Abstract
For organisms with complex life cycles, climate change can have both direct effects and indirect effects that are mediated through plastic responses to temperature and that carry over beyond the developmental environment. We examined multiple responses to environmental warming in a dragonfly, a species whose life history bridges aquatic and terrestrial environments. We tested larval survival under warming and whether warmer conditions can create carry-over effects between life history stages. Rearing dragonfly larvae in an experimental warming array to simulate increases in temperature, we contrasted the effects of the current thermal environment with temperatures +2.5°C and +5°C above ambient, temperatures predicted for 50 and 100 years in the future for the study region. Aquatic mesocosms were stocked with dragonfly larvae (Erythemis collocata) and we followed survival of larvae to adult emergence. We also measured the effects of warming on the timing of the life history transition to the adult stage, body size of adults, and the relative size of their wings, an aspect of morphology key to flight performance. There was a trend toward reduced larval survival with increasing temperature. Warming strongly affected the phenology of adult emergence, advancing emergence by up to a month compared with ambient conditions. Additionally, our warmest conditions increased variation in the timing of adult emergence compared with cooler conditions. The increased variation with warming arose from an extended emergence season with fewer individuals emerging at any one time. Altered emergence patterns such as we observed are likely to place individuals emerging outside the typical season at greater risk from early and late season storms and will reduce effective population sizes during the breeding season. Contrary to expectations for ectotherms, body size was unaffected by warming. However, morphology was affected: at +5°C, dragonflies emerging from mesocosms had relatively smaller wings. This provides some of the first evidence that the effects of climate change on animals during their growth can have carry-over effects in morphology that will affect performance of later life history stages. In dragonflies, relatively smaller wings are associated with reduced flight performance, creating a link between larval thermal conditions and adult dispersal capacity.
Keywords: carry-over effects, environmental warming, flight morphology, freshwater systems, Libellulidae, phenology, thermal performance
Introduction
Climate change is altering temperature in both aquatic and terrestrial environments, and will influence the conditions in which organisms develop and function. Developing in warmer environments can cause organisms to respond in ways that alter their traits and performance in ways that may affect population dynamics, potentially threatening the persistence of populations and species (Thomas et al. 2004, Parmesan 2006). Increased temperatures during development have been shown to increase mortality (McCauley et al. 2015, Tseng and O’Connor 2015), cause faster developmental rates which can result in reductions in adult body size (Daufresne et al. 2009, Gardner et al. 2011, Sheridan and Bickford 2011), and to advance seasonal phenologies (Parmesan 2006, Diez et al. 2012). Additionally, many organisms have shifted their ranges to spatially track their shifting thermal niche (Parmesan 2006). While some organismal responses to climate change likely have negative consequences for population persistence (e.g., increased mortality), others may be protective (e.g., range shifts can allow species to persist in the climatic conditions to which they are adapted) or have mixed effects. For example, advanced phenologies can either have negative effects (Inouye 2008, Augspurger 2013), or similar to range shifts, advanced phenologies can allow species to temporally shift their life-history to maintain populations in habitats with new thermal regimes (O’Regan et al. 2014). Responses to climate change can also interact; such interactions may be especially common in organisms such as insects with complex life-histories where transition to the adult stage fixes many adult traits. For example, adult skeletal body size is fixed at the transition to the adult size in insects. If adults are smaller as a result of developing in warmer conditions, this may affect the ability of species to shift their ranges in response to climate change by altering the dispersal capacity of individuals (McCauley and Mabry 2011). Therefore, measuring multiple responses to predicted climate change scenarios will provide critical insights into the effects of warming on organismal performance.
While much of the earth’s biota will be affected to varying extents by climate change, ectotherms whose internal temperatures track external conditions may be especially sensitive to warming (Gillooly et al. 2001, Forster et al. 2011), although their capacity for plasticity may also serve to mitigate some of these impacts (Seebacher et al. 2015). Given the dominance of ectotherms in aquatic systems, organisms in these habitats may be at especially high risk from climate change. Additionally, many aquatic insects have complex life-cycles with aquatic juveniles and terrestrial adults, but relatively little research has examined how warming affects the connections between these stages and the studies that have been done have variable results. Higgins et al. (2015) found that warming of lepidopteran larvae accelerated development through the middle instars of the caterpillar phase, but did not change the time to pupation or growth rate. Increased temperatures were, however, associated with decreased pupal mass. Potter et al. (2011) found that warming lepidopteran eggs resulted in more rapid development through the egg stage and more rapid initial larval growth, but no persistent effects on later life-history stages. These results suggest that the developmental stage in which warming is experienced and species’ differences may affect the impact warming has across an organisms’ life-history. We know little, however, about how long lasting the effects of warmer developmental conditions may be. Freshwater insects undergo their final molt at the transition to the adult stage and the exoskeleton is fixed at this point with no potential for change in skeletal measures, and changes in the larval period to patterns of growth or allocation should influence adult phenotypes.
We examined warming applied throughout the majority of the larval period in an aquatic insect, measuring its effects at the transition to the adult stage. This also allows us to assess whether the animals we examined exhibit the relationship between temperature and adult body size predict by the temperature-size rule (TSR). The TSR describes the negative relationship between temperature and adult body size commonly observed in ectotherms (i.e., hotter is smaller; Atkinson 1994, 1995, Kingsolver and Huey 2008). Aquatic insects typically, although not universally, exhibit this pattern of being smaller as adults when they have developed in warmer temperatures (Atkinson 1995). This relationship generates a strong potential for carry-over effects between larval responses to thermal conditions in the developmental environment and adult size, which has implications for their performance (Sokolovska et al. 2000).
