Abstract
Pseudomonas chlororaphis PA23 was isolated from the rhizosphere of soybeans and identified as a biocontrol bacterium against Sclerotinia sclerotiorum, a fungal plant pathogen. This bacterium produces a number of secondary metabolites, including phenazine-1-carboxylic acid, 2-hydroxyphenazine, pyrrolnitrin (PRN), hydrogen cyanide, proteases, lipases and siderophores. It also synthesizes and accumulates polyhydroxyalkanoate (PHA) polymers as carbon and energy storage compounds under nutrient-limited conditions. Pseudomonads like P. chlororaphis metabolize glucose via the Entner-Doudoroff and Pentose Phosphate pathways, which provide precursors for phenazine production. Mutants defective in phenazine (PHZ; PA23-63), PRN (PA23-8), or both (PA23-63-1) accumulated higher concentrations of PHAs than the wild-type strain (PA23) when cultured in Ramsay’s Minimal Medium with glucose or octanoic acid as the carbon source. Expression levels of six pha genes, phaC1, phaZ, phaC2, phaD, phaF, and phaI, were compared with wild type PA23 by quantitative real time polymerase chain reaction (qPCR). The qPCR studies indicated that there was no change in levels of transcription of the PHA synthase genes phaC1 and phaC2 in the phz- (PA23-63) and phz- prn- (PA23-63-1) mutants in glucose medium. There was a significant increase in expression of phaC2 in octanoate medium. Transcription of phaD, phaF and phaI increased significantly in the phz- prn- (PA23-63-1) mutant. Mutations in regulatory genes like gacS, rpoS, and relA/spoT, which affect PHZ and PRN production, also resulted in altered gene expression. The expression of phaC1, phaC2, phaF, and phaI genes was down-regulated significantly in gacS and rpoS mutants. Thus, it appears that PHZ, PRN, and PHA production is regulated by common mechanisms. Higher PHA production in the phz- (PA23-63), prn- (PA23-8), and phz- prn- (PA23-63-1) mutants in octanoic medium could be correlated with higher expression of phaC2. Further, the greater PHA production observed in the phz- and prn- mutants was not due to increased transcription of PHA synthase genes in glucose medium, but due to more accessibility of carbon substrates and reducing power, which were otherwise used for the synthesis of PHZ and PRN.
Keywords: Pseudomonas chlororaphis PA23, Biocontrol, Phenazine, Pyrrolnitrin, phz- and prn- mutants, Regulatory mutants, gacS, rpoS, relA/spoT, Polyhydroxyalkanoates, pha gene expression
1. Introduction
Pseudomonas chlororaphis strain PA23 is a soybean rhizosphere isolate that has been developed as a biocontrol agent to protect canola from stem rot caused by Sclerotinia sclerotiorum [1,2]. Strain PA23 produces a number of compounds, including phenazines (PHZ), pyrrolnitrin (PRN), hydrogen cyanide (HCN), proteases, lipases and siderophores that contribute to its biological control potential [3,4]. PHZs are bacterial secondary metabolites that have long been recognized for their broad-spectrum antibiotic activity and ability to antagonize a range of fungal phytopathogens [5,6,7]. Phenazine-1-carboxylic acid (PCA), one of the major PHZs produced by the fluorescent pseudomonads, was commercially named as Shenqinmycin. A 1% Shenqinmycin suspension was registered as a new biopesticide to prevent rice sheath blight, pepper blight and cucumber seedling damping-off [8].
Glucose and glycerol favor the production of PHZ in P. chlororaphis PCL1391 [9], while mannitol and fructose favor the production of PRN in P. protegens strain CHA0 [10]. PHZs are synthesized from glucose via the shikimic acid pathway [11]. A number of mutants defective in PHZ or PRN production, or both, were isolated from P. chlororaphis PA23 [12]. These mutations affected P. chlororaphis antibiotic production as well as biocontrol activity [13,14]. PHZ production imparts deep orange pigmentation to P. chlororaphis PA23 in glucose medium, while growth on octanoate results in colonies with a pale-yellow color, indicating reduced PHZ production.
Fluorescent pseudomonads are known to produce biodegradable polyhydroxyalkanoates (PHAs) in excess carbon conditions when other nutrients such nitrogen, phosphorus, or oxygen are limiting. The PHAs are stored as granules in the cytoplasm and used as a carbon and energy source under carbon-limited conditions [15]. PHA and PHZ synthesis both appear to be important traits for root colonization and plant growth promotion in rhizosphere bacteria [16]. P. chlororaphis can produce medium-chain-length (mcl-) PHAs consisting of C6 to C14 carbon chain monomers when grown on vegetable oils [17,18,19,20] and fatty acids extracted from glycerol bottom [21] as sole carbon sources. In contrast, P. putida, which also produces mcl-PHA polymers, cannot utilize vegetable oils directly for PHA production because it does not secrete extracellular lipase [22]. The P. chlororaphis PA23 genome encodes a pha operon containing seven PHA genes, which are highly conserved among Pseudomonas species.
PHA can be synthesized from related (fatty acids) or unrelated carbon substrates (glucose and gluconate). Both de novo syntheses of fatty acids and degradation of fatty acids supply precursors for PHA biosynthesis. PHZs and PHAs are secondary metabolites that accumulate during stationary phase and compete with each other for carbon partitioning or electrons or for reducing power. Production of different secondary metabolites is tightly regulated in terms of transcription and translation of specific genes as well as for partitioning of carbon. Earlier experiments have shown higher production of PHAs in P. chlororaphis PA23 mutants defective in PHZ production [23] and rhlB and rhlR mutants of P. aerugenosa defective in rhamnolipid biosynthesis [24], indicating that different secondary metabolites compete for precursors and reducing power.
A number of mutations in regulatory gene systems, including the gacA/gacS two-component system, the stationary phase sigma factor rpoS, and the stringent response (relA/spoT), are known to affect biocontrol activity of P. chlororaphis PA23 by altering antibiotic synthesis [3,25,26,27,28]. Further positive and negative interactions among these genes regulate secondary metabolite production and fungal antagonism [25,26,27,28,29,30].
Scattered information is available on the effect of these mutations on PHA production in different Pseudomonas species. Mutations in the global regulatory system gacA/gacS in P. putida CA-3 and P. aeruginosa down-regulated the expression of pha genes, and therefore synthesis and accumulation of PHA [30]. In P. putida and P. chlororaphis 1391, the rpoS gene is expressed in stationary phase and positively regulates PHA synthesis after the onset of the stationary phase [26,31,32]. In Ralstonia eutropha H16 spoT2 (pppGpp synthase), mutants synthesized only minor amounts of PHB [33,34]. However, in P. putida KT2440, there was no effect of relA/spoT mutations on the accumulation of PHAs [35].
In an earlier study examining the relationship between PHZ and PHA production, we discovered that the PHZ-minus mutant P. chlororaphis PA23-63 accumulated greater concentrations of mcl-PHAs compared to the wild type [23]. In the current study, we sought to determine whether changes in carbon partitioning and/or altered pha gene expression account for the higher PHA production observed for PHZ and PRN mutants. Further, we investigated whether regulatory genes that control PHZ/PRN also affect PHA production and transcription of pha genes.
2. Materials and Methods
2.1. Bacterial Strain/Mutants and Primers
Pseudomonas chlororaphis PA23 was isolated from the soybean rhizosphere [2]. PA23 mutants defective in PHZ and PRN production, together with primers used in the current study are listed in Table 1. The P. chlororaphis cultures (Wt and 6 mutants) used in the present study were described earlier for PHZs, PRN, and fungal antagonistic activity [3,12,13,25]. Mutant PA23-63 has a Tn5 insertion in the phzE gene and is not able to synthesize PHZ. Mutant PA23-8 has prnBC deletion and is not able to produce PRN. Mutant PA23-63-1 was derived from PA23-63 and contains a deletion of the prnBC genes. The other three strains have mutations in regulatory genes gacS, rpoS and SR (stringent response relS/spoT). P. chlororaphis strains PA23, PA23-8 (prn-), rpoS, and SR produced orange colonies on LB agar plates while P. chlororaphis strains PA23-63, PA23-63-1, and gacS produced white colonies on LB agar plates. Primers were designed based on the PA23 genome sequence [36] and synthesized by Alpha DNA (Montreal, QC, Canada).
Table 1.
