Abstract
Objective:
Despite a growing prevalence of hypertension, young adults (18–39 year-olds) have lower hypertension control rates compared to older adults. The purpose of this study was to evaluate the role of sociodemographic factors in hypertension control among young adults with regular primary care access.
Methods:
A retrospective analysis included 3208 patients, 18–39 years old, who met clinical criteria for an initial (incident) hypertension diagnosis in a large, Midwestern, academic practice from 2008 to 2011. Patients with a prior antihypertensive medication prescription were excluded. Kaplan-Meier analysis was used to estimate the probability of achieving hypertension control over 24 months by gender. Cox proportional hazard models were fit to identify sociodemographic predictors of delays in hypertension control.
Results:
Among the 3208 young adults with incident hypertension, 48% achieved hypertension control within 24 months. Kaplan-Meier analysis demonstrated that young women had a higher hypertension control rate at 24 months (57%) compared to young men (41%). According to adjusted hazard models, young men had a 39% lower rate of hypertension control (hazard ratio 0.61; 95% confidence interval 0.55–0.69) compared to women. Being unmarried (0.87; 0.78–0.98) and a non-English primary language speaker (0.47; 0.37–0.60) also predicted lower hypertension control rates.
Conclusions:
Gender disparities, being unmarried, and non-English primary language are important barriers to hypertension control among young adults with regular primary care use. Interventions tailored to sociodemographic characteristics may improve hypertension control in this challenging population.
Keywords: Hypertension, Young Adult, Ambulatory Care, Healthcare Disparities, Cox Proportional Hazards Models, Kaplan Meier Analysis
INTRODUCTION
It is well-established that hypertension is the leading preventable cause of death in the United States, associated with premature cardiovascular disease (CVD), stroke, heart failure, and chronic kidney disease [1,2]. According to the American Heart Association’s Heart Disease and Stroke Statistics, almost 15% of young adults (18–39 years old) have prevalent hypertension [3], using the definition of ≥140/90 mmHg, with an expected rise in prevalence with the new U.S. hypertension guidelines [2]. Achievement of hypertension control reduces an individual’s risk for hypertension-related morbidity and mortality. However, even with the prior hypertension definition (≥140/90 mmHg), young adults had lower hypertension control rates (35%) when compared to adults ≥40 years old (56%) [4].
The majority of young adults with uncontrolled hypertension see their providers more than once a year, signifying barriers to hypertension control beyond access to care [5,6]. Understanding barriers specific to this young adult population is an important step to develop effective hypertension interventions. Other researchers have identified sociodemographic characteristics (e.g., gender, race, ethnicity, language) as a hypertension control barrier [7–14]. However, research has been primarily limited to middle-aged and older adults [11–13] and younger adults without health insurance and/or without a primary care home [15,16]. Therefore, the objective of this study was to evaluate the relationship of sociodemographic characteristics to hypertension control rates among young adults with incident hypertension and regular primary care access. There are multiple current initiatives to improve hypertension control for patients with primary care access, focusing on the dissemination of clinical best practices such as the Million Hearts initiative by the U.S. Department of Health and Human Services [17,18]. Our research facilitates these efforts by identifying barriers unique to subsets of young adults, providing a foundation for healthcare systems to develop improvement projects in this important population.
METHODS
Sample
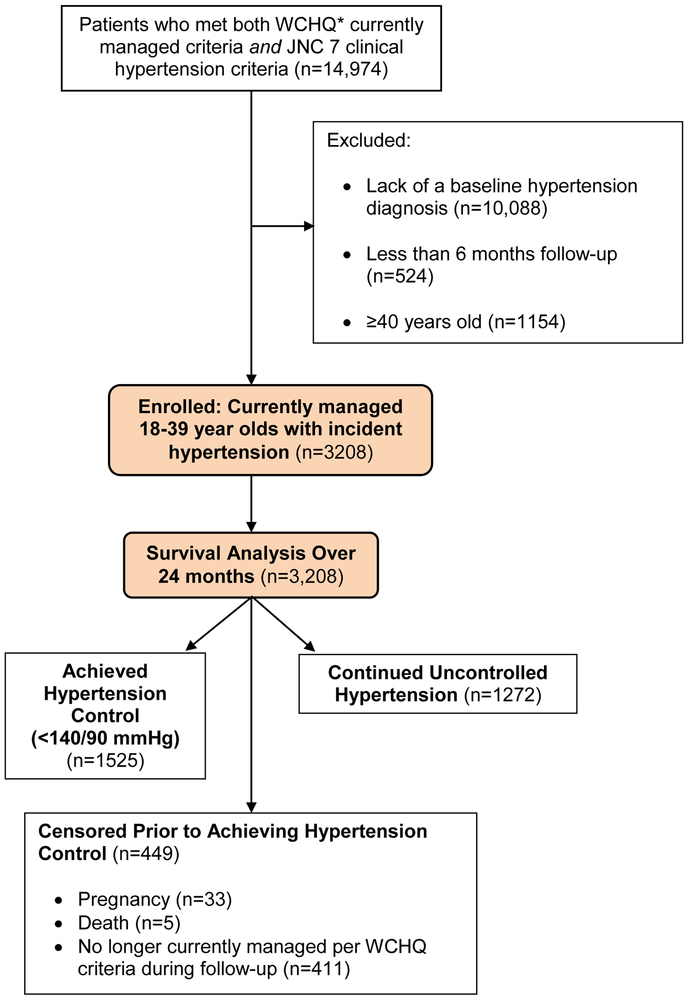
The University of Wisconsin-Madison Health Sciences Institutional Review Board approved this study with a waiver of written informed consent. This retrospective cohort analysis used electronic health record data of patients with uncontrolled hypertension from a large, Midwestern, multi-disciplinary academic group practice. To construct the sample (Figure 1), we identified all patients who met criteria defined by the Wisconsin Collaborative for Healthcare Quality (WCHQ) [19,20] for being “currently managed” in the healthcare system between January 1, 2008 and December 31, 2011. WCHQ is a voluntary consortium of Wisconsin healthcare organizations committed to publicly reporting performance measures of quality and affordability of healthcare services [21]. Per WCHQ criteria, eligible “currently managed” patients had to have ≥2 billable office encounters in an outpatient, non-urgent, primary care setting, or one primary care and one office encounter in an urgent care setting, in the three years prior to study enrollment, with at least one visit in the prior two years [22]. Electronic health records were assessed for the date that a patient met the Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC 7) criteria for a new diagnosis of hypertension [1] (incident hypertension), meaning they had not received a previous diagnosis of or treatment for hypertension. JNC 7 criteria were used since they were the established U.S. hypertension guidelines during the reporting period. A patient was determined as meeting hypertension eligibility criteria based on electronic health record data if there were: a) ≥3 elevated outpatient blood pressure measurements from three separate dates, ≥30 days apart, but within a two-year span (systolic blood pressure ≥140 mmHg or diastolic blood pressure ≥90 mmHg) or b) two elevated blood pressures [23,24] (systolic blood pressure ≥160 mmHg or diastolic blood pressure ≥100 mmHg), ≥30 days apart within a two-year period [25–29]. The blood pressures within the administrative data were acquired using a guideline-based [30] protocol, automated blood pressure machines, and appropriate sized cuffs. Patients had to be seated quietly for at least 5 minutes in a back-supported chair, with feet flat on the floor and arm supported at heart level. If the initial blood pressure was ≥140/90 mmHg, an additional measurement was acquired at least 2 minutes later during the same visit and the average blood pressure was used [25,30]. Hospital and emergency department blood pressures were excluded to avoid falsely elevated blood pressures.
