Abstract
Multiple myeloma remains an incurable disease, and continued efforts are required to develop novel agents and novel drug combinations with more effective anti-myeloma activity. Here, we show that the pan-PIM kinase inhibitors SGI1776 and CX6258 exhibit significant anti-myeloma activity and that combining a pan-PIM kinase inhibitor with the immunomodulatory agent lenalidomide in an in vivo myeloma xenograft mouse model resulted in synergistic myeloma cell killing without additional hematologic or hepatic toxicities. Further investigations indicated that treatment with a pan-PIM kinase inhibitor promoted increased ubiquitination and subsequent degradation of IKZF1 and IKZF3, two transcription factors crucial for survival of myeloma cells. Combining a pan-PIM kinase inhibitor with lenalidomide led to more effective degradation of IKZF1 and IKZF3 in multiple myeloma cell lines as well as xenografts of myeloma tumors. We also demonstrated that treatment with a pan-PIM kinase inhibitor resulted in increased expression of cereblon, and that knockdown of cereblon via a shRNA lentivirus abolished the effects of PIM kinase inhibition on the degradation of IKZF1 and IKZF3 and myeloma cell apoptosis, demonstrating a central role of cereblon in pan-PIM kinase inhibitor-mediated down-regulation of IKZF1 and IKZF3 and myeloma killing. These data elucidate the mechanism of pan-PIM kinase inhibitor mediated anti-myeloma effect and the rationale for the synergy observed with lenalidomide co-treatment, and provide justification for a clinical trial of the combination of pan-PIM kinase inhibitors and lenalidomide for the treatment of multiple myeloma.
Keywords: multiple myeloma, Pan-PIM kinase inhibitor, lenalidomide, IKZF1/3, cereblon
1. INTRODUCTION
Multiple myeloma (MM) is a malignant plasma cell disorder with no curative therapy[1]. MM is the second most common hematologic malignancy, with an estimated 30,770 new cases in the United States in 2018[2]. Symptomatic MM is characterized by a clonal proliferation of plasma cells and evidence of end-organ damages that include anemia, hypercalcemia, lytic bone lesions, and renal failure[3], resulting in significant mortality and morbidity. Over the last 2–3 decades, the response to treatment and survival of patients with MM have significantly improved largely due to the incorporation of several new biological and molecular targeted agents which have been used along with autologous hematopoietic stem cell transplant. However, relapse is almost universal with nearly all patients eventually developing resistance to currently available agents even after achievement of a complete remission[4], therefore continued effort should be dedicated to developing novel therapies and novel drug combinations with more effective antimyeloma activity.
Recently, many new drugs have been developed for the treatment of MM, including immunomodulatory drugs (IMiDs), proteasome inhibitors, monoclonal antibodies, and HDAC inhibitors[4]. IMiDs, including thalidomide, lenalidomide and pomalidomide, are one of the mainstays in the treatment of MM. Lenalidomide is the most commonly used IMiD in the treatment of MM, and is used in newly diagnosed myeloma patients, in the relapsed setting, and for maintenance therapy after induction or autologous hematopoietic stem cell transplant. Given this universality, the development of agents with the potential to synergize with lenalidomide can lead to a drastic improvement in treatment response and survival in patients with MM.
PIM (proviral insertion in murine malignancies) kinases are a small family of serine/threonine kinases with 3 isoforms (PIM1, PIM2 and PIM3)[5, 6]. PIM kinases are overexpressed and play important roles in tumorigenesis in various human cancers, including prostate cancer[7], B-cell lymphoma[8, 9], leukemias[10, 11], and breast cancer[12, 13]. The expression of PIM2 kinase was found to be higher in MM than in any other cancer type[14]. Recent research showed that inhibition of PIM2 kinase has significant anti-tumor efficacy in multiple myeloma[15] and that targeting PIM3 may have activity against adult T-cell leukemia[16]. It has also been reported that PIM1 mRNA expression is a potential prognostic biomarker in acute myeloid leukemia[17]. Furthermore, accumulating lines of evidence have suggested that PIM kinases are important in the pathogenesis of MM. PIM kinases play a wide range of roles in MM pathogenesis including cell proliferation[18], survival[14], cell cycle dysregulation, oncogenic collaboration with c-Myc, DNA damage repair[19], and bone destruction[20, 21]. A phase 1 study of the novel pan-PIM kinase inhibitor (LGH447) showed encouraging single-agent activities in heavily pre-treated relapsed/refractory MM[22].
Despite growing interest in pan-PIM kinase inhibitors as therapeutic agents in MM, the molecular pathways through which these agents kill myeloma cells, and their ability to be combined with other anti-myeloma agents has not been characterized. In the current study, we investigated the anti-myeloma effects of pan-PIM kinase inhibitors, their combinatorial effects with lenalidomide, and their mechanism of action in both of these settings.
2. MATERIALS AND METHODS
2.1. Reagents and antibodies
CX6528, SGI1776, and lenalidomide were purchased from Selleckchem (Houston, TX). SGI1776 used for in vivo studies was provided by Tolero Pharmaceuticals, Inc (Lehi, Utah). Cycloheximide, bortezomib, and MG132 were purchased from Sigma-Aldrich (St. Louis, MO). Antibodies against PIM1, PIM2, PIM3 and ubiquitin were purchased from Abcam (Cambridge, MA). Antibodies against c-Myc, IRF4, pS6, and PARP were purchased from Cell Signaling (Danvers, MA). Annexin V and caspase 3 antibodies were purchased from BD Biosciences (San Jose, CA). IKZF1 antibody was purchased from Santa Cruz Biotechnology, Inc (Dallas, TX). IKZF3 antibody was purchased from Novus Biologicals LLC (Littleton, CO). Cereblon antibody was obtained from Sigma-Aldrich (St. Louis, MO).
2.2. Primary human CD138+ myeloma cells and myeloma cell lines
Primary human CD138+ cells were isolated from the bone marrow aspirates of patients with myeloma with patients’ informed consent and IRB approval. The study was performed in compliance with the guidelines of the Ethical Committee of Duke University Medical Center. MM cell lines used in this study included MM1R, MM1S, RPMI8226, RPMI8226-Dox40, NCIH929, OPM1, JK6L and U266. MM1R cells stably co-expressing eGFP and luciferase were generated by transducing with lentiviruses encoding eGFP and luciferase as reported previously [23].
2.3. Cell proliferation assay
MM cell lines or primary human CD138+ myeloma cells were treated with various concentrations of SGI1776 or CX6528 as indicated in the text. For thiazolyl blue tetrazolium bromide (MTT) cell proliferation assay, 5 × 104 myeloma cells were plated in triplicate in a well of a 96-well plate in a final volume of 100µl that contained SGI1776 or CX6528. The cells were incubated at 370C in 5% CO2 incubator for various durations as indicated after which 20µl of the combined MTS/PMS solution (5mg/ml MTT) were added into each well and the plate was incubated for and additional 3–4 hrs at 370C in 5% CO2 incubator. Absorbances at 490 nm were then measured using a plate reader. For live cell number measurement, MM cells were plated in 12-well plates (0.5×105 cells/well) and treated with various concentration of SGI1776 or CX6528, then stained with trypan blue and counted at the timepoints indicated.
2.4. Western blot analysis
MM cells were harvested, washed with PBS, and re-suspended in lysis buffer containing 50mM Tris-HCl pH 7.4, 150mM NaCl, 1mM EDTA, 1% Triton x100, 1% Sodium deoxycholate, and 0.1% SDS. The cells were further lysed by brief sonication. The lysates were centrifuged at 12,000rpm for 10min to remove cell debris. Total protein was quantified using the Dc protein estimation kit (Bio Rad). Approximately 20µg protein was loaded and run on SDS-PAGE then transferred onto PVDF membrane. The membrane was blocked with 5% milk in Tris-Buffered Saline containing 0.05% of Tween 20 (TBST) for 1hr then primary antibodies were applied with 1% BSA in TBST and incubated overnight at 40C. The membrane was then probed with HRP-conjugated secondary antibody for 1hr at room temperatue and developed using Pierce ECL substrate. Data shown is representative of at least 3 separate sets of experiments.