Bacterial strains and oligonucleotide primers used in the present study.
| Strain/Mutant | Relevant Genotype, Phenotype or Sequence | Reference |
|---|---|---|
| P. chlororaphis PA23 | RifR; WT [soybean root tip isolate] PHZ+PRN+ | [2] |
| PA23-63 | PHZ- RifR phzE::Tn5-OT182 genomic fusion | [12] |
| PA23-8 | PRN- RifR prnBC deletion mutant | [12] |
| PA23-63-1 | PHZ- PRN- RifR phzE::Tn5-OT182 genomic fusion; prnBC deletion mutant | [12] |
| PA23rpoS | PA23 with pKNOCK inserted into rpoS | [25] |
| PA23gacS | PHZ- RifR gacS::Tn5-OT182 genomic fusion | [3] |
| PA23relA/spoT | PA23 with pKNOCK-Gm inserted into relA; TetR cassette inserted into spoT | [25] |
| PA23rpoS | PA23 with pKNOCK-Tc inserted into rpoS | [13] |
| Primers | ||
| phzA-FOR | 5′-GACTGGCAATGGCACAAC-3′ | [14] |
| phzA-REV | 5′-GCAATAACCTTCGGGATAACC-3′ | [14] |
| phzI-FOR | 5′-CGATGCCGTTGTTCTGG-3′ | [14] |
| phzI-REV | 5′-AGCCGTTCGTAGTGGACTC-3′ | [14] |
| phaF-FOR | 5′-GAAAAAGAAGGCAGCTCGTG-3′ | This study |
| phaF-REV | 5′-ATCGACTTTCTTGCCGACAG-3′ | This study |
| phaI-FOR | 5′-CTACACCAAGGTCGGTCAGG-3′ | This study |
| phaI-REV | 5′-ATCCAGCTGCACTTCGACTT-3′ | This study |
| phaD-FOR | 5′-CTGGGTATCGCTGACCAGTT-3′ | This study |
| phaD-REV | 5′-ACTACCGCTTCCTGTTCCAG-3′ | This study |
| phaC2-FOR | 5′-ATTCCAGATCAGGTCGTTGG-3′ | This study |
| phaC2-REV | 5′-GGTCAGCCTGCTGGATAGTC-3′ | This study |
| phaZ-FOR | 5′-CCTGCCCATAGTCGAGGTAA-3′ | This study |
| phaZ-REV | 5′-CTGGAGCTGGTGTTTCCATT-3′ | This study |
| phaC1-FOR | 5′-GCCAGGTAGGTTTGCAGGTA-3′ | This study |
| phaC1-REV | 5′-TCTGCTCGTATGGTGCTGAC-3′ | This study |
2.2. Culture Media and Growth Conditions
Luria–Bertani (LB) medium, with 2% agar, was used to maintain P. chlororaphis PA23 cultures. LB agar plates containing gentamicin or rifampicin and tetracycline were streaked with P. chlororaphis mutants and incubated at 30 °C for 24 hours (h). Single colonies were then picked and used to inoculate liquid LB medium. Ramsay’s Minimal Medium (RMM) was used in all the experiments: 6.7 g of Na2HPO4·7H2O, 1.5 g of KH2PO4, 1.0 g of (NH4)2SO4, 0.2 g of MgSO4·7H2O, 60 mg of ferrous ammonium citrate, 10 mg of CaCl2·2H2O, 1 mL of trace element solution, pH 7.0. Each liter of trace element solution contained the following: 0.3 g of H3BO3, 0.2 g of CoCl2·6H2O, 0.1 g of ZnSO4·7H2O, 30 mg of MnCl2·4H2O, 30 mg of NaMoO4·2H2O, 20 mg of NiCl2·6H2O, 10 mg of CuSO4·5H2O. Glucose (20 g/L) or octanoic acid (20 mmol/L) was added to the RMM as the sole carbon source in the mcl-PHA production studies [23].
2.3. Growth of P. chlororaphis Strains and Analyses of PHA Synthesis
Single colonies of P. chlororaphis PA23 and derivative strains grown on LB agar plates were used to inoculate cultures with 10 mL of LB broth. Pre-experimental inoculum cultures grown in LB medium for 18 hours (h) on a rotary shaker (150 rev/min) at 30 °C were used to inoculate overnight cultures in RMM (50 mL) with 20 g/L glucose or 20 mmol/L octanoic acid as substrates, and incubated for 24 h at 30 °C. Experimental cultures containing RMM (100 mL) with glucose or octanoic acid in 500 mL baffled-flasks were inoculated with 2% (v/v) of the overnight inoculum cultures and incubated for up to 48 h on a rotary shaker (150 rev/min) at 30 °C. Cultures were harvested by centrifugation at 4190× g at 4 °C for 30 min.
All tests were conducted with three independently replicated cultures (i.e., 3 biological replicates). The harvested cells were washed twice in phosphate-buffered saline and dried at 60 °C for 48 h to estimate cell dry mass. PHA subunit composition was determined by gas chromatography (Agilent Technologies Canada Inc., Mississauga, ON, Canada, Model 7890A) analysis, as described by Braunegg et al. [37]. Samples of dried cell biomass (5–10 mg) were placed in 15 mL screw-cap tubes and analyzed for percent cell dry mass (% cdm) accumulation of PHA and subunit composition. To this biomass, 1 mL of chloroform containing 1 mg of benzoic acid [internal standard], and 1 mL of methanol containing 15% concentrated sulfuric acid were added. Tubes were boiled in a water bath for 6 h, and then 0.5 mL of water was added to each tube. The chloroform (lower) layer was transferred to a 2 mL GC vial and analyzed by GC fitted with a DB-23 capillary column and flame ionization detector. The initial oven temperature was maintained at 60 °C for 5 min, which was increased to 250 °C at a ramping rate of 15 °C/min. The peaks were identified by their retention time, and concentration of subunits was estimated by comparison with known concentrations of different 3-hydroxy fatty acid standards.
2.4. PHA Production by P. chlororaphis PA23 at Different Time Points
Cultures of P. chlororaphis PA23 and derivative strains were prepared as described above (Section 2.3). The experimental cultures were incubated for up to 96 h on a rotary shaker [150 rev/min] at 30 °C. Samples for cell dry mass and PHA production were analyzed at t = 12, 24, 36, 48, 72, and 96 h post-inoculation (h pi), as described earlier. After harvesting the cells, supernatants were used for estimating residual glucose, octanoic acid and ammonium nitrogen in the medium [23]. For glucose, estimation, supernatants (1 mL) were centrifuged at 21,000× g for 10 min and glucose was estimated using High Performance Liquid Chromatography (Water Breeze HPLC; System, Water Incorporation, Milford, MA, USA). For octanoic acid, supernatants were vortexed for 3 min and 1 mL of supernatant in a 15-mL culture tube was dried in an oven for 48 h at 60 °C. The residues were methanolyzed and octanoic acid concentrations were determined by GC analysis, described above (Section 2.3). Ammonium nitrogen was measured using the Quikchem method 10-107-06-1-I for determination of ammonium in wastewater by flow injection analysis (Lachat Instrument, Loveland, CO, USA).
2.5. RNA Isolation, cDNA Synthesis and Gene Expression
To monitor the expression of metabolite and regulatory genes involved in PHZ and PHA production, quantitative real-time PCR (qPCR) was employed. The expression of phaC1, phaZ, phaC2, phaD, phaF and phaI (all associated with PHA production) was determined in P. chlororaphis PA23 and its derivative strains. The housekeeping gene, rpoB, was chosen to normalize expression values of the target genes. Expression of these genes in RMM glucose medium (supporting PHZ production) or RMM octanoic acid medium (supporting PHA production) was also compared. Relative gene expression in comparison to Wt P. chlororaphis PA23 was calculated. Primers used for gene expression analysis are listed in Table 1. Primers were designed based on the sequences of the respective genes obtained from P. chlororaphis PA23 (GenBank accession no. NZ_CP008696).
Cultures were grown in RMM (2% glucose) or RMM octanoic (20 mmol/L) at 30 °C for 24 h or 20 h, respectively. A sample (0.2 mL) for each time point was mixed with 0.2 mL RNA protect, vortexed, and then stored at −80 °C until required for RNA isolation. Total RNA was extracted from the cell pellets using a PureLink RNA Mini Kit (Ambion, Life Technologies, Carlsbad, CA, USA) following the supplier’s protocol. Residual DNA was removed through on column DNase treatment using the Pure link DNase system (Thermo Fisher Scientific, Waltham, MA, USA). The concentration of RNA was determined using a Nanodrop 1000 spectrophotometer (Thermo fisher Scientific, Waltham, MA, USA), and RNA integrity was analyzed by electrophoresis using an Experion system (Bio-Rad Laboratories Canada, Mississauga, ON, Canada). cDNA was generated from 1 μg RNA by reverse transcription using the Maxima First-Strand cDNA Synthesis Kit (Thermo Scientific, Waltham, MA, USA) and the following conditions were employed: initial heating at 25 °C for 10 min, reverse transcription at 50 °C for 15 min, and enzyme denaturation at 85 °C for 5 min.