Figure 1.
Study Sample: Enrollment and Analysis
*WCHQ: Wisconsin Collaborative for Healthcare Quality
After meeting criteria for incident hypertension, patients were then excluded if they did not receive an electronic health record diagnosis of hypertension based on the Tu criteria [31], had less than 6 months of follow-up, or were ≥40 years old (Figure 1). The Tu algorithm for administrative data was used to define patients who have been diagnosed with hypertension using the following ICD-9 codes [32]: 401.x (essential hypertension), 402.x (hypertensive heart disease), 403.x (hypertensive renal disease), 404.x (hypertensive heart and renal disease), and 405.x (secondary hypertension).
Each patient meeting all eligibility criteria received an “index date” (the first date all criteria were met). A 365-day period prior to this index date was the “baseline period” to assess patients’ comorbidities and healthcare utilization. Patients were followed for 24-months to account for less frequent ambulatory visits within 1 year among younger populations [25]. Patients continued to accrue time in the study from the index date until they achieved the primary outcome (hypertension control) or were censored (death, end of primary care management, pregnancy, or end of study [24 months]). Censoring for “end of primary care management” accounted for disruptions in healthcare access in this young population (e.g., change in insurance, residence). Patients who were pregnant during the study were excluded one year before, during, and one year following pregnancy using a modified Manson approach [33] (n=33; 1.03%).
Primary Outcome Variable
The primary outcome was time (days) from the index date to achieving hypertension control over 24 months, defined as the first of three consecutive normal blood pressures (<140/90 mmHg) on three separate health system dates [28]. To account for blood pressure variability, multiple clinic blood pressures were used to define hypertension control since 24-hour ambulatory blood pressure monitoring data was not available. Results are reported in months.
Primary Explanatory Variables
Patient sociodemographic characteristics were obtained from the electronic health record during the baseline study period. Based upon prior research [34,35], gender was our primary explanatory variable. Patients self-identified their gender; male or female were the electronic health record choices available at the time of the study. Other sociodemographic variables included age at cohort entry, marital status (single, married, divorced, or widowed), ever receiving Medicaid (yes or no), and race/ethnicity. In the U.S., Medicaid is a joint federal and state program that provides funding for medical and health-related services for U.S. citizens and permanent residents with low income and/or disabilities. Prior studies have used Medicaid coverage as a proxy for individual socioeconomic status in the U.S. [36–38]. Race/ethnicity was included because of the increased prevalence of hypertension among young African-Americans [39]. All of the patients self-classified their race/ethnicity in the electronic health record (White, Black, Asian, Hispanic/Latino, Other [Native Hawaiian, Pacific Islander, Multi-racial], or Unknown).
Other Explanatory Variables
Patient and provider variables representing barriers to hypertension control were selected based on an established conceptual model for clinical inertia [40]. Other patient-related factors included body mass index, baseline tobacco status, and comorbidities. Patients’ self-reported tobacco status was updated at every face-to-face ambulatory, urgent care, and emergency department visit per health system policy. Current tobacco use was defined as any use of cigarette, cigar, or pipe use over the past 12 months [41]. Former tobacco use was the cessation of any inhaled tobacco products for ≥12 months [41]. The classification of “Never” tobacco use was defined as no lifetime use of inhaled tobacco product [41]. This analysis did not include passive tobacco use or vaping products. Patient comorbidities were assessed at baseline using the following established algorithms: hyperlipidemia (ICD-9 codes: 272.0–272.4) [42], diabetes mellitus with/without complications (ICD-9 codes: 250.00–250.93, 357.2, 362.0–362.02, 366.41) [43], chronic kidney disease (ICD-9 codes: 016.0, 095.4,189.0, 189.9, 223.0, 236.91, 250.4, 271.4, 274.1, 283.11, 403.X1, 404.X2, 404.X3, 440.1, 442.1, 447.3, 572.4, 580–588, 591, 642.1, 646.2, 753.12–753.17, 753.19, 753.2, 794.4) [44], and mental health conditions (depression [ICD-9 codes: 296.2X, 296.3X, 300.4X] [45] and/or anxiety [ICD-9 codes: 300.0–300.02, 300.09, 300.21–300.23, 300.3, 309.24, 309.81]) [45]. Elixhauser and the Medicare Chronic Condition Data Warehouse Administrative algorithms were used to identify: chronic pulmonary disease [46], stroke/transient ischemic attack [47], collagen vascular diseases [48], thyroid diseases [46], congestive heart failure, and deficiency anemias [46]; due to their low prevalence we created an indicator variable for the presence of any of these conditions.
