Abstract
Background
Little is known about respiratory tract infection (RTI) severity in children following consultation.
Objectives
To investigate post-consultation symptom trajectories in children with acute cough and RTI and whether baseline characteristics predict trajectory group.
Methods
Prospective cohort study of 2296 children (3 months–16 years) whose parents were invited to report cough severity and duration using a 7-point Likert scale. Longitudinal latent class analysis (LLCA) was used to identify post-consultation symptom trajectories in the first 15 days, and multinomial models to predict class membership.
Results
Complete data were available for 1408 children (61%). The best LLCA model identified five post-consultation symptom trajectory groups: ‘very rapid recovery’ (28.5%), ‘rapid recovery’ (37.7%), ‘intermediate recovery’ (18.2%), ‘persistent symptoms’ (9.5%) and ‘initial deterioration with persistent symptoms’ (6.0%). Compared with very rapid recovery, parent-reported severe cough in the 24 hours prior to consultation increased the likelihood of rapid recovery (OR 1.79 [95% CI 1.23, 2.60]), intermediate recovery (OR 2.13 [1.38, 3.30] and initial deterioration with persistent symptoms (OR 2.29 [1.26, 4.16]). Initial deterioration was also associated with ‘severe barking cough’ (OR 3.64 [1.50, 8.82]), ‘severely reduced energy in the 24 hours prior to consultation’ (OR 3.80 [1.62, 8.87] and higher parent-assessed illness severity at consultation (OR 2.21 [1.17, 4.18]).
Conclusion
We identified five distinct symptom trajectory groups showing the majority of children improved post-consultation, with only one group experiencing illness deterioration. The few characteristics associated with group membership did not fall into a pattern that seemed clinically useful.
Keywords: Anti-bacterial agents, child, decision making, general practice, prognosis, respiratory tract infections
Introduction
Respiratory tract infections (RTI) are frequent in children and a common reason for primary care consultations (1). They are mostly self-limiting with low risks of complications, but represent the major indication for antibiotics prescribed for children internationally (2–4), and this despite antimicrobial resistance being high on public health agendas (5,6).
Systematic reviews have demonstrated that diagnostic and prognostic uncertainty, and poor clinician–parent communication are major drivers of antibiotic prescribing for children (7–9), suggesting that managing prognostic uncertainty and improving communication could reduce antibiotic use. Indeed, an internet-based training program for family physicians providing information on symptoms and the natural history of infections was found to be effective in reducing antibiotic prescribing for RTI in adults (10). Interventions are also more effective when focused on specific symptoms (rather than generic messages about antibiotic overuse and resistance) (11) and when providing information about the natural history of RTIs (12). The National Institute for Health and Care Excellence recommends that delayed antibiotic prescribing should include advice only to use antibiotics if symptoms worsen or do not settle in accordance with the expected course of the illness (13).
While there is good evidence regarding overall RTI symptom duration in children (14), we are not aware of any research investigating post-consultation symptom trajectories—and specifically whether children’s symptoms worsen before improving, how commonly this occurs, or whether these children can be identified at the baseline consultation.
The aims of this study were to investigate (i) whether modelling prospective symptom severity and duration data in children presenting to primary care with acute RTI could identify distinct and clinically useful post-consultation symptom trajectories, and (ii) whether baseline characteristics can be used to predict trajectory group membership. In addition, we sought to describe the change in symptom severity the day after the consultation.
Methods
Participants
Participants were part of the ‘TARGET’ multi-centre prospective cohort study of children aged 3 months to under 16 years presenting to general practice with acute cough and RTI between July 2011 and May 2013. The study design (15) and main results (16) have been reported in detail elsewhere, but briefly, eligible children were recruited by prescribing ‘clinicians’ (family physicians and nurse practitioners) across four centres when they presented with an acute cough as the most prominent symptom, combined with other symptoms or signs suggestive of RTI. Here, we report a secondary analysis based on data from symptom diaries that the parents of children (n = 2296) recruited to the lead centre only (Bristol, UK) were asked to provide.
Symptom diary
Parents were invited to complete a symptom diary, the design of which was based on a previously validated diary (17) and successfully used in a previous study (18). The presence of six symptoms (cough, shortness of breath, impaired sleep, being unwell, reduced level of activity and temperature) was recorded once daily in the diary (Supplementary Material S1) for the first 28 days post-consultation, or until all symptoms were scored ‘normal’ for two consecutive days, whichever was soonest. Symptom severity was scored from zero (normal) to six (as bad as it could be). Day of recruitment was defined as ‘day 1’.
For the current analysis, we selected only the symptom ‘cough’ as it was the only diary item also required for study inclusion (and therefore the most prevalent symptom), and we considered it most representative of the overall illness.
Parents could complete the symptom diary either online or on a paper version. Online data entry had the advantage that validation rules were applied to prevent parents entering invalid data. Study team administrators telephoned parents up to three times to support diary completion during the first 7 days and then at least once a week until day 28 if no data had been obtained that week. On symptom diary completion, parents received a £5 high street voucher and a thank you letter. All diaries were checked for accuracy and completion, and two attempts were made to contact parents to clarify any data queries.