Gene expression was studied by qPCR using the CFX96 C1000 Touch Thermocycler Real-Time System (Bio-Rad Laboratories Canada, Mississauga, ON, Canada) and SsoFast SYBR Green Supermix (CFX96 C1000 Touch Thermocycler Real-Time System (Bio-Rad Laboratories Canada, Mississauga, ON, Canada)) using Hard Shell PCR 96-well thin walled plates (CFX96 C1000 Touch Thermocycler Real-Time System (Bio-Rad Laboratories Canada, Mississauga, ON, Canada)). PCR reactions were performed in a 10-μL reaction volume containing 5 μL SsoFast SYBR Green Supermix, 0.5 μL each of forward and reverse primer, 1 μL cDNA, and 3 μL of RNAse/DNAse free PCR water. PCR reaction conditions included an initial denaturation at 98 °C for 3 min, followed by 40 cycles of 98 °C for 10 seconds (s) and 60 °C for 30 s. The reactions were performed in triplicate and experiments were repeated with three biological replicates. Relative gene expression was determined using the ΔΔCt method [38]. The data was analyzed using Cfx96 Manager software version 3.1 (Bio-Rad Laboratories Canada, Mississauga, ON, Canada).
2.6. Statistical Analysis
Cell dry mass and PHA production data was analyzed by two-way analysis of variance (ANOVA) using SAS analytic software (SAS Institute Incorporation, Cary, NC, USA (https://www.sas.com). Least significant differences (LSD) for interactions between substrate and treatments were calculated and are shown on the histograms.
3. Results
3.1. pha Cluster in P. chlororaphis PA23
Expression of six pha genes of P. chlororaphis PA23 encoded by EY04_01515, EY04_01520, EY04_01525, EY04_01530, EY04_01535, and EY04_01540 was studied in the present investigation. Two Class II PHA synthase genes, phaC1 and phaC2, are encoded in the P. chlororaphis PA23 genome. A PHA depolymerase gene, phaZ, is located in between phaC1 and phaC2. A putative polyhydroxyalkanoic acid protein (EY04_01510), identified as a hypothetical protein, resides in the P. chlororaphis PA23 pha operon together with three regulatory genes, phaD, phaF, and phaI. The nucleotide sequence of these genes from the whole genome sequence from NCBI (Accession No. CP008696) was used to design primers for qPCR to study gene expression. In P. putida the phaC1, phaZ and phaC2, phaF and phaI genes have their own promoter, which is regulated by regulated by phaD as well as phaFI products [39]. The phaF and phaI genes are transcribed divergently to other pha genes [39]. The two phaC1 and phaC2 gene products encoding PHA synthases have different substrate specificity [40].
3.2. Growth and Cell Dry Mass of P. chlororaphis 23 and It Mutant Derivatives
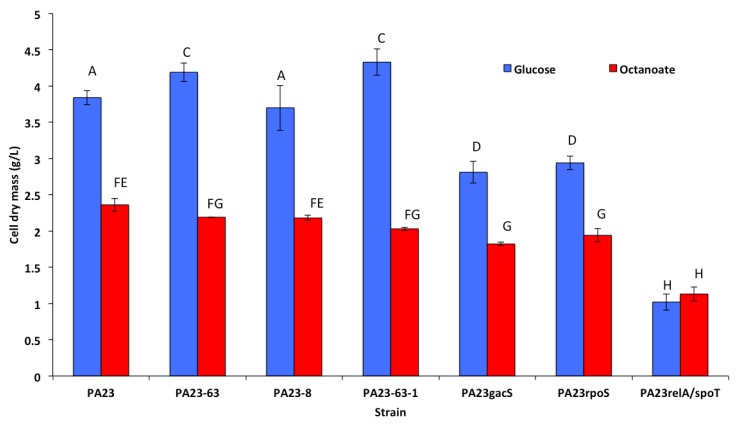
P. chlororaphis PA23 and its mutant derivatives were able to grow in RMM with glucose and octanoate as a sole carbon source. The overall growth of bacteria was better in RMM with glucose compared to RMM with octanoic acid. In glucose medium, P. chlororaphis PA23 accumulated 3.86 g/L cell dry mass, while in octanoic acid medium, it accumulated only 2.36 g/L cell dry mass, which was significantly different (Figure 1). Mutants defective in PHZ production produced more cell dry mass than the other mutants on glucose medium. There was a non-significant difference in cell dry mass of PHZ and PRN mutants in octonaic acid as a substrate. Cell dry mass produced by the P. chlororaphis gacS, rpoS, and SR mutants was significantly less than wild type P. chlororaphis PA23 in both RMM glucose and RMM octanoic acid media.
Figure 1.
Cell dry mass production by P. chlororaphis PA23 and it mutant derivatives in RMM medium with glucose versus octanoic acid as the carbon source. Bars with same letter are non-significantly different at the 5% level.
3.3. PHA Production by P. chlororaphis PA23 and It Mutant Derivatives
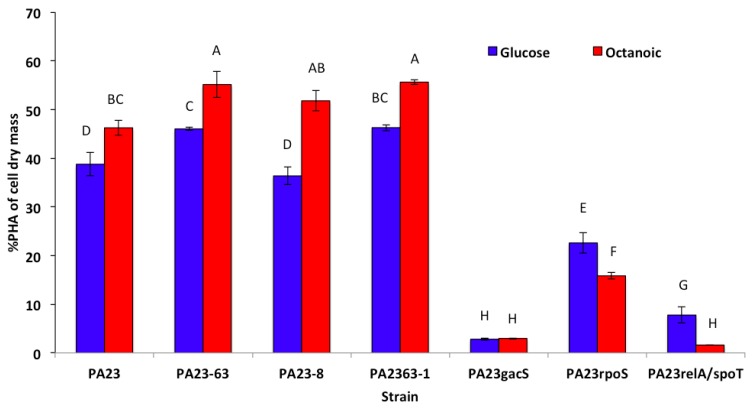
PHA production was studied in RMM media containing glucose or octanoic acid as a sole carbon source. Among the three mutants defective in PHZ and PRN synthesis, only two mutants P. chlororaphis PA23-63 and P. chlororaphis PA23-63-1 produced significantly greater concentrations of mcl-PHAs than Wt P. chlororaphis PA23 (Figure 2). In the case of mutants defective in PRN synthesis, there was no significant increase in PHA accumulation in P. chlororaphis PA23. PHA accumulation in RMM octanoic acid medium was also affected significantly in the PHZ and PRN mutants. PHA accumulation increased by 19.2%, 11.97%, and 20.28% over the Wt in P. chlororaphis PA23-63, PA23-63-1, and PA23-8 respectively. In the gacS mutant, PHA accumulation was only 2.81% and 2.97% of cell dry mass as compared to 34.16% and 46.25% for the Wt in RMM glucose and RMM octanoate media, respectively. PHA accumulation in the P. chlororaphis rpoS mutant decreased significantly to 22.59% and 15.83% in RMM glucose and RMM octanoic acid media, respectively. In the P. chlororaphis SR mutant, PHA accumulation was only 7.78% and 1.62% in RMM glucose and octanoic acid medium, respectively, which was significantly less than Wt P. chlororaphis PA23.
Figure 2.
PHA accumulation by Wt P. chlororaphis PA23 and its mutant derivatives in RMM media with glucose versus octanoic acid as the carbon source. Bars with same letter are non-significantly different at the 5% level.
3.4. Monomer Composition of PHAs Produced by P. chlororaphis PA23 and It Derivative Mutants
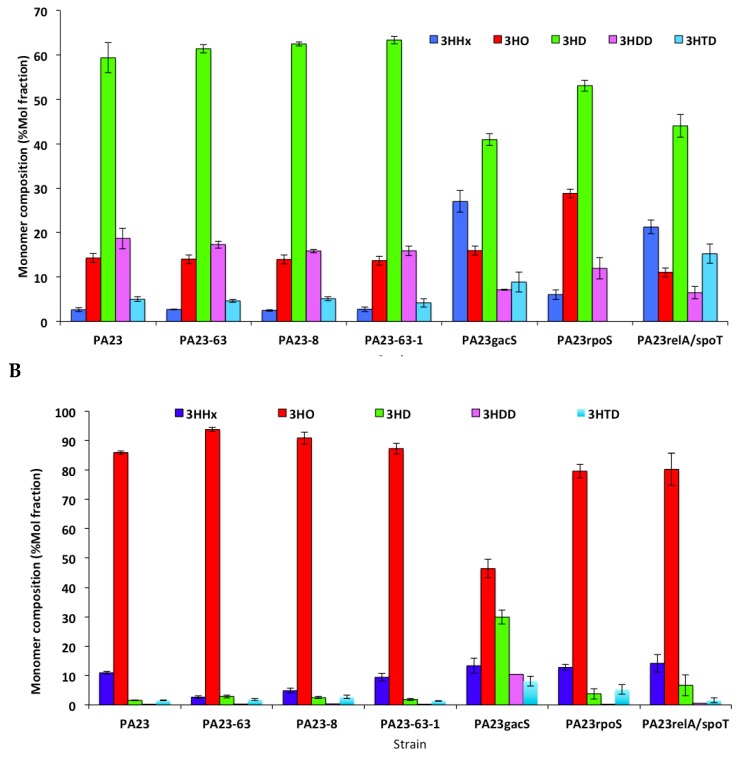
In RMM medium with glucose, P. chlororaphis PA23 synthesized PHAs with 3-hydroxydecanoate as a major component (app. 60 mol%; Figure 3). The other monomers included 3-hydroxyhexanoate (2.6 mol%), 3-hydroxyoctanoate (14.2 mol%), 3-hydroxydodecanoate (18.7 mol%), and 3-hydroxytetradecanoate (5.0 mol%). The PHZ and PRN defective mutants synthesized PHA polymers with subunit compositions similar to those of the Wt. For the three regulatory mutants (gacS, rpoS, SR), the PHAs contained higher amounts of 3-hydroxyhexanoate and/or 3-hydroxyoctanoate compared to the parent. The monomer composition of PHAs synthesized by P. chlororaphis PA23 in RMM octanoate medium was different from that of RMM glucose medium.