Patients’ morbidity burden can predict healthcare utilization, which may influence diagnosis and antihypertensive medication initiation rates [49,50]. Therefore, we used the Johns Hopkins Adjusted Clinical Group (ACG) Case-Mix System (version 10.0), which assesses morbidity burden to predict future healthcare utilization [50,51]. The ACG risk score was selected because our study sample contains a diverse mix of government-insured and privately-insured ambulatory young adults. An ACG risk score of 1.0 represents expected healthcare utilization on an individual-level according to the patient’s age and gender [51]. The number of primary care, specialty, and urgent care visits were measured in the baseline period. Primary care visits included physician, nurse practitioner, and physician assistant visits in Family Medicine/Family Practice, Internal Medicine, and lower prevalence primary care specialties (Obstetrics/Gynecology, Pediatrics/Adolescent Medicine) to reflect broader primary care options in this younger population.
Patients were assigned to the primary care provider they saw most frequently in outpatient face-to-face Evaluation & Management visits, as reported in professional service billing [22]. Statistical models also controlled for providers’ specialty (Internal Medicine, Family Medicine/Family Practice, Other) and gender, which were obtained from human resource data.
Statistical Analysis
Analyses were conducted using SAS 9.1.3 (SAS Institute, Inc., Cary, NC) and Stata 13.1 (Stata-Corp, College Station, TX). Categorical variables were summarized using percentages; continuous variables were summarized using means (standard deviations). Kaplan-Meier survival curves were computed by gender to evaluate the probability of achieving hypertension control as a function of time since meeting criteria. A multivariable Cox proportional hazards regression analysis was conducted using robust estimates of the variance to obtain adjusted hazard ratios (HRs) and 95% confidence intervals (CIs) for explanatory variables associated with achieving hypertension control by gender. Tests were considered significant at p<0.05. The proportional-hazards assumption for the model was tested using a generalized linear regression of the scaled Schoenfeld residuals on functions of time [52]. Interaction testing between gender and race/ethnicity was performed.
RESULTS
Descriptive Data
Overall, 3208 patients met inclusion criteria (Figure 1). Table 1 summarizes this study population by gender. Among young adults with incident hypertension (mean age 31, SD 5.5 years; 58% male), 78% had stage 1 (mild) hypertension [1], and 58% were obese (BMI ≥30 kg/m2). At baseline, compared to men, women were more likely to be obese, have diabetes mellitus, anxiety and/or depression, a low prevalence condition, and/or reported Medicaid use. Young women were also more likely to have a female provider, higher rates of urgent care use, and a higher ACG healthcare utilization score. Young men were more likely to be married, have higher baseline blood pressures, and current or former tobacco use.
Table 1.
Baseline Demographics of Young Adults (18-39 years old) with Incident Hypertension, By Self-Reported Gender (n=3208)
| Total Population n=3208 |
Gender |
P value | ||
|---|---|---|---|---|
| Male n=1874 (58%) |
Female n=1334 (42%) |
|||
| PATIENT CHARACTERISTICS | ||||
| Age, m (SD) | 31 (5.5) | 31 (5.5) | 31 (5.5) | <0.001 |
| Lowest age tertile (18-29), n (%) | 1167 (36) | 684 (37) | 483 (36) | 0.90 |
| Middle age tertile (30-35), n (%) | 1146 (36) | 673 (36) | 473 (35) | 0.90 |
| Highest age tertile (36-39), n (%) | 895 (28) | 517 (28) | 378 (28) | 0.90 |
| Race/Ethnicity, n (%) | 0.001 | |||
| White | 2665 (83) | 1592 (85) | 1073 (80) | |
| Non-white* | 543 (17) | 282 (15) | 261 (20) | |
| Marital Status, n (%) | <0.001 | |||
| Married | 1405 (44) | 880 (47) | 525 (39) | |
| Not married (single/divorced/widowed) | 1803 (56) | 994 (53) | 809 (61) | |
| Primary Spoken Language, n (%) | <0.001 | |||
