Abstract
Background
Piperacillin-tazobactam-nonsusceptible (TZP-NS) Enterobacteriaceae are typically also resistant to ceftriaxone. We recently encountered bacteremias due to Escherichia coli (Ec) and Klebsiella pneumoniae (Kp) that were TZP-NS but ceftriaxone-susceptible (CRO-S).
Methods
We reviewed all Ec and Kp bacteremias from 2011 to 2015 at our center and assessed the prevalence, antimicrobial susceptibilities, genetic profiles, patient characteristics, treatments, and outcomes of TZP-NS/CRO-S infections. We identified risk factors for TZP-NS/CRO-S infections compared with Ec and Kp bacteremias that were TZP-S and CRO-S (Control Group 1) and compared outcomes of patients with TZP-NS/CRO-S bacteremias, Control Group 1, and patients bacteremic with extended-spectrum β-lactamase (ESBL)–producing Ec and Kp.
Results
There were 1857 Ec and Kp bacteremia episodes, of which 78 (4.2%) were TZP-NS/CRO-S (Ec: 50/1227 [4.1%]; Kp: 28/630 [4.4%]). All TZP-NS/CRO-S isolates were also ampicillin-sulbactam-NS. Of 32 TZP-NS/CRO-S isolates that were sequenced, 28 (88%) harbored blaTEM-1 or blaSHV-1, none had an ESBL or AmpC β-lactamase gene, and many sequence types were represented. Independent risk factors for TZP-NS/CRO-S bacteremia were exposure to β-lactam/β-lactamase inhibitors (BL/BLIs; adjusted odds ratio [aOR], 5.5; P < .001) and cephalosporins (aOR, 3.0; P = .04). Thirty-day mortality after TZP-NS/CRO-S bacteremia was 25%, which was similar to control groups and was similar in patients treated empirically with BL/BLIs compared with those treated with cephalosporins or carbapenems. Targeted therapy with cephalosporins did not yield a higher 30-day mortality rate than carbapenem therapy.
Conclusions
TZP-NS/CRO-S Ec and Kp are emerging causes of bacteremia, and further research is needed to better understand the epidemiology, resistance mechanisms, and clinical impact of these strains.
Keywords: bacterial resistance, Enterobacteriaceae, piperacillin-tazobactam
Enterobacteriaceae, such as Escherichia coli (Ec) and Klebsiella pneumoniae (Kp), are common pathogens, causing approximately 30% of health care–associated infections [1]. Bloodstream infections due to Enterobacteriaceae are associated with substantial morbidity and mortality [2]. Compounding this problem, antibiotic resistance among these organisms is increasing, leading to fewer available therapies and worse clinical outcomes [3].
Piperacillin-tazobactam (TZP) is a β-lactam/β-lactamase inhibitor (BL/BLI) that is commonly relied upon to treat infections caused by Enterobacteriaceae. Resistance to TZP among these bacteria is primarily caused by enzymes such as carbapenemases, AmpC β-lactamases, and some extended-spectrum β-lactamases (ESBLs) that hydrolyze piperacillin and are not sufficiently inhibited by tazobactam. Such enzymes typically also hydrolyze third-generation cephalosporins, and thus most Enterobacteriaceae that are resistant to TZP are also resistant to ceftriaxone (CRO) [4].At our medical center, we encountered Ec and Kp bloodstream infections that tested nonsusceptible to TZP in vitro but susceptible to CRO. Infections with similar phenotypes have recently been reported by others, indicating that these bacteria are not unique to our institution [5, 6]. However, little is known regarding the mechanisms of resistance or the prevalence, epidemiology, risk factors, and optimal therapies of bloodstream infections caused by these bacteria.
Given the extensive use of TZP in hospitals across the world, a new mechanism of resistance to TZP could have important consequences. We therefore conducted an epidemiologic study to assess the prevalence of bacteremia due to TZP-NS/CRO-S Ec and Kp, identify β-lactamases harbored by these bacteria, characterize their genetic diversity, identify risk factors for these infections, and compare treatments and outcomes of these infections by the type of antimicrobial therapy used.