Baseline and follow-up variables
At the recruitment (baseline) consultation, clinicians completed a baseline case report form (Supplementary Material S2), which included 8 sociodemographic and 4 past medical history items, 24 parent-reported symptoms (including symptom severity; mild, moderate or severe in the 24 hours prior to consultation), 14 physical examination signs, and whether antibiotics were prescribed (immediate or delayed). Children’s medical records were checked for chronic illness and RTIs in the 12 months prior to recruitment. Variables were excluded from analysis if they were rare in this population (present in <50 individuals). Symptom severity (mild/moderate/severe) was dichotomized between severe and other categories if more than 5% had severe symptoms, or between moderate/severe and mild if 5% or less had severe symptoms.
Statistical analyses
First, characteristics of children with and without symptom diaries were compared using the chi-square test for categorical variables and unpaired t-test for continuous variables. Subsequently, two sets of analyses were performed: (i) an analysis of initial change and (ii) a longitudinal trajectory model that utilized repeated symptom data. All analyses were carried out using Stata/MP 14.1 for Windows (StataCorp. 2015. Stata Statistical Software: Release 14. College Station, TX: StataCorp LP) with the exception of the derivation of the trajectory model that employed Mplus version 7.4 (19).
Analysis of initial change
For the analyses of initial change in symptom severity, we compared the severity of ‘cough’ reported in the symptom diary on the day of recruitment (day 1) and the severity reported on day 2. We accepted a difference of one point reflecting real change as we considered this to adequately discriminate severity on adjacent days. Associations in univariable analyses were tested by chi-square test.
Latent class models for longitudinal patterns of change
Longitudinal latent class analysis (LLCA) was used to derive distinct trajectories of cough (20,21). LLCA assumes that variability in response is due to a latent (unobserved) grouping. Starting with a single class, additional classes were added incrementally until the resulting model was deemed acceptable based on a range of statistical and substantive criteria. The resulting latent grouping was then used as the dependent variable in a set of multinomial regression models to study potential risk factors for class membership. For these analyses, we assigned each child to the class for which their assignment probabilities were greatest. Simulation studies indicate that such an approach may downwardly bias results when entropy (class separation) is low; however, in this instance entropy was 0.9, which is above the currently recommended guidelines for using this ‘standard three-step’ approach.
Two restrictions were made to maintain a reasonable ratio of sample size to number of parameters. First, we restricted our data analyses to the first 2 weeks post-consultation (when we anticipated most of the symptom severity change to occur), giving 15 days’ repeated data. Second, the seven-category ordinal responses were collapsed into three-category variables indicating increasing severity: ‘normal’, ‘very little problem’ and ‘slight problem’ were combined and coded 0, ‘moderately bad’ and ‘bad’ were coded 1, and ‘very bad’ and ‘as bad as can be’ coded 2.
Preliminary results for the LLCA utilizing 15 consecutive days of data showed that the model was unable to capture the strong association between adjacent measures, even when many classes were included. This problem was most marked at the start and end of the 2-week timespan—that is, when most respondents are consistently ill or consistently well from one day to the next. To address this, we reduced the number of measures by taking only alternate days (days 1, 3, etc.) giving 8 days in all, resulting in no dramatic changes in either the class sizes or within-class patterns of change (Supplementary Material S3, LLCA models—model fit and comparisons).
Missing data
Parents were asked to record symptoms until they scored ‘normal’ on all symptoms for two consecutive days. As a consequence, there were missing values (by design) for all days after this. We inserted the value 0, ‘normal’, for all days following two consecutive days of ‘normal’. In addition, a small amount of sporadic missingness was retained as the trajectory modelling can incorporate partially incomplete data using maximum likelihood (ML) estimation. As with multiple imputations, ML-based estimation is based on the missing at random assumption—namely, that conditional on the data in the model, there are no systematic differences between respondents and nonrespondents with reference to the variables with missing data.