2.5. Real-time PCR detection
Total RNA was isolated by Trizol (Invitrogen). The first-strand cDNA was synthesized with the iScript cDNA synthesis kit (Bio-Rad). RT-PCR was performed on Bio-Rad iQ5 using the following primers: IKZF1: 5’-CCCCTGTAAGCGATACTC CA-3’ and 5’CCACGACTCTGTCACTCTTGG-3’; IKZF3: 5’-ATCAACAAGGAAGGGGAGGT-3’ and 5’-CAGGGCTCTGTGTTCTCCTC-3’; cereblon: 5’-TCCAGCAAGCTAAAGTGCAA-3’ and 5’-AGCGAGGCCATGAAGTTAGA-3’; β-Actin: 5’-ACCTTCTACAATGAGCTG-3’ and 5’-CCTGGATAGCAACGTACAGG-3’.
2.6. Apoptosis assay
MM cells were treated with SGI1776 or CX6528 for various durations, then harvested and stained with Annexin V antibody, and analyzed by flow cytometry analysis or lysed and immunoblotted for the levels of PARP cleavage and caspase-3 cleavage.
2.7. Lentivirus production and gene transduction
The production and gene transduction of lentiviruses encoding shRNA against human cereblon (CRBN) and control shRNA were performed as described previously [24]. Briefly, oligonucleotides encoding CRBN specific shRNA, or control shRNA were cloned into GIPZ lentiviral vectors. Lentiviruses were produced after transient transfection of HEK 293T cells with individual lentiviral vector along with the packaging plasmids (VSV-G and psPax2) according to the manufacturer’s instructions (Trans- GIPZ Lentiviral shRNA Packaging Kit). The supernatants containing viral particles were collected 48h after transfection. Cells were transduced with lentiviruses by cocentrifugation at 3,000rpm for 3h at 370C in the presence of 8µg/ml polybrene.
2.8. Syngeneic transplanted VK*MYC myeloma mouse model
All our animal studies were conducted in accordance with guidelines approved by the Institutional Animal Care and Use Committees at Duke University. Syngeneic transplanted VK*MYC myeloma mouse model was used for in vivo studies[25–28]. Cryopreserved splenocytes of VK*MYC mice were kindly provided by Dr. P. Leif Bergsagel at Mayo Clinic, Arizona. The cells were expanded by injecting 1 vial of the cells into 10 C57Bl/6 mice of 10–12 weeks old. The splenocytes were harvested once the intensity of the monoclonal (M) spike reached >1/2 of the albumin intensity and used for experiments. To generate syngeneic transplanted VK*MYC myeloma mouse model, cryopreserved splenocytes were injected via tail vein into 10–12 weeks old, non-irradiated C57Bl/6 mice (10 recipient mice per one vial of cells, that is approximately 1 × 106 splenocytoes per mouse). Blood samples were collected weekly beginning 3 weeks after cell injection. Myeloma development was monitored by measurement of the M spike on serum protein electrophoresis. When the myeloma developed (~ day 26 after cell injection), the mice were treated with SGI1776 (75mg/kg body weight) or control buffer daily given by oral gavage. In a separate set of experiments, when myeloma was established the mice were treated with control buffer, SGI1776 (35mg/kg), lenalidomide (25mg/kg) or a combination of SGI1776 and lenalidomide via daily oral gavage. Animal survival was monitored.
2.9. Myeloma xenograft mouse modeles
For myeloma xenograft mouse experiments, two tumor inoculation approaches were used: subcutaneous injection and intravenous administration. MM1R cells stably expressing luciferase (1.5×106 cells/mouse) were injected subcutaneously or intravenously into sublethally irradiated (1.5Gy for subcutaneous model and 1.75Gy for intravenous model) NSG mice. Tumor growth was followed weekly by bioluminescence imaging using Living Imaging Software (Xenogen) after intraperitoneal administration of luciferin. When tumors were established as determined by bioluminescence imaging, the mice were treated with control buffer, SGI1776 (35mg/kg), lenalidomide (25mg/kg) or a combination of SGI1776 and lenalidomide via daily oral gavage. Blood samples were collected for the measurement of whole blood cell counts and liver alanine transaminase enzyme (ALT). Tumor growth was monitored by measuring tumor size (in the subcutaneous model) and/or quantifying bioluminescence intensity. Animal survival was monitored daily. In the subcutaneous model, at the end of experiments the mice were euthanized and the tumors were harvested and weighed. Protein was extracted from the tumors and the expression of IKZF1 and IKZF3 was measured by immunoblot analysis.
2.10. Statistical analysis
Statistical analyses were performed with GraphPad Prism software. Comparisons of gene expresson were performed using one-way ANOVA. Student’s t-test was also used to compare paired samples. The differences in survival were determined by Log-Rank sum test. Differences were considered statisticaly significant at p<0.05.
3. RESULTS
3.1. Pan-PIM kinase inhibitors show effective anti-myeloma activity in human myeloma cells in vitro
Two pan-PIM kinase inhibitors, SGI1776 and CX6258, were used to test the antimyeloma activity of PIM kinase inhibition [11, 29]. We found that both SGI1776 and CX6258 effectively inhibited the growth of 8 human myeloma cell lines with an average IC50 of ~7.5µM (Figure 1A and Supplemental Figure S1) and induced apoptosis as demonstrated by PARP cleavage, Caspase 3 activation, and positive Annexin V staining (Figure 1B-C and Supplemental Figure S2 ). Additionally, SGI1776 and CX5268 demonstrated significant anti-myeloma activity for primary human myeloma cells isolated from patients’ bone marrow aspirates (Figure 1D).
Figure 1. Pan-PIM kinase inhibitors showed effective anti-myeloma activity in vitro and in vivo.
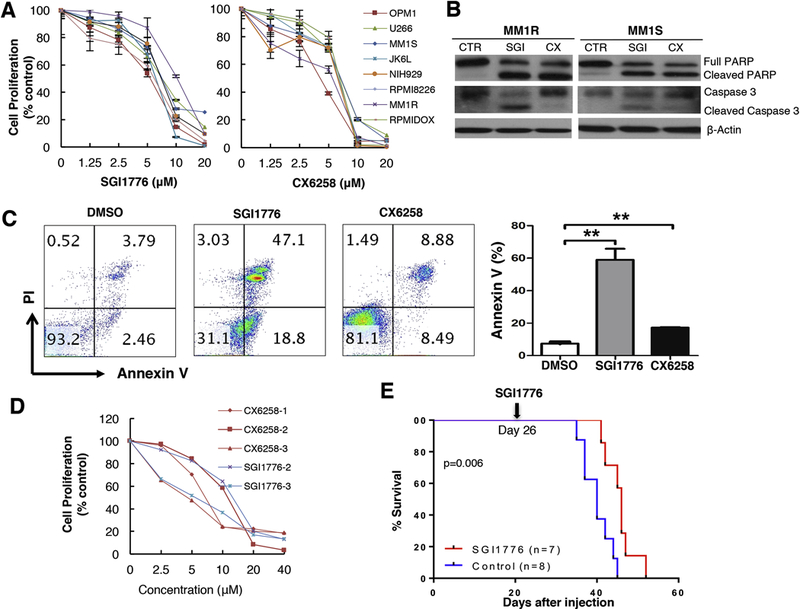
A. Dose-dependent inhibition of cell viability by SGI1776 and CX6258. Cell proliferation was measured by MTT assay. B. PAPR cleavage and Caspase 3 activation were analyzed by western blot. MM1R and MM1S cells were treated with 7.5µM of SGI1776 (SGI), 5µM CX6258 (CX), or DMSO (CTR) for 12h. C. Increased Annexin V+ cells by SGI1776 and CX6258 were analyzed by flow cytometry. MM1R cells were treated with 7.5µM SGI1776, 5µM CX6258, or DMSO for 12h. Percentages of Annexin V+ cells from 3 separate sets of experiments were shown (Mean ± SEM. **: p< 0.01). D. Inhibition of primary human CD138+ myeloma cells by SGI1776 and CX6528. Cell proliferation was measured by MTT assay. Numbers represent different human derived samples. E. Improved animal survival by treatment of SGI1776 in transplanted VK*MYC mice.