METHODS
Identification of Cases and Controls
This single-center, retrospective, case-control-control study was performed at NewYork-Presbyterian Hospital/Weill Cornell Medical Center. Initially, we identified all episodes of bacteremia due to Ec or Kp from 2011 to 2015. Subsequent episodes of bacteremia in a given patient were only included if >30 days elapsed between episodes. All episodes caused by Ec or Kp that were TZP-NS but CRO-S were designated cases, and the proportion of all episodes due to this phenotype was calculated. We utilized antimicrobial susceptibility testing (AST) results that were performed for routine clinical care using 2015 Clinical and Laboratory Standards Institute (CLSI) breakpoints [7]. The AST platform was Vitek2 (bioMérieux Inc., Durham, NC) from January 2011 to September 2014 and the MicroScan Walkaway plus system (Beckman Coulter Inc., Brea, CA) from October 2014 to December 2015.
Control Group 1 (susceptible controls) was comprised of bacteremia episodes caused by Ec or Kp that were susceptible to both TZP and CRO (TZP-S/CRO-S). Control Group 2 (ESBL-like controls) was comprised of episodes of bacteremia caused by Ec or Kp that were susceptible to meropenem but resistant to ceftriaxone (MEM-S/CRO-R). This susceptibility profile was used as a surrogate marker for ESBL production because formal testing for the presence of ESBLs was not routinely performed at our institution. Among eligible controls, we selected the episode of bacteremia that was closest in time to each case, matched by species, in a 1:1 ratio. Control groups did not include duplicate patients.
Data Collection and Definitions
The following data were collected from the electronic medical record: age, sex, comorbidities of the Charlson Comorbidity Index [8], Pitt bacteremia score [9], inpatient and outpatient antimicrobial therapies before and after the onset of bacteremia, hospital exposures, source of bacteremia, mortality, need for transfer to the intensive care unit (ICU), length of hospital stay, duration of bacteremia, and recurrence of infection. Institutional Review Board approval was obtained from Weill Cornell Medicine (protocol #1506016256).
Ec or Kp bacteremia was defined as growth of either organism in a blood culture, and bacteremia onset was defined as the day that the first positive blood culture was collected. Infections occurring >72 hours after admission to the hospital were considered hospital-onset, and those diagnosed within the first 72 hours of hospitalization were classified as community-onset. Prior exposure to an antimicrobial agent was defined as receiving >48 hours of the agent in the 30 days before bacteremia onset. Recent hospitalization was defined as any hospital admission in the 90 days before bacteremia onset. The source of infection was determined by review of the medical record by 2 infectious diseases physicians.
Empirical therapy was defined as the antimicrobial agent with potential activity against Enterobacteriaceae that was administered for the greatest portion of the first 48 hours after bacteremia onset. Targeted therapy was defined as the antimicrobial agent with in vitro activity against Enterobacteriaceae that was administered for the greatest portion of time between days 3 and 7 after bacteremia onset. Recurrence of bacteremia was defined as another documented episode of bacteremia due to the same organism with the same resistance phenotype >30 days after the initial episode.
β-Lactamase Gene Identification
Thirty-two (41%) of the 78 PTZ-NS/CRO-S Ec and Kp bloodstream isolates were available for additional testing. We performed whole-genome sequencing on these isolates, as follows: The Wizard Genomic DNA Purification Kit (Promega, Madison, WI) was used to yield large quantities of high-quality DNA suitable for whole-genome sequencing. Libraries were prepared for sequencing using Illumina NexteraXT kits (Illumina, Inc., San Diego, CA) and sequenced on an Illumina NextSeq platform with paired 150-base sequence reads. Chromosomal point mutations and antimicrobial resistance genes in sequenced isolates were identified by BLAST using the ResFinder 3.0 (https://cge.cbs.dtu.dk/services/ResFinder/). In silico multilocus sequence typing (MLST) analysis was performed using MLST 2.0 (https://cge.cbs.dtu.dk/services/MLST/) [10].
Statistical Analyses
All statistical analyses were performed using Stata, version 12.0 (StataCorp, College Station, TX). Independent t tests and Wilcoxon rank-sum tests were used for continuous variables with and without normal distribution, respectively, and chi-square tests were used for categorical variables. Univariate and multivariate conditional logistic regression analysis were used to assess potential risk factors for the development of TZP-NS/CRO-S bacteremia, compared with Control Group 1. Variables with P < .2 on univariate analyses were included in multivariate analyses. All P values were 2-tailed, and P values <.05 were considered statistically significant.