Results
Baseline characteristics and comparison of symptom diary responders and nonresponders
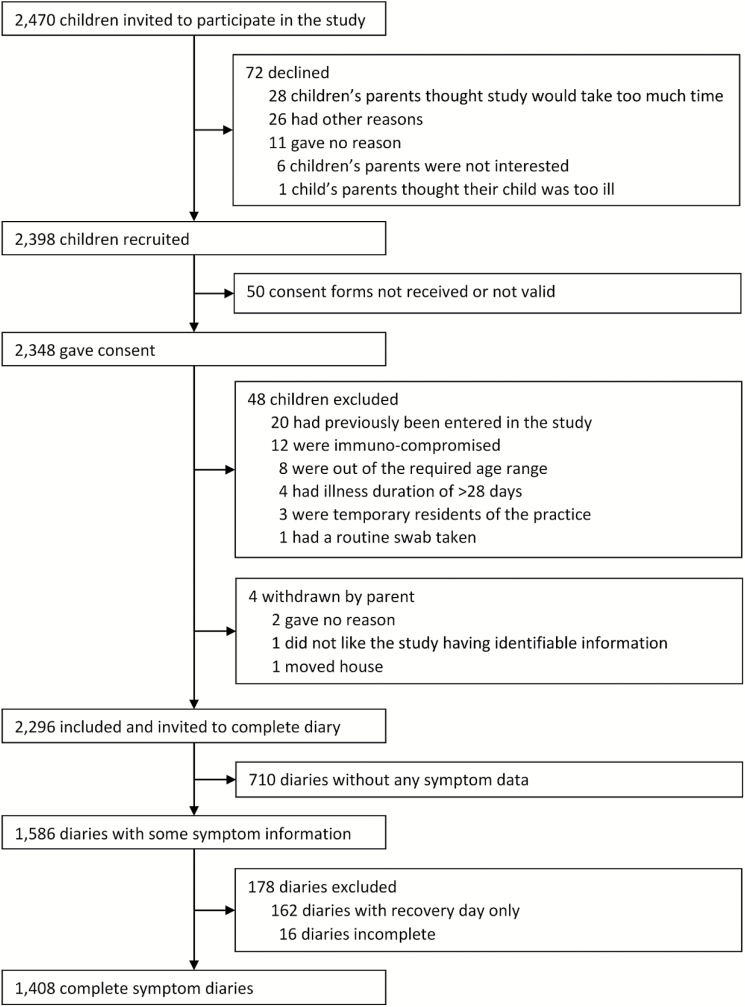
Of the 2296 children recruited to the Bristol centre, 1408 (61.3%) had complete symptom diary data [1214 (86%) paper, 194 (14%) online; Fig. 1]. Of these, 742 (53%) were male, mean age was 4.2 years, 1251 (89%) were White and the mean pre-consultation illness duration was 7.7 days (Table 1). Children with and without complete symptom diaries did not differ with respect to these baseline characteristics, parent or clinician global illness severity scores or antibiotic prescribing (Table 1). However, compared with nonresponders, responding mothers were more likely to be older, live in less deprived areas, have breastfed at 3 months and not smoke (Table 1).
Figure 1.
Flowchart showing the recruitment of children presenting to primary care with respiratory tract infection between July 2011 and May 2013 and invited to complete symptom diaries.
Table 1.
Baseline characteristics of 2296 children presenting to primary care with respiratory tract infection between July 2011 and May 2013 (Children with and without post-consultation symptom diary data are compared.)
| Children with diary data n = 1408 |
Children without diary data n = 888 |
P Value | |
|---|---|---|---|
| Child baseline characteristics | |||
| Male gender, n (%) | 742 (52.7) | 446 (50.2) | 0.248a |
| Mean age, year | 4.1 | 4.2 | 0.772b |
| Ethnicity, White, n (%) | 1251 (89.2) | 767 (86.6) | 0.061a |
| Chronic illness, n (%) | 271 (19.3) | 167 (18.8) | 0.803a |
| Current asthma, n (%) | 147 (10.4) | 86 (9.7) | 0.559a |
| Previous asthma, n (%) | 67 (4.8) | 34 (3.8) | 0.290a |
| Diabetes, n (%) | 2 (0.1) | 1 (0.1) | 0.849a |
| Pre-consultation illness duration, mean days | 7.7 | 7.4 | 0.176b |
| Global illness severity score clinician (range 0–10), mean | 2.9 | 2.9 | 0.861b |
| Global illness severity score carer (range 0–10), mean | 5.3 | 5.3 | 0.460b |
| Family/background data | |||
| Mother’s mean age, year | 35.0 | 32.4 | <0.001b |
| Number of children in home (mean) | 1.9 | 2.1 | <0.001b |
| Only child, n (%) | 501 (35.7) | 289 (32.8) | 0.150a |
| Breastfed at 3 months, n (%) | 676 (51.3) | 325 (40.5) | <0.001a |
| Mother smoker, n (%) | 217 (15.6) | 249 (28.9) | <0.001a |
| Index of multiple deprivation, mean | 20.7 | 24.8 | <0.001b |
| Living in 20% most deprived areas, n (%) | 223 (15.9) | 238 (27.1) | <0.001a |
| Clinical management | |||
| Antibiotics prescribed, n (%) | 568 (40.3) | 366 (41.2) | 0.678a |
| Immediate, n (%) | 446 (31.7) | 299 (33.7) | 0.457a |
| Delayed, n (%) | 122 (8.7) | 67 (7.6) | |
aPearson’s chi-square test.
bUnpaired t-test.
Analysis of initial change
There were missing diary data on severity of cough for the day of recruitment in 21 children, and they were excluded for the analyses on initial change in severity.
On the day after consultation (day 2), the vast majority of parents reported children had the same (61%) or improving (31%) cough severity, with 7.5% reporting a deterioration (Table 2). Younger age (<2 years) was associated with worsening cough severity from day 1 to day 2, whereas moderate-to-severe shortness of breath in the 24 hours prior to consultation, severely disturbed sleep in the 24 hours prior to consultation, or inter-/subcostal recession observed by clinician were associated with improved cough severity (Supplementary Table S1, univariable analyses of change in cough severity).
Table 2.