We have focused on the effects of warming on odonates (dragonflies and damselflies), an important group of predatory freshwater insects, because there is increasing evidence that odonates are affected by climate change (Hassall and Thompson 2008, Hassall 2015, Suhling et al. 2015). Evidence of these responses includes advancing phenologies, both in natural populations (Hassall et al. 2007, Dingemanse and Kalkman 2008, Richter et al. 2008) and in response to experimental warming (Richter et al. 2008, McCauley et al. 2015), as well as northerly range expansions in many European species (Hickling et al. 2005, Flenner and Sahlén, 2008, Grewe et al. 2013). However, within odonates, species responses to environmental change can be highly variable and depend on both the context of the warming and the presence of additional stressors (e.g., competitors, potential intraguild predators, food limitation), and on the specific responses quantified (e.g., growth rate, mortality, size at or timing of emergence; Suhling and Suhling 2013, Nilsson- Örtman et al. 2014, Suhling et al. 2015). Field studies assessing the effects of warming across sites or years provide evidence that warmer conditions are affecting odonate populations, but the context dependence inherent to these systems can limit our ability to extend these results and make general predictions with respect to the effects of climate change on odonates in other contexts. Additionally, most of the experimental results assessing the responses of odonate larvae to warming are from studies comparing growth and survival responses across temperatures which vary between treatments, but are held constant within rearing conditions (e.g., growth at 20°C versus 26°C). While this can provide valuable insights into the effects of temperature on these responses, this approach does not capture the natural thermal variation these animals would experience on temporal scales ranging from diurnal to seasonal (Paaijmans et al. 2013, Colinet et al. 2015). Because organisms frequently exhibit non-linear response to temperature, it is difficult to make accurate predictions about the effects of climate change based on experiments that use constant temperatures (Lawson et al. 2015, Nadeau et al. 2017). Fluctuating temperatures, such as those organisms encounter in natural habitats, can be more energetically demanding than constant temperatures, but can increase performance when they occur within the permissive range for an ectotherm (Colinet et al. 2015). However, when temperature variation results in the thermal environment exceeding their upper thermal optima and become stressful, even if non-lethal, they can result in cumulative damage that decreases individual performance (Colinet et al. 2015). By experimentally manipulating mean temperatures under a naturalistic context in which variation is maintained across treatments, we can gain insights into the effects of warming that may not be provided by experiments comparing performance at constant temperatures, and which may either under- or over-estimate the effects of warming depending on how that warming fits within the thermal performance curve of the animal.
We conducted a mesocosm experiment to assess how environmental warming during the aquatic larval phase of a libellulid odonate (Erythemis collocata) affected performance and individual level traits. Larvae were reared in mesocosms that provide a naturalistic context but with a thermostat and warming set-up that increased mean temperatures while also tracking ambient levels of temperature variation. Our two warmed treatments raised temperature to a set level above ambient (based on 50- and 100-year climate predictions for the region, Cayan et al. 2009), but with the same level of thermal variation across all treatments. We measured larval survival, date of emergence to the adult stage, and the body size and morphology of adults emerging from different thermal conditions. These measures were chosen as response metrics because they are all potentially affected by the thermal conditions in which organisms develop, and can have fitness consequences. We hypothesized that warming would negatively affect larval survival, as the increasing stress imposed by conditions outside the thermal optima can increase larval mortality (McCauley et al. 2015, Tseng and O’Conner 2015) and these effects may be greater when the environment varies in temperature (Paaijmans et al. 2013). We also expected to see accelerated development rates, leading to early emergence into the adult stage with warming. By measuring adults, we were able to assess whether conditions in the larval developmental environment had carry-over effects on adult traits including body size and relative wing size. Based on the temperature size rule (Atkinson 1994, 1995, Kingsolver and Huey 2008) we expected to see reduced body size in adults emerging from warmed mesocosms. We also expected that animals under thermal stress might decrease their allocation to the relatively costly structures associated with flight and therefore emerge from warmed tanks with relatively smaller wings. This experiment allowed us to assess these multiple responses to increased mean temperatures which have implications for both individual performance and population dynamics.
Methods
Study species
We studied responses to environmental warming in the dragonfly species Erythemis collocata (Hagen 1861). This species is distributed across the western half of North America and is abundant in the region of Northern California where we conducted this research. While species in this genus have been recorded to be both uni- and bi-voltine in their development (reviewed in Corbet et al. 2006), our observations indicate that a uni-voltine development strategy is typical in this region.
Effects of increasing mean temperatures
Erythemis collocata eggs were collected between 9 and 26 July, 2013, by using aerial insect nets to catch adult females flying at ponds on the Wantrup Wildlife Sanctuary (Napa County, California, 38°35′58.07″ N, 122°22′13.10″ W) and then dipping the female’s abdomen into water until eggs were released. Females and any males they were captured with were marked on the wings with permanent ink to avoid resampling individuals. Cups containing eggs were taken to our rearing set-up 40 km southeast of the Wantrup Reserve, located at the Quail Ridge Reserve (Napa County, California, 38°28′58.72″ N, 122°8′58.17″ W, hereafter: QRR). Cups were housed in a climate-controlled room overnight at QRR and then the following morning checked for tanning, an indication that eggs were fertilized. A total of 32 fertilized clutches were collected in this way.
Once we established that clutches were fertile, the cups containing eggs were gently emptied into one of several 1,153-L cattle tanks, clutches were spread across these tanks. Tanks were filled with well water, leaf litter (primarily oak, Quercus spp. the dominant trees in this region, which form an important component of the allochthonous debris entering local ponds), and structure provided by polypropylene rope tied to stainless steel washers to weight one end to the bottom of the tank, creating floating strands. Eggs hatched in these tanks and on 18 August, 2013, larvae were collected and transferred to rearing tanks (416-L cattle tanks). We randomly assigned fifty larvae to each rearing tank. Rearing tanks were filled with well water which was allowed to age for one week. Each tank contained inert materials to provide structural complexity (polypropylene rope and aquarium rocks). Tanks also received a standard volume of leaf litter (again primarily oak), which also provided structure and slowly released nutrients. To provide a food base for larvae, all tanks received a standard inoculum from a zooplankton culture and a small volume of rabbit chow for nutrients.
Experimental rearing tanks were established in 8 blocks with three tanks per block, for a total of 24 tanks in the experiment. Tanks were set up with an insulating wrap made of fiberglass insulation covered in Tyvek HomeWrap (DuPont, Wilmington, Delaware, USA) to decrease variation in temperature and prevent the development of thermal gradients within tanks. Each tank was also covered with 70% shade cloth attached to a wood frame that kept the cloth from contacting the water surface, to stabilize temperatures and prevent colonization by other aquatic organisms, while allowing rainwater to enter the tanks. Precipitation maintained water levels through the winter rainy season; well water was added periodically to keep tank water levels stable during periods without rainfall. These additions of water never exceeded 10% of a tank’s total volume. Well water is cooler than water in the tanks and caused small dips in temperature directly after being added, but temperatures re-equilibrate within a few hours and this represents a very small proportion of the total experimental time period.