RESULTS
Prevalence
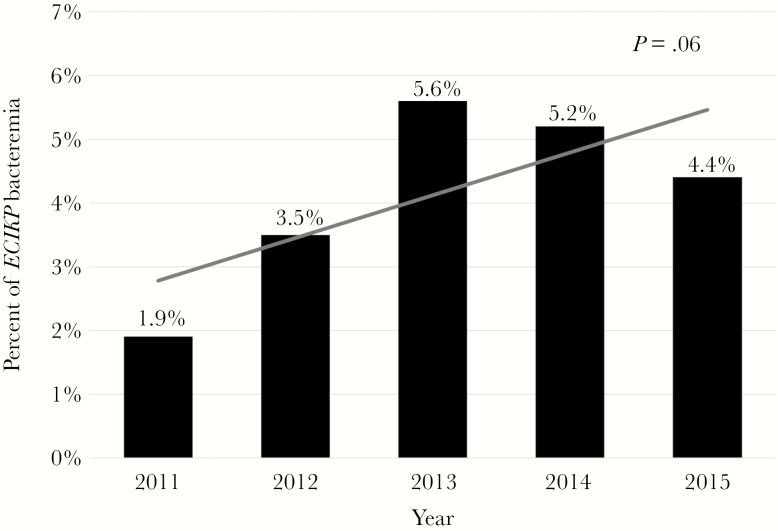
There were 1857 episodes of Ec (n = 1227) and Kp (n = 630) bacteremia from 2011 to 2015, of which 78 (4.2%) were TZP-NS/CRO-S (Ec: 50 [4.1%]; Kp: 28 [4.4%]). There was a nonsignificant increase in the percentage of bacteremias with this phenotype during the study period (P = .06), with the highest percentage of 5.6% in 2013 (Figure 1).
Figure 1.
Prevalence of the piperacillin-tazobactam-nonsusceptible/ceftriaxone-susceptible phenotype among Ec and Kp bloodstream isolates from 2011 to 2015.
Antimicrobial Susceptibility Profiles, β-Lactamase Genes, and Sequence Types
Of the TZP-NS/CRO-S isolates, 58% were TZP-resistant (minimum inhibitory concentration [MIC], >64/4 μg/mL) and 42% were TZP-intermediate (MIC, 32/4–64/4 μg/mL). All TZP-NS/CRO-S isolates were also nonsusceptible to ampicillin-sulbactam, and 89% were nonsusceptible to amoxicillin-clavulanate (Supplementary Figure 1). All TZP-NS/CRO-S isolates were susceptible to aztreonam, ceftazidime, and cefepime, and 83% were susceptible to cefuroxime. TZP MIC data were available for 76 of the 78 TZP-S/CRO-S strains (Control Group 1), and all 76 had TZP MICs ≤8/4 μg/mL (the lowest Microscan TZP dilution).
Among the TZP-NS/CRO-S isolates, 20 Ec and 12 Kp were available for whole-genome sequencing. Fifteen (75%) of the 20 Ec harbored blaTEM-1 only, 1 harbored blaSHV-1 only, 1 harbored blaOXA-1 only, 2 harbored at least 2 of these genes, and 1 did not harbor a β-lactamase gene (Supplementary Table 1). Five (40%) of the 12 Kp harbored blaSHV-1 only, 1 harbored blaLEN-12 only, 5 harbored blaSHV-1 with another narrow-spectrum β-lactamase gene, and 1 harbored blaSHV-11 with blaOXA-1 (Supplementary Table 2). None of the isolates possessed ESBL enzymes, inhibitor-resistant enzymes, plasmid-mediated AmpC β-lactamases, or carbapenemases. There was extensive genetic diversity among both Ec and Kp strains (Supplementary Tables 1 and 2). The most common Ec multilocus sequence types (STs) were ST131 (n = 7), ST73 (n = 4), and ST1193 (n = 3), and the most common Kp STs were ST45 (n = 5) and ST14 (n = 2).
Risk Factors for Infection
The demographic and clinical characteristics of the 3 groups are described in Table 1. The case and control groups did not differ in age, gender, or location where the patients were admitted; >75% of patients were admitted from home in all 3 groups. The Charlson Comorbidity Index (CCI) and Pitt bacteremia score did not differ between cases and controls. Compared with ESBL-like controls (Group 2), patients with TZP-NS/CRO-S infection were more likely to have been recently hospitalized, to have a hematologic malignancy, and to have their source of infection be translocation from the gastrointestinal tract.
Table 1.