| English | 2904 (91) | 1664 (89) | 1240 (93) | |
| Other | 304 (9.5) | 210 (11) | 94 (7.1) | |
| On Medicaid ever,† n (%) | 578 (18) | 241 (13) | 337 (25) | <0.001 |
| Baseline Tobacco Status, n (%) | 0.002 | |||
| Current tobacco use | 742 (23) | 457 (24) | 285 (21) | |
| Former tobacco use | 648 (20) | 403 (22) | 245 (18) | |
| Never tobacco use | 1818 (57) | 1014 (54) | 804 (60) | |
| Body mass index, kg/m2, m (SD) | 33 (8.5) | 32 (7.1) | 35 (9.8) | 0.024 |
| BMI <30 kg/m2, n (%) | 1351 (42) | 886 (47) | 465 (35) | <0.001 |
| BMI ≥30 kg/m2, n (%) | 1857 (58) | 988 (53) | 869 (65) | <0.001 |
| JNC 7 Stage of Hypertension§, n (%) | 0.05 | |||
| Stage 1: 140-159/90-99 mmHg | 2495 (78) | 1435 (77) | 1060 (79) | |
| Stage 2: ≥160/100 mmHg | 713 (22) | 439 (23) | 274 (21) | |
| Systolic blood pressure, mmHg, m (SD) | 142 (9.1) | 143 (9.2) | 141 (9.0) | 0.030 |
| Diastolic blood pressure, mmHg, m (SD) | 89 (8.0) | 89 (8.5) | 90 (7.3) | |
| Baseline Comorbid Conditions, n (%) | ||||
| Hyperlipidemia | 265 (8.3) | 162 (8.6) | 103 (7.7) | 0.35 |
| Diabetes mellitus | 107 (3.3) | 46 (2.5) | 61 (4.6) | 0.001 |
| Anxiety and/or depression | 768 (24) | 365 (19) | 403 (30) | <0.001 |
| Low prevalence condition‡ | 292 (9.1) | 114 (6.1) | 178 (13) | <0.001 |
| ACG§ Risk Score, young, m (SD) | 1.1 (1.3) | 0.95 (1.2) | 1.3 (1.3) | 0.12 |
| Lowest ACG tertile (≤0.61), n (%) | 1075 (34) | 788 (42) | 287 (22) | <0.001 |
| Middle ACG tertile (0.62-1.00), n (%) | 1064 (33) | 586 (31) | 478 (36) | <0.001 |
| Highest ACG tertile (≥1.01), n (%) | 1069 (33) | 500 (27) | 569 (43) | <0.001 |
| Baseline Ambulatory Visits, m (SD) | ||||
| Primary Care Visits | 2.8 (2.7) | 2.2 (2.2) | 3.5 (3.1) | 0.29 |
| Specialty Care Visits | 2.0 (2.7) | 1.8 (2.3) | 2.4 (3.1) | 0.76 |
| Urgent Care Visits | 0.84 (1.4) | 0.73 (1.2) | 1.0 (1.6) | <0.001 |
| PROVIDER FACTORS | ||||
| Primary Care Provider, n (%) | 2810 (88) | 1675 (89) | 1135 (85) | <0.001 |
| Female Provider, n (%)‖ | 1458 (45) | 544 (29) | 914 (69) | <0.001 |
n, numerator; %, percent; m, mean; SD, standard deviation; BMI, body mass index; kg/m2, kilograms per meters squared
Non-white: Black n=258 (8.0%), Asian n=53 (1.7%), Hispanic/Latino n=102 (3.2%), American Indian/Alaska Native n=13 (0.4%), Native Hawaiian/Pacific Islander n=31 (1.0%)
On Medicaid during the baseline or study period
Due to low prevalence, an indicator variable was created for the presence of any of the following: atrial fibrillation, chronic pulmonary disease, stroke/transient ischemic attack, collagen vascular disease, deficiency anemias, congestive heart failure, chronic kidney disease, thyroid disorders
ACG = Adjusted Clinical Group Case-Mix Assessment System
n=32 (1.0%) missing provider gender
Table. P-value bold represents significant at <0.05
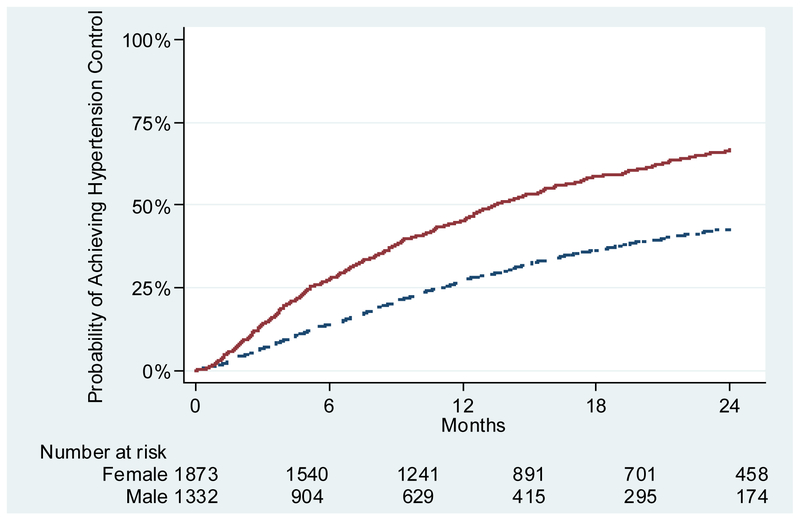
Incident Hypertension Control Rates by Gender
Among all patients 18 to 39 years old, only 48% (n=1525) achieved hypertension control within 24 months after meeting criteria for incident hypertension. Kaplan-Meier curves (Figure 2) demonstrated that female young adults had a higher rate of hypertension control (57%; n=765) compared to males (41%; n=760). Among patients who achieved control, the median (25th-75th percentile) time to control among females was 6.9 (3.2–13.0) months compared to males at 9.4 (4.5–16.7) months.
Figure 2.
Probability of Achieving Hypertension Control by Gender

Sociodemographic Predictors and Time to Hypertension Control
Unadjusted Cox proportional hazards models (Table 2) demonstrated that young men with incident hypertension had a significantly lower rate of achieving hypertension control (HR 0.52; 95% CI, 0.47–0.58) compared to women. After adjustment, young men had a 39% lower rate of hypertension control compared to women (HR 0.61; 95% CI, 0.55–0.69). Among other sociodemographic variables, being unmarried (HR 0.87; 95% CI, 0.78–0.98) and a non-English primary language speaker (HR 0.47; 95% CI, 0.37–0.60) predicted lower hypertension control rates among young adults.
Table 2.
