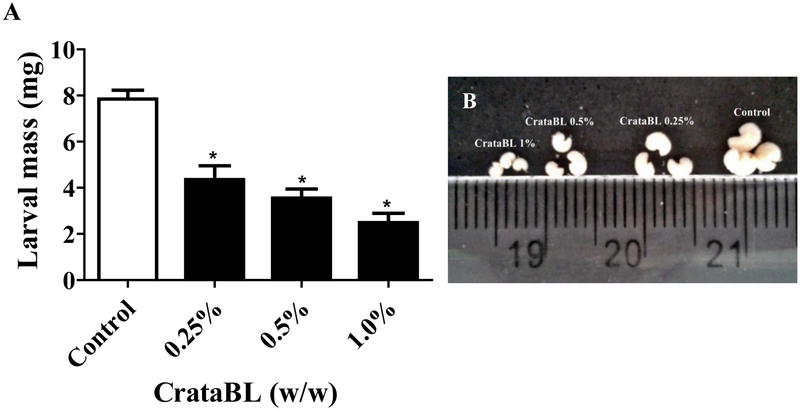
Previous studies have shown that plant lectins interfere with the development of insect larvae, in particular those of C. maculatus.37–41 The mass of the larvae was significantly lower in all CrataBL-fed groups compared to controls, demonstrating the efficiency of this protein as a deleterious component for C. maculatus. A mass reduction of about 45% and 70% was observed when the seeds used as the feed incorporated 0.25% or 1% (w/w) of CrataBL, respectively (Figure 3). No mortality among the larvae was found at the tested doses. The effect was similar to the effects of some other lectins. For example, the BmoLL lectin, purified from Bauhinia monandra leafs, 42 reduced the larval mass of C. maculatus by 50% when incorporated at a concentration of 0.4% in artificial seeds,18 whereas the TEL lectin purified from Talassia esculenta seeds reduced the larval mass by 50% when applied at a concentration of 1% (w/w).43
Figure 3.
Effects of CrataBL on the development of C. maculatus larvae in artificial seeds. (A) Mass of surviving larvae at 18 days. Values represent mean (±SEM) and the statistical treatments were performed using One-way ANOVA (Tukey test), p-value ˂ 0.001(*), n=20. (B) Photographs of C. maculatus larvae developed in artificial seeds with the incorporation of 0.25%, 0.5%, 1% (w/w) CrataBL, and without this protein (control).
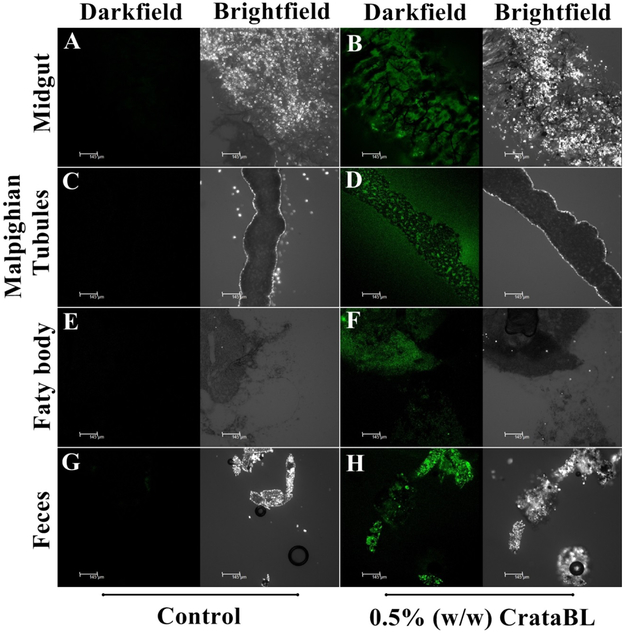
Investigation of CrataBL accumulation in C. maculatus.