Demographics and Clinical Characteristics of Cases and Control Groups
| Variable | Cases TZP-NS/CRO-S (n = 78) |
Control Group 1 TZP-S/CRO-S (n = 78) |
P a | Control Group 2 MEM-S/CRO-R (n = 78) |
P b |
|---|---|---|---|---|---|
| Age, median (IQR), y | 65 (49–74) | 65 (55–77) | .39 | 58 (46–72) | .30 |
| Male gender | 46 (59) | 36 (46) | .11 | 35 (45) | .08 |
| Location before admission | |||||
| Home | 66 (85) | 69 (88) | .48 | 59 (76) | .16 |
| Subacute rehabilitation center | 4 (5) | 3 (4) | .70 | 5 (6) | .73 |
| Acute rehabilitation center | 1 (1) | 2 (3) | .56 | 1 (1) | 1.00 |
| Outside hospital | 7 (9) | 4 (5) | .35 | 13 (17) | .15 |
| Comorbidities | |||||
| Hematologic malignancy | 33 (42) | 22 (28) | .07 | 21 (27) | .04 |
| Diabetes mellitus | 14 (18) | 18 (23) | .43 | 16 (21) | .69 |
| CKD (Cr > 2 mg/dL or hemodialysis) | 13 (17) | 8 (10) | .24 | 8 (10) | .24 |
| Solid tumor | 12 (15) | 22 (28) | .052 | 13 (17) | .83 |
| HSCT in last 6 mo | 7 (9) | 4 (5) | .35 | 6 (8) | .77 |
| Congestive heart failure | 7 (9) | 9 (12) | .60 | 14 (18) | .10 |
| Cirrhosis | 3 (4) | 3 (4) | 1.00 | 2 (3) | .65 |
| HIV infection | 3 (4) | 4 (5) | .70 | 2 (3) | .65 |
| SOT in last 6 mo | 1 (1) | 0 | .32 | 3 (4) | .31 |
| Charlson Comorbidity Index, median (IQR) | 5 (3–6) | 5 (3–7) | .77 | 5 (2–6) | .77 |
| Hospital exposures | |||||
| Hospitalized in last 90 d | 47 (60) | 31 (40) | .01 | 31 (40) | .01 |
| CVC at time of bacteremia | 40 (50) | 27 (35) | .035 | 35 (45) | .42 |
| Surgery in last 30 d | 19 (24) | 16 (21) | .57 | 23 (29) | .47 |
| Endoscopy in last 30 d | 18 (23) | 9 (12) | .06 | 10 (13) | .10 |
| ICU >48 h in last 30 d | 11 (14) | 10 (13) | .82 | 13 (17) | .66 |
| Hemodialysis | 7 (9) | 1 (1) | .06 | 7 (9) | 1.00 |
| Enteric tube feeds | 6 (8) | 4 (5) | .51 | 8 (10) | .58 |
| Mechanical ventilation >48 h in last 30 d | 4 (5) | 4 (5) | 1.00 | 10 (13) | .09 |
| Antimicrobial agents in the last 30 d | 57 (73) | 22 (28) | <.001 | 46 (59) | .06 |
| BL/BLI | 34 (44) | 9 (12) | <.001 | 23 (29) | .07 |
| TZP | 27 (35) | 9 (12) | <.001 | 22 (28) | .39 |
| Amoxicillin-clavulanate | 8 (10) | 0 | .006 | 1 (1) | .03 |
| Cephalosporin | 17 (22) | 7 (9) | .026 | 16 (21) | .85 |
| Fluoroquinolone | 14 (18) | 8 (10) | .17 | 11 (14) | .51 |
| Carbapenem | 8 (10) | 2 (3) | .09 | 14 (18) | .17 |
| Aztreonam | 1 (1) | 1 (1) | 1.002 | 2 (3) | .56 |
| Aminoglycoside | 0 | 1 (1) | .32 | 1 (1) | .32 |
| Hospital-onset bacteremiac | 41 (53) | 25 (32) | .001 | 36 (46) | .42 |
| Hospital-days from admission until bacteremia onset, median (IQR) | 4 (0–14) | 0 (0–8) | .005 | 1.5 (0–14) | .65 |
| Presumed source of infection | |||||
| Translocation from gastrointestinal tract | 32 (41) | 23 (29) | .13 | 10 (13) | .001 |
| Urinary | 18 (23) | 28 (36) | .08 | 32 (41) | .02 |
| Biliary | 17 (22) | 12 (15) | .30 | 9 (12) | .09 |
| Vascular catheter–related | 10 (13) | 5 (6) | .17 | 11 (14) | .82 |
| Intraabdominal abscess | 1 (1) | 5 (6) | .10 | 6 (8) | .053 |
| Pulmonary | 0 (0) | 3 (4) | .08 | 7 (9) | .007 |
| Surgical site infection | 0 (0) | 2 (3) | .16 | 0 (0) | N/A |
| Skin and soft tissue infection | 0 (0) | 0 (0) | N/A | 3 (4) | .08 |
| Pitt bacteremia score, median (IQR) | 3 (1–4) | 2 (0–3) | .18 | 2 (1–4) | .10 |
Values are represented as No. (% of total) or median (IQR). Statistically significant P values are in bold.