Changeain cough severity from day 1 (recruitment) until day 2 in 1385 children presenting to primary care with respiratory tract infection between July 2011 and May 2013
| Cough severity on day 1 (recruitment) | Number of children | Cough severity on day 2 | |||||
|---|---|---|---|---|---|---|---|
| Improved | Same | Worse | |||||
| n | n | % | n | % | n | % | |
| Normal | 9 | NA | 7 | 77.8 | 2 | 22.2 | |
| Very little problem | 23 | 2 | 8.7 | 19 | 82.6 | 2 | 8.7 |
| Slight problem | 138 | 27 | 19.6 | 90 | 65.2 | 21 | 15.2 |
| Moderately bad | 389 | 101 | 26.0 | 245 | 63.0 | 43 | 11.1 |
| Bad | 460 | 151 | 32.8 | 280 | 60.1 | 29 | 6.3 |
| Very bad | 290 | 112 | 38.6 | 171 | 59.0 | 7 | 2.4 |
| As bad as can be | 76 | 37 | 48.7 | 39 | 51.3 | NA | |
| Total | 1385b | 430 | 31.0 | 851 | 61.4 | 104 | 7.5 |
NA, not applicable, impossible change.
a‘Improved’ and ‘worse’ are defined by change of ≥1 point on a 7-point Likert scale.
bNumber of children <1408 due to missing data.
Analyses of longitudinal patterns of change
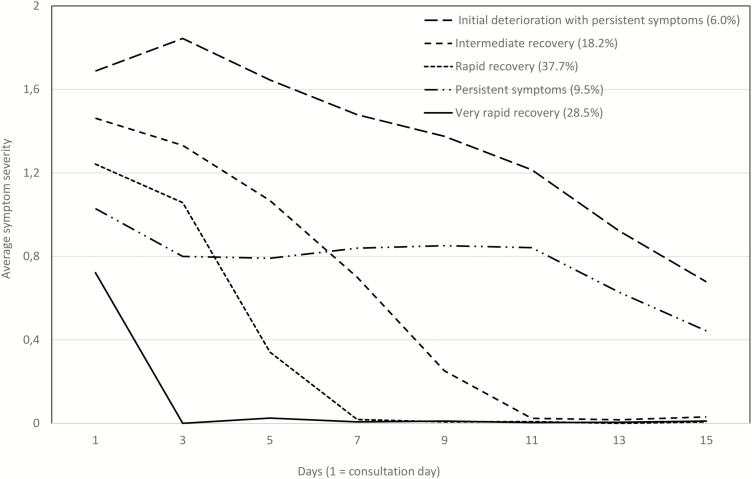
Five post-consultation symptom trajectories were identified (Fig. 2), showing distinct patterns of cough severity and duration, which varied with regard to (i) initial symptom severity, (ii) severity change immediately following consultation, and (iii) symptom duration. ‘Very rapid recovery’ (to which 28.5% of children belonged) had the lowest initial symptom severity (0.8/2) and had reached average severity scores of zero by day 3. ‘Rapid recovery’ (37.7%) and ‘intermediate recovery’ (18.2%) had slightly higher initial severities (1.2/2 and 1.5/2), with longer recovery times—7 and 11 days respectively. ‘Persistent symptoms’ (9.5%) started with moderate severity and showed little change for 10 days. Finally, ‘initial deterioration with persistent symptoms’ [the group to which the smallest percentage (6.0%) belonged] had both the highest severity starting point and the slowest recovery rate.
Figure 2.
Post-consultation symptom trajectories derived by longitudinal latent class analysis in 1408 children presenting to primary care with respiratory tract infection between July 2011 and May 2013 (percentage of children assigned to each class). Symptom severity scores have been collapsed from range 0–6.
Multinomial modelling
A total of 33 explanatory variables were investigated (Table 3). Children whose parents reported severe cough in the 24 hours prior to consultation were more likely to be assigned to trajectory groups with more severe and longer lasting cough in a ranked manner, but this pattern was not observed when differentiating between different types of cough (dry, productive, barking). Assignment to the initial deterioration with persistent symptoms group was associated with severe barking cough and severely reduced energy in the 24 hours prior to consultation, along with parental assessment of illness severity in the top quartile at the consultation. Children assessed by the clinician to be in the top quartile were more likely to be in the intermediate recovery group. Also, moderate-to-severe wheeze in the 24 hours prior to consultation, and fever, pallor or crackles observed by the clinician were associated with trajectories with more severe and longer lasting cough than very rapid recovery. However, there was no systematic assignment to one or more trajectory groups.
Table 3.