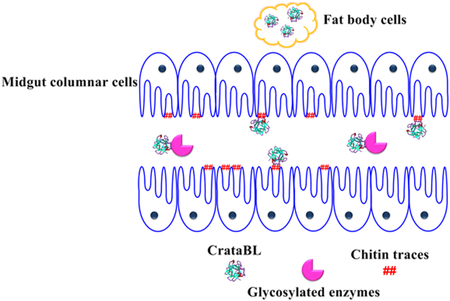
In order to verify biodistribution, clearance, or specific retention of CrataBL in the C. maculaus larvae we used CrataBL-FITC conjugated with FITC. Trafficking of FITC-labeled CrataBL was investigated using a Leica confocal microscope (488 nm excitation, emission detected at 490–550 nm). CrataBL was found in the midgut (Figure 4 A-B), Malpighian tubules (Figure 4 C-D), fat body (Figure 4 E-F), and feces (Figure 4 G-H). Retention of CrataBL in these tissues may be attributed to its lectin properties, primarily to its capacity for binding to N-acetylglucosamine (GlcNAc). Toxic activity of CrataBL on the caste of soldiers and workers of N. corniger insect (termite) was previously reported.14
Figure 4.
Detection of CrataBL in C. maculatus larvae. Magnification 10x. A-B midgut, C-D Malpighian tubules, E-F fat body, G-H feces of larvae. Bar = 145µm. Images obtained with a confocal microscope Leica TCS SP8.
Whereas millimolar levels of GlcNAc binding have been shown to inhibit hemagglutinating activity of CrataBL, the structural basis of this phenomenon has not yet been established, since the crystal structure of CrataBL has been determined only for the free lectin,22 while attempts to cocrystallize it with bound carbohydrates have not been successful as yet.
Lectins capable of specific binding of GlcNAc residues appear to be frequently insecticidal, including targets such as C. maculatus.44,45 The mechanism by which insecticidal lectins exert their function is not well elucidated, but may involve the binding to glycoproteins present in midgut epithelial cells, thereby disrupting cellular function.
Glycoproteins are major constituents of insect digestive tract membranes, i.e, the gut of these insects contains specific binding molecules that are targets of plant lectins.18,46–48 The lectin wheat germ agglutinin was demonstrated to interfere with the formation and integrity of the peritrophic membrane of the insect midgut.49 The peritrophic membrane is a barrier that protects the midgut epithelium from abrasive food particles. This membrane is present in most phytophagous insects and is composed primarily of chitin (containing GlcNAc residues). It is noteworthy that C. maculatus does not have a defined peritrophic membrane;50 however, the presence of chitin located in the apical part of microvilli in the midgut epithelium was demonstrated by chemical and immunocytochemical methods.51
Other proteins, for example vicilin, act by a mechanism similar to that of lectins, by binding to the midgut structures which are major components of the insect peritrophic membranes rich in GlcNAc.52–55 It was demonstrated that vicilin is concentrated in microvilli of enterocytes, interfering with the digestive physiology of larvae.51 Moreover, these proteins also cross the intestinal epithelium and are found in the cells of the fat body,56,57 destabilizing the internal organs of the larvae.58 Based on the results of this one and previous studies we may suggest that, similarly to the case of vicilin, broad biodistribution of the glycoprotein/lectin CrataBL might be related to its high affinity to GlcNAc.
Enzyme activity assay.
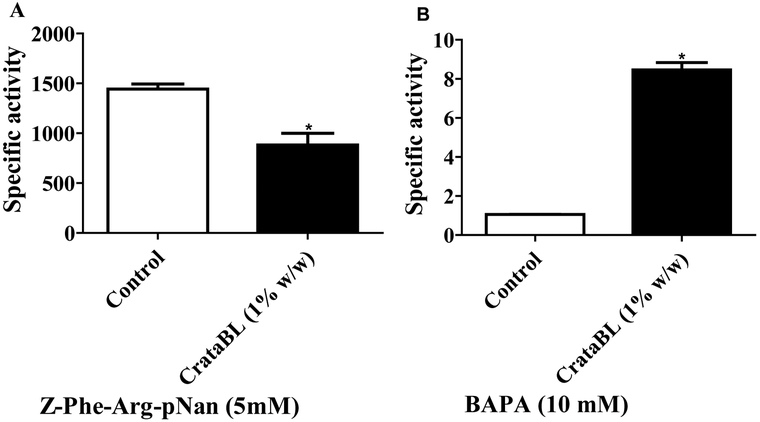
It has been shown that lectins, in addition to binding to glycoconjugates, can also bind to glycosylated digestive enzymes, interfering with their activity. Such binding results in a decrease of chitin hydrolysis in peritrophic membrane of the insect midgut, interfering with uptake of proteins and thereby inhibiting the digestion and absorption of food.25,43