Abbreviations: BL/BLI, β-lactam/β-lactamase inhibitor; CKD, chronic kidney disease; Cr, creatinine; CRO-R, ceftriaxone-resistant; CRO-S, ceftriaxone-susceptible; CVC, central venous catheter; HSCT, hematopoietic stem cell transplant; ICU, intensive care unit; IQR, interquartile range; MEM-S, meropenem-susceptible; N/A, not applicable; SOT; solid organ transplant; TZP-NS, piperacillin-tazobactam-nonsusceptible; TZP-S, piperacillin-tazobactam-susceptible.
a P value comparing Cases with Control Group 1.
b P value comparing Cases with Control Group 2.
cInfection onset >72 hours after admission to the hospital.
When compared with TZP-S/CRO-S controls (Group 1), univariate analysis identified recent hospitalization, presence of a central venous catheter, hospital onset of bacteremia, BL/BLI exposure, and cephalosporin exposure as factors associated with TZP-NS/CRO-S bacteremia (Table 1). The median number of days that patients were in the hospital before the onset of bacteremia was significantly higher in the TZP-NS/CRO-S group than in susceptible controls. In multivariate analysis, only exposure to BL/BLI (adjusted odds ratio [aOR], 5.52; P < .001) and exposure to cephalosporins (aOR, 3.02; P = .04) were independent factors associated with bacteremia due to the TZP-NS/CRO-S phenotype compared with TZP-S/CRO-S controls (Table 2).
Table 2.
Univariate and Multivariate Analysis of Risk Factors for TZP-NS/CRO-S Bacteremia Compared With TZP-S/CRO-S Bacteremia
| Potential Risk Factors | Univariate Odds Ratio (95% CI) |
P | Multivariate Adjusted Odds Ratio (95% CI) |
P |
|---|---|---|---|---|
| BL/BLI within 30 d | 5.92 (2.59–13.54) | <.001 | 5.52 (2.19–13.91) | <.001 |
| Cephalosporin within 30 d | 2.83 (1.10–7.27) | .026 | 3.02 (1.02–8.85) | .04 |
| Recent hospitalization | 2.30 (1.21–4.37) | .010 | - | - |
| CVC at time of bacteremia | 1.99 (1.04–3.79) | .035 | - | - |
| Hospital-onset bacteremia | 2.34 (1.23–4.50) | .001 | - | - |
Abbreviations: BL/BLI, β-lactam/β-lactamase inhibitor; CI, confidence interval; CRO-S, ceftriaxone-susceptible; CVC, central venous catheter; TZP-NS, piperacillin-tazobactam-nonsusceptible; TZP-S, piperacillin-tazobactam-susceptible.
Antibacterial Therapy and Outcomes
The most commonly chosen empirical antibacterial therapy across all 3 groups was TZP (TZP-NS/CRO-S, 53%; TZP-S/CRO-S controls, 51%; ESBL-like controls, 58%). However, significantly more patients were placed empirically on carbapenems in the TZP-NS/CRO-S and ESBL-like groups (both 24%) when compared with TZP-S/CRO-S controls (12%; P = .04).