3.2. Pan-PIM kinase inhibitors have in vivo anti-myeloma activity in a syngeneic transplanted VK*MYC myeloma mouse model
We next investigated the anti-myeloma activity of pan-PIM kinase inhibitors in vivo using a syngeneic transplanted VK*MYC myeloma mouse model[25–28, 30]. Cryopreserved splenocytes of VK*MYC mice were injected via tail vein into 10–12 weeks old, non-irradiated syngeneic C57Bl/6 mice (~1 × 106 splenocytes/recipient mouse). Myeloma development was monitored by measurement of the monoclonal M spike on serum protein electrophoresis weekly starting at week 3 after cell injection. At day 26 of cell injection when myeloma was established as evidenced by M spike (Supplemental Figure S3), the mice were treated with daily SGI1776 (75mg/kg body weight) or control buffer. The transplanted VK*MYC myeloma model is a very aggressive mouse model. Daily treatment of transplanted VK*MYC mice with established myeloma with SGI1776 extended their survival (median survival of 40 days for mice receiving control buffer versus 46 days for mice treated with SGI1776; p=0.006, Figure 1E). These data confirm the single agent anti-myeloma activity of pan-PIM inhibitors previously seen in both preclinical and clinical studies[14, 22, 31].
3.3. Combination of pan-PIM kinase inhibitor and lenalidomide has enhanced anti-myeloma activity in vivo in myeloma xenograft mouse models
The effectiveness of pan-PIM kinase inhibitors in killing human myeloma cells in vitro and their ability to prolong the survival of syngeneic transplanted VK*MYC myeloma mouse model prompted us to investigate the combinatorial effects of pan-PIM kinase inhibitors with lenalidomide in vivo. Two myeloma xenograft mouse models were used: subcutaneous tumor cell implantation and tail vein administration of tumor cells. The subcutaneous xenograft mouse model allows us to make tumor size measurement and harvest tumors at the end of treatment for molecular analyses. The tail vein administration model results in systemic spread of tumor cells and allows for detection of a survival benefit with treatment.
In the subcutaneous xenograft mouse model, MM1R cells stably expressing luciferase (1.5×106 cells/mouse) were injected subcutaneously into the back of sublethally irradiated (1.5Gy) NSG mice. Tumor growth was followed weekly by bioluminescence imaging. Beginning at day 10 after xenograft implantation (when the tumors were established and could be detected by bio-luminescence imaging), mice were treated daily with either: DMSO, SGI1776 (35mg/kg body weight), lenalidomide (25mg/kg body weight), or the combination of SGI1776 and lenalidomide by oral gavage. While both SGI1776 and lenalidomide alone were able to inhibit tumor growth compared to the control group (Figure 2A-D), the combination of SGI1776 and lenalidomide had enhanced anti-myeloma effect, resulting in near complete inhibition of myeloma cell growth after 3 weeks of treatment (Figure 2A-D).
Figure 2. Pan-PIM kinase inhibitor and lenalidomide combination resulted in more effective anti-myeloma activity in vivo in subcutaneous xenograft myeloma model.
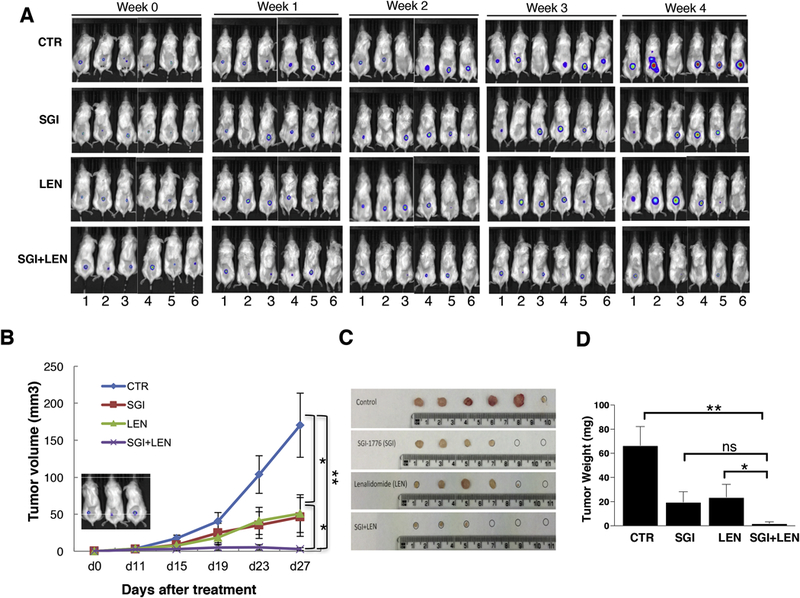
MM1R cells stably expressing luciferase (1.5×106 cells/mouse) were injected subcutaneously into the back of sublethally irradiated (1.5Gy) NSG mice. Tumor growth was followed weekly by bioluminescence imaging. At day 10 of cell injection, mice were treated (indicated as Week 0 in A and day 0 in B) with either: DMSO, SGI1776 (35mg/kg body weight), lenalidomide (25mg/kg body weight), or the combination of SGI1776 and lenalidomide by daily oral gavage. A and B: The combination of SGI1776 and lenalidomide is more effective in anti-myeloma activity. A: Bio-luminescence imaging; B: Tumor volume. The inset panel showed myeloma tumor establishment measured by bioluminescent imaging just before the treatment. C and D: Tumor was significantly reduced in mice treated with a combination of SGI1776 and lenalidomide. C: Tumor size; D: tumor weight. (ns: no statistically significant; *: p<0.05; **: p< 0.01)
In the systemic myeloma xenograft mouse model, NSG mice were sublethally irradiated (1.75Gy) and injected via tail vein MM1R cells stably expressing luciferase (1.5×106 cells/mouse). Tumor growth was followed weekly by bioluminescence imaging after intraperitoneal administration of luciferin. Beginning at day 19 after intravenous cell injection (when the tumors were established and could be detected by bio-luminescence imaging), mice were treated daily with either: DMSO, SGI1776 (35mg/kg) alone, lenalidomide (25mg/kg) alone, or the combination of SGI1776 and lenalidomide through oral gavage. The SGI1776 and lenalidomide combination led to improved disease control compared to either agent alone (Figure 3A). Tumor bio-luminescence intensities were significantly reduced in the combination group compared to the control or single agent groups. Furthermore, combination treatment resulted in a 3 week survival advantage compared to the DMSO control group (median survival of 73 days vs 52 days for the combination treatment group vs the DMSO control group, respectively; p<0.01, Figure 3B). Importantly, no additional hematologic or hepatic toxicities were observed in the combination group (Figure 3C).
Figure 3. Pan-PIM kinase inhibitor and lenalidomide combination resulted in more effective anti-myeloma activity in vivo in systemic xenograft myeloma model.