To investigate if CrataBL affects the activity of digestive enzymes we measured the specific activity of two classes of enzymes from the midgut of C. maculatus larvae. The use of inhibitors of cysteine proteinases for Coleoptera plague control has also being attributed to the inhibition of exogenous activity of this class of proteinases.56,58,59 It should be noted, however, that CrataBL does not inhibit papain, the enzyme used most commonly as the model of cysteine proteinases.22 Our kinetic results indicate the activity of cysteine proteases in C. maculatus larvae, since the midgut extract cleaves better a more specific substrate for this class of enzymes, and the conditions used for the hydrolysis, such 0.1 M sodium phosphate buffer, pH 6.3 with 0.01 M EDTA, 0.4 M NaCl and 0.005 M DTT, are very suitable for cysteine proteinases. This activity decreased by 39% in the larvae fed for 18 days with artificial seeds containing CrataBL (1% w/w) when compared with control (Figure 5A). Conversely, specific activity of serine proteinases (Figure 5B) increased approximately 8-fold when compared with control. Previous studies suggested that, by binding to a sugar moiety on the enzyme surface, lectins decrease proteolysis, thus impairing larval development.60 A significant increase of the serine proteinase-like activity can be explained by the fact that, in order to overcome the deleterious effects of the lectin, the larvae upregulate other classes of proteolytic enzymes; however, this effect was not sufficient to overcome the toxicity of the protein. We can suggest that CrataBL, functionally active as both a lectin and/or as an inhibitor, interferes directly in production in the C. maculatus midgut of proteolytic enzymes necessary for larval development.
Figure 5.
Specific activity of the midgut of larvae of C. maculatus treated with CrataBL. The larvae were kept for 18 days in artificial seeds containing CrataBL (1% w/w). (A) Specific activity of cysteine proteinases. (B) Specific activity of serine proteinases. The values represents means (± SEM) and the statistical treatments were performed using t-test, p-value˂0.05 (*). Specific activity (U/mg), U – unit of enzyme activity (U=1 µmol/min).
In conclusion, our results indicate that the biological activity of CrataBL involves its binding to specific proteins in the intestinal epithelium, resulting in anti-nutritional effects that may be considered in the development of biopesticides.
Acknowledgments
Funding
This work was supported by FAPESP (2009/17058–6 and 2009/53766–5), CAPES and CNPq (470275/2012), and in part by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research,
REFERENCES
- (1).De Azevedo FR; Leitão ACL; Lima MAA; Guimarães JA Efficacy of natural products to control Callosobruchus maculatus (Fab.) in stores cowpea (Vigna unguiculata (L.) Walp). Ver. Ciênc. Agron 2007, 28, 182–187. [Google Scholar]
- (2).War AR; Paulraj MG; Ahmad T; Buhroo AA; Hussain B; Ignacimuthu S; Sharma HC Mechanisms of plant defense against insect herbivores. Plant Signal. Behav 2012, 7, 1306–1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Carlini CR; Oliveira AEA; Azambuja P; Xavier-Filho J; Wells MA Biological effects of canatoxin in different insect models: evidence for a proteolytic activation of the toxin by insect cathepsin-like enzymes. J. Econ. Entomol 1997, 90, 340–348. [DOI] [PubMed] [Google Scholar]