Each of three temperature treatments were represented by a single randomly assigned tank per block. Temperature treatments were: ambient, + 2.5°C above the temperature of the ambient tank in that block (medium), and + 5°C above the temperature of the ambient tank in that block (high; mean °C ± 1 SE over the course of the experiment: ambient 14.4 ± 0.3, medium 17.0 ± 0.3, high 19.4 ± 0.3; Appendix S1). Each tank had a temperature sensor that continuously monitored water temperature (CR1000; Campbell Scientific, Logan, Utah, USA). Medium and high treatments were created using an aquarium heater designed for large volumes (JBJ 500Watt True Temp for 378–606 L) that were turned on or off by the CR1000 system to adjust water temperatures to maintain the temperature differential of the treatments relative to the temperature reading from the ambient tank. Ambient tank temperatures were monitored once/hour. Our warming regime was designed to simulate the 50- and 100-year climate projections for the study region (Cayan et al. 2009). This experimental set-up allowed us to simulate warming in a realistic fashion, incorporating both daily and seasonal variation in temperature. Experimental warming began when larvae were placed into tanks.
In April 2014, shade cloth was removed and each tank was covered with a mosquito net (Bryne nets, Inter IKEA Sytems, Delft, The Netherlands) suspended above the tank. These nets captured adult dragonflies as they emerged into their adult stage. Nets were checked twice per day, at approximately 1000 and 1300 h, and adults with exoskeletons sufficiently hardened to be handled safely were removed from nets and placed in a mesh cage (Minifångst, Inter IKEA Systems, Delft, The Netherlands). Adults that had not hardened sufficiently for safe handling were left in the nets until the next net check, something which was most common in the morning so that few individuals were ever left in nets overnight. During each net check, we also searched for dragonflies that had begun the process of emergence but failed to complete metamorphosis successfully. These animals were included in counts of larval survivors. Hardening cages were brought to a climate controlled room (21°C) in the main field station at QRR and individuals were left overnight before further handling. The following day adults were sexed, marked on the wing with a unique number in permanent ink (Sharpie Fine Point), and photographed. Digital calipers (accurate to ± 0.001 mm) were used to measure head-width, thorax length, and forewing length (haphazardly chosen, either the right or left wing).
Adult emergence occurred from April into September. On 2 September 2014, tanks were searched for dragonflies that had not yet emerged. Five tanks were found to have a total of 9 larvae still remaining (0.75% of individuals in the experiment). We calculated survival as the proportion of individuals that successfully emerged as adults.
The experiment was initially set up with a block effect as a factor. Each row had all three treatments and was considered a block. To assess whether retaining this block term in the model was appropriate, we tested for both block and treatment effects on temperatures in tanks across the duration of the experiment. Both block (rmANOVA: F6,11 = 7.9, P = 0.002) and treatment (rmANOVA: F2,11 = 4700, P < 0.0001; Appendix S1) effects were present. However, because the treatment effect was so large in relation to the block effect, and because the sensors and heaters were so precise in generating temperatures that different treatments did not overlap, we focused on individual tanks as replicates and did not consider the block effect in further analyses. This procedure should result in a more conservative statistical analysis because the small variation among blocks is included in the treatment effect. Two temperature sensors malfunctioned during the experiment, which resulted in the removal of one entire row of the experiment and one medium replicate. In the first instance the entire row was removed because unfortunately the malfunctioning sensor was in the ambient tank for that row. As the ambient tank is used to set temperatures for the medium and high tanks within a row, we cannot have confidence in the level of warming in the tanks in that row and so all three tanks were removed from the analysis. The other malfunctioning sensor was in a medium treatment tank, which was removed from the experiment while retaining the ambient and high treatment tanks in the block to preserve as much replication as possible. Finally, we also removed one tank that had less than 15% survival, an outlier with respect to survival in other tanks, due to concerns about whether the few remaining individuals were representative of the entire replicate or whether this high rate of mortality was due to some unmeasured disturbance to the tank (McCauley et al. 2015).
We applied a MANOVA statistical framework to examine the effects of temperature, sex, and their interaction on larval survival, timing of and variation in emergence phenology, and flight performance (quantified as the ratio of forewing length to head width; McCauley et al. 2015). For interpretation, we than conducted univariate ANOVAs for all of the variables included in the overarching MANOVA, except for the three body size measures, which were analyzed in a separate MANOVA. We also used post hoc least squared means tests to differentiate between treatments. All the response variables were non-normal and were rank transformed, with the data being approximately homogeneous for variances. All data analysis was conducted in SAS 9.4.
Results
Effects of increasing mean temperatures
Both temperature (MANOVA, F14, 50 = 3.62, P = 0.0004) and sex (MANOVA, F7, 25 = 3.77, P = 0.006) had an overall effect on the responses of individuals. However, there was no interaction between temperature and sex (MANOVA, F14, 50 = 0.38, P = 0.97). Below, we separate the responses variables into groupings based on specific types of performance.
Survival
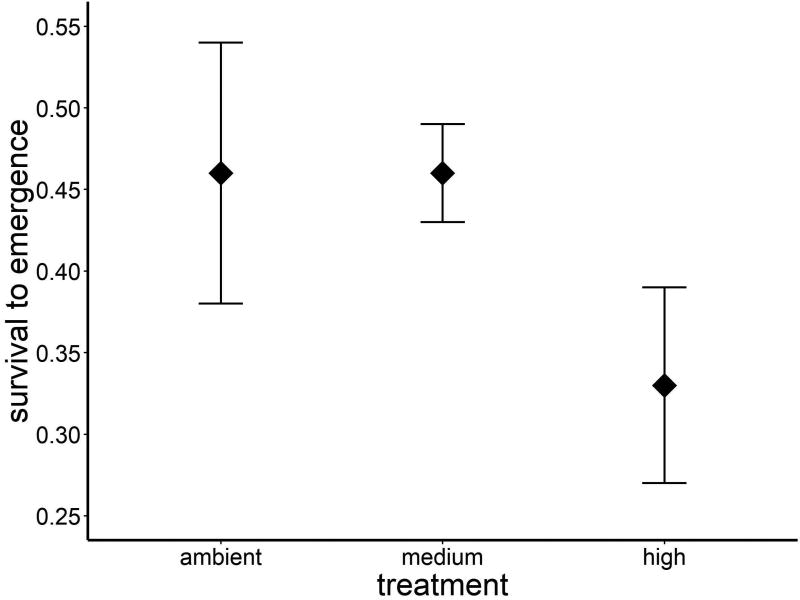
Warming affected larval survival, with decreased survival in the warmest treatment (ANOVA, F2, 31 = 2.95, P = 0.07; proportion surviving, mean ± SE: ambient 0.46 ± 0.08; medium, 0.46 ± 0.03; high, 0.33 ± 0.06; Fig. 1). Neither sex (ANOVA, F1, 31 = 0.64, P = 0.43) nor the interaction between temperature and sex (ANOVA, F2, 31 = 0.97, P = 0.39) affected larval survival.