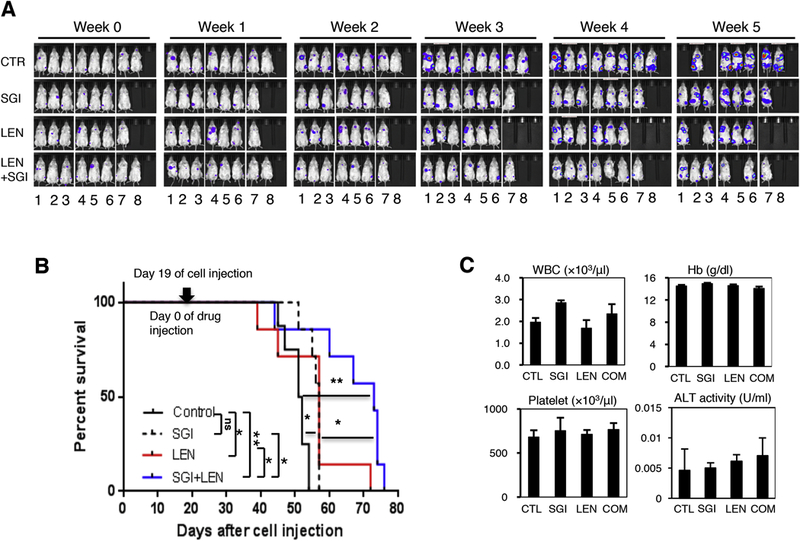
MM1R cells stably expressing luciferase (1.5×106 cells/mouse) were injected via tail vein into sublethally irradiated (1.75Gy) NSG mice. Tumor growth was followed weekly by bioluminescence imaging. At day 19 of cell injection, mice were treated (indicated as Week 0 in A) with either: DMSO, SGI1776 (35mg/kg body weight), lenalidomide (25mg/kg body weight), or the combination of SGI1776 and lenalidomide by daily oral gavage. A. Tumor growth was significantly reduced in mice treated with a combination of SGI1776 and lenalidomide. B. Combination of SGI1776 and lenalidomide significantly prolonged animal survival. C. No added hematologic or hepatic toxicities were observed in SGI1776 and lenalidomide combination treatment. Liver alanine transaminase (ALT) activity was measured by colorimetric ELISA. (ns: no statistically significant; *: p<0.05; **: p< 0.01)
3.4. Pan-PIM kinase inhibitors promote proteasome degradation of IKZF1 and IKZF3
We performed studies to understand the downstream molecular events following the treatment of pan-PIM kinase inhibitors. Specifically, we focused on the expression and the degradation of IKAROS Family Zinc Finger (IKZF) 1 and 3, because these two zincfinger transcriptional factors play a pivotal role in both the generation of plasma cells [32, 33] and the survival of myeloma cells [34, 35]. IKZF1 and IKZF3 regulate the expression of c-Myc and interferon regulatory factor 4 (IRF4). Furthermore, recent studies have demonstrated that IKZF1 and IKZF3 play an important role in IMiDs’ anti-myeloma activity [34–38]. We first examined if pan-PIM kinase inhibitors would affect the expression of IKZF1 and IKZF3 in MM cells. Treatment with pan-PIM kinase inhibitors (SGI1776 or CX5268) consistently caused down-regulation of IKZF1 and IKZF3 as well as their downstream targets, i.e., c-Myc and IRF4, in all myeloma cell lines we tested including MM1R and MM1S shown in Figure 4A (Supplementary Figure S4 for other myeloma cell lines). Additionally, the protein levels of IKZF1 and IKZF3 in tumor samples from SGI1776 treated subcutaneous xenograft mice dramatically decreased compared to the control (Figure 4B).
Figure 4. Pan-PIM kinase inhibitors promote IKZF1 and IKZF3 degradation.
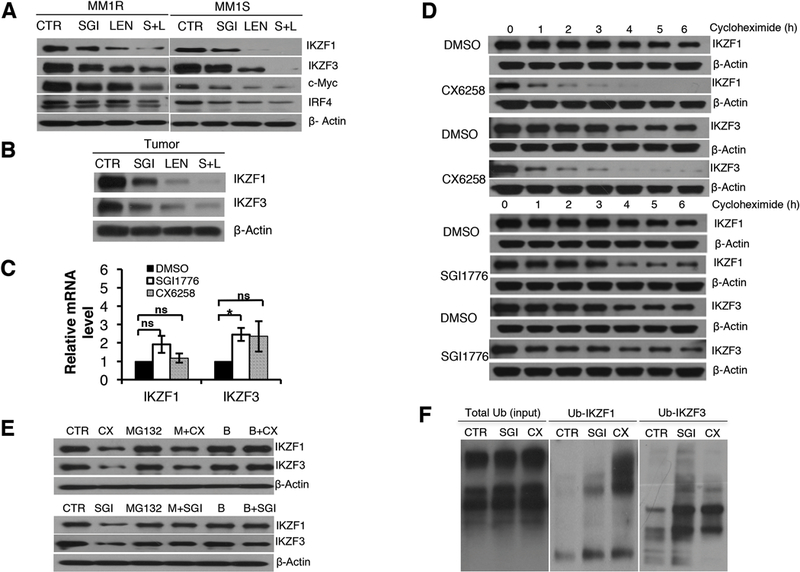
A. Pan-PIM kinase inhibitors decreased the protein levels of IKZF1, IKZF3, c-Myc and IRF4, and the combination of pan-PIM inhibitors and lenalidomide resulted in more effective down-regulation of IKZF1, IKZF3, c-Myc and IRF4. MM1S and MM1R cells were treated with DMSO, SGI1776 (7.5µM for MM1R and 5µM for MM1S), lenalidomide (L, 1µM for both MM1R and MM1S), or a combination of SGI1776 and lenalidomide. The expression of IKZF1, IKZF3, c-Myc, IRF4, and β-actin was measured by immunoblot analysis. B. Pan-PIM kinase inhibitor SGI1776 decreased the protein levels of IKZF1 and IKZF3 in vivo in xenograft tumor, and the combination of SGI1776 and lenalidomide resulted in more effective down-regulation of IKZF1 and IKZF3 in vivo. The expression of IKZF1 and IKZF3 from grinded tumor was measured by immunoblot analysis. C. Pan-PIM kinase inhibitors, SGI1776 (10µM), CX6258 (5µ), did not decrease the gene transcription of IKZF1 and IKZF3 in MM1R and MM1S cells. IKZF1 and IKZF3 mRNAs from MM cells after Pan-PIM inhibitor treatment were quantitated by Real-time PCR. D. Pan-PIM kinase inhibitors increased IKZF1 and IKZF3 protein degradation. MM1R cells were treated with CX6528 (5µM), SGI1776 (10µM), or DMSO 3h and then treated with cycloheximide (100mg/ml). Cells were collected at each hour. E. Proteasome inhibitor (MG132 and bortezomib) prevented the degradation of IKZF1 and IKZF3 by pan-PIM kinase inhibitors. MM1R cells were treated with DMSO (CTR), proteasome inhibitor MG132 (M, 1µM) or Bortezomib (B, 50nM) for 1h, followed by treatment with DMSO, CX6528 (5µM) or SGI1776 (10µM) for additional 6h. F. Pan-PIM inhibitors increased the ubiquitination of IKZF1 and IKZF3. MM1R cells were treated with DMSO (CTR), 10µM SGI1776 (SGI) or 5µM CX6258 (CX) for 16 hours. Total ubiquitinated proteins were captured using immunoprecipitation and then the elute was immunoblotted for ubiquitinated IKZF1 and IKZF3. (ns: no statistically significant; *: p<0.05)
We hypothesized that combining pan-PIM kinase inhibitors and lenalidomide would lead to more effective down-regulation of IKZF1 and IKZF3. Indeed, as shown in Figure 4A this combination resulted in further decreased protein level of IKZF1 and IKZF3 as well as their downstream targets c-Myc and IRF4 in vitro in human MM cell lines. This augmented downregulation of IKZF1 and IKZF3 with the combination treatment was also observed in vivo in tumors from mice implanted with subcutaneous xenografts of MM cells (Figure 4B).