Unadjusted and Adjusted Hazard Ratios and 95% CIs of Achieving Incident Hypertension Control Among Young Adults (18-39 years-old)
| Variable | Unadjusted HR (95% CI) |
P value* | Adjusted HR (95% CI) |
P value* |
|---|---|---|---|---|
| Gender† | ||||
| Male | 0.52 (0.47-0.58) | <0.001 | 0.62 (0.55-0.69) | <0.001 |
| Female (Reference) | 1.00 – | – | 1.00 – | -- |
| Age, tertile | ||||
| Lowest age tertile (18-29 years old) | 0.99 (0.86-1.15) | 0.93 | ||
| Middle age tertile (30-35 years old) | 1.09 (0.96-1.24) | 0.17 | ||
| Highest age tertile (36-39 years old) (Reference) | 1.00 – | -- | ||
| Race/Ethnicity | ||||
| White | 0.91 (0.79-1.04) | 0.18 | ||
| Non-white (Reference) | 1.00 – | -- | ||
| Marital Status | ||||
| Married (Reference) | 1.00 – | -- | ||
| Not married | 0.87 (0.78-0.98) | 0.023 | ||
| Primary Spoken Language, n (%) | ||||
| English (Reference) | 1.00 – | -- | ||
| Other | 0.47 (0.37-0.60) | <0.001 | ||
| Body mass index, kg/m2 | ||||
| Not obese (BMI ≤29.9 kg/m2) (Reference) | 1.00 – | -- | ||
| Obese/Morbidly obese (BMI ≥30 kg/m2) | 0.86 (0.77–0.95) | 0.004 | ||
| Baseline Tobacco Status | ||||
| Current tobacco use | 0.89 (0.77–1.02) | 0.09 | ||
| Former tobacco use | 0.94 (0.83–1.08) | 0.40 | ||
| Never tobacco use (Reference) | 1.00 – | -- | ||
| On Medicaid ever‡ | 1.35 (1.18–1.54) | <0.001 | ||
| JNC 7 Stage of Hypertension | ||||
| Stage 1: 140-159/90-99 mmHg | 1.35 (1.18–1.54) | <0.001 | ||
| Stage 2: ≥160/100 mmHg (Reference) | 1.00 – | -- | ||
| Baseline Comorbid Conditions | ||||
| Hyperlipidemia | 1.18 (0.97–1.45) | 0.10 | ||
| Diabetes mellitus | 0.96 (0.71–1.31) | 0.81 | ||
| Anxiety and/or depression | 1.21 (1.07–1.37) | 0.002 | ||
| ACG§ Risk Score | 1.09 (1.04–1.13) | <0.001 | ||
| Baseline Ambulatory Visits | ||||
| Primary Care Visits | 1.08 (1.06–1.10) | <0.001 | ||
| Specialty Care Visits | 1.11 (1.08–1.14) | <0.001 | ||
| Urgent Care Visits | 1.03 (0.99–1.08) | 0.14 |
CI, Confidence Interval; HR, Hazard Ratio; BMI, body mass index; kg/m2, kilograms per meters squared
Global p-value for proportional hazards assumption p=0.80
Gender is the primary explanatory variable. Models were adjusted for age, race/ethnicity, marital status, primary spoken language, BMI, tobacco status, Medicaid use, chronic comorbid conditions, morbidity burden (ACG score), and ambulatory encounter frequency.
On Medicaid during the baseline or study period
ACG = Adjusted Clinical Group Case-Mix Assessment System
Higher body mass index (HR 0.86; 95% CI, 0.77–0.95) predicted lower rates of hypertension control. Predictors of higher hypertension control rates were any previous Medicaid use (HR 1.35; 95% CI, 1.18–1.54), mild (Stage 1) hypertension (HR 1.35; 95% CI, 1.18–1.54), presence of anxiety and/or depression (HR 1.21; 95% CI, 1.07–1.37), higher ACG co-morbidity scores (HR 1.09; 95% CI, 1.04–1.13), and more frequent primary care (HR 1.08; 95% CI, 1.06–1.10) and specialty clinic visits (HR 1.11; 95% CI, 1.08–1.14). There was not a significant interaction between gender and race/ethnicity (HR 0.97; 95% CI, 0.74–1.27; p=0.814). Provider factors (gender and specialty) were not significant predictors for hypertension control among young adults.
DISCUSSION
Our findings demonstrate significant gender disparities in hypertension control rates among young adults with incident hypertension and regular primary care use. Despite the higher prevalence of hypertension among young men [53], young women achieved higher rates of control over 2 years. This gender difference remained a significant predictor of hypertension control even after adjusting for co-morbidities, behavior risk factors such as tobacco use, and healthcare use. Interestingly, data from the National Longitudinal Study of Adolescent to Adult Health (Add Health Study) [54] demonstrated that young women are more likely to be aware of their hypertension and have more frequent healthcare access for birth control and gynecological services [55]. In our young adult population, there was not a significant difference in primary and specialty care use between young men and women; yet, women had higher rates of urgent care use. Our analysis also demonstrated that young women had higher ACG healthcare utilization scores demonstrating greater co-morbidity burden including diabetes and mental health conditions. Therefore, this study highlights that gender is an independent contributor to lower hypertension control among young adults beyond primary care clinic visits. One explanation may be the higher prevalence of more severe (Stage 2) hypertension among young men in our population compared to women. A pathophysiologic contributor may be the higher prevalence of current and former tobacco use among young men compared to women; however, other biological gender differences have been suggested, such as sex steroid hormone profiles [35,56,57]. We did not find a significant interaction between gender and race/ethnicity; however, we had limited power and larger studies are needed for more robust interaction analysis.
In our study, marital status was another independent sociodemographic predictor of lower hypertension control rates among young adults, which is supported by earlier research [12]. Prior studies in predominantly older adults and/or populations outside the Unites States have also suggested that marital status modifies gender differences in hypertension awareness and control [34]. These studies have demonstrated that single individuals are less likely to be aware of hypertension and support our findings that they are also less likely to achieve control [34]. Our data strengthens prior hypotheses that marriage may be protective against adverse health outcomes [58]; however, additional research is needed in the young adult population.
Another concerning finding is that young adults with a non-English primary language were 53% less likely to achieve hypertension control. Our findings are supported by Multi-Ethnic Study of Atherosclerosis (MESA) data which demonstrated that participants who spoke Spanish at home had lower control of multiple cardiovascular risk factors, including hypertension [59]. This finding is also partly explained by our prior analysis among undiagnosed young adults, which demonstrated that a non-English primary language was associated with a slower rate of receiving a hypertension diagnosis [25]. Our current analysis only included young adults with a prior hypertension diagnosis, demonstrating that additional barriers exist beyond awareness of hypertension.