- (4).Macedo MLR; Coelho MB; Freire MGM; Machado OLT; Marangoni S; Novello JC Effect of a toxic protein isolated from Zea mays seeds on the development and survival of the cowpea weevil, Callosobruchus maculatus. Protein. Peptide. Lett 2000, 17, 25–31. [Google Scholar]
- (5).Sumikawa JT; Brito MV; Macedo MLR; Uchoa AF; Miranda A; Araujo APU; Silva-Luca RA; Sampaio MU; Oliva MLV The defensive functions of plant inhibitors are not restricted to insect inhibition enzyme. Phytochemistry. 2010, 71, 214–220. [DOI] [PubMed] [Google Scholar]
- (6).Peumans WJ; Van Damme EJM Lectins as plant defense proteins. Plant Physiol. 1995, 109, 347–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Van Damme EJM; Lannoo N; Fouquaert E; Peumans WJ The identification of inducible cytoplasmatic/nuclear carbohydrate-binding proteins urges to develop novel concepts about the role of plant lectins. Glycoconj. J 2004, 20, 449–460. [DOI] [PubMed] [Google Scholar]
- (8).Sharma A; Ng TB; Wong JH; Lin P Purification and characterization of a lectin from Phaseolus vulgaris cv. (Anasazi Beans). J. Biomed. and Biotechnol 2009, 2009, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Vandenborre G; Smagghe G; Van Damme EJM Plant lectins as defense proteins against phytophagous insects. Phytochemistry. 2011, 72, 1538–1550. [DOI] [PubMed] [Google Scholar]
- (10).Vijayan M; Chandra N Lectins. Curr. Opin. Struct. Biol 1999, 9, 707–714. [DOI] [PubMed] [Google Scholar]
- (11).Imberty A; Mitchell EP; Wimmerova M Structural basis of high-affinity glycan recognition by bacterial and fungal lectins. Curr. Opin. Struct. Biol 2005, 15, 525–534. [DOI] [PubMed] [Google Scholar]
- (12).Correia MTS; Coelho LCBB; Paiva PMG Lectins, carbohydrate recognition molecules: are they toxic? in: Siddique YH (Ed.), Recent Trends in Toxicology, Transworld Research Network, Kerala: 2008, 37, 47–59. [Google Scholar]
- (13).Li YR; Liu QH; Wang HX; Ng TB A novel lectin with potent antitumor, mitogenic and HIV-1 reverse transcriptase inhibitory activities from the edible mushroom Pleurotus citrinopileatus. Biochim. Biophys. Acta: Gen. Subjects 2008, 1780, 51–57. [DOI] [PubMed] [Google Scholar]
- (14).Araújo RM; Ferreira RS; Napoleão TH; Carneiro-da-Cunha MG; Coelho LC; Correia MT; Oliva ML; Paiva PM; Crataeva tapia bark lectin is an affinity adsorbent and insecticidal agent. Plant Science. 2012, 183, 20–26. [DOI] [PubMed] [Google Scholar]
- (15).Carlini CR; Grossi-de-Sá MF Plant toxic proteins with insecticidal properties. A review on their potentialities as bioinsecticides. Toxicon. 2002, 40, 1515–1539. [DOI] [PubMed] [Google Scholar]
- (16).Gatehouse AMR; Powell KS; Peumans WJ; Van Damme EJM; Gatehouse JA Insecticidal properties of plant lectins; their potential in plant protection In Lectins: Biomedical PerspectiVes; Pusztai AJ; Bardocz S, Eds.; Taylor and Francis: Hants, U.K., 1995, 35–38. [Google Scholar]
- (17).Vasconcelos IM; Oliveira JTA; Antinutritional properties of plant lectins. Toxicon. 2004, 44, 1737–1747. [DOI] [PubMed] [Google Scholar]
- (18).Macedo MLR; Freire MGM; Silva MBR; Coelho LCBB; Insecticidal action of Bauhinia monandra leaf lectin (BmoLL) against Anagasta kuehniella (Lepidoptera: Pyralidae), Zabrotes subfasciatus and Callosobruchus maculatus (Coleoptera: Bruchidae). Comp. Biochem. Phys. A 2007, 146, 486–498. [DOI] [PubMed] [Google Scholar]
- (19).Sa RA; Napoleão TH; Santos NDL; Gomes FS; Albuquerque AC; Xavier HS; Coelho LCBB; Bieber LW; Paiva PMG Induction of mortality Nasutitermes corninger (Isoptera, Termitidae) by Myracrondruon unrundeuva heartwood lectin. Int. Biodeterior. Biodegradation 2007, 62, 460–464. [Google Scholar]