To determine the mechanism by which PIM-kinase inhibition decreases IKZF1 and IKZF3 protein levels, we first determined if pan-PIM kinase inhibitors affected gene transcription of IKZF1 and IKZF3. MM cell lines were treated with SGI1776 or CX6258 for 16hrs and harvested for RNA. Real-time PCR did not show decrease in IKZF1 and IKZF3 mRNA levels after pan-PIM kinase inhibitor treatment (Figure 4C and Supplemental Figure S5). In fact, IKZF1 and IKZF3 mRNA levels may increase with pan-PIM kinase inhibitor treatment. This increase in IKZF1 and IKZF3 mRNA level could be due to the compensatory feedback from the down-regulation of IKZF1 and IKZF3 protein. These data suggested that pan-PIM kinase inhibitors down-regulate IKZF1 and IKZF3 expression at the post-transcription level.
We then set out to determine if inhibition of PIM kinases increased the rate of IKZF1 and IKZF3 degradation. To this end, we treated MM1R cells with DMSO, SGI1776, or CX6258 for 3hrs, and then added cycloheximide to inhibit new protein synthesis. Cells were harvested every hour for 6 hours post cycloheximide treatment, and IKZF1 and IKZF3 protein levels were measured by immunoblot. As shown in Figure 4D, treatment with the pan-PIM kinase inhibitors SGI1776 or CX6258 significantly increased the protein degradation of IKZF1 and IKZF3.
We further examined if this decrerase in IKZF1 and IKZF3 protein with PIM kinase inhibition was dependant on proteasome mediated degradation by treating MM1R cells with a PIM inhibitor, a proteasome inhibitors (either MG132 or bortezomib) or the combination of a PIM inhibitor and a proteasome inhibitor. MG132 and bortezomib protected IKZF1 and IKZF3 from pan-PIM kinase inhibitor-induced degradation (Figure 4E), suggesting that pan-PIM kinase inhibitors increased proteasome degradation of IKZF1 and IKZF3.
Specific ubiquitinations were measured to determine the ubiquitin-proteasome cascade. As shown in Figure 4F, treatment of pan-PIM kinase inhibitors increased the ubiquitination of IKZF1 and IKZF3, further supporting that pan-PIM kinase inhibitors down-regulate IKZF1 and IKZF3 by the ubiquitin-proteasome pathway.
3.5. Increased cereblon expression is associated with pan-PIM kinase inhibitor induced down-regulation of IKZF1 and IKZF3
Cereblon (CRBN) is a substrate receptor of the E3 ubiquitin ligase complex which mediates the degradation of target proteins including IKZF1 and IKZF3 through the ubiquitin-proteasome system [39]. Importantly, CRBN is the common primary target of all IMiDs and the interaction of CRBN and IMiDs is critical for induction of apoptosis of myeloma cells [34]. Binding of IMiDs increases the affinity of CRBN to IKZF1 and IKZF3 [36–38, 40], leading to their rapid ubiquitination and subsequent degradation. We investigated whether CRBN is involved in the regulation of pan-PIM kinase inhibitor-induced IKZF1 and IKZF3 protein ubiquitination and degradation.
We found that both SGI1776 and CX6258 increased mRNA expression of CRBN in a time dependent manner (Figure 5A), and also increased CRBN protein level (Figure 5B). To determine if CRBN is required for pan-PIM kinase inhibitor-induced degradation of IKZF1 and IKZF3 and for the anti-myeloma activity of pan-PIM kinase inhibitors, we knocked down CRBN with a CRBN-specific shRNA lentivirus and then treatment with pan-PIM kinase inhibitors. CRBN-specific shRNA reduced CRBN gene expression by >80% (Figure 5C). With knockdown of CRBN, both SGI1776 and CX6258 were unable to induce down-regulation of IKZF1 and IKZF3 (Figure 5D), indicating increased CRBN expression is associated with pan-PIM inhibitors induced down-regulation of IKZF1 and IKZF3. Importantly, when CRBN was knocked down, the effects of SGI1776 and CX6258 on inducing myeloma cell apoptosis were significantly attenuated (Figure 5E), demonstrating the critical role of CRBN upregulation in the anti-myeloma activity of pan-PIM kinase inhibitor.
Figure 5. Pan-PIM kinase inhibitors up-regulate CRBN and promote the degradation of IKZF1 and IKZF3.
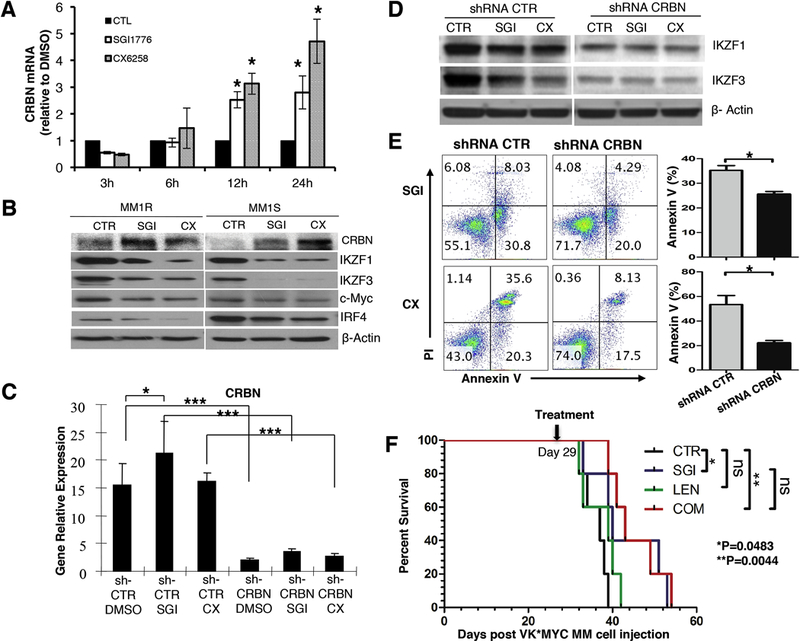
A. SGI176 (10µM) and CX6258 (5µM) upregulated CRBN mRNA expression. B. SGI176 (10µM) and CX6258 (5µM) increased CRBN protein expression level and down-regulated protein expression of IKZF1, IKZF3, c-Myc and IRF4 measured at 24h after treatment. C. MM1R cells were transduced with lentiviral vector encoding control shRNA (shCTR) or CRBN-specific shRNA (shCRBN) lentivirus for 48h and CRBN mRNA expression was measured by real-time PCR. D. IKZF1 and IKZF3 protein expression was measured after sh CRBN or sh CTR lentivirus infections. E. Knockdown of CRBN significantly reduced the anti-myeloma effects of pan-PIM kinase inhibitors. MM1R cells were transduced with lentiviral vector encoding control shRNA (shCTR) or CRBN-specific shRNA (shCRBN) lentivirus for 48h and treated with SGI1776 (10µM) and CX6258 (5µM). Annexin V+ cells were measured by flow cytometry. Percentages of Annexin V+ cells from 3 separate sets of experiments were shown (Mean ± SEM). F. The survival of the mice treated with SGI1776 and lenalidomide combination was almost the same as that of the mice treated with SGI1776 alone. At day 29 after cell injection, transplanted VK*MYC myeloma mice were treated with either: DMSO, SGI1776 alone, lenalidomide alone, or the combination of SGI1776 and lenalidomide. (ns: no statistically significant; *: p<0.05; **: p< 0.01; ***:p<0.001)
Due to a single amino acid change in murine CRBN (isoleucine instead of valine at position 391), lenalidomide does not bind to murine CRBN to induce IKZF1/IKZF3 degradation[38, 41]. To further determine the role of CRBN-IKZF1/3 pathway in the anti-myeloma activity of pan-PIM kinase inhibitor and the synergy between pan-PIM kinase inhibitor and lenalidomide, we treated transplanted VK*MYC myeloma mice with either: DMSO, SGI1776 alone, lenalidomide alone, or the combination of SGI1776 and lenalidomide. As expected, lenalidomide is ineffective in VK*MYC myeloma mice (Figure 5F). Moreover, the survival of the mice treated with the combination was almost the same as that of the mice treated with SGI1776 alone (Figure 5F). This finding suggests that pan-PIM kinase inhibitors enhance lenalidomide’s anti-myeloma activity via the cereblon-IKZF1/3 cascade and that the synergy observed is a result of PIM inhibition on this axis.