Young adults have persistently low hypertension control rates [39]; however, there is a paucity of effective and sustainable interventions. This is concerning given the increased prevalence of hypertension with new U.S. hypertension guidelines [2]. Our findings demonstrate that within young adult populations, addressing gender disparities, non-English language speakers, and those with limited social support (single) may improve control. It is important to note that this population already had a hypertension diagnosis and routine primary care access, with multiple contact visits. Additional hypertension outreach beyond healthcare access is critical to address sociodemographic barriers to hypertension control.
An important strength of this study was the ability to analyze a large sample of young adults with incident hypertension with regular primary care in a large multispecialty group practice. The findings may not be generalizable to young adults without healthcare access due to lack of insurance or transition states (e.g., move, employment). The use of data from a single healthcare system may also limit the generalizability since treatment patterns may differ across systems and regions. However, this healthcare system is one of the 10 largest physician practices in the United States, including over 300 primary care physicians and 43 primary care clinics. Our sociodemographic analysis lacked some variables including income, education, and alcohol use which may be related to our findings; however, this analysis included numerous covariates including comorbidities, patient utilization, and provider data, which improves the validity and clinical applicability of our study. The use of retrospective administrative data has known limitations, including the potential for misclassification of diagnoses. However, validated algorithms were used to identify hypertension and other comorbidities. Finally, we had a small sample size of young adults across different races/ethnicities, limiting our power to analyze gender and race interactions.
Conclusions
Persistently low hypertension control rates among young adults, with an increasing prevalence of hypertension, highlights an urgent need to develop sustainable, effective interventions for this challenging population. Gender disparities, language barriers, and home support (represented by marital status) are important barriers to hypertension control in young adults, even with regular primary care access. Tailoring interventions to young adults that address these barriers may improve hypertension control in this challenging population.
Acknowledgments:
The authors gratefully acknowledge Katie Ronk, BS and Patrick Ferguson, MPH for data preparation, and Jamie LaMantia, BS for manuscript preparation.
Sources of Funding:
This original research was supported by the Clinical and Translational Science Award program, previously through the National Center for Research Resources (NCRR – UL1RR025011), and now by the National Center for Advancing Translational Sciences of the National Institutes of Health (NIH) under award number UL1TR000427. Heather M. Johnson is supported by the National Heart, Lung, and Blood Institute of the NIH (K23HL112907). During this study, Christie M. Bartels was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the NIH (K23AR062381).
Additional funding for the project was provided by the UW Health Innovation Program and the UW School of Medicine and Public Health from The Wisconsin Partnership Program.
Footnotes
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The funders did not play any role in the study design; in the collection, analysis, or interpretation of the data; in the writing of the manuscript; or in the decision to submit the article for publication.
Prior Presentation: Part of the data was presented in poster format by R. Haggart at the University of Wisconsin-Madison 16th Annual Medical Student Research Forum in Madison, WI on November 20, 2017.
Conflicts of Interest: C. Bartels and H. Johnson have a clinical appointment with the academic group practice that has a financial interest in delivering care to the general population from which study subjects were drawn. C. Bartels and H. Johnson also receive grant funding from Independent Grants for Learning and Change (Pfizer). For the remaining authors, none were declared.
REFERENCES
- 1.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL Jr., et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA 2003; 289:2560–2572. [DOI] [PubMed] [Google Scholar]
- 2.Whelton PK, Carey RM, Aronow WS, Casey DE Jr., Collins KJ, Dennison Himmelfarb C, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol 2018; 71:e127–e248. [DOI] [PubMed] [Google Scholar]
- 3.Benjamin EJ, Virani SS, Callaway CW, Chamberlain AM, Chang AR, Cheng S, et al. Heart Disease and Stroke Statistics-2018 Update: A Report From the American Heart Association. Circulation 2018; 137:e67–e492. [DOI] [PubMed] [Google Scholar]
- 4.Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, et al. Heart Disease and Stroke Statistics-2016 Update: A Report From the American Heart Association. Circulation 2016; 133:e38–e360. [DOI] [PubMed] [Google Scholar]
- 5.King CC, Bartels CM, Magnan EM, Fink JT, Smith MA, Johnson HM. The importance of frequent return visits and hypertension control among US young adults: a multidisciplinary group practice observational study. J Clin Hypertens (Greenwich) 2017; 19:1288–1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nwankwo T, Yoon SS, Burt V, Gu Q. Hypertension among adults in the United States: National Health and Nutrition Examination Survey, 2011–2012. NCHS Data Brief 2013; 1–8. [PubMed] [Google Scholar]
- 7.Cook S, Drum ML, Kirchhoff AC, Jin L, Levie J, Harrison JF, et al. Providers’ assessment of barriers to effective management of hypertension and hyperlipidemia in community health centers. J Health Care Poor Underserved 2006; 17:70–85. [DOI] [PubMed] [Google Scholar]