Potential risk factors for post-consultation symptom trajectory group membership of 1408 children presenting to primary care between July 2011 and May 2013 with respiratory tract infection (Estimates are odds ratios derived from a multinomial logistic regression model.)
| Variable | Reference group | ‘Exposed’ group | Very rapid recovery | Rapid recovery | Intermediate recovery | Persistent symptoms | Initial deterioration | P Value |
|---|---|---|---|---|---|---|---|---|
| OR | OR [95% CI] | OR [95% CI] | OR [95% CI] | OR [95% CI] | ||||
| Age | 2–15 years | 0–1 year | 1.00 ref | 0.84 [0.60, 1.17] | 0.85 [0.56, 1.29] | 0.86 [0.52, 1.44] | 0.70 [0.36, 1.36] | 0.775 |
| Gender | Female | Male | 1.00 ref | 0.95 [0.74, 1.23] | 0.96 [0.70, 1.32] | 0.89 [0.60, 1.31] | 0.93 [0.58, 1.49] | 0.982 |
| Ethnicity | White | Non-White | 1.00 ref | 0.96 [0.63, 1.46] | 1.01 [0.60, 1.68] | 0.97 [0.51, 1.83] | 1.40 [0.70, 2.79] | 0.870 |
| Mother smoking | No | Yes | 1.00 ref | 1.04 [0.73, 1.48] | 0.91 [0.58, 1.44] | 0.87 [0.49, 1.53] | 1.57 [0.87, 2.84] | 0.482 |
| Living in 20% most deprived areas | No | Yes | 1.00 ref | 0.70 [0.49, 1.00] | 1.05 [0.69, 1.59] | 0.73 [0.42, 1.28] | 1.38 [0.78, 2.45] | 0.063 |
| No of consultations in the last year | 0–4 consultations | ≥5 consultations | 1.00 ref | 0.95 [0.58, 1.56] | 0.88 [0.47, 1.65] | 1.33 [0.67, 2.64] | 1.89 [0.91, 3.95] | 0.287 |
| Current asthma | No | Yes | 1.00 ref | 1.30 [0.83, 2.03] | 1.29 [0.75, 2.23] | 2.08 [1.16, 3.73] | 1.71 [0.83, 3.54] | 0.149 |
| Previous asthma | No | Yes | 1.00 ref | 1.23 [0.64, 2.37] | 1.12 [0.49, 2.53] | 2.08 [0.91, 4.75] | 2.38 [0.94, 6.02] | 0.213 |
| Pre-consultation illness duration | >3 days | ≤3 days | 1.00 ref | 1.08 [0.81, 1.45] | 0.79 [0.54, 1.15] | 0.91 [0.58, 1.43] | 1.07 [0.63, 1.82] | 0.505 |
| Severe dry coughb | No | Yes | 1.00 ref | 1.63 [0.93, 2.85] | 1.34 [0.67, 2.69] | 1.80 [0.83, 3.88] | 2.14 [0.91, 5.08] | 0.306 |
| Severe productive coughb | No | Yes | 1.00 ref | 1.74 [1.03, 2.92] | 2.74 [1.56, 4.82] | 2.02 [1.00, 4.06] | 2.10 [0.93, 4.74] | 0.010 |
| Severe barking coughb | No | Yes | 1.00 ref | 2.17 [1.14, 4.14] | 1.71 [0.78, 3.74] | 2.15 [0.90, 5.16] | 3.64 [1.50, 8.82] | 0.041 |
| Any severe coughb | No | Yes | 1.00 ref | 1.79 [1.23, 2.60] | 2.13 [1.38, 3.30] | 1.60 [0.93, 2.76] | 2.29 [1.26, 4.16] | 0.003 |
| Severe blocked or runny noseb | No | Yes | 1.00 ref | 1.33 [0.81, 2.17] | 1.25 [0.68, 2.28] | 1.50 [0.75, 2.99] | 0.89 [0.33, 2.37] | 0.669 |
| Moderate-to-severe change in cryb | No | Yes | 1.00 ref | 1.00 [0.67, 1.49] | 1.13 [0.70, 1.83] | 0.86 [0.46, 1.62] | 1.25 [0.63, 2.47] | 0.892 |
| Moderate-to-severe shortness of breathb | No | Yes | 1.00 ref | 1.30 [0.96, 1.76] | 1.45 [1.00, 2.10] | 1.13 [0.72, 1.80] | 1.31 [0.76, 2.25] | 0.303 |
| Moderate-to-severe wheezeb | No | Yes | 1.00 ref | 1.49 [1.10, 1.03] | 1.51 [1.04, 2.19] | 1.58 [1.01, 2.46] | 1.85 [1.10, 3.11] | 0.035 |
| Moderate-to-severe diarrhoeab | No | Yes | 1.00 ref | 0.72 [0.38, 1.37] | 1.18 [0.58, 2.30] | 1.54 [0.70, 3.38] | 0.23 [0.03, 1.76]a | 0.124 |
| Moderate-to-severe vomitingb | No | Yes | 1.00 ref | 1.07 [0.70, 1.64] | 1.29 [0.78, 2.13] | 1.23 [0.66, 2.27] | 1.84 [0.95, 3.56] | 0.413 |
| Moderate-to-severe reduced fluid intakeb | No | Yes | 1.00 ref | 1.25 [0.87, 1.80] | 1.36 [0.87, 2.10] | 0.93 [0.52, 1.67] | 0.98 [0.49, 1.97] | 0.508 |
| Severe reduced food intakeb | No | Yes | 1.00 ref | 0.90 [0.50, 1.63] | 1.90 [1.03, 3.52] | 0.85 [0.33, 2.14] | 1.93 [0.82, 4.51] | 0.065 |
| Severe reduced energyb | No | Yes | 1.00 ref | 1.17 [0.59, 2.31] | 2.37 [1.17, 4.82] | 1.31 [0.49, 3.48] | 3.80 [1.62, 8.87] | 0.010 |
| Severe disturbed sleepb | No | Yes | 1.00 ref | 1.27 [0.89, 1.82] | 1.76 [1.17, 2.65] | 1.49 [0.90, 2.47] | 1.76 [0.98, 3.14] | 0.063 |
| Moderate-to-severe reduction in urine passedb | No | Yes | 1.00 ref | 1.37 [0.76, 2.47] | 2.25 [1.19, 4.26] | 1.54 [0.67, 3.51] | 1.08 [0.36, 3.28] | 0.156 |
| Severe feverb | No | Yes | 1.00 ref | 0.90 [0.53, 1.53] | 1.61 [0.90, 2.87] | 0.80 [0.34, 1.88] | 1.13 [0.49, 2.83] | 0.299 |
| Temperature ≥37.8°C | No | Yes | 1.00 ref | 1.19 [0.80, 1.78] | 1.87 [1.19, 2.93] | 0.73 [0.37, 1.47] | 1.51 [0.77, 2.95] | 0.024 |