4. DISCUSSION
This study confirms the anti-myeloma effect of pan-PIM kinase inhibitors using in vivo and in vitro models. Importantly, we demonstrated that the novel combination of pan-PIM kinase inhibitors and lenalidomide has a synergistic anti-myeloma effect. Furthermore, our data showed that pan-PIM kinase inhibitors increase the expression of CRBN and subsequently promote the degradation of IKZF1 and IKZF3. Our study is the first to report a synergistic anti-myeloma effect of the combination of pan-PIM kinase inhibitors and lenalidomide, and the enhanced degradation of IKZF1 and IKZF3 through the regulation of CRBN expression.
IMiDs, including thalidomide, lenalidomide and pomalidomide, are widely used for the treatment of MM, and nearly all myeloma patients will receive IMiDs at some point during their course of treatment. IMiDs bind to CRBN, leading to the recruitment and targeted ubiquitination and degradation of IKZF1 and IKZF3[34–36, 38]. However, lenalidomide has no effect on the expression of CRBN [34]. Our study indicated that the mechanism of pan-PIM kinase inhibitors on MM is different from that of lenalidomide. Specifically, our data showed that pan-PIM kinase inhibitors increase CRBN expression, providing IMiDs with more CRBN targets to bind to, and resulting in enhanced recruitment and targeted ubiquitination and degradation of IKZF1 and IKZF3. Based on our findings, CRBN is not only critical for IMiDs’ anti-myeloma activity but also plays a central role in pan-PIM kinase inhibitor-mediated IKZF1 and IKZF3 degradation and in pan-PIM kinase inhibitors’ anti-myeloma activity. In the setting of combining pan-PIM kinase inhibitor and lenalidomide, the enhanced degradation of IKZF1 and IKZF3 comes from two sources: the lenalidomide induced increased capability of CRBN for the recruitment of IKZF1 and IKZF3, and the increased CRBN levels induced by Pan-PIM inhibitors. Based on these findings we conclude that lenalidomide and pan-PIM kinase inhibitors induced down-regulation of IKZF1 and IKZF3 through complimentary, nonoverlapping mechanisms resulting in a synergistic anti-myeloma effect when used in combination (Figure 6). Additionally, we hypothesize that the addition of pan-PIM kinase inhibitors may delay development of acquired resistance to IMiDs as several studies have shown that the expression of CRBN correlates with sensitivity to IMiDs [42–44].
Figure 6. Schematic diagram of our working model.
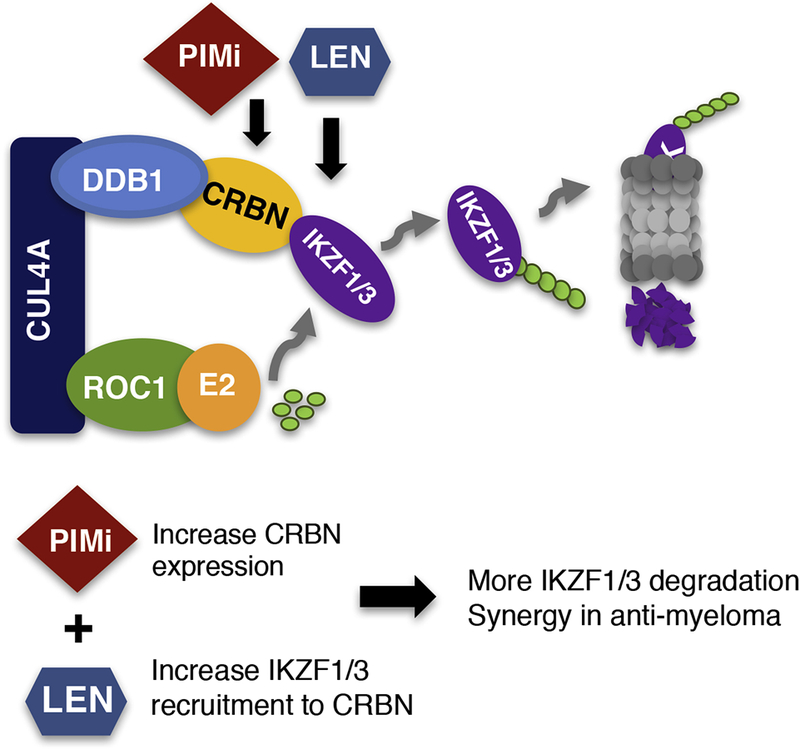
Schematic diagram showing the different, yet complimentary mechanisms of pan-PIM kinase inhibitors and lenalidomide. Lenalidomide binds directly to CRBN, promoting the recruitment of IKZF1 and IKZF3 to CRBN and subsequent degradation of IKZF1 and IKZF3. Pan-PIM kinase inhibitors increase the expression of CRBN, resulting in enhanced recruitment of IKZF1 and IKZF3 and effective anti-myeloma activity. The combination of pan-PIM kinase inhibitors with lenalidomide provide complimentary mechanisms of action for more effective degradation of IKZF1 and IKZF3 and synergy in anti-myeloma activity.
Despite many advances, management of MM is still a challenge. Our study provides justification for clinical trials combining pan-PIM inhibitors and IMiD for the treatment of MM and has important ramifications in the care of patients with MM.
Supplementary Material
HIGHLIGHTS.
Pan-PIM kinase inhibitors (SGI1776 and CX6258) exhibited significant antimyeloma activity
Combination of a pan-PIM kinase inhibitor and lenalidomide showed synergistic anti-myeloma effects without additional hematologic or hepatic toxicities
Combining pan-PIM kinase inhibitors with lenalidomide enhanced the degradation of IKZF1 and IKZF3
Elevated expression of cereblon by pan-PIM kinase inhibitors prompted the degradation of IKZF1 and IKZF3
ACKNOWLEDGEMENTS
The authors thank Dr. P. Leif Bergsagel at Mayo Clinic Arizona for providing the VK*MYC myeloma cells; Dr. David Bearss and Mr. Adam Siddiqui at Tolero Pharmaceuticals, Inc for providing SGI1776; Dr. Renate Burger at the University of Kiel for providing JK6L; Dr. Luigi Racioppi for valuable discussion, and Dr. Benny Chen and his lab members for technical support.
FUNDING
This work is supported by MUSC Hollings Cancer Center Startup Fund (to YK), Duke Cancer Institute Fund (to NC and YK), Hollings Cancer Center ACS IRG (to YK), ASCO Conquer Cancer Foundation Career Development Award (to YK), NIH 1K08HL 10378001A1 (to YK), NIH 3P30CA138313–01S3 (to YK), a research grant from Genentech Inc (to YK), NIH R44CA199767 (to YK), and NIH R01CA197792 (to YK). The project is supported in part by the National and Fujian Provincial Key Clinical Specialty Discipline Construction Program, China (to JZ and JH).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICTS OF INTEREST
The authors declare no conflict of interest.