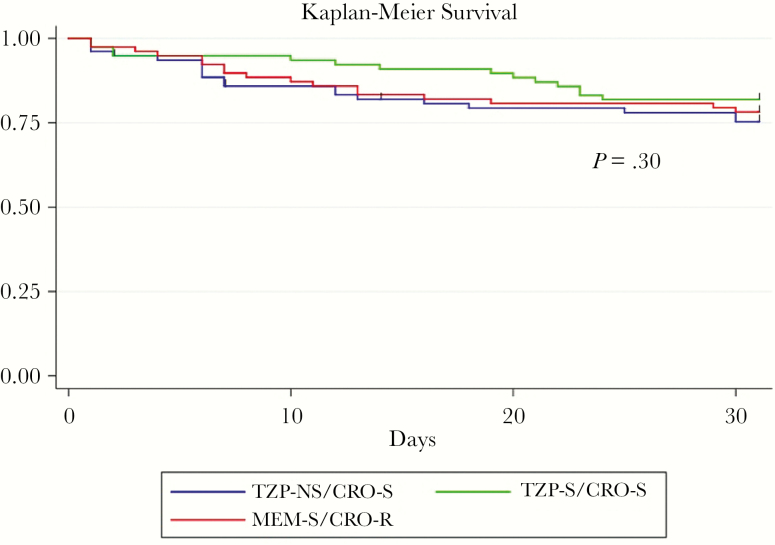
Thirty-day mortality in the TZP-NS/CRO-S group was 25%, which did not significantly differ from TZP-S/CRO-S controls (18%) or ESBL-like (22%) controls. The Kaplan-Meier survival curve showed no significant difference in mortality between the 3 groups over 30 days (P = .30) (Figure 2).
Figure 2.
Kaplan-Meier survival curves for subjects with bacteremia caused by Ec and Kp that are piperacillin-tazobactam-nonsusceptible (TZP-NS)/ceftriaxone-susceptible (CRO-S; blue), susceptible to TZP and CRO (green), and extended-spectrum β-lactamase–like (CRO-resistant, meropenem-susceptible; red). Abbreviations: CRO-R, ceftriaxone-resistant; MEM-S, meropenem-susceptible; TZP-S, piperacillin-tazobactam-susceptible.
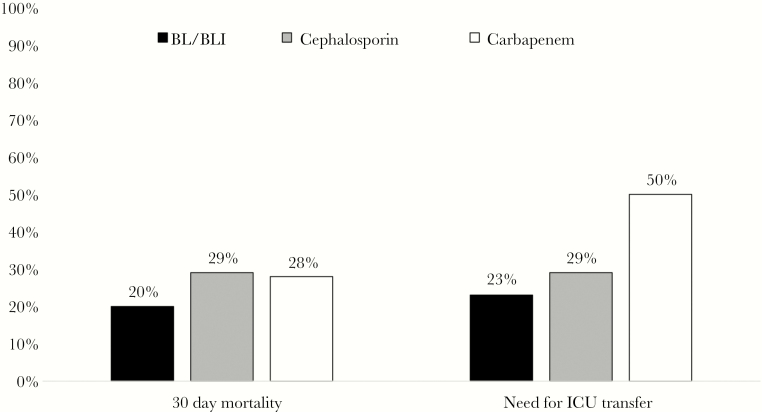
Within the TZP-NS/CRO-S group, 30-day mortality and need for ICU transfer were not significantly different in patients treated empirically with BL/BLIs, cephalosporins, or carbapenems (Figure 3). The most commonly chosen targeted therapies were cephalosporins (40%) and carbapenems (35%). The 30-day mortality rate was greater in patients who received carbapenems for targeted therapy compared with a cephalosporin (33% vs 17%), but this difference was not statistically significant. Recurrence of TZP-NS/CRO-S bacteremia after 30 days occurred in only 3% of cases.
Figure 3.
Thirty-day mortality rates and need for intensive care unit transfer in patients with piperacillin-tazobactam-nonsusceptible/ceftriaxone-susceptible Ec and Kp bacteremia treated empirically with a β-lactam/β-lactamase inhibitor (BL/BLI; black), cephalosporin (gray), or carbapenem (white).
DISCUSSION
We found that between 2011 and 2015, >4% of all Ec and Kp bloodstream infections at our institution displayed a TZP-NS/CRO-S resistance phenotype. Furthermore, of TZP-NS/CRO-S bloodstream isolates that were available for genotyping, none harbored an ESBL, AmpC-β-lactamase, or carbapenemase, and no single sequence type predominated. There have been few published analyses of bacterial infections caused by organisms with this unique resistance profile. Stainton and colleagues recently reported that 1.3% of Ec and Kp isolates from their institution were TZP-resistant but susceptible to all cephalosporins, monobactams, and carbapenems [5]. However, few of these bacteria were bloodstream isolates. To our knowledge, our study is the first to document and characterize the emergence of bloodstream infections due to Ec and Kp that possess this atypical phenotype.