- 8.Holland N, Segraves D, Nnadi VO, Belletti DA, Wogen J, Arcona S. Identifying barriers to hypertension care: implications for quality improvement initiatives. Dis Manag 2008; 11:71–77. [DOI] [PubMed] [Google Scholar]
- 9.Ogedegbe G Barriers to optimal hypertension control. J Clin Hypertens (Greenwich) 2008; 10:644–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Walsh JM, Sundaram V, McDonald K, Owens DK, Goldstein MK. Implementing effective hypertension quality improvement strategies: barriers and potential solutions. J Clin Hypertens (Greenwich) 2008; 10:311–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van Rossum CT, van de Mheen H, Witteman JC, Hofman A, Mackenbach JP, Grobbee DE. Prevalence, treatment, and control of hypertension by sociodemographic factors among the Dutch elderly. Hypertension 2000; 35:814–821. [DOI] [PubMed] [Google Scholar]
- 12.He J, Muntner P, Chen J, Roccella EJ, Streiffer RH, Whelton PK. Factors associated with hypertension control in the general population of the United States. Arch Intern Med 2002; 162:1051–1058. [DOI] [PubMed] [Google Scholar]
- 13.Jo I, Ahn Y, Lee J, Shin KR, Lee HK, Shin C. Prevalence, awareness, treatment, control and risk factors of hypertension in Korea: the Ansan study. J Hypertens 2001; 19:1523–1532. [DOI] [PubMed] [Google Scholar]
- 14.Psaltopoulou T, Orfanos P, Naska A, Lenas D, Trichopoulos D, Trichopoulou A. Prevalence, awareness, treatment and control of hypertension in a general population sample of 26,913 adults in the Greek EPIC study. Int J Epidemiol 2004; 33:1345–1352. [DOI] [PubMed] [Google Scholar]
- 15.Hill MN, Bone LR, Kim MT, Miller DJ, Dennison CR, Levine DM. Barriers to hypertension care and control in young urban black men. Am J Hypertens 1999; 12:951–958. [DOI] [PubMed] [Google Scholar]
- 16.Fang J, Wang G, Ayala C, Lucido SJ, Loustalot F. Healthcare Access Among Young Adults: Impact of the Affordable Care Act on Young Adults With Hypertension. Am J Prev Med 2017; 53:S213–S219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wall HK, Hannan JA, Wright JS. Patients with undiagnosed hypertension: hiding in plain sight. JAMA 2014; 312:1973–1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Frieden TR, Berwick DM. The “Million Hearts” initiative--preventing heart attacks and strokes. N Engl J Med 2011; 365:e27. [DOI] [PubMed] [Google Scholar]
- 19.Hatahet MA, Bowhan J, Clough EA. Wisconsin Collaborative for Healthcare Quality (WCHQ): lessons learned. WMJ 2004; 103:45–48. [PubMed] [Google Scholar]
- 20.Sheehy A, Pandhi N, Coursin DB, Flood GE, Kraft SA, Johnson HM, Smith MA. Minority status and diabetes screening in an ambulatory population. Diabetes Care 2011; 34:1289–1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wisconsin Collaborative for Healthcare Quality. WCHQ website. Wisconsin Collaborative for Healthcare Quality, Inc, Middleton, WI: Available at: http://www.wchq.org/. Accessed May 23, 2018. [Google Scholar]
- 22.Thorpe CT, Flood GE, Kraft SA, Everett CM, Smith MA. Effect of patient selection method on provider group performance estimates. Med Care 2011; 49:780–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Myers MG, Tobe SW, McKay DW, Bolli P, Hemmelgarn BR, McAlister FA. New algorithm for the diagnosis of hypertension. Am J Hypertens 2005; 18:1369–1374. [DOI] [PubMed] [Google Scholar]
- 24.Schmittdiel J, Selby JV, Swain B, Daugherty SL, Leong TK, Ho M, et al. Missed opportunities in cardiovascular disease prevention?: low rates of hypertension recognition for women at medicine and obstetrics-gynecology clinics. Hypertension 2011; 57:717–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Johnson HM, Thorpe CT, Bartels CM, Schumacher JR, Palta M, Pandhi N, et al. Undiagnosed hypertension among young adults with regular primary care use. J Hypertens 2014; 32:65–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Johnson HM, Thorpe CT, Bartels CM, Schumacher JR, Palta M, Pandhi N, et al. Antihypertensive medication initiation among young adults with regular primary care use. J Gen Intern Med 2014; 29:723–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ho AK, Bartels CM, Thorpe CT, Pandhi N, Smith MA, Johnson HM. Achieving Weight Loss and Hypertension Control Among Obese Adults: A US Multidisciplinary Group Practice Observational Study. Am J Hypertens 2016; 29:984–991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ho AK, Thorpe CT, Pandhi N, Palta M, Smith MA, Johnson HM. Association of anxiety and depression with hypertension control: a US multidisciplinary group practice observational study. J Hypertens 2015; 33:2215–2222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wallace ML, Magnan EM, Thorpe CT, Schumacher JR, Smith MA, Johnson HM. Diagnosis and treatment of incident hypertension among patients with diabetes: a U.S. multi-disciplinary group practice observational study. J Gen Intern Med 2015; 30:768–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Weber MA, Schiffrin EL, White WB, Mann S, Lindholm LH, Kenerson JG, et al. Clinical practice guidelines for the management of hypertension in the community: a statement by the American Society of Hypertension and the International Society of Hypertension. J Clin Hypertens (Greenwich) 2014; 16:14–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tu K, Chen Z, Lipscombe LL. Prevalence and incidence of hypertension from 1995 to 2005: a population-based study. CMAJ 2008; 178:1429–1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tu K, Campbell NR, Chen ZL, Cauch-Dudek KJ, McAlister FA. Accuracy of administrative databases in identifying patients with hypertension. Open Med 2007; 1:e18–26. [PMC free article] [PubMed] [Google Scholar]