| Pallor | No | Yes | 1.00 ref | 1.57 [1.15, 2.15] | 1.68 [1.15, 2.45] | 1.26 [0.78, 2.03] | 1.77 [1.04, 3.03] | 0.022 |
| Inter-/subcostal recession | No | Yes | 1.00 ref | 1.61 [0.82, 3.15] | 1.57 [0.70, 3.49] | 1.16 [0.41, 3.32] | 0.74 [0.16, 3.34] | 0.541 |
| Inflamed pharynx/tonsils | No | Yes | 1.00 ref | 0.92 [0.68, 1.24] | 1.15 [0.80, 1.65] | 0.86 [0.54, 1.37] | 1.28 [0.76, 2.16] | 0.520 |
| Wheeze assessed by clinician | No | Yes | 1.00 ref | 1.54 [1.10, 2.16] | 1.40 [0.92, 2.12] | 0.91 [0.52, 1.58] | 1.41 [0.78, 2.57] | 0.067 |
| Crackles assessed by clinician | No | Yes | 1.00 ref | 1.75 [1.26, 2.42] | 1.88 [1.28, 2.77] | 1.00 [0.59, 1.70] | 1.41 [0.79, 2.53] | 0.002 |
| Severity by parent | Quartiles 1–3 | Top quartile | 1.00 ref | 1.38 [0.91, 2.09] | 1.74 [1.08, 2.81] | 0.83 [0.41, 1.68] | 2.21 [1.17, 4.18] | 0.027 |
| Severity by clinician | Quartiles 1–3 | Top quartile | 1.00 ref | 1.29 [0.91, 1.83] | 1.81 [1.21, 2.70] | 0.72 [0.39, 1.31] | 1.06 [0.55, 2.03] | 0.010 |
aOnly one in cell.
bIn the 24 hours prior to consultation.
Discussion
Summary
We found novel evidence of five distinct classes of post-consultation symptom trajectory in children presenting to primary care with acute cough and RTI, and four could be ranked according to severity and duration of symptoms. Only a few baseline characteristics were associated with trajectory group membership, but for most of them the association was weak and did not correspond with how the trajectory groups could be ranked. Severe cough in the 24 hours prior to consultation was associated with trajectory groups with more severe and longer lasting cough in a ranked manner.
The majority of children showed no evidence of symptom deterioration post-consultation. Increased symptom severity on the day following consultation was reported in 7.5% of the children and 6% were assigned to the initial deterioration with persistent symptoms group.
Strengths and limitations
To our knowledge, this is the first study to use LLCA to identify different symptom trajectories in RTI in any age group. The study was rigorously conducted and used a large, prospective cohort study to measure severity and duration of symptoms following consultation for acute cough and RTI in children. This is a secondary analysis and therefore no formal power calculation was performed. Multiple variables were analysed to explore possible associations, and the size of some of the groups and rarity of some symptoms limit our interpretation.
We are aware of two main weaknesses. The first is the relatively high symptom data attrition at 39%, which is comparable with other studies relying on patient-reported outcomes (22,23). There is a risk that symptom trajectories could differ in the groups with and without symptom diaries, even when children’s baseline characteristics, including illness severity and clinician’s management, were similar in the two groups. Parents were more likely to respond if they lived in affluent areas and the results could therefore be vulnerable to bias as children residing in deprived areas could have different symptom trajectories, for example as a result of less adequate nutrition or other factors. That said, we did not find evidence of deprivation being associated with trajectory group membership or initial change in cough severity, but may lack power to reliably rule out an effect.
The second is the potential for confounding and performance bias effects of antibiotic treatment. Previous studies have shown limited benefit of antibiotics for children with acute cough and RTI (24). It is still possible that clinicians’ selection of children for antibiotics could have altered their subsequent outcome, so-called confounding by indication. Indeed, a separate analysis that used propensity scoring to adjust for confounding by indication (paper submitted for publication) has shown that delayed (but not immediate) antibiotic prescribing reduced subsequent consultations for illness deterioration. Moreover, parents knew whether their children were given antibiotics, and it is possible that this knowledge changed, perhaps improved, their perception of symptom severity.