Figure 1.
There is a trend (P = 0.07) towards decreased survival of E. collocata larvae in the high treatment (+5°C) compared to ambient and medium treatments (mean proportion surviving ± 1 SE).
Development time and phenology
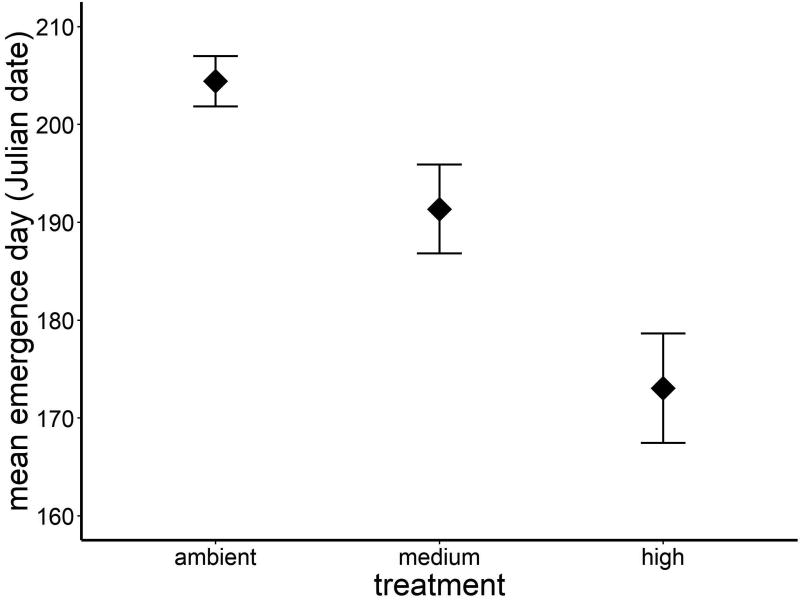
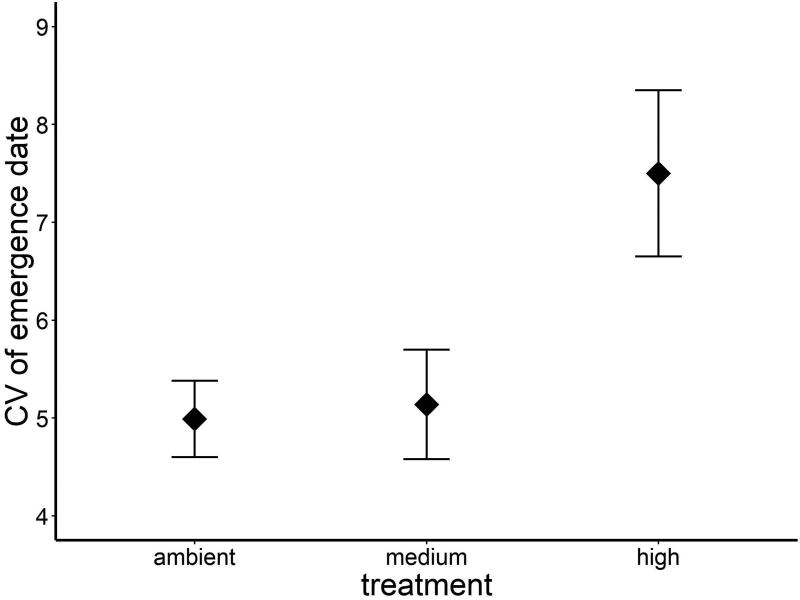
Temperature affected both time to emergence (ANOVA, F2, 31 = 12.46, P = 0.0001; Fig. 2) and variation in emergence phenology (see below). Post hoc tests indicated no difference in emergence phenology between the ambient (mean ± 1 SE Julian day of emergence: 204 ± 3) and medium treatments (Julian day 191 ± 5), but the high temperature treatment had earlier emergence than either of the other treatments (Julian day 173 ± 6). The difference in mean emergence date between the ambient and high temperature treatments was 31 days, a full month earlier in high temperature tanks. Warming also affected the shape of the emergence distribution through time, which we quantified using the coefficient of variation in emergence for each treatment (ANOVA, F2, 31 = 3.70, P = 0.04; Fig. 3). Warmer conditions were associated with more variation in relation to the treatment mean; that is, a proportionally extended period over which individuals emerged through time (mean ± 1 SE in CV: high, 7.5 ± 0.8), compared with proportionally shorter emergence periods for the ambient and medium temperature treatments (ambient, 5.0 ± 0.4; medium, 5.1 ± 0.6). There were no effects of sex (ANOVA, F1, 31 = 0.04, P = 0.85) or the interaction between temperature and sex (ANOVA, F2, 31 = 0.01, P = 0.97) on variation in emergence date.
Figure 2.
Warming significantly advanced the timing of emergence into the adult stage in E. collocata (P = 0.0001). Emergence of adults from these mesocosms occurred on average a month earlier in the high treatment (+5°C) compared to ambient treatment (mean Julian date of emergence ± 1 SE).
Figure 3.
Warming also increased the co-efficient of variation of the timing of emergence with higher levels of variation in emergence timing in the warmest treatment compared with the ambient and medium treatment.
Body size
There was a trend toward an effect of temperature across the three body measures in the multivariate analysis, with individuals from the high temperature treatment being somewhat different (MANOVA, F6, 58 = 2.05, P = 0.07), but the direction of that difference depended on the trait measured. Generally, this result is a product of shorter forewings and longer thoraces with higher temperatures, but when morphological measures were examined independently the effects were minor and not significant (all P >0.2). Females were also somewhat smaller than males in general (MANOVA, F3, 29 = 2.41, P = 0.09). There was no interaction between temperature and sex (MANOVA, F6, 58 = 0.32, P = 0.92).