Figure 3.
Monomer composition of PHAs synthesized by P. chlororaphis PA23 and its mutant derivatives in (A) RMM glucose medium and (B) RMM octanoate medium as the carbon source.
The major subunit components of PHAs synthesized in RMM octanoate medium were 3-hydroxyoctanoate (86 mol%), followed by 3-hydroxyhexanoate (11 mol%). Other monomers like 3-hydroxydecanoate, 3-hydroxydodecanoate, and 3-hydroxytetradecanoate were also present in small quantities. PHAs produced by PHZ and PRN mutants had greater 3-hydroxoctanoate content and lower 3-hydroxyhexanoate content than the Wt P. chlororaphis PA23 strain. In this carbon source, the gacS mutant showed a different monomer composition of all five PHA monomers compared to PA23.
3.5. Growth and PHA Production by P. chlororaphis PA23 in Glucose and Octanoic Acid Medium at Different Time Points
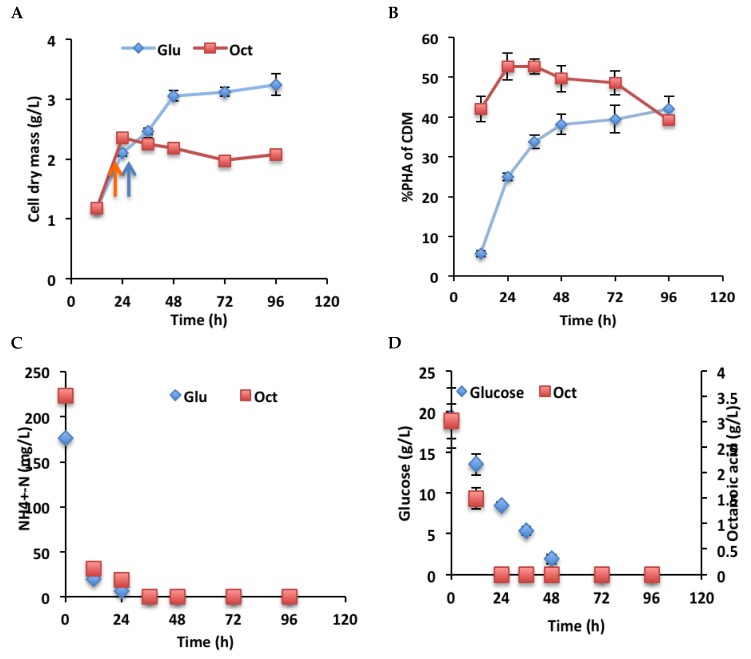
P. chlororaphis PA23 was grown in RMM glucose or RMM octanoic acid media for 96 h and samples were analyzed for cell dry mass, PHA accumulation, residual ammonium-N, and residual glucose or octanoic acid (Figure 4). Cell dry mass in RMM glucose medium increased up to 48 h pi and then stabilized with no further increase in cell dry mass. In RMM octanoic acid medium, cell dry mass increased up to 24 h pi, with no further increase in cell dry mass thereafter. PHA production in RMM glucose medium increased up to 96 h pi, but in RMM octanoic acid medium, maximum PHA accumulated at 24 h pi with no further increase up to 72 h pi. In RMM glucose and RMM octanoic media, maximum ammonium-N was consumed within 24 h pi and no detectable ammonium nitrogen was present in the medium after 36 h pi. Samples for qPCR were taken at 24 h pi in RMM glucose medium and at 16 h pi in RMM octanoic acid medium, respectively. At these time points, both cultures had started to accumulate PHA polymers (Figure 4B).
Figure 4.
Growth and PHA accumulation, with substrate and nitrogen consumption by P. chlororaphis PA23 in RMM glucose versus RMM octanoic acid media. (A) Cell dry mass production; (B) PHA accumulation; (C) Residual glucose and octanoic acid concentrations; and (D) Residual ammonium-nitrogen concentrations. Samples for RNA isolation from RMM glucose cultures were taken at 24 h pi and from RMM octanoic acid cultures at 16 h pi.
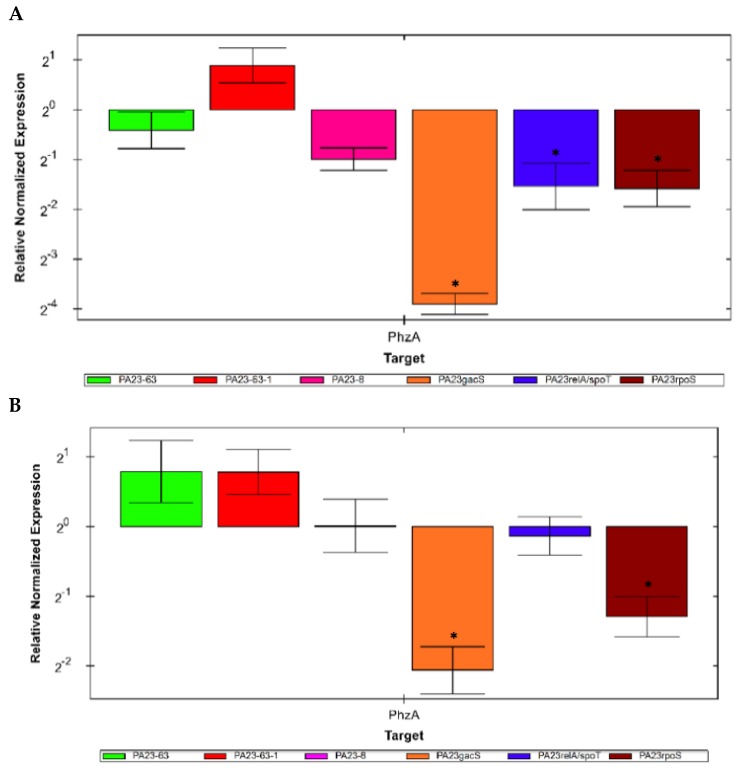
3.6. Expression of the phzA Gene in Mutants Defective in PHZ and/or PRN Production
The first gene in the PHZ biosynthetic operon is phzA (EY04_26270, annotated as PHZ biosynthesis protein). Expression of phzA was studied by qPCR in P. chlororaphis PA23 (Wt), PA23-63 (phz-), PA23-8 (prn-), and PA23-63-1 (phz- prn-), as well as the gacS, rpoS, and SR mutants. There was no change in phzA gene expression in the phz-, prn-, or phz- prn- P. chlororaphis mutants in either RMM glucose or RMM octanoic acid cultures (Figure 5). In the gacS, rpoS, and relA/spoT regulatory mutants, there was a significant decrease in the expression of phzA in all three strains grown in glucose; in octanoate, phzA expression was reduced in PA23gacS and PA23rpoS.
Figure 5.
Expression of phzA in P. chlororaphis PA23 mutants defective in the PHZ and/or PRN structural genes or in genes that regulate PHZ and PRN synthesis in cultures containing Ramsay’s Minimal Medium (RMM) plus (A) glucose or (B) octanoic acid as the carbon source. The housekeeping gene rpoB was chosen to normalize expression values of target genes. Expression levels in the Wt were normalized to 1. Data was normalized and expression relative to wild type PA23 was calculated. Differentially expressed genes are indicated with an asterisk (* p < 0.01).