Interpretation/comparison with existing literature
We have applied a novel approach to an extensive data set on symptom severity in children following consultations for RTIs. Previous studies have focused on duration only and the results from studies up to 2012 have been summarized (14). The findings of this study complement the existing knowledge on symptom duration by also incorporating symptom severity. We have made some concessions to be able to use latent class analyses to model trajectories. The values on the Likert scale are collapsed from seven to three, and we restricted the analysis to the first 15 days post-consultation. Most RTI-related symptoms (earache, sore throat, croup and symptoms related to bronchiolitis and the common cold) resolve within 15 days, the exception being cough which can last up to 25 days, although 80% resolve within 15 days (14). The duration described in the model is therefore not directly comparable to previous studies on duration.
Implications for clinical care and research
Numerous interventions have been designed in an attempt to reduce unnecessary antibiotic prescriptions in primary care (11). Those that aim to direct prescribing, such as computer decision support ‘pop-ups’ or feedback reports, are popular among health authorities and policy makers, but show little effect (25). There is also emphasis on developing and implementing better guidelines. However, research shows that a major implication of new guidelines has been for clinicians to shift towards more unspecific diagnoses where treatment decisions are not strongly regulated (2). This suggests that other strategies are required to reduce antibiotic prescribing.
Increased knowledge of the natural history of infections will aid patient assessment, communication with patients and carer, treatment decisions and safety netting information (8,9). We have found that distinct symptom trajectory groups exist, but it is not possible for clinicians to differentiate between groups using clinical presentation at the point of care. Parents and clinicians can be reassured that a minority (around 1 in 14) of children deteriorate post-consultation and that the vast majority show steady improvement, albeit at different rates. This provides support for clinicians to more often watch and wait rather than to prescribe antibiotics for children with RTIs.
The UK guidelines recommend that delayed antibiotic prescribing should include advice to only use antibiotics if symptoms worsen or do not settle in accordance with the expected course of the illness (13). Our results suggest illness deterioration should be a relatively low contributor to the cashing of delayed antibiotics, and as children with initial deterioration show later improvement advice about using antibiotics should also include information about signs suggesting bacterial and more serious infection.
Conclusions
Five distinct symptom trajectory groups were identified showing the vast majority of children improve post-consultation, albeit from different levels of baseline severity. Only one group (6% of the patient population) showed any post-consultation illness deterioration, and baseline characteristics were not strongly associated with membership of this trajectory. Knowledge of the existence of different trajectories will aid clinicians in the management of children with acute cough and RTI.
Declaration
Funding: This paper presents independent research funded by the National Institute for Health Research (NIHR) under its Programme Grants for Applied Research Programme (grant reference number RP-PG-0608-10018) and led by researchers at the University of Bristol and NHS Bristol Clinical Commissioning Group. KAW is funded by a post-doctoral grant from the Research Council of Norway (project number 228939). ADH is funded by NIHR Research Professorship (NIHR-RP-02-12-012). ADH and HC are supported by the NIHR Health Protection Research Unit in Evaluation of Interventions at University of Bristol in partnership with Public Health England. NR’s time is supported by the National Institute for Health Research (NIHR) Collaboration for Leadership in Applied Health Research and Care West (CLAHRC West) at University Hospitals Bristol NHS Foundation Trust. The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR, the Department of Health or Public Health England.
Ethical approval: The study was approved by the South West Central Bristol Research Ethics Committee, UK (reference number 10/H0102/54), and research governance approvals were obtained for all areas before the start of recruitment. Informed consent was obtained from parents (and assent from children aged ≥11 years).
Conflict of interest: HC reports receiving honoraria from Sanofi Pasteur, and consultancy fees from IMS Health, AstraZeneca and GSK all paid to her employer. The other authors have no conflicts of interest to disclose.
Supplementary Material
Acknowledgements
We are extremely grateful to the children, parents or carers, and families who have participated in the study, all family physician practices including recruiting clinicians, administrative and research contacts, and all other staff whose participation made this study possible. We thank all our colleagues from the TARGET Programme, the TARGET Programme Management Group, and the TARGET Programme Steering Committee for their time, expertise and support. Index of Multiple Deprivation data: ©Crown Copyright 2006. Source: National Statistics/Ordnance Survey Extracts are Crown Copyright and may only be reproduced by permission.