REFERENCES:
- [1].Ludwig H, Bolejack V, Crowley J, Blade J, Miguel JS, Kyle RA, Rajkumar SV, Shimizu K, Turesson I, Westin J, Sonneveld P, Cavo M, Boccadoro M, Palumbo A, Tosi P, Harousseau JL, Attal M, Barlogie B, Stewart AK, Durie B, Survival and years of life lost in different age cohorts of patients with multiple myeloma, J Clin Oncol, 28 (2010) 1599–1605. [DOI] [PubMed] [Google Scholar]
- [2].Siegel RL, Miller KD, Jemal A, Cancer Statistics, 2017, CA Cancer J Clin, 67 (2017) 7–30. [DOI] [PubMed] [Google Scholar]
- [3].Anderson KC, Multiple myeloma: a clinical overview, Oncology (Williston Park), 25 Suppl 2 (2011) 3–9. [PubMed] [Google Scholar]
- [4].Chim CS, Kumar SK, Orlowski RZ, Cook G, Richardson PG, Gertz MA, Giralt S, Mateos MV, Leleu X, Anderson KC, Management of relapsed and refractory multiple myeloma: novel agents, antibodies, immunotherapies and beyond, Leukemia, (2017). [DOI] [PMC free article] [PubMed]
- [5].Brault L, Gasser C, Bracher F, Huber K, Knapp S, Schwaller J, PIM serine/threonine kinases in the pathogenesis and therapy of hematologic malignancies and solid cancers, Haematologica, 95 (2010) 1004–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Cuypers HT, Selten G, Quint W, Zijlstra M, Maandag ER, Boelens W, van Wezenbeek P, Melief C, Berns A, Murine leukemia virus-induced T-cell lymphomagenesis: integration of proviruses in a distinct chromosomal region, Cell, 37 (1984) 141–150. [DOI] [PubMed] [Google Scholar]
- [7].Chen WW, Chan DC, Donald C, Lilly MB, Kraft AS, Pim family kinases enhance tumor growth of prostate cancer cells, Mol Cancer Res, 3 (2005) 443–451. [DOI] [PubMed] [Google Scholar]
- [8].Wang Z, Bhattacharya N, Weaver M, Petersen K, Meyer M, Gapter L, Magnuson NS, Pim-1: a serine/threonine kinase with a role in cell survival, proliferation, differentiation and tumorigenesis, J Vet Sci, 2 (2001) 167–179. [PubMed] [Google Scholar]
- [9].Cohen AM, Grinblat B, Bessler H, Kristt D, Kremer A, Schwartz A, Halperin M, Shalom S, Merkel D, Don J, Increased expression of the hPim-2 gene in human chronic lymphocytic leukemia and non-Hodgkin lymphoma, Leuk Lymphoma, 45 (2004) 951955. [DOI] [PubMed] [Google Scholar]
- [10].Lin YW, Beharry ZM, Hill EG, Song JH, Wang W, Xia Z, Zhang Z, Aplan PD, Aster JC, Smith CD, Kraft AS, A small molecule inhibitor of Pim protein kinases blocks the growth of precursor T-cell lymphoblastic leukemia/lymphoma, Blood, 115 (2010) 824–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Chen LS, Redkar S, Bearss D, Wierda WG, Gandhi V, Pim kinase inhibitor, SGI-1776, induces apoptosis in chronic lymphocytic leukemia cells, Blood, 114 (2009) 4150–4157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Braso-Maristany F, Filosto S, Catchpole S, Marlow R, Quist J, Francesch-Domenech E, Plumb DA, Zakka L, Gazinska P, Liccardi G, Meier P, Gris-Oliver A, Cheang MC, Perdrix-Rosell A, Shafat M, Noel E, Patel N, McEachern K, Scaltriti M, Castel P, Noor F, Buus R, Mathew S, Watkins J, Serra V, Marra P, Grigoriadis A, Tutt AN, PIM1 kinase regulates cell death, tumor growth and chemotherapy response in triple-negative breast cancer, Nat Med, 22 (2016) 1303–1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Horiuchi D, Camarda R, Zhou AY, Yau C, Momcilovic O, Balakrishnan S, Corella AN, Eyob H, Kessenbrock K, Lawson DA, Marsh LA, Anderton BN, Rohrberg J, Kunder R, Bazarov AV, Yaswen P, McManus MT, Rugo HS, Werb Z, Goga A, PIM1 kinase inhibition as a targeted therapy against triple-negative breast tumors with elevated MYC expression, Nat Med, 22 (2016) 1321–1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Asano J, Nakano A, Oda A, Amou H, Hiasa M, Takeuchi K, Miki H, Nakamura S, Harada T, Fujii S, Kagawa K, Endo I, Yata K, Sakai A, Ozaki S, Matsumoto T, Abe M, The serine/threonine kinase Pim-2 is a novel anti-apoptotic mediator in myeloma cells, Leukemia, 25 (2011) 1182–1188. [DOI] [PubMed] [Google Scholar]
- [15].Nair JR, Caserta J, Belko K, Howell T, Fetterly G, Baldino C, Lee KP, Novel inhibition of PIM2 kinase has significant anti-tumor efficacy in multiple myeloma, Leukemia, 31 (2017) 1715–1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Ishikawa C, Senba M, Hashimoto T, Imaizumi A, Mori N, Expression and significance of Pim-3 kinase in adult T-cell leukemia, Eur J Haematol, 99 (2017) 495504. [DOI] [PubMed] [Google Scholar]
- [17].Cheng H, Huang C, Xu X, Hu X, Gong S, Tang G, Song X, Zhang W, Wang J, Chen L, Yang J, PIM-1 mRNA expression is a potential prognostic biomarker in acute myeloid leukemia, J Transl Med, 15 (2017) 179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Lu J, Zavorotinskaya T, Dai Y, Niu XH, Castillo J, Sim J, Yu J, Wang Y, Langowski JL, Holash J, Shannon K, Garcia PD, Pim2 is required for maintaining multiple myeloma cell growth through modulating TSC2 phosphorylation, Blood, 122 (2013) 1610–1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Ramachandran J, Santo L, Siu KT, Panaroni C, Raje N, Pim2 is important for regulating DNA damage response in multiple myeloma cells, Blood cancer journal, 6 (2016) e462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Paino T, Garcia-Gomez A, Gonzalez-Mendez L, San-Segundo L, Hernandez-Garcia S, Lopez-Iglesias AA, Algarin EM, Martin-Sanchez M, Corbacho D, Ortizde-Solorzano C, Corchete LA, Gutierrez NC, Maetos MV, Garayoa M, Ocio EM, The Novel Pan-PIM Kinase Inhibitor, PIM447, Displays Dual Antimyeloma and Bone-Protective Effects, and Potently Synergizes with Current Standards of Care, Clin Cancer Res, 23 (2017) 225–238. [DOI] [PubMed] [Google Scholar]
- [21].Hiasa M, Teramachi J, Oda A, Amachi R, Harada T, Nakamura S, Miki H, Fujii S, Kagawa K, Watanabe K, Endo I, Kuroda Y, Yoneda T, Tsuji D, Nakao M, Tanaka E, Hamada K, Sano S, Itoh K, Matsumoto T, Abe M, Pim-2 kinase is an important target of treatment for tumor progression and bone loss in myeloma, Leukemia, 29 (2015) 207–217. [DOI] [PubMed] [Google Scholar]
- [22].Raab M, Ocio EM, Thomas SK, Gunther A, Lebovic D, Kumar SK, Jakubowiak AJ, Song D, Xiang F, Hynds D, Vanasse KG, Goh YT, Phase 1 Study Of The Novel Pan-Pim Kinase Inhibitor LGH447 In Patients With Relapsed/ Refractory Multiple Myeloma, Blood (ASH 2013 Annual Meeting Abstract), (2013) 3186.