- 33.Manson JM, McFarland B, Weiss S. Use of an automated database to evaluate markers for early detection of pregnancy. Am J Epidemiol 2001; 154:180–187. [DOI] [PubMed] [Google Scholar]
- 34.Abu-Saad K, Chetrit A, Eilat-Adar S, Alpert G, Atamna A, Gillon-Keren M, et al. Blood pressure level and hypertension awareness and control differ by marital status, sex, and ethnicity: a population-based study. Am J Hypertens 2014; 27:1511–1520. [DOI] [PubMed] [Google Scholar]
- 35.Sandberg K, Ji H. Sex differences in primary hypertension. Biol Sex Differ 2012; 3:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Harnick DJ, Cohen JL, Schechter CB, Fuster V, Smith DA. Effects of practice setting on quality of lipid-lowering management in patients with coronary artery disease. Am J Cardiol 1998; 81:1416–1420. [DOI] [PubMed] [Google Scholar]
- 37.Shen JJ, Wan TT, Perlin JB. An exploration of the complex relationship of socioecologic factors in the treatment and outcomes of acute myocardial infarction in disadvantaged populations. Health Serv Res 2001; 36:711–732. [PMC free article] [PubMed] [Google Scholar]
- 38.Foraker RE, Rose KM, Whitsel EA, Suchindran CM, Wood JL, Rosamond WD. Neighborhood socioeconomic status, Medicaid coverage and medical management of myocardial infarction: atherosclerosis risk in communities (ARIC) community surveillance. BMC Public Health 2010; 10:632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Benjamin EJ, Blaha MJ, Chiuve SE, Cushman M, Das SR, Deo R, et al. Heart Disease and Stroke Statistics-2017 Update: A Report From the American Heart Association. Circulation 2017; 135:e146–e603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.O’Connor PJ. Overcome clinical inertia to control systolic blood pressure. Arch Intern Med 2003; 163:2677–2678. [DOI] [PubMed] [Google Scholar]
- 41.Majernick TG, Zacker C, Madden NA, Belletti DA, Arcona S. Correlates of hypertension control in a primary care setting. Am J Hypertens 2004; 17:915–920. [DOI] [PubMed] [Google Scholar]
- 42.Borzecki AM, Wong AT, Hickey EC, Ash AS, Berlowitz DR. Identifying hypertension-related comorbidities from administrative data: what’s the optimal approach? Am J Med Qual 2004; 19:201–206. [DOI] [PubMed] [Google Scholar]
- 43.Hebert PL, Geiss LS, Tierney EF, Engelgau MM, Yawn BP, McBean AM. Identifying persons with diabetes using Medicare claims data. Am J Med Qual 1999; 14:270–277. [DOI] [PubMed] [Google Scholar]
- 44.Foley RN, Murray AM, Li S, Herzog CA, McBean AM, Eggers PW, Collins AJ. Chronic kidney disease and the risk for cardiovascular disease, renal replacement, and death in the United States Medicare population, 1998 to 1999. J Am Soc Nephrol 2005; 16:489–495. [DOI] [PubMed] [Google Scholar]
- 45.Marciniak MD, Lage MJ, Dunayevich E, Russell JM, Bowman L, Landbloom RP, Levine LR. The cost of treating anxiety: the medical and demographic correlates that impact total medical costs. Depress Anxiety 2005; 21:178–184. [DOI] [PubMed] [Google Scholar]
- 46.Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care 1998; 36:8–27. [DOI] [PubMed] [Google Scholar]
- 47.Chronic Conditions Data Warehouse. 2011. Chronic Condition Reference List. Buccaneer, West Des Moines, IA: Available at: https://www.ccwdata.org/web/guest/condition-categories. Accessed May 23, 2018. [Google Scholar]
- 48.MacLean CH, Louie R, Leake B, McCaffrey DF, Paulus HE, Brook RH, Shekelle PG. Quality of care for patients with rheumatoid arthritis JAMA 2000; 284:984–992. [DOI] [PubMed] [Google Scholar]
- 49.Campbell NR, So L, Amankwah E, Quan H, Maxwell C. Characteristics of hypertensive Canadians not receiving drug therapy. Can J Cardiol 2008; 24:485–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Starfield B, Weiner J, Mumford L, Steinwachs D. Ambulatory care groups: a categorization of diagnoses for research and management. Health Serv Res 1991; 26:53–74. [PMC free article] [PubMed] [Google Scholar]
- 51.Weiner JP, Starfield BH, Steinwachs DM, Mumford LM. Development and application of a population-oriented measure of ambulatory care case-mix. Medical Care 1991; 29:452–472. [DOI] [PubMed] [Google Scholar]
- 52.Grambsch PM, Therneau TM. Proportional hazards tests and diagnostics based on weighted residuals. Biometrika 1994; 81:515–526. [Google Scholar]
- 53.Zhao B, Jose PO, Pu J, Chung S, Ancheta IB, Fortmann SP, Palaniappan LP. Racial/ethnic differences in hypertension prevalence, treatment, and control for outpatients in northern California 2010–2012. Am J Hypertens 2015; 28:631–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Everett B, Zajacova A. Gender differences in hypertension and hypertension awareness among young adults. Biodemography Soc Biol 2015; 61:1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bertakis KD, Azari R, Helms LJ, Callahan EJ, Robbins JA. Gender differences in the utilization of health care services. J Fam Pract 2000; 49:147–152. [PubMed] [Google Scholar]
- 56.Vitale C, Fini M, Speziale G, Chierchia S. Gender differences in the cardiovascular effects of sex hormones. Fundam Clin Pharmacol 2010; 24:675–685. [DOI] [PubMed] [Google Scholar]
- 57.Ong KL, Tso AW, Lam KS, Cheung BM. Gender difference in blood pressure control and cardiovascular risk factors in Americans with diagnosed hypertension. Hypertension 2008; 51:1142–1148. [DOI] [PubMed] [Google Scholar]
- 58.Schwandt HM, Coresh J, Hindin MJ. Marital Status, Hypertension, Coronary Heart Disease, Diabetes, and Death Among African American Women and Men: Incidence and Prevalence in the Atherosclerosis Risk in Communities (ARIC) Study Participants. J Fam Issues 2010; 31:1211–1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Eamranond PP, Legedza AT, Diez-Roux AV, Kandula NR, Palmas W, Siscovick DS, Mukamal KJ. Association between language and risk factor levels among Hispanic adults with hypertension, hypercholesterolemia, or diabetes. Am Heart J 2009; 157:53–59. [DOI] [PMC free article] [PubMed] [Google Scholar]