References
- 1. Hay AD, Heron J, Ness A; ALSPAC study team The prevalence of symptoms and consultations in pre-school children in the Avon Longitudinal Study of Parents and Children (ALSPAC): a prospective cohort study. Fam Pract 2005; 22: 367–74. [DOI] [PubMed] [Google Scholar]
- 2. Thompson PL, Spyridis N, Sharland M et al. . Changes in clinical indications for community antibiotic prescribing for children in the UK from 1996 to 2006: will the new NICE prescribing guidance on upper respiratory tract infections just be ignored?Arch Dis Child 2009; 94: 337–40. [DOI] [PubMed] [Google Scholar]
- 3. Hersh AL, Shapiro DJ, Pavia AT, Shah SS. Antibiotic prescribing in ambulatory pediatrics in the United States. Pediatrics 2011; 128: 1053–61. [DOI] [PubMed] [Google Scholar]
- 4. Hellman J, Grape M, Ternhag A. Antibiotic consumption among a Swedish cohort of children born in 2006. Acta Paediatr 2015; 104: 1035–8. [DOI] [PubMed] [Google Scholar]
- 5. Department of Health. UK Five Year Antimicrobial Resistance Strategy 2013 to 2018. London, UK: Department of Health, 2013. [Google Scholar]
- 6. World Health Organisation. Antimicrobial Resistance: Global Report on Surveillance. France: World Health Organization, 2014. [Google Scholar]
- 7. Lucas PJ, Cabral C, Hay AD, Horwood J. A systematic review of parent and clinician views and perceptions that influence prescribing decisions in relation to acute childhood infections in primary care. Scand J Prim Health Care 2015; 33: 11–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Horwood J, Cabral C, Hay AD, Ingram J. Primary care clinician antibiotic prescribing decisions in consultations for children with RTIs: a qualitative interview study. Br J Gen Pract 2016; 66: e207–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cabral C, Horwood J, Hay AD, Lucas PJ. How communication affects prescription decisions in consultations for acute illness in children: a systematic review and meta-ethnography. BMC Fam Pract 2014; 15: 63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Little P, Stuart B, Francis N et al. ; GRACE consortium. Effects of internet-based training on antibiotic prescribing rates for acute respiratory-tract infections: a multinational, cluster, randomised, factorial, controlled trial. Lancet 2013; 382: 1175–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Andrews T, Thompson M, Buckley DI et al. . Interventions to influence consulting and antibiotic use for acute respiratory tract infections in children: a systematic review and meta-analysis. PLoS One 2012; 7: e30334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Macfarlane J, Holmes W, Gard P et al. . Reducing antibiotic use for acute bronchitis in primary care: blinded, randomised controlled trial of patient information leaflet. BMJ 2002; 324: 91–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. National Institute for Health and Care Excellence (NICE). Respiratory Tract Infections (Self-Limiting): Prescribing Antibiotics. London: NICE, 2008. [PubMed] [Google Scholar]
- 14. Thompson M, Vodicka TA, Blair PS et al. ; TARGET Programme Team. Duration of symptoms of respiratory tract infections in children: systematic review. BMJ 2013; 347: f7027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Redmond NM, Davies R, Christensen H et al. ; TARGET team. The TARGET cohort study protocol: a prospective primary care cohort study to derive and validate a clinical prediction rule to improve the targeting of antibiotics in children with respiratory tract illnesses. BMC Health Serv Res 2013; 13: 322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hay AD, Redmond NM, Turnbull S et al. . Development and internal validation of a clinical rule to improve antibiotic use in children presenting to primary care with acute respiratory tract infection and cough: a prognostic cohort study. Lancet Respir Med 2016; 4: 902–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Watson L, Little P, Moore M, Warner G, Williamson I. Validation study of a diary for use in acute lower respiratory tract infection. Fam Pract 2001; 18: 553–4. [DOI] [PubMed] [Google Scholar]
- 18. Hay AD, Wilson A, Fahey T, Peters TJ. The duration of acute cough in pre-school children presenting to primary care: a prospective cohort study. Fam Pract 2003; 20: 696–705. [DOI] [PubMed] [Google Scholar]
- 19. Muthén LK, Muthén BO.. Mplus User’s Guide. 7th edn. Los Angeles, CA: Muthén & Muthén, 2012. [Google Scholar]
- 20. Croudace TJ, Jarvelin MR, Wadsworth ME, Jones PB. Developmental typology of trajectories to nighttime bladder control: epidemiologic application of longitudinal latent class analysis. Am J Epidemiol 2003; 157: 834–42. [DOI] [PubMed] [Google Scholar]
- 21. Collins LM, Lanza ST.. Latent Class and Latent Transition Analysis. 1st edn. Hoboken, NJ: John Wiley & Sons, Inc, 2010. [Google Scholar]
- 22. Kiezebrink K, Crombie IK, Irvine L et al. . Strategies for achieving a high response rate in a home interview survey. BMC Med Res Methodol 2009; 9: 46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. MacLennan G, McDonald A, McPherson G, Treweek S, Avenell A; RECORD Trial Group Advance telephone calls ahead of reminder questionnaires increase response rate in non-responders compared to questionnaire reminders only: the RECORD phone trial. Trials 2014; 15: 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Fahey T, Stocks N, Thomas T. Systematic review of the treatment of upper respiratory tract infection. Arch Dis Child 1998; 79: 225–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Vodicka TA, Thompson M, Lucas P et al. ; TARGET Programme team. Reducing antibiotic prescribing for children with respiratory tract infections in primary care: a systematic review. Br J Gen Pract 2013; 63: e445–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.