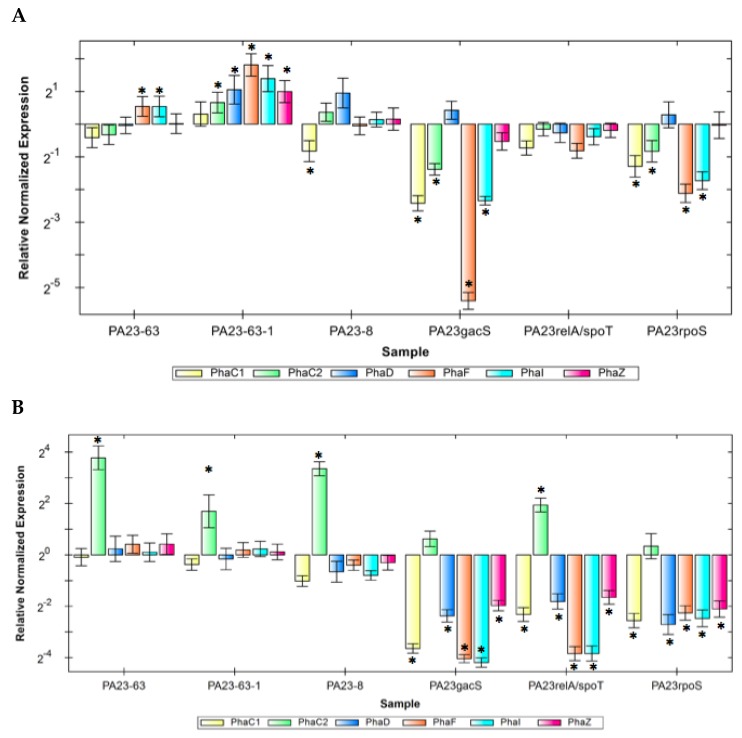
3.7. Expression of pha Genes in the PA23 PHZ and/or PRN Mutants
Six pha genes, phaC1, phaZ, phaC2, phaD, phaF, and phaI were studied in P. chlororaphis PA23, PA23-63 (phz-), PA23-8 (prn-), and PA23-63-1 (phz- prn-). In PA23-63 (phz-) grown in RMM glucose, no change in expression levels of phaC1, phaZ, phaC2, and phaD were observed (Figure 6). However, two pha genes, phaF and phaI, were up-regulated significantly. In PA23-8 (prn-), no change in the pha genes was observed, except for the phaC1 gene, which was down-regulated significantly. In PA23-63-1 (phz- prn-), expression levels of phaZ, phaD, phaF, and phaI genes were significantly up-regulated. In RMM octanoic acid, expression levels of the phaC2 gene were significantly increased in PA23-8 (prn-) and the double mutant PA23-63-1 (phz- prn-).
Figure 6.
Expression of pha genes in P. chlororaphis PA23 mutants defective in the PHZ and/or PRN structural genes or in genes that regulate PHZ and PRN synthesis in cultures containing Ramsay’s Minimal Medium (RMM) plus (A) glucose or (B) octanoic acid as the carbon source. The housekeeping gene rpoB was chosen to normalize expression values of target genes. Expression levels in the WT were normalized to 1. Data was normalized and expression relative to wild type PA23 was calculated. Differentially expressed genes are indicated with an asterisk (* p < 0.01).
Three regulatory mutants PA23gacS, PA23rpoS, and SR (PA23relA/spoT) affecting PHZ and PRN production were studied for expression of pha genes in glucose and octanoic medium (Figure 7). When grown in RMM glucose, the gacS and rpoS mutants both exhibited reduced expression of phaC1, phaC2, phaF, and phaI genes, while phaZ and phaD remained unaffected. In the P. chlororaphis SR (PA23relA/spoT) mutant, there was no significant change in the expression of any of the pha genes. By way of comparison, expression of phaC1, phaD, phaF, phaI, and phaZ was down-regulated in P. chlororaphis PA23gacS, PA23rpoS and the SR (PA23relA/spoT) mutants grown in octanoic acid medium. Surprisingly, phaC2 was significantly upregulated in the SR mutant.
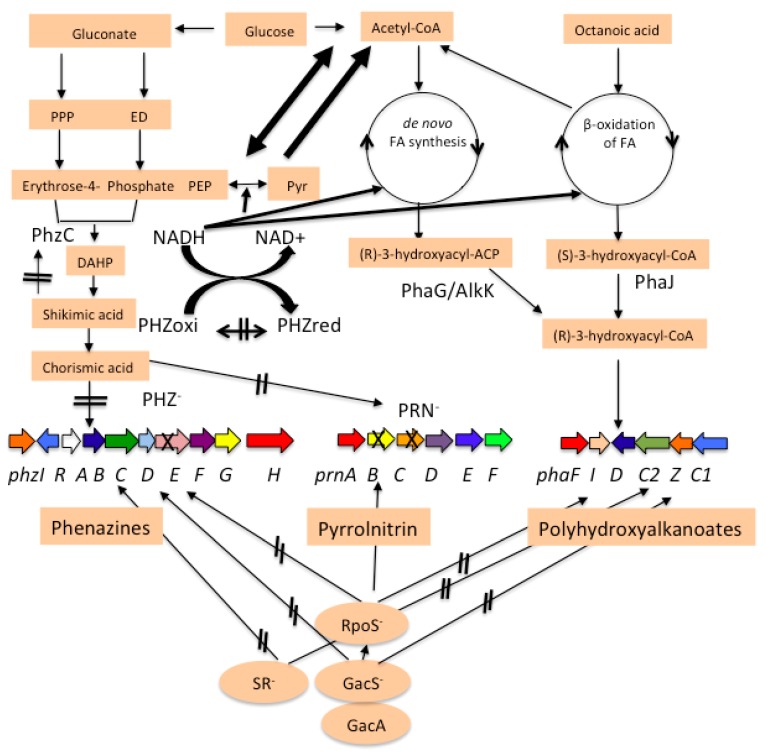
Figure 7.
Pathway of PHZ/ PRN and PHA production. Blocking PHZ and PRN production provides more precursors for PHA production. X indicates the gene mutated or deleted in phz and prn gene clusters. Thick arrows indicate higher carbon and reducing power flow for PHA production.
4. Discussion
Fluorescent pseudomonads are known to produce more than 100 phenazine-like compounds, which have been classified as antibiotics. Phenazines have a role in the survival of bacteria under low oxygen tension, iron acquisition, redox homeostatis, and transition to biofilm growth, which give producers an ecological advantage in the environment [6]. P. chlororaphis PA23 was identified as a biocontrol bacterium due to the production of PHZ and PRN antibiotics [4]. This bacterium was also able to use vegetable oils as a sole carbon source to produce mcl-PHAs [23]. PHA is an energy and carbon storage polymer, which acts as food reserve for bacteria. The polymer is degraded under starving condition when nitrogen is present in the environment. Presence of PHAs in rhizosphere bacteria enhances their colonization and promotes plant growth. PHA producing bacteria can survive under starving conditions and under hostile environments. Therefore, both PHZ/PRN and PHA provide ecological benefits to the producing bacteria.
A number of mutants defective in the production of PHZ and PRN antibiotics are available [12,13,14,25]. Our preliminary studies indicated that mutants of P. chlororaphis PA23 defective in PHZ production accumulated more PHA than the PA23 parent strain. Both PHZ/PRN and PHA are accumulated in the stationary phase of P. chlororaphis growth and have common global regulators like GacA/GacS, RpoS, and the stringent response (SR). The precursors for PHZ and PRN production are acetyl-CoA and erythrose-4-phosphate, which are provided by β-oxidation and by the ED pathway, respectively [41] (Figure 7).
Glucose is a preferred carbon source for cell growth and PHZ synthesis, but not for PHA production. P. chlororaphis PA23 produced a deep orange color and 38.5% more cell dry mass in glucose medium with 34% PHAs. In octanoic medium, it produced pale yellow color with low cell dry mass but with higher PHAs (46.25%). Moreover, PHA accumulation was higher in P. chlororaphis phz-, prn-, and phz- prn- mutants cultured on RMM octanoate medium than RMM glucose medium. Higher PHA production in cultures containing fatty acids as the carbon source is correlated with higher gene expression levels of phaC1, phaC2, and phaZ, which was at least 15-fold greater in cells grown on fatty acid than those in cells grown on glucose in P. putida KT2440 [42]. Whether the enhanced PHA production was due to a higher expression of pha genes in PHZ/PRN null mutants or change in metabolic flux for PHA production by supplying more carbon expression of pha genes was studied.
To explore the first possibility, we studied the expression of pha genes in P. chlororaphis phz-, prn-, and phz- prn- mutants (PHZ/PRN null mutants). No significant changes in the levels of phaC1 and phaC2 gene transcription were observed in P. chlororaphis phz-, prn-, and phz- prn- mutants, compared with the expression levels of these genes in P. chlororaphis PA23 cultured in RMM glucose medium. However, when the P. chlororaphis phz-, prn-, and phz- prn- mutants were cultured in RMM octanoic acid medium, expression levels of the phaC2 gene increased significantly [4–8 times] compared to P. chlororaphis PA23 cultured in the same medium. The greater level of PHA production and accumulation in the P. chlororaphis phz-, prn- and phz- prn- mutants cultured in RMM octanoic acid could be due to higher phaC2 expression levels. Earlier, the expression of phaC2 along with phaC1 was associated with greater PHA production and accumulation than the phaC1 gene alone in Pseudomonas corrugata [43]. Moreover, PhaC1 and PhaC2 have different substrate specificity to use different carbon sources efficiently [40].