A recent surveillance study suggests that TZP-NS/CRO-S Ec and Kp may be widespread, as these bacteria were detected in 29 out of 40 participating US hospitals [6]. Given that resistance to third-generation cephalosporins and carbapenems, but not TZP, is often used as a marker for the presence of expanded resistance mechanisms, it is possible that bacteria with this resistance profile are underrecognized and underreported. The emergence of resistance to TZP among Ec and Kp that is not mediated by ESBLs or carbapenemases is particularly worrisome because TZP is 1 of the 3 most commonly prescribed antimicrobial agents in US hospitals [11]. Adding to this concern is that the most common MLST type among sequenced TZP-R/CRO-S Ec strains was ST131, an Ec lineage that is highly successful and virulent and has a propensity to acquire ESBL genes, such as blaCTX-M, and fluoroquinolone resistance determinants [12].
We found that exposure to BL/BLIs and exposure to cephalosporins were both independent risk factors for having a bloodstream infection caused by a TZP-NS/CRO-S isolate. It is possible that BL/BLI exposure selects for TZP-NS Ec and Kp to become dominant members of the patient’s gastrointestinal microbiota, leading to an increased risk that these organisms will be isolated during infection. However, this rationale does not explain why cephalosporin exposure was identified as an independent risk factor. Furthermore, the majority of patients with bacteremia due to TZP-NS/CRO-S organisms did not have prior BL/BLI exposure. Moreover, as with bacteremias due to ESBL-producing Ec and Kp, these infections were not limited to hospital settings. In fact, nearly one-half of bacteremias due to TZP-NS/CRO-S organisms were community onset, and 40% occurred in patients without recent hospitalization.
The timely administration of antimicrobials agents with in vitro activity against bloodstream pathogens has been consistently associated with improved outcomes in patients with severe sepsis and those with Gram-negative bacteremia [2, 13, 14]. TZP is used extensively as empirical therapy in hospitalized patients while awaiting blood culture results, and Ec and Kp are common bloodstream pathogens [1, 11]. Thus, the emergence of bacteremias due to TZP-NS Ec and Kp has potentially grave consequences.
Despite these concerns, we did not identify an increase in 30-day mortality or need for ICU transfer in patients with TZP-NS/CRO-S Ec and Kp bacteremia who were empirically treated with TZP compared with those empirically treated with cephalosporins and carbapenems. This unexpected finding is consistent with those of murine pneumonia models of infection with TZP-NS/CRO-S Ec and Kp [15, 16]. In these in vivo studies, TZP exposure resulted in bacterial killing that was similar in mice infected with TZP-NS/CRO-S strains compared with those infected with TZP-S strains.
These findings, combined with the findings from our clinical study, question the clinical significance of in vitro TZP nonsusceptibility among Ec and Kp that are CRO-S. The explanation for this discordance is unclear and requires further investigation to elucidate the mechanism of TZP resistance in these strains. Unfortunately, despite performing whole-genome sequencing on 32 of the isolates and demonstrating that all but 1 harbored a narrow-spectrum β-lactamase, we were unable to definitively determine the TZP resistance mechanism. If this resistance is caused by in vitro hyperexpression of SHV or TEM β-lactamase [17, 18], then perhaps these enzymes are not expressed to the same degree in vivo. Alternatively, in vitro antimicrobial susceptibility testing for TZP utilizes 2-fold serial dilutions of piperacillin but a fixed concentration of 4 mg/L of tazobactam. However, achievable serum concentrations of tazobactam in vivo may be as high as 25–35 mg/L, exceeding those used during in vitro testing [19]. Thus, isolates that appear resistant in vitro may still be inhibited in vivo due to higher attainable bloodstream concentrations of tazobactam. Lastly, a prior analysis demonstrated that some TZP-NS/CRO-S isolates harbor deleted or dysfunctional outer membrane porins [20], which have previously been associated with reduced in vivo fitness and virulence [21].
Clinicians caring for patients with bacteremia due to TZP-NS Ec and Kp may be tempted to use carbapenems for targeted therapy because carbapenems are the treatments of choice for infections caused by ESBL- and AmpC-producing Enterobacteriaceae [22, 23]. We found no significant difference in 30-day mortality between patients treated with a cephalosporin compared with those who received a carbapenem as targeted therapy. Although the number of patients is small in this retrospective analysis, this finding, combined with the absence of ESBL and AmpC enzymes in these organisms, supports the use of cephalosporins for infections caused by TZP-NS/CRO-S Ec and Kp. Establishing the effectiveness of cephalosporins for these infections has important implications for antimicrobial stewardship because carbapenem use could then be avoided.