Flight performance morphology
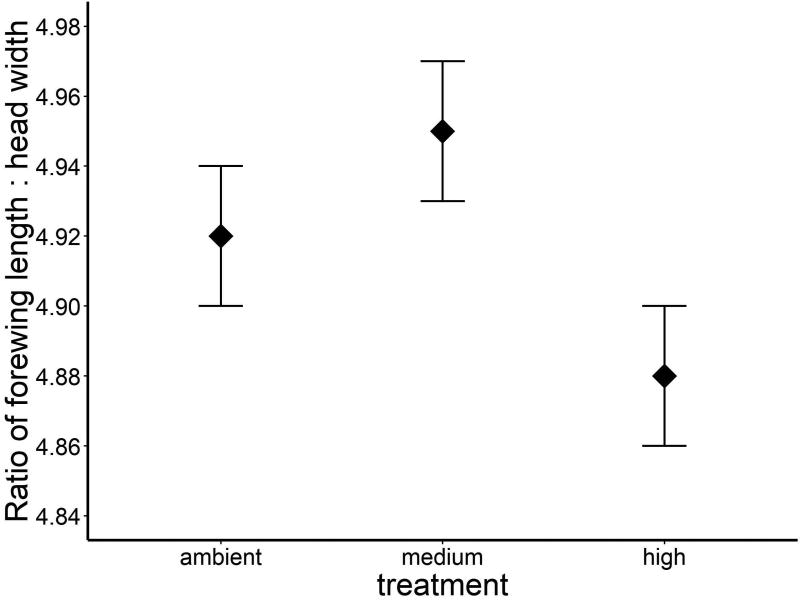
Temperature affected the ratio of forewing length to head width (ANOVA, F2, 31 = 4.01, P = 0.03; Fig. 4), with individuals from the high temperature treatment having proportionally smaller forewings (4.88 ± 0.02) than individuals from the medium (4.95 ± 0.02) or ambient treatments (4.92 ± 0.02), which were not different from each other. There was no effect of sex (ANOVA, F1, 31 = 0.01, P = 0.91) or the interaction between temperature and sex (ANOVA, F2, 31 = 0.04, P = 0.68).
Figure 4.
Adult E. collocata emerging from the high treatment (+5°C) had relatively smaller wings (forewing to head width ratio ± 1 SE) compared with adults from ambient and medium mesocosms.
Discussion
We manipulated the temperatures of experimental aquatic habitats in which larval dragonflies (E. collocata) developed to investigate the consequences of higher temperatures on several aspects of dragonfly performance and growth patterns. We increased the average temperatures experienced by larvae, mimicking the conditions predicted for this region in 50 (+2.5°C) and 100 (+5°C) years from now (Cayan et al. 2009), and reared dragonflies from early larval stages through to the adult stage. Developmental temperatures affected multiple aspects of dragonfly biology, including survival, flight morphology, and most dramatically, emergence phenology. Our results provide some insights into the impacts climate change will have on dragonfly populations.
Effects on mortality
Based on previous work (McCauley et al. 2015, Tseng and O’Connor 2015), we expected that larval mortality would be higher in warmed treatments compared with ambient conditions. While we found a trend towards increased mortality under the highest level of warming (P = 0.07), the effect of temperature on mortality was weaker than previously observed in another dragonfly species. In our previous work with Pachydiplax longipennis larvae, collected as eggs from the same site where we collected E. collocata, exhibited a significant and sharp decline in survival at the highest rearing temperatures (McCauley et al. 2015). In neither experiment does it appear that we exceeded the upper lethal temperature limits for these species. Our maximum temperatures in this experiment were at ~30°C, while dragonfly larvae lethal limits appear to be commonly above 40°C (Dallas and Rivers-More 2012, Stewart et al. 2013). This suggests that mortality in both studies was related to temperature stress rather than directly lethal temperatures. Differential responses by species to warming conditions are the basis for patterns that are beginning to emerge of winners and losers in response to climate change (Somero 2010, Domisch et al. 2011). The two species of dragonfly which we have tested for mortality effects in response to warming both experience an increase in mortality with warming, but the strength and potential impact of these responses differs. These species commonly co-occur in ponds throughout our study region, and the greater sensitivity of P. longipennis to warming may give E. collocata an advantage in these communities under future climatic conditions (but see Chavez et al. 2015). However, experiments combining these species under realistic levels of stressors are necessary to determine how communities will be structured in the future.
Timing and pattern of emergence
Warming advanced the timing of emergence to the adult stage. In the +5°C treatment, adult emergence occurred on average one month earlier than in the ambient treatment (Fig. 2). This pattern matched our predictions, and was expected because increased developmental rates and resulting earlier phenology of emergence in response to warming are common (Hassall et al. 2007, Dingemanse and Kalkman, 2008, Richter et al. 2008, McCauley et al. 2015) and are considered one of the dominant responses by plants and animals to climate change (Parmesan 2006). The pattern of the phenological timing of emergence to the adult stage may advance either because temperature cues that trigger life history events occur earlier, or because development proceeds more rapidly with warming, leading to more rapid completion of earlier life-history stages (Tang et al. 2016). In our system, the latter mechanism is likely to be the largest factor contributing to this pattern, as the life history transition to the adult stage relies on completion of growth and development in the larval stage, which are both temperature dependent (Suhling et al. 2015). The consequences of shifts in phenology will depend on both species and the environmental context. In a seasonal environment, earlier emergence can be risky, exposing early emergers to unsuitable weather including late frosts or late season storms (Inyoue 2008, Augspurger 2013). Our research site is in a Mediterranean climate with winter rains and little precipitation from late spring to summer (UCD-NRS 2004) when dragonflies are in their adult stage. While dragonflies are capable of handling rainy conditions, adult dragonflies do not typically fly in the rain and exposure to rainier and cooler conditions may decrease foraging and result in costs in adult survival and breeding success, particularly in populations such as this one where adult conditions are typically warm and dry. Given that climate change is also likely to change patterns of precipitation, conclusions about the consequences of early emergence are speculative and will depend on interactions between the biology of the organisms affected and the total set of changes in weather conditions including not only temperature but also rainfall patterns. Nonetheless, our results highlight the need to look at how earlier phenologies will affect exposure of vulnerable life history stages to weather conditions outside the range normally experienced.