Common global regulators like GacS/GacA, RpoS and SR are involved in PHZ/PRN and PHA production. The GacA/GacS and RpoS either abolished or decreased the PHZ/PRN and PHA production, while the SR system had little or no effect. These systems also regulated the transcription of phz, prn and pha genes [30]. The transcription of phaC1, phaZ, phaD, phaF and phaI was significantly downregulated in octanoate medium in gacA/gacS and rpoS. SR mutants (relA/spoT) of P. chlororaphis PA23 were impaired in PHA production with an effect on transcription of pha genes in glucose medium. In this respect, P. chlororaphis PA23 behaved like Ralstonia eutropha in PHA production [33,34]. The reduced phzA transcriptional fusion expression and decreased PHZ production in P. chlororaphis was identical to pha gene expression and decreased PHA production [12]. Regulation of PHZ/PRN and PHA synthesis by the GacA/GacS, RpoS and SR systems suggests that the expression of the phzA and pha genes is under the influence of global regulatory networks, but limited information is available regarding how these regulatory pathways are interlinked.
Blocking of one secondary metabolite can enhance the production of other secondary metabolites. PHZ and PRN in P. chlororaphis are synthesized via the shikimic pathway and precursors are provided by the ED and fatty acid biosynthesis/degradation pathways [44]. phz-/prn- mutants of P. chlororaphis are blocked to produce PHZ/PRN and shikimic acid and chorsimic acid accumulated in the medium, Shikimic acid and chorismic acid, which inhibited their production by feedback and allosteric effects [45]. Blocking of aromatic amino acids synthesis by deleting shikimic acid kinases was earlier reported to accumulate shikimic acid in the medium [46] and the excess carbon can be diverted for other secondary metabolites like PHAs. In the phz- mutant P. chlororaphis PA23-63, higher levels of PRN production are observed compared to Wt PA23. The defect in PHZ production resulted in greater carbon flow (C3 and C4 organic phosphates) into the shikimic acid pathway for PRN synthesis, which led to increased PRN production [12]. Blocking of metabolic pathway for pyocyanin, pyochelin, anthranilate and prephenate production in P. aeruginosa PA1201 increased PHZ production by 46%, 14%, 15%, and 23% respectively [47]. The phzC gene encodes a type II DAHP synthase in PHZ-producing bacteria. This step is tightly regulated by regulated feedback inhibition; otherwise, it will deplete the metabolites for amino acid biosynthesis. Therefore, it is possible that a mutation in the phz and prn genes, which resulted in defective PHZ and PRN proteins, shunted more carbon to the PHA synthesis pathway, resulting in greater PHA accumulation. Phenazine production in P. fluorescens 2–79 was reported as high as 0.31 g/g cell biomass, which is comparable to 0.25–0.4 g/g of cell biomass PHA production [48].
Metabolic engineering has been used to increase PHZ or PHA production in Pseudomonas species. Blocking of metabolic pathways, which diverts precursors to other futile pathways, overexpressing the genes of feeding pathways or deleting regulatory genes are common strategies to enhance PHZ production in P. aeruginosa and P. chlororaphis [49,50]. Deletion of the prn operon showed that PHZ production increased 2.5 times in P. chlororaphis, which could be due to the higher availability of precursors for PHZ production [12]. Phenazines and PRNs are redox active compounds modulating the intracellular redox potential and serving as intracellular redox buffers [51]. PHZ- mutants of P. aeruginosa PA14 have higher NADH/NAD+ redox ratio than wild type PHZ producing cultures, which was correlated with oxygen limitation. The ratio of NADH/NAD+ plays a major role in central metabolism [44]. Oxygen limitation has been earlier reported to improve PHA production in P. putida [52]. These conditions are more suitable for PHA accumulation in PHZ- mutants. In P. putida acetyl-CoA/free CoA and NADH/NAD+ ratios regulate PHA storage/mobilization [53]. Phenazine reduction was at the peak during the stationary phase when PHA production started. Higher reducing power in PHZ- mutants could lead to higher PHA production in P. chlororaphis. Overproducing PHA strains of P. putida have low NADPH/NADP+ ratios because more NADPH and NADH are consumed to convert 3-ketoacyl-ACP into (R)-3-hydroxyacyl-ACP [54]. High NADH/NAD+ ratio supports high PHA production. PHAs act not only as carbon and energy reservoirs, but also as a sink for reducing power. Similar observations of the importance of the NADH/NAD+ ratio for PHA synthesis from fatty acids in P. putida have been published [55,56,57]. Recently PHZ-producing recombinants of P. putida KT2440 have been developed. These recombinants produced up to 424 mg/L PHZ. However, PHA production in these recombinants was not studied [58]. It will be interesting to see how PHA production was affected in these recombinants or how the deletion of pha genes in these recombinants affects PHZ production.
5. Conclusions
Pseudomonas chlororaphis PA23 produces a number of compounds, including phenazines (PHZ), pyrrolnitrin (PRN), hydrogen cyanide (HCN), proteases, lipases and siderophores, which contribute to its biological control potential. It can also use vegetable oils to accumulate medium chain length polyhydroxyalkanoates (PHAs). Phenazine/pyrrolnitrin defective mutants produced more PHAs from glucose and octanoic acid than P. chlororaphis PA23. Both PHA and PHZs are accumulated during stationary phase and have common regulators like GacA/GacS, RpoS, and the stringent response, which affect their expression. The possible reason for enhanced PHA production and expression of pha genes was studied in these mutants. Mutants defective in PHZ and PRN production showed an increase in expression of pha genes. However, regulatory mutants i.e., gacS, rpoS and SR mutants had significant effect on pha gene expression and PHA production. It was speculated that increased PHA production in these mutants could be due to availability of more carbon and reducing power in PHZ and PRN mutants. How PHZ/PRN and PHA production pathways are connected is not very clear. Recombinants of P. putida producing PHZ/PRN will throw some light on production of these two important secondary metabolites.
Author Contributions
D.B.L. and T.d.K. conceived and designed the experiments; P.K.S., R.I.M. and J.P. performed the experiments; N.S. and P.K.S. analyzed the data. P.K.S., T.d.K. and D.B.L. wrote the paper.
Funding
This work was funded by the Natural Sciences and Engineering Research Council of Canada (NSERC) through an NSERC Discovery grant (RGPIN-5961-2016), and by Genome Canada, through the Genome Applications Partnership Program (GAPP) grant titled, “Fibre composite and biomatrix genomics (FiCoGen: Applications to the ground transportation industry”.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Fernando W.G.D., Nakkeeran S., Zhang Y., Savchuk S. Biological control of Sclerotinia sclerotiorum (Lib.) de Bary by Pseudomonas and Bacillus species on canola petals. Crop Prot. 2007;26:100–107. doi: 10.1016/j.cropro.2006.04.007. [DOI] [Google Scholar]
- 2.Savchuk S.C., Dilantha Fernando W.G. Effect of timing of application and population dynamics on the degree of biological control of Sclerotinia sclerotiorum by bacterial antagonists. FEMS Microbiol. Ecol. 2004;49:379–388. doi: 10.1016/j.femsec.2004.04.014. [DOI] [PubMed] [Google Scholar]
- 3.Poritsanos N., Selin C., Fernando W.G.D., Nakkeeran S., de Kievit T.R. A GacS deficiency does not affect Pseudomonas chlororaphis PA23 fitness when growing on canola, in aged batch culture or as a biofilm. Can. J. Microbiol. 2006;52:1177–1188. doi: 10.1139/w06-079. [DOI] [PubMed] [Google Scholar]