- (20).Coelho JS; Santos NDL; Napoleão TH; Gomes FS; Ferreira RS; Zingali RB; Coelho LCBB; Leite SP; Navarro DMAF; Paiva PMG Effect of Moringa oleifera lectin on development and mortality of Aedes aegypti larvae. Chemosphere. 2009, 77, 934–938. [DOI] [PubMed] [Google Scholar]
- (21).Pratissoli D; Polanczyk RA; Dalvi LP; Cocheto JG; Melo DG Occurrence of Ascia monuste orseis (Lepidoptera: Pieridae) in Crataeva tapia seedlings. Cienc. Rural 2007, 37, 874–875. [Google Scholar]
- (22).Ferreira RS; Zhou D; Ferreira JG; Silva MCC; Silva-Lucca RA; Reinhard M, Paredes-Gamero EJ, Berlonin TC, Correa MT, Paiva PM, Gutchina A; Wlodawer A; Oliva MLV Crystal Structure of Bark Protein (CrataBL) and Its Effect in Human Prostate Cancer Cell Lines. PloS ONE. 2013, 8, e64426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Zhang F; Walcott B; Zhou D; Gustchina A; Lasanajak Y; Smith DF; Ferreira RS; Correia MTS; Paiva PM; Bovin NV; Wlodawer A; Oliva MLV; Linhardt RJ Structural studies of the interaction of Crataeva tapia bark proteien with heparina and other glycosaminoglycans. Biochemistry. 2013, 52, 2148–2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Araújo RM; Vaz AF; Santos ME; Zingali RB; Coelho LC; Paiva PM; Correa MT; Oliva ML; Ferreira RS A new exogen anticoagulante with high selectivity to intrinsic pathway of coagulation. Thromb. Res 2011, 128, 395–397. [DOI] [PubMed] [Google Scholar]
- (25).Salu BS; Ferreira RS; Brito MV; Ottaiano TF; Cruz JWMC; Silva MCC; Correia MTS; Paiva PMG; Maffei FHA; Oliva MLV CrataBL, a lectin and fator Xa inhibitor, plays a role in blood coagulation and impairs thrombus formation. Biol. Chem 2014, 395, 1027–1035. [DOI] [PubMed] [Google Scholar]
- (26).Araújo RM; Aguiar JS; Coelho LCBB; Paiva PMG; Melo AMM; Silva TG; Correa MTS; Vaz AFM Lectin from Crataeva tapia bark exerts antitumor, anti-inflammatory and analgesic activities. Nat. Prod. Bioprospect 2011, 1, 97–100. [Google Scholar]
- (27).Oliva LV; Almeida-Reis R; Theodoro-Junior O; Oliveira BM; Leick EA; Prado CM; Brito MV; Correia MTS; Paiva PMG; Martins MA; Oliva MLV; Tibério IFLC A plant proteinase inhibitor from Crataeva tapia (CrataBL) attenuates elastase-induced pulmonar inflammatory, remodeling, and mechanical alterations in mice. Process Biochem. 2015, http//dx.doi.org/10.1016/j.procbio.2015.06.004 [Google Scholar]
- (28).Sreerama N; Woody RW Estimation of protein secondary structure from circular dichroism spectra: comparison of CONTIN, SELCON, and CDSSTR methods with an expanded reference set. Anal. Biochem 2000, 287, 252–60. [DOI] [PubMed] [Google Scholar]
- (29).Sreerama N; Venyaminov SY; Woody RW Analysis of protein circular dichroism spectra based on the tertiary structure classification. Anal. Biochem 2001, 299, 271–274. [DOI] [PubMed] [Google Scholar]
- (30).Macedo MLR; Fernandes KVS; Sales MP; Xavier-Filho J Vicilins variants and the resistance of cowpea (Vigna unguiculata) seeds to the cowpea weevil Callosobruchus maculatus). Comp. Biochem. Physiol 1993, 105C, 89–94. [Google Scholar]
- (31).Venyaminov SY; Yang JT Determination of protein secondary structure, in: Fasman GD (Ed.), Circular Dichroism and the Conformational Analysis of Biomolecules, Plenum Press; New York, 1996, 69–108. [Google Scholar]
- (32).Woody RW; Dunker AK Aromatic and cystine side-chain circular dichroism in proteins, in: Fasman GD (Ed.), Circular Dichroism and the Conformational Analysis of Biomolecules, Plenum Press, New York, 1996, 109–158. [Google Scholar]