Warming changed not only the average emergence dates but also the pattern of emergence, increasing the coefficient of variation in emergence date in our warmest treatment compared with the others (Fig. 3), and changing the shape of the distribution of emergence dates. Under ambient thermal conditions, the distribution of individuals emerging across the season is roughly normal, while in the warmest conditions the emergence distribution is flatter and peaks earlier than under ambient conditions (Appendix S2). We did not anticipate this pattern, in part because while the literature suggests that climate change will alter species’ phenologies, most studies focus on the mean timing of life history events and little attention has been paid to the distribution curves of these events.
The consequences of changes to the distribution of individuals emerging across the season are unknown, but we can propose hypotheses about the potential effects. The pattern may increase the number of individuals emerging during the margins of the normal flight season, when weather conditions fall outside the normal weather fluctuations they experience, placing them at risk. Additionally, warming may change effective population size across the season as emergence distributions become more drawn out. An adult odonate will rarely live through the entirety of the flight season; across the season, newly emerged individuals replace previous emergers that have died. In detailed mark-recapture studies with damselflies that indicate that adults may live a few weeks, Sherratt et al. (2010) found evidence of physiological senescence after 15 days as an adult, while Hassall et al. (2015) recaptured fewer than 2% of individuals at 20 days post marking. In our own mark-resight studies of dragonflies in ponds in the region of this study (California’s Coast Range), the longest period across which a marked adult was observed was 24 days (McCauley 2010). At our highest level of warming, the phenology of emergence was drawn out, with a lower peak number of individuals emerging (Appendix S2). This could alter patterns of effective population size across the season, as the number of potentially reproductive individuals present in the population at any given time is dependent on how many have emerged within a few weeks prior to that time. The population dynamic consequences of these changes in the emergence distribution remain to be determined, but potential implications are large. For males, in which there is intense competition for mates, particularly during the normal peak of the flight season (Munguía-Steyer et al. 2016), this may increase their chances of finding a mate and reduce the variance in male fitness. It may also reduce the opportunity for female choice and decrease encounter rates between males and females; however, these hypotheses remain to be tested.
Carry-over effects: contrasting effects of rearing temperature on body size and morphology
Contrary to our initial predictions, we found no effect of thermal conditions on body size. Although we observed a trend towards altered body size in our MANOVA, when examined separately, no single measure of size (head width, wing length, or thorax length) showed a significant difference between treatments (all P > 0.2). Our expectation that body size would decline with temperature, is based on a well-established relationship between body size and temperature, the hotter is smaller rule for ectotherms (Atkinson 1994, 1995, Angilleta et al. 2002, Angilleta and Dunham 2003, Kingsolver and Huey 2008), and reviews that suggest that declines in body size may be a common response to climate change (Gardner et al. 2011, Sheridan and Bickford 2011). However, our previous study testing for this relationship in a dragonfly also did not find an effect of warmer conditions on body size (McCauley et al. 2015), and there is little evidence that warmer developmental conditions lead to decreased body size in odonates (Hassall and Thompson 2008). It is not clear why odonates do not appear to exhibit declines in body size with higher temperatures. In this study and our previous research (McCauley et al. 2015) we did not follow individuals and body size at emergence may reflect processes in addition to growth, including differential mortality that obscures declines in body size. If those individuals that are on a growth trajectory to reach the adult stage at a smaller size are also more likely to die prior to emergence to the adult stage, then we would not detect an effect of temperature on body size. This, however, has yet to be tested. While the pattern we observed appears to be common across the dragonflies we studied, the mechanism creating this pattern is unknown and would be best explored in studies that follow individual growth curves under different temperature conditions.
Although warming did not affect overall body size, it did affect flight morphology. In our +5°C warming treatment, emerging adults had smaller wings relative to head size (a standard metric of odonate body size). Therefore, the effects of warming in the larval stage carry-over to the adult stage, affecting adult performance. Smaller wings for a given body size are likely to lead to higher wing loading, with more weight being carried for a given area of wing (Dudley 2002). While insect flight, including that of odonates, is complex and involves multiple factors that affect flight kinematics (Wootton 1991, 1992), changes in morphology that decrease relative wing size are likely to affect flight performance. Longer wings are associated with a higher probability of dispersal in some odonates (Conrad et al. 2002), and relative wing size is related to range size in North American damselflies (Rundle et al. 2007). Previously, we hypothesized that if warming reduced body size at emergence, it might reduce the capacity of populations to respond to climate change by shifting their distributions to track a suitable climate envelope (McCauley and Mabry 2011). We have found no evidence for reductions in body size at emergence in response to warming, but changes in morphology may have similar consequences. This change in morphology but not body size in response to warming is a novel result and deepens our understanding of how climate change may affect organisms with complex life-cycles. Carry-over effects from warming could alter performance of later life history stages and for organisms that switch habitats between life history stages, change the connections that exist across ecosystem boundaries.
Summary
Results from this study indicate that warming that simulates expected environmental changes within the next 100 years can affect responses by animals that will affect individual performance and potentially population dynamics including mortality, the time of emergence to the adult stage, and morphology of individuals exposed to warming. These effects may arise from direct effects of temperature on developing larvae or from indirect effects of warming on their prey. We did not quantify differences in the zooplankton community in our mesocosms, although visual inspection confirmed that all tanks had zooplankton in densities high enough to be readily detected in this way. Nonetheless, it is possible that warming could affect the zooplankton community and indirectly influence growth and mortality in larvae in these tanks. Additionally, tank covers made of shade cloth are not impermeable to colonization by zooplankton or aquatic insects (Caceres and Soluk 2002). The mesh size of these covers (ca. 1×1mm) suggests that zooplankton would be the most likely colonists (Caceres and Soluk 2002), and insect colonization was not observed in mesocosms. Further consideration of the indirect effects of warming on dragonflies, or other predators, is worth further consideration. In our study, we cannot distinguish the direct and indirect effects, but previous work suggests that direct effects are likely to be strong (Chavez et al. 2016) and the net effects of the direct effects of warming and possible indirect effects through the zooplankton communities are clearly important in the survival and development in these dragonflies.
Comparing the results of this study to previous work we have done with an ecologically similar species provides an interesting contrast. In a previous study with P. longipennis, we found a sharp decrease in survival with warming and no effect of warming on morphology (McCauley et al. 2015). Here, we observed a trend towards decreased survival with increased temperature in E. collocata, but the effect was weaker than in P. longipennis. Further, E. collocata exhibited changes in flight morphology with warming that were not seen in P. longipennis. The variation in responses between ecologically similar species with high levels of habitat overlap suggests that generalizing from a single species may be problematic. Species can exhibit idiosyncratic responses to warming that will affect their performance and ultimately determine which species will be able to persist in the context of warming and which will be negatively affected.