- 4.Zhang Y., Fernando W.G.D., de Kievit T.R., Berry C., Daayf F., Paulitz T.C. Detection of antibiotic-related genes from bacterial biocontrol agents with polymerase chain reaction. Can. J. Microbiol. 2006;52:476–481. doi: 10.1139/w05-152. [DOI] [PubMed] [Google Scholar]
- 5.Pierson L.S., III, Pierson E.A. Metabolism and function of phenazines in bacteria: Impacts on the behavior of bacteria in the environment and biotechnological processes. Appl. Microbiol. Biotechnol. 2010;86:1659–1670. doi: 10.1007/s00253-010-2509-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mavrodi D.V., Parejko J.A., Mavrodi O.V., Kwak Y.S., Weller D.M., Blankenfeldt W., Thomashow L.S. Recent insights into the diversity, frequency and ecological roles of phenazines in fluorescent Pseudomonas spp. Environ. Microbiol. 2013;15:675–686. doi: 10.1111/j.1462-2920.2012.02846.x. [DOI] [PubMed] [Google Scholar]
- 7.Thomashow L. Phenazines in the environment: Microbes, habitats, and ecological relevance. In: Chincholkar S., Thomashow L., editors. Microbial Phenazines. Springer; Berlin/Heidelberg, Germany: 2013. pp. 199–216. [Google Scholar]
- 8.Xu Y. Genomic features and regulation of phenazine biosynthesis in the rhizosphere strain Pseudomonas aeruginosa M18. In: Chincholkar S., Thomashow L., editors. Microbial Phenazines. Springer; Berlin/Heidelberg, Germany: 2013. pp. 177–198. [Google Scholar]
- 9.Van Rij E.T., Wesselink M., Chin-A-Woeng T.F., Bloemberg G.V., Lugtenberg B.J. Influence of environmental conditions on the production of phenazine-1-carboxamide by Pseudomonas chlororaphis PCL1391. Mol. Plant-Microbe Interact. 2004;17:557–566. doi: 10.1094/MPMI.2004.17.5.557. [DOI] [PubMed] [Google Scholar]
- 10.Duffy B.K., Defago G. Controlling instability in gacS-gacA regulatory genes during inoculant production of Pseudomonas fluorescens biocontrol strains. Appl. Environ. Microbiol. 2000;66:3142–3150. doi: 10.1128/AEM.66.8.3142-3150.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Blankenfeldt W. The Biosynthesis of phenazines. In: Chincholkar S., Thomashow L., editors. Microbial phenazines: Biosynthesis, Agriculture and Health. Springer; Heidelberg, Germany: 2013. pp. 1–18. [Google Scholar]
- 12.Selin C., Habibian R., Poritsanos N., Sarangi N.P., Dilantha Fernando W.G., de Kievit T.R. Phenazines are not essential for Pseudomonas chlororaphis PA23 biocontrol of Sclerotinia sclerotiorum, but do play a role in biofilm formation. FEMS Microbiol. Ecol. 2010;71:73–83. doi: 10.1111/j.1574-6941.2009.00792.x. [DOI] [PubMed] [Google Scholar]
- 13.Selin C., Dilantha Fernando W.G., de Kievit T.R. The PhzI/PhzR quorum-sensing system is required for pyrrolnitrin and phenazine production, and exhibits cross-regulation with RpoS in Pseudomonas chlororaphis PA23. Microbiology. 2012;158:896–907. doi: 10.1099/mic.0.054254-0. [DOI] [PubMed] [Google Scholar]
- 14.Nandi M., Selin C., Brawerman G., Dilantha Fernando W.G., de Kievit T.R. The global regulator ANR is essential for Pseudomonas chlororaphis strain PA23 biocontrol. Microbiology. 2016;162:2159–2169. doi: 10.1099/mic.0.000391. [DOI] [PubMed] [Google Scholar]
- 15.Kumar P., Kim B.S. Valorization of polyhydroxyalkanoates production process by co-synthesis of value-added products. Bioresour. Technol. 2018;269:544–556. doi: 10.1016/j.biortech.2018.08.120. [DOI] [PubMed] [Google Scholar]
- 16.Pierson L.S., III, Pierson E.A. Phenazine antibiotic production in Pseudomonas aureofaciens: Role in rhizosphere ecology and pathogen suppression. FEMS Microbiol. Lett. 1996;136:101–108. doi: 10.1111/j.1574-6968.1996.tb08034.x. [DOI] [Google Scholar]
- 17.Yun H.S., Kim D.Y., Chung C.W., Kim H.W., Yang Y.K., Rhee Y.H. Characterization of a tacky poly[3-hydroxyalkanoate] produced by Pseudomonas chlororaphis HS21 from palm kernel oil. J. Microbiol. Biotechnol. 2003;13:64–69. doi: 10.1139/cjm-2017-0412. [DOI] [Google Scholar]
- 18.Muhr A., Rechberger E.M., Salerno A., Reiterer A., Malli K., Strohmeier K., Shober S., Mittlebach M., Koller M. Novel description of mcl-PHA biosynthesis by Pseudomonas chlororaphis from animal-derived waste. J. Biotechnol. 2013;165:45–51. doi: 10.1016/j.jbiotec.2013.02.003. [DOI] [PubMed] [Google Scholar]
- 19.Solaiman D.K.Y., Ashby R.D., Crocker N., Lai B.H., Zerkowski J.A. Rhamnolipid and poly[hydroxyalkanoate] biosynthesis in 3-hydroxyacyl-ACP: CoA transacylase [phaG]-knockouts of Pseudomonas chlororaphis. Biocatal. Agric. Biotechnol. 2014;3:159–166. doi: 10.1016/j.bcab.2013.09.009. [DOI] [Google Scholar]
- 20.Sharma P.K., Fu J., Cicek N., Sparling R., Levin D.B. Kinetics of medium-chain-length polyhydroxyalkanoate production by a novel isolate of Pseudomonas putida LS46. Can. J. Microbiol. 2012;58:982–989. doi: 10.1139/w2012-074. [DOI] [PubMed] [Google Scholar]
- 21.Walsh M., O’Connor K., Babu R., Woods T., Kenny S. Plant oils and products of their hydrolysis as substrates for polyhydroxyalkanoate synthesis. Chem. Biochem. Eng. Q. 2015;29:123–133. doi: 10.15255/CABEQ.2014.2252. [DOI] [Google Scholar]
- 22.Sharma P.K., Fu J., Zhang X., Fristensky B., Sparling R., Levin D.B. Genome features of Pseudomonas putida LS46, a novel polyhydroxyalkanoate producer and its comparison with other P. putida strains. AMB Express. 2014;4:37. doi: 10.1186/s13568-014-0037-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sharma P.K., Munir R.I., de Kievit T., Levin D.B. Synthesis of polyhydroxyalkanoates [PHAs] from vegetable oils and free fatty acids by wild-type and mutant strains of Pseudomonas chlororaphis. Can. J. Microbiol. 2017;63:1009–1024. doi: 10.1139/cjm-2017-0412. [DOI] [PubMed] [Google Scholar]
- 24.Choi M.H., Xu J., Gutierrez M., Yoo T., Cho Y.H., Yoon S.C. Metabolic relationship between polyhydroxyalkanoic acid and rhamnolipid synthesis in Pseudomonas aeruginosa: Comparative 13C NMR analysis of the products in wild-type and mutants. J. Biotechnol. 2011;151:30–42. doi: 10.1016/j.jbiotec.2010.10.072. [DOI] [PubMed] [Google Scholar]
- 25.Manuel J., Selin C., Fernando W.G., de Kievit T. Stringent response mutants of Pseudomonas chlororaphis PA23 exhibit enhanced antifungal activity against Sclerotinia sclerotiorum in vitro. Microbiology. 2012;158:207–216. doi: 10.1099/mic.0.053082-0. [DOI] [PubMed] [Google Scholar]
- 26.Girard G., van Rij E.T., Lugtenberg B.J.J., Bloemberg G.V. Regulatory roles of psrA and rpoS in phenazine-1-carboxamide synthesis by Pseudomonas chlororaphis PCL1391. Microbiology. 2006;152:43–58. doi: 10.1099/mic.0.28284-0. [DOI] [PubMed] [Google Scholar]
- 27.Selin C., Manuel J., Fernando W.G.D., de Kievit T. Expression of the Pseudomonas chlororaphis strain PA23 Rsm system is under control of GacA, RpoS, PsrA, quorum sensing and the stringent response. Biol. Control. 2014;69:24–33. doi: 10.1016/j.biocontrol.2013.10.015. [DOI] [Google Scholar]
- 28.Wang D., Lee S.H., Seeve C., Yu J.M., Pierson L.S., III, Pierson E.A. Roles of the Gac-Rsm pathway in the regulation of phenazine biosynthesis in Pseudomonas chlororaphis 30–84. MicrobiologyOpen. 2013;2:505–524. doi: 10.1002/mbo3.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ge Y., Yang S., Fang Y., Yang R., Mou D., Cui J., Wen L. RpoS as an intermediate in RsmA-dependent regulation of secondary antifungal metabolites biosynthesis in Pseudomonas sp. M18. FEMS Microbiol. Lett. 2006;268:81–87. doi: 10.1111/j.1574-6968.2006.00562.x. [DOI] [PubMed] [Google Scholar]
- 30.Ryan W.J., O’Leary N.D., O’Mahony M., Dobson A.D.W. GacS-dependent regulation of polyhydroxyalkanoate synthesis in Pseudomonas putida CA-3. Appl. Environ. Microbiol. 2013;79:1795–1802. doi: 10.1128/AEM.02962-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Raiger-Lustman L.J., Ruiz J.A. The alternative sigma factor, rpoS affects polyhydroxyalkanoate metabolism in Pseudomonas putida. FEMS Microbiol. Lett. 2008;284:218–224. doi: 10.1111/j.1574-6968.2008.01203.x. [DOI] [PubMed] [Google Scholar]