- (33).Azarkan M; Dibiani R; Goormaghtigh E; Raussens V; Baeyens-Volant D The papaya Kunitz-type trypsin inhibitor is a highly stable beta-sheet glycoprotein. Biochim. Biophys. Acta 2006, 1764, 1063–1072. [DOI] [PubMed] [Google Scholar]
- (34).Bhattacharyya A; Babu C; Purification and biochemical characterization of a serine proteinase inhibitor from Derris trifoliata Lour. seeds: insight into structural and antimalarial features. Phytochemistry. 2009, 70, 703–12. [DOI] [PubMed] [Google Scholar]
- (35).Silva-Lucca RA; Faneca HM; de Lima MC; De Caroli FP; Assis ML; Sampaio MU; Oliva ML Interaction of proteinase inhibitors with phospholipid vesicles is modulated by pH. Int. J. Biol. Macromol 2010, 47, 551–557. [DOI] [PubMed] [Google Scholar]
- (36).Silva CP; Xavier-Filho J; Comparison between the levels of aspartic and cysteine proteinases of the larval midguts of Callosobrucus maculatus (F.) and Zabrotes subfasciatus (Both.)(Coleoptera: Bruchidae). Comp. Biochem. Phys. B 1991, 99, 529–533. [Google Scholar]
- (37).Gatehouse AMR; Boutler D; Hilder VA Potential of plant derived genes in the genetic manipulation of crops for insect resistance In: Gatehouse AR; Hilder VA; Boutler D (Eds.), Plant Genetic Manipulation for Crop Protection. CAB International Wallingford, England: 1992, 155–181. [Google Scholar]
- (38).Huesing JE; Murdock LL; Shade RE Effect of wheat germ isolectins on development of cowpea weevil. Phytochemistry. 1991, 30, 785–788. [Google Scholar]
- (39).Janzen DH; Juster HB; Liener IE Insecticidal action of phytohemagglutinin in black beans on a bruchid beetle. Science. 1976, 192, 795–796. [DOI] [PubMed] [Google Scholar]
- (40).Machuka JS; Okeola OG; Chrispeels MJ; Jackai LEN African yam beans seed lectin affects the development of the cowpea weevil but does not affect the development of larvae of legume pod borer. Phytochemistry. 2000, 53, 667–674. [DOI] [PubMed] [Google Scholar]
- (41).Murdock LL; Huesing JE; Nielson SS; Pratt RC; Shade RE Biological effects of plant lectins on the cowpea weevil. Phytochemistry. 1990, 29, 85–89. [Google Scholar]
- (42).Andrade CA; Baszkin A; Santos-Magalhães NS ; Coelho LC; de Melo CP Dielectric properties of Bauhinia monandra and concanavalin A lectin monolayers, part I. J. Colloid. Interface. Sci. 2005, 289, 371–378. [DOI] [PubMed] [Google Scholar]
- (43).Macedo MLR; Freire MGM; Novello JC; Marangoni S Talassia esculenta lectin and larval developemnt of Callosobruchus maculatus and Zabrotes subfasciatus (Coleoptera: Brichidae). Biochim. Biophys. Acta 2002, 1572, 83–88. [DOI] [PubMed] [Google Scholar]
- (44).Chrispeels MJ; Raikhel NV Lectins, lectin genes and their role in plant defense. Plant Cell. 1991, 3, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (45).Macedo MLR; Castro MM; Freire MG Mechanisms of insecticidal action of TEL (Talisia esculenta Lectin) against Callosobruchus maculatus (Coleoptera: Bruchidae). Arch. Insect. Biochem. Phys 2004, 56, 84–96. [DOI] [PubMed] [Google Scholar]
- (46).Zhu-Salzman K; Shade RE; Koiwa H; Salzman RA; Narashimhan M,; Bressan A; Hasegawa PM; Murdock LL Carbohydrate binding and resistance to proteolysis control insecticidal activity of Griffonia simplicifolia lectin II. Proc. Nat. Acad. Sci. USA 1998, 95, 15123–15128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (47).Macedo MLR; Freire MGM Insect digestive enzymes as a target for pest control. Invertebrate Surviv. J 2011, 8, 190–198. [Google Scholar]