Supplementary Material
Acknowledgments
We thank Shane Waddell, Virginia Boucher, Chris Hill, Marge Hill, the University of California Natural Reserve System, the Quail Ridge Reserve, and the Wantrup Wildlife Refuge of the Napa Land Trust for research support and access to research sites. Thanks to Dachin Frances, Michelle Rampulla, Maria Chavez, Jackson Deen, Renee Pardee, Grant Reed, Jerome Peters, Serena Corona, and the students of NMSU’s Fall 2013 Field Ecology class for research assistance in the field. We had permits from the California Department of Fish and Game (SC-9036) to conduct this work as well as permission from the UC-NRS system and the Napa Land Trust. J. I. Hammond was supported by Grant Number K12GM088021 from the National Institute of General Medical Sciences. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of General Medical Sciences or the National Institutes of Health. We received financial support for this project from a NMSU College of Arts and Sciences Mini-grant and the National Science Foundation (DEB 1245415). All applicable institutional and/or national guidelines for the care and use of animals were followed.
Literature Cited
- Angilletta MJ, Dunham AE. The temperature-size rule in ectotherms: simple evolutionary explanations may not be general. American Naturalist. 2003;162:332–342. doi: 10.1086/377187. [DOI] [PubMed] [Google Scholar]
- Angilletta MJ, Niewiarowski PH, Navas CA. The evolution of thermal physiology in ectotherms. Journal of Thermal Biology. 2002;27:249–268. [Google Scholar]
- Atkinson D. Temperature and organism size: a biological law for ectotherms. In: Begon M, Fitter AH, editors. Advances in Ecological Research. Vol. 25 1994. pp. 1–58. [Google Scholar]
- Atkinson D. Effects of temperature on the size of aquatic ectotherms: exceptions to the general rule. Journal of Thermal Biology. 1995;20:61–74. [Google Scholar]
- Augspurger CK. Reconstructing patterns of temperature, phenology, and frost damage over 124 years: spring damage risk is increasing. Ecology. 2013;94:41–50. doi: 10.1890/12-0200.1. [DOI] [PubMed] [Google Scholar]
- Caceres CE, Soluk DA. Blowing in the wind: a field test of overland dispersal and colonization by aquatic invertebrates. Oecologia. 2002;131:402–408. doi: 10.1007/s00442-002-0897-5. [DOI] [PubMed] [Google Scholar]
- Chavez MY, Mabry KE, McCauley SJ, Hammond JI. Differential larval responses of two ecologically similar insects (Odonata) to temperature and resource variation. International Journal of Odonatology. 2015;18:297–304. doi: 10.1080/13887890.2015.1082946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colinet H, Sinclair BJ, Vernon P, Renault D. Insects in fluctuating thermal environments. In: Berenbaum MR, editor. Annual Review of Entomology. Vol. 60. 2015. pp. 123–140. [DOI] [PubMed] [Google Scholar]
- Conrad KF, Willson KH, Whitfield K, Harvey IF, Thomas CJ, Sherratt TN. Characteristics of dispersing Ischnura elegans and Coenagrion puella (Odonata): age, sex, size, morph and ectoparasitism. Ecography. 2002;25:439–445. [Google Scholar]
- Dallas HF, Rivers-Moore NA. Critical thermal maxima of aquatic macroinvertebrates: towards identifying bioindicators of thermal alteration. Hydrobiologia. 2012;679:61–76. [Google Scholar]
- Daufresne M, Lengfellner K, Sommer U. Global warming benefits the small in aquatic ecosystems. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:12788–12793. doi: 10.1073/pnas.0902080106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diez JM, Ibanez I, Miller-Rushing AJ, Mazer SJ, Crimmins TM, Crimmins MA, Bertelsen CD, Inouye DW. Forecasting phenology: from species variability to community patterns. Ecology Letters. 2012;15:545–553. doi: 10.1111/j.1461-0248.2012.01765.x. [DOI] [PubMed] [Google Scholar]
- Dingemanse NJ, Kalkman VJ. Changing temperature regimes have advanced the phenology of Odonata in the Netherlands. Ecological Entomology. 2008;33:394–402. [Google Scholar]
- Domisch S, Jahnig SC, Haase P. Climate-change winners and losers: stream macroinvertebrates of a submontane region in Central Europe. Freshwater Biology. 2011;56:2009–2020. [Google Scholar]
- Flenner I, Sahlen G. Dragonfly community re-organisation in boreal forest lakes: rapid species turnover driven by climate change? Insect Conservation and Diversity. 2008;1:169–179. [Google Scholar]
- Forster J, Hirst AG, Atkinson D. How do organisms change size with changing temperature? The importance of reproductive method and ontogenetic timing. Functional Ecology. 2011;25:1024–1031. [Google Scholar]
- Gardner JL, Peters A, Kearney MR, Joseph L, Heinsohn R. Declining body size: a third universal response to warming? Trends in Ecology & Evolution. 2011;26:285–291. doi: 10.1016/j.tree.2011.03.005. [DOI] [PubMed] [Google Scholar]
- Gillooly JF, Brown JH, West GB, Savage VM, Charnov EL. Effects of size and temperature on metabolic rate. Science. 2001;293:2248–2251. doi: 10.1126/science.1061967. [DOI] [PubMed] [Google Scholar]
- Grewe Y, Hof C, Dehling DM, Brandl R, Brandle M. Recent range shifts of European dragonflies provide support for an inverse relationship between habitat predictability and dispersal. Global Ecology and Biogeography. 2013;22:403–409. [Google Scholar]
- Hassall C. Odonata as candidate macroecological barometers for global climate change. Freshwater Science. 2015;34:1040–1049. [Google Scholar]
- Hassall C, Sherratt TN, Watts PC, Thompson DJ. Live fast, die old: no evidence of reproductive senescence or costs of mating in a damselfly (Odonata: Zygoptera) Journal of Animal Ecology. 2015;84:1542–1554. doi: 10.1111/1365-2656.12407. [DOI] [PubMed] [Google Scholar]
- Hassall C, Thompson DJ. The effects of environmental warming on Odonata: a review. International Journal of Odonatology. 2008;11:131–153. [Google Scholar]