- [23].Kummetha Venkata J, An N, Stuart R, Costa LJ, Cai H, Coker W, Song JH, Gibbs K, Matson T, Garrett-Mayer E, Wan Z, Ogretmen B, Smith C, Kang Y, Inhibition of sphingosine kinase 2 down-regulates the expression of c-Myc and Mcl-1 and induces apoptosis in multiple myeloma, Blood, 124 (2014) 1915–1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Song JH, Kraft AS, Pim kinase inhibitors sensitize prostate cancer cells to apoptosis triggered by Bcl-2 family inhibitor ABT-737, Cancer research, 72 (2012) 294–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Cooke RE, Gherardin NA, Harrison SJ, Quach H, Godfrey DI, Prince M, Koldej R, Ritchie DS, Spontaneous onset and transplant models of the Vk*MYC mouse show immunological sequelae comparable to human multiple myeloma, Journal of translational medicine, 14 (2016) 259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Guillerey C, Harjunpaa H, Carrie N, Kassem S, Teo T, Miles K, Krumeich S, Weulersse M, Cuisinier M, Stannard K, Yu Y, Minnie SA, Hill GR, Dougall WC, Avet-Loiseau H, Teng MWL, Nakamura K, Martinet L, Smyth MJ, TIGIT immune checkpoint blockade restores CD8(+) T cell immunity against multiple myeloma, Blood, (2018). [DOI] [PubMed]
- [27].Chesi M, Robbiani DF, Sebag M, Chng WJ, Affer M, Tiedemann R, Valdez R, Palmer SE, Haas SS, Stewart AK, Fonseca R, Kremer R, Cattoretti G, Bergsagel PL, AID-dependent activation of a MYC transgene induces multiple myeloma in a conditional mouse model of post-germinal center malignancies, Cancer Cell, 13 (2008) 167–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Chesi M, Matthews GM, Garbitt VM, Palmer SE, Shortt J, Lefebure M, Stewart AK, Johnstone RW, Bergsagel PL, Drug response in a genetically engineered mouse model of multiple myeloma is predictive of clinical efficacy, Blood, 120 (2012) 376–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Haddach M, Michaux J, Schwaebe MK, Pierre F, O’Brien SE, Borsan C, Tran J, Raffaele N, Ravula S, Drygin D, Siddiqui-Jain A, Darjania L, Stansfield R, Proffitt C, Macalino D, Streiner N, Bliesath J, Omori M, Whitten JP, Anderes K, Rice WG, Ryckman DM, Discovery of CX-6258. A Potent, Selective, and Orally Efficacious pan-Pim Kinases Inhibitor, ACS medicinal chemistry letters, 3 (2012) 135–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Matthews GM, Lefebure M, Doyle MA, Shortt J, Ellul J, Chesi M, Banks KM, Vidacs E, Faulkner D, Atadja P, Bergsagel PL, Johnstone RW, Preclinical screening of histone deacetylase inhibitors combined with ABT-737, rhTRAIL/MD5–1 or 5azacytidine using syngeneic Vk*MYC multiple myeloma, Cell Death Dis, 4 (2013) e798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Cervantes-Gomez F, Chen LS, Orlowski RZ, Gandhi V, Biological effects of the Pim kinase inhibitor, SGI-1776, in multiple myeloma, Clinical lymphoma, myeloma & leukemia, 13 Suppl 2 (2013) S317–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Morgan B, Sun L, Avitahl N, Andrikopoulos K, Ikeda T, Gonzales E, Wu P, Neben S, Georgopoulos K, Aiolos, a lymphoid restricted transcription factor that interacts with Ikaros to regulate lymphocyte differentiation, Embo j, 16 (1997) 2004–2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Cortes M, Georgopoulos K, Aiolos is required for the generation of high affinity bone marrow plasma cells responsible for long-term immunity, J Exp Med, 199 (2004) 209–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Kronke J, Udeshi ND, Narla A, Grauman P, Hurst SN, McConkey M, Svinkina T, Heckl D, Comer E, Li X, Ciarlo C, Hartman E, Munshi N, Schenone M, Schreiber SL, Carr SA, Ebert BL, Lenalidomide causes selective degradation of IKZF1 and IKZF3 in multiple myeloma cells, Science, 343 (2014) 301–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Lu G, Middleton RE, Sun H, Naniong M, Ott CJ, Mitsiades CS, Wong KK, Bradner JE, Kaelin WG Jr., The myeloma drug lenalidomide promotes the cereblondependent destruction of Ikaros proteins, Science, 343 (2014) 305–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Ito T, Ando H, Suzuki T, Ogura T, Hotta K, Imamura Y, Yamaguchi Y, Handa H, Identification of a primary target of thalidomide teratogenicity, Science, 327 (2010) 1345–1350. [DOI] [PubMed] [Google Scholar]
- [37].Fischer ES, Bohm K, Lydeard JR, Yang H, Stadler MB, Cavadini S, Nagel J, Serluca F, Acker V, Lingaraju GM, Tichkule RB, Schebesta M, Forrester WC, Schirle M, Hassiepen U, Ottl J, Hild M, Beckwith RE, Harper JW, Jenkins JL, Thoma NH, Structure of the DDB1-CRBN E3 ubiquitin ligase in complex with thalidomide, Nature, 512 (2014) 49–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Chamberlain PP, Lopez-Girona A, Miller K, Carmel G, Pagarigan B, Chie-Leon B, Rychak E, Corral LG, Ren YJ, Wang M, Riley M, Delker SL, Ito T, Ando H, Mori T, Hirano Y, Handa H, Hakoshima T, Daniel TO, Cathers BE, Structure of the human Cereblon-DDB1-lenalidomide complex reveals basis for responsiveness to thalidomide analogs, Nature structural & molecular biology, 21 (2014) 803–809. [DOI] [PubMed] [Google Scholar]
- [39].Watkin LB, Jessen B, Wiszniewski W, Vece TJ, Jan M, Sha Y, Thamsen M, Santos-Cortez RL, Lee K, Gambin T, Forbes LR, Law CS, Stray-Pedersen A, Cheng MH, Mace EM, Anderson MS, Liu D, Tang LF, Nicholas SK, Nahmod K, Makedonas G, Canter DL, Kwok PY, Hicks J, Jones KD, Penney S, Jhangiani SN, Rosenblum MD, Dell SD, Waterfield MR, Papa FR, Muzny DM, Zaitlen N, Leal SM, Gonzaga-Jauregui C, Boerwinkle E, Eissa NT, Gibbs RA, Lupski JR, Orange JS, Shum AK, COPA mutations impair ER-Golgi transport and cause hereditary autoimmune-mediated lung disease and arthritis, Nat Genet, 47 (2015) 654–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Gandhi AK, Kang J, Havens CG, Conklin T, Ning Y, Wu L, Ito T, Ando H, Waldman MF, Thakurta A, Klippel A, Handa H, Daniel TO, Schafer PH, Chopra R, Immunomodulatory agents lenalidomide and pomalidomide co-stimulate T cells by inducing degradation of T cell repressors Ikaros and Aiolos via modulation of the E3 ubiquitin ligase complex CRL4(CRBN.), Br J Haematol, 164 (2014) 811–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Serdar Dilan, Köpff Simon, Scheffold Annika, Kronke J, Generation of a Lenalidomide-Sensitive Mouse Model for Multiple Myeloma By Expression of the Crbn I391V Mutant in Mopc-315.BM Cells, Blood, 130 (2017) 3071. [Google Scholar]
- [42].Heintel D, Rocci A, Ludwig H, Bolomsky A, Caltagirone S, Schreder M, Pfeifer S, Gisslinger H, Zojer N, Jager U, Palumbo A, High expression of cereblon (CRBN) is associated with improved clinical response in patients with multiple myeloma treated with lenalidomide and dexamethasone, Br J Haematol, 161 (2013) 695–700. [DOI] [PubMed] [Google Scholar]
- [43].Broyl A, Kuiper R, van Duin M, van der Holt B, el Jarari L, Bertsch U, Zweegman S, Buijs A, Hose D, Lokhorst HM, Goldschmidt H, Sonneveld P, High cereblon expression is associated with better survival in patients with newly diagnosed multiple myeloma treated with thalidomide maintenance, Blood, 121 (2013) 624–627. [DOI] [PubMed] [Google Scholar]
- [44].Zhu YX, Braggio E, Shi CX, Kortuem KM, Bruins LA, Schmidt JE, Chang XB, Langlais P, Luo M, Jedlowski P, LaPlant B, Laumann K, Fonseca R, Bergsagel PL, Mikhael J, Lacy M, Champion MD, Stewart AK, Identification of cereblon-binding proteins and relationship with response and survival after IMiDs in multiple myeloma, Blood, 124 (2014) 536–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