- 32.Ruiz J.A., López N.I., Méndez B.S. rpoS gene expression in carbon-starved cultures of the polyhydroxyalkanoate-accumulating species Pseudomonas oleovorans. Curr. Microbiol. 2004;48:396–400. doi: 10.1007/s00284-003-4183-5. [DOI] [PubMed] [Google Scholar]
- 33.Juengert J.R., Christoph M., Borisava M., Mayer C., Wolz C., Brigham C.J., Sinskey A.J., Jendrossek D. Absence of ppGpp leads to increased mobilization of intermediately accumulated poly [3-hydroxybutyrate] in Ralstonia eutropha H16. Appl. Environ. Microbiol. 2017;83:13–16. doi: 10.1128/AEM.00755-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brigham C.J., Speth D.R., Rha C., Sinskey A.J. Whole-genome microarray and gene deletion studies reveal regulation of the polyhydroxyalkanoate production cycle by the stringent response in Ralstonia eutropha H16. Appl. Environ. Microbiol. 2012;78:8033–8044. doi: 10.1128/AEM.01693-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mozejko-Ciesielska J., Dabrowska D., Szalewska-Palasz A., Ciesielski S. Medium-chain-length polyhydroxyalkanoates synthesis by Pseudomonas putida KT2440 relA/spoT mutant: Bioprocess characterization and transcriptome analysis. AMB Express. 2017;7:92. doi: 10.1186/s13568-017-0396-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Loewen P.C., Villenueva J., Dilantha Fernando W.G., de Kievit T. Genome Sequence of Pseudomonas chlororaphis strain PA23. Genome Annoc. 2014;2:e00689-14. doi: 10.1128/genomeA.00689-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Braunegg G., Sonnleitner B., Lafferty R.M. A rapid gas chromatographic method for the determination of polyhydroxybutyric acid in microbial biomass. Eur. J. Appl. Microbiol. Biotechnol. 1978;6:29–37. doi: 10.1007/BF00500854. [DOI] [Google Scholar]
- 38.Livak K.J., Schmittgen T.D. Analysis of Relative gene expression data using real time quantitative PCR and the 2−δδCT method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 39.Prieto A., Escapa I.F., Martínez V., Dinjaski N., Herencias C., Peña F., Natalia Tarazona N., Revelles O. A holistic view of polyhydroxyalkanoate metabolism in Pseudomonas putida. Environ. Microbiol. 2016;18:341–357. doi: 10.1111/1462-2920.12760. [DOI] [PubMed] [Google Scholar]
- 40.Chen J.Y., Liu T., Zheng Z., Chen J.C., Chen G.Q. Polyhydroxyalkanoate synthases PhaC1 and PhaC2 from Pseudomonas stutzeri 1317 had different substrate specificities. FEMS Microbiol. Lett. 2004;234:231–237. doi: 10.1111/j.1574-6968.2004.tb09538.x. [DOI] [PubMed] [Google Scholar]
- 41.Mavrodi D.V., Blankenfeldt W., Thomashow L.S., Mentel M. Phenazine compounds in fluorescent Pseudomonas spp. biosynthesis and regulation. Ann. Rev. Phytopathol. 2006;44:417–445. doi: 10.1146/annurev.phyto.44.013106.145710. [DOI] [PubMed] [Google Scholar]
- 42.De Eugenio L.I., Galán B., Escapa I.F., Maestro B., Sanz J.M., García J.L., Prieto M.A. The PhaD regulator controls the simultaneous expression of the pha genes involved in polyhydroxyalkanoate metabolism and turnover in Pseudomonas putida KT2442. Environ. Microbiol. 2010;12:1591–1603. doi: 10.1111/j.1462-2920.2010.02199.x. [DOI] [PubMed] [Google Scholar]
- 43.Conte E., Catara V., Greco S., Russo M., Alicata R., Strano L., Lombardo A., Di Silvestro S., Catara A. Regulation of polyhydroxyalkanoate synthases (phaC1 and phaC2) gene expression in Pseudomonas corrugata. Appl. Microbiol. Biotechnol. 2006;72:1054–1062. doi: 10.1007/s00253-006-0373-y. [DOI] [PubMed] [Google Scholar]
- 44.Price-Whelan A., Newman D.K. Rethinking ‘secondary’ metabolism: Physiological roles for phenazine antibiotics. Nat. Chem. Biol. 2006;2:71–78. doi: 10.1038/nchembio764. [DOI] [PubMed] [Google Scholar]
- 45.Liu D.F., Ai G.M., Zheng Q.X., Liu C., Jiang C.Y., Liu L.X., Liu Y.M., Yang C., Liu S.J. Metabolic flux responses to genetic modification for shikimic acid production by Bacillus subtilis strains. Microb. Cell Fact. 2014;13:40. doi: 10.1186/1475-2859-13-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Krämer M., Bongaerts J., Bovenberg R., Kremer S., Müller U., Orf S., Wubbolts M., Raeven L. Metabolic engineering for microbial production of shikimic acid. Metab. Eng. 2003;5:277–283. doi: 10.1016/j.ymben.2003.09.001. [DOI] [PubMed] [Google Scholar]
- 47.Jin K., Zhou L., Jiang H., Sun S., Fang Y., Liu J., Zhang X., He Y.W. Engineering the central biosynthetic and secondary metabolic pathways of Pseudomonas aeruginosa strain PA1201 to improve phenazine-1-carboxylicacid production. Metab. Eng. 2015;32:30–38. doi: 10.1016/j.ymben.2015.09.003. [DOI] [PubMed] [Google Scholar]
- 48.Blankenfeldt W., Parsons J.F. The structural biology of phenazine biosynthesis. Curr. Opin. Struct. Biol. 2014;29:26–33. doi: 10.1016/j.sbi.2014.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bilal M., Guo S., Iqbal H.M.N., Hu H., Wang W., Zhang X. Engineering Pseudomonas for phenazine biosynthesis, regulation, and biotechnological applications: A review. World J. Microbiol. Biotechnol. 2017;33:191. doi: 10.1007/s11274-017-2356-9. [DOI] [PubMed] [Google Scholar]
- 50.Shen X., Wang Z., Huang X., Hu H., Wang W., Zhang X. Developing genome-reduced Pseudomonas chlororaphis strains for the production of secondary metabolites. BMC Genom. 2017;18:715. doi: 10.1186/s12864-017-4127-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Price-Whelan A., Dietrich L.E.P., Newman D.K. Pyocyanin alters redox homeostasis and carbon flux through central metabolic pathways in Pseudomonas aeruginosa PA14. J. Bacteriol. 2007;189:6372–6381. doi: 10.1128/JB.00505-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Blunt W., Dartiailh C., Sparling R., Gapes D., Levin D.B., Cicek N. Microaerophilic environments improve the productivity of medium chain length polyhydroxyalkanoate biosynthesis from fatty acids in Pseudomonas putida LS46. Process Biochem. 2017;59:18–25. doi: 10.1016/j.procbio.2017.04.028. [DOI] [Google Scholar]
- 53.Poblete-Castro I., Escapa I., Jager C., Puchalka J., Lam C., Schomburg D., Prieto M., Santos M.D. The metabolic response of P. putida KT2442 producing high levels of polyhydroxyalkanoate under single and multiple-nutrient-limited growth: Highlights from a multi-level omics approach. Microb. Cell Fact. 2012;11:34. doi: 10.1186/1475-2859-11-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Acuña J.M.B., Bielecka A., Häussler S., Schobert M., Jahn M., Wittmann C., Jahn D., Poblete-Castro I. Production of medium chain length polyhydroxyalkanoate in metabolic flux optimized Pseudomonas putida. Microb. Cell Fact. 2014;13:88. doi: 10.1186/1475-2859-13-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lee I.Y., Kim M.K., Park Y.H., Lee S.Y. Regulatory effects of cellular nicotinamide nucleotides and enzyme activities on poly(3-hydroxybutyrate) synthesis in recombinant Escherichia coli. Biotechnol. Bioeng. 1996;52:707–712. doi: 10.1002/(SICI)1097-0290(19961220)52:6<707::AID-BIT8>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 56.Escapa I.F., García J.L., Bühler B., Blank L.M., Prieto M.A. The polyhydroxyalkanoate metabolism controls carbon and energy spillage in Pseudomonas putida. Environ. Microbiol. 2012;14:1049–1063. doi: 10.1111/j.1462-2920.2011.02684.x. [DOI] [PubMed] [Google Scholar]
- 57.Ren Q., de Roo G., Ruth K., Witholt B., Zinn M., Thöny-Meyer L. Simultaneous accumulation and degradation of polyhydroxyalkanoates: Futile cycle or clever regulation? Biomacromolecules. 2009;10:916–922. doi: 10.1021/bm801431c. [DOI] [PubMed] [Google Scholar]
- 58.Domröse A., Weihmann R., Thies S., Jaeger K.E., Drepper T., Loeschck A. Rapid generation of recombinant Pseudomonas putida secondary metabolite producers using yTREX. J. Synth. Syst. Biotechnol. 2017;2:310–319. doi: 10.1016/j.synbio.2017.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]