- Hassall C, Thompson DJ, French GC, Harvey IF. Historical changes in the phenology of British Odonata are related to climate. Global Change Biology. 2007;13:933–941. [Google Scholar]
- Hickling R, Roy DB, Hill JK, Thomas CD. A northward shift of range margins in British Odonata. Global Change Biology. 2005;11:502–506. [Google Scholar]
- Higgins JK, Maclean HJ, Buckley LB, Kingsolver JG. Growth, developmental and stress responses of larvae of the clouded sulphur butterfly Colias eriphyle to repeated exposure to high, sub-lethal temperatures. Physiological Entomology. 2015;40:189–195. [Google Scholar]
- Inouye DW. Effects of climate change on phenology, frost damage, and floral abundance of montane wildflowers. Ecology. 2008;89:353–362. doi: 10.1890/06-2128.1. [DOI] [PubMed] [Google Scholar]
- Kingsolver JG, Huey RB. Size, temperature, and fitness: three rules. Evolutionary Ecology Research. 2008;10:251–268. [Google Scholar]
- Lawson CR, Vindenes Y, Bailey L, van de Pol M. Environmental variation and population responses to global change. Ecology Letters. 2015;18:724–736. doi: 10.1111/ele.12437. [DOI] [PubMed] [Google Scholar]
- McCauley SJ. Body size and social dominance influence breeding dispersal in male Pachydiplax longipennis (Odonata) Ecological Entomology. 2010;35:377–385. [Google Scholar]
- McCauley SJ, Hammond JI, Frances DN, Mabry KE. Effects of experimental warming on survival, phenology, and morphology of an aquatic insect (Odonata) Ecological Entomology. 2015;40:211–220. doi: 10.1111/een.12175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCauley SJ, Mabry KE. Climate change, body size, and phenotype dependent dispersal. Trends in Ecology & Evolution. 2011;26:554–555. doi: 10.1016/j.tree.2011.06.017. [DOI] [PubMed] [Google Scholar]
- Munguia-Steyer R, Cordoba-Aguilar A, Maya-Garcia J. Rubyspot territorial damselflies behave as "Nasty Neighbors". Journal of Insect Behavior. 2016;29:143–152. [Google Scholar]
- Nadeau CP, Urban MC, Bridle JR. Climates past, present, and yet-to-come shape climate change vulnerabilities. Trends in Ecology & Evolution. 2017;32:786–800. doi: 10.1016/j.tree.2017.07.012. [DOI] [PubMed] [Google Scholar]
- O'Regan SM, Palen WJ, Anderson SC. Climate warming mediates negative impacts of rapid pond drying for three amphibian species. Ecology. 2014;95:845–855. doi: 10.1890/13-0916.1. [DOI] [PubMed] [Google Scholar]
- Paaijmans KP, Heinig RL, Seliga RA, Blanford JI, Blanford S, Murdock CC, Thomas MB. Temperature variation makes ectotherms more sensitive to climate change. Global Change Biology. 2013;19:2373–2380. doi: 10.1111/gcb.12240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parmesan C. Ecological and evolutionary responses to recent climate change. Annual Review of Ecology Evolution and Systematics. 2006;37:637–669. [Google Scholar]
- Potter KA, Davidowitz G, Woods HA. Cross-stage consequences of egg temperature in the insect Manduca sexta. Functional Ecology. 2011;25:548–556. [Google Scholar]
- Richter O, Suhling F, Mueller O, Kern D. A model for predicting the emergence of dragonflies in a changing climate. Freshwater Biology. 2008;53:1868–1880. [Google Scholar]
- Rundle SD, Bilton DT, Abbott JC, Foggo A. Range size in North American Enallagma damselflies correlates with wing size. Freshwater Biology. 2007;52:471–477. [Google Scholar]
- Seebacher F, White CR, Franklin CE. Physiological plasticity increases resilience of ectothermic animals to climate change. Nature Climate Change. 2015;5:61–66. [Google Scholar]
- Sheridan JA, Bickford D. Shrinking body size as an ecological response to climate change. Nature Climate Change. 2011;1:401–406. [Google Scholar]
- Sherratt TN, Laird RA, Hassall C, Lowe CD, Harvey IF, Watts PC, Cordero-Rivera A, Thompson DJ. Empirical evidence of senescence in adult damselflies (Odonata: Zygoptera) Journal of Animal Ecology. 2010;79:1034–1044. doi: 10.1111/j.1365-2656.2010.01719.x. [DOI] [PubMed] [Google Scholar]
- Sokolovska N, Rowe L, Johansson F. Fitness and body size in mature odonates. Ecological Entomology. 2000;25:239–248. [Google Scholar]
- Somero GN. The physiology of climate change: how potentials for acclimatization and genetic adaptation will determine 'winners' and 'losers'. Journal of Experimental Biology. 2010;213:912–920. doi: 10.1242/jeb.037473. [DOI] [PubMed] [Google Scholar]
- Stewart BA, Close PG, Cook PA, Davies PM. Upper thermal tolerances of key taxonomic groups of stream invertebrates. Hydrobiologia. 2013;718:131–140. [Google Scholar]
- Suhling F, Suhling I, Richter O. Temperature response of growth of larval dragonflies: an overview. International Journal of Odonatology. 2015;18:15–30. [Google Scholar]
- Suhling I, Suhling F. Thermal adaptation affects interactions between a range-expanding and a native odonate species. Freshwater Biology. 2013;58:705–714. [Google Scholar]
- Tang JW, Korner C, Muraoka H, Piao SL, Shen MG, Thackeray SJ, Yang X. Emerging opportunities and challenges in phenology: a review. Ecosphere. 2016;7 [Google Scholar]
- Therry L, Nilsson-Ortman V, Bonte D, Stoks R. Rapid evolution of larval life history, adult immune function and flight muscles in a poleward-moving damselfly. Journal of Evolutionary Biology. 2014;27:141–152. doi: 10.1111/jeb.12281. [DOI] [PubMed] [Google Scholar]
- Thomas CD, et al. Extinction risk from climate change. Nature. 2004;427:145–148. doi: 10.1038/nature02121. [DOI] [PubMed] [Google Scholar]
- Tseng M, O'Connor MI. Predators modify the evolutionary response of prey to temperature change. Biology Letters. 2015;11 doi: 10.1098/rsbl.2015.0798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wootton RJ. Functional morphology of insect wings. Annual Review of Entomology. 1992;37:113–140. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.