- (48).Macedo MLR; Damico DCS; Freire MGM; Toyama MH; Marangoni S; Novello JC Purification and characterization of N-acetylglucosamine-binding lectin from Koelreuteria paniculata seeds and its effect on the larval development Callosobruchus maculatus (Coleoptera: Bruchidae) and Anagasta kuehniela (Lepidoptera: Pyralidae). J. Agric. Food Chem 2003, 51, 2980–2986. [DOI] [PubMed] [Google Scholar]
- (49).Harper SM; Crenshaw RW,; Mullins MA; Privalle LS Lectin biding to insect brush border membranes. J. Ecan. Entomol 1998, 5, 1197–1202. [Google Scholar]
- (50).Vats LK Alimentary canal in bruchid larvae (Bruchidae: Coleoptera). Res. Bull. Panjab Univ.Sci 1976, 27, 103–106. [Google Scholar]
- (51).Sales MP; Pimenta PP; Paes NS; Grossi-de-Sa MF; Xavier-Filho J Vicilins (7S storage globulins) of cowpea (Vigna unguiculata) seeds bind to chitinious structures of the midgut of Callosobruchus maculatus (Coleoptera: Bruchidae) larvae. Brazilian J. Med. Biol. Res 2001, 34, 27–34. [DOI] [PubMed] [Google Scholar]
- (52).Firmino F; Fernandes KVS; Sales MP; Gomes VM; Miranda MRA; Domingues SJS; Xavier-Filho J Cowpea (Vigna unguiculata) vicilins associate with putative chitinous structures in midgut and feces of the bruchid beetles Callosobruchus maculatus and Zabrotes subfasciatus. Braz. J. Med. Biol. Res 1996, 29, 749–756. [Google Scholar]
- (53).Gomes VM; Blanco-Labra A; Sales MP; Fernandes KVS; Cordeiro RA; Xavier-Filho J Vicilin storage proteins from cowpea (legume) seeds inhibit fungal development. J. Agric. Food Chem 1997, 45, 4110–4115. [Google Scholar]
- (54).Sales MP; Gomes VM; Fernandes KVS; Xavier-Filho J Chitin-binding proteins from cowpea (Vigna unguiculata) seeds. Braz. J. Med. Biol. Res 1996, 29, 319–326. [PubMed] [Google Scholar]
- (55).Yunes ANA; Andrade MT; Sales MP; Moraes RA; Fernandes KVS; Gomes VM; Xavier-Filho J Legume seed vicilins (7S storage proteins) interfere with the development of the cowpea weevil [Callosobruchus maculatus (F.)]. Sci. Food Agr 1998, 76, 111–116. [Google Scholar]
- (56).Uchoa AF; daMatta RA; Retamall CA; Albuquerque-Cunha JM; Souza SM; Samuels RI; Silva CP; Xavier-Filho J Presence of the storage seed protein vicilin in internal organs of larval Callosobruchus maculatus (Coleoptera: Bruchidae). J. Insect Physiol 2006, 52, 169–178. [DOI] [PubMed] [Google Scholar]
- (57).Souza SM; Uchôa AF; Silva JR; Samuels RI; Oliveira AEA; Oliveira EM; Linhares RT; Alexandre D; Silva CP; The fate of vicilins, 7S storage globulins, in larvae and adult Callosobruchus maculatus (Coleoptera: Chrysomelidae: Bruchinae). J. Insect Physiol 2010, 56, 1130–1138. [DOI] [PubMed] [Google Scholar]
- (58).Fitches E; Ilett C; Gatehouse AMR; Gatehouse LN; Greene R; Edwards JP; Gatehouse JA The effects of Phaseolus vulgaris erythro- and leucoagglutinating isolectins (PHA-E and PHA-L) delivered via artificial diet and transgenic plants on the growth and development of tomato moth (Lacanobia oleracea) larvae lectin binding to gut glycoproteins in vitro and in vivo. J. Insect Physiol 2001, 47, 1389–1398. [DOI] [PubMed] [Google Scholar]
- (59).Dixon RA; Sumner LW Legume natural products: understanding and manipulating complex pathways for human and animal health. Plant Physiology. 2003, 131, 878–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (60).Macedo MLR; Oliveira CFR; Oliveira CT Insecticidal activity of plant lectins and potential application in crop protection. Molecules. 2015, 20 2014–2033. [DOI] [PMC free article] [PubMed] [Google Scholar]