This study has several limitations. First, this was a retrospective analysis from a single center. Thus, the findings reported here might not be applicable to other institutions. Second, although this is the largest analysis of TZP-NS/CRO-S Ec and Kp bacteremias, we likely had limited statistical power to detect significant differences between case and control groups and to detect differences in outcomes by antimicrobial therapy. A larger multicenter study would provide more answers regarding prevalence, risk factors, and outcomes of patients with infections due to organisms with this resistance profile. The results of the MERINO study highlight the importance of randomized trials in comparing the effectiveness of different antimicrobial therapies and the potential perils of reaching definitive conclusions from observational data [24]. Additionally, we did not have access to the majority of study isolates, and thus were not able to repeat susceptibility testing or perform genomic analyses on most of the TZP-NS/CRO-S isolates. Lastly, despite performing whole-genome sequencing on many of the isolates, this study did not identify the mechanism(s) of resistance for this unique phenotype. Work exploring the molecular resistance mechanisms using transcriptome analyses is underway, and the in vivo potency of BL/BLIs, cephalosporins, and carbapenems is being investigated in animal models. This work will hopefully provide valuable mechanistic knowledge to our current clinical understanding of these infections.
In conclusion, TZP is a heavily relied upon antimicrobial agent. Thus, the emergence of Ec and Kp strains that are TZP-NS but do not harbor ESBL or AmpC enzymes has important clinical implications. We found that a substantial portion of bloodstream infections due to Ec or Kp were TZP-NS/CRO-S and that these infections had similar outcomes when compared with TZP-susceptible and ESBL-like counterparts. Multicenter clinical and molecular studies are needed to better understand the clinical epidemiology, resistance mechanisms, and comparative effectiveness of treatment options for these infections.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Acknowlegments
Financial support. This work was supported by the National Institute of Allergy and Infectious Diseases (T32 AI007613 to R.G. and K23 AI114994 to M.J.S.) and by the National Center for Advancing Translational Sciences (UL1 TR000457 to J.I.M.).
Potential conflicts of interest. M.J.S. has received consulting fees from Achaogen and research grants through Weill Cornell Medicine from Allergan, Merck, and bioMérieux. All other authors report no potential conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Sievert DM, Ricks P, Edwards JR, et al. Antimicrobial-resistant pathogens associated with healthcare-associated infections: summary of data reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2009–2010. Infect Control Hosp Epidemiol 2013; 34:1–14. [DOI] [PubMed] [Google Scholar]
- 2. Kumar A, Roberts D, Wood KE, et al. Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit Care Med 2006; 34:1589–96. [DOI] [PubMed] [Google Scholar]
- 3. Ben-Ami R, Rodríguez-Baño J, Arslan H, et al. A multinational survey of risk factors for infection with extended-spectrum beta-lactamase-producing Enterobacteriaceae in nonhospitalized patients. Clin Infect Dis 2009; 49:682–90. [DOI] [PubMed] [Google Scholar]
- 4. Paterson DL, Bonomo RA. Extended-spectrum beta-lactamases: a clinical update. Clin Microbiol Rev 2005; 18:657–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Stainton SM, Thabit AK, Kuti JL, et al. Prevalence, patient characteristics and outcomes of a novel piperacillin/tazobactam-resistant, pan-β-lactam-susceptible phenotype in Enterobacteriaceae: implications for selective reporting. Clin Microbiol Infect 2017; 23:581–2. [DOI] [PubMed] [Google Scholar]
- 6. Monogue ML, Tanner LK, Brecher SM, et al. Detection of piperacillin-tazobactam-resistant/pan-β-lactam-susceptible Escherichia coli with current automated susceptibility test systems. Infect Control Hosp Epidemiol 2017; 38:379–80. [DOI] [PubMed] [Google Scholar]
- 7. Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing; 25thInformational Supplement. CLSI M100-S25. Wayne, PA: Clinical and Laboratory Standards Institute; 2015. [Google Scholar]
- 8. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987; 40:373–83. [DOI] [PubMed] [Google Scholar]
- 9. Korvick JA, Bryan CS, Farber B, et al. Prospective observational study of Klebsiella bacteremia in 230 patients: outcome for antibiotic combinations versus monotherapy. Antimicrob Agents Chemother 1992; 36:2639–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Larsen MV, Cosentino S, Rasmussen S, et al. Multilocus sequence typing of total-genome-sequenced bacteria. J Clin Microbiol 2012; 50:1355–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Magill SS, Edwards JR, Beldavs ZG, et al. Prevalence of antimicrobial use in US acute care hospitals, May-September 2011. JAMA 2014; 312:1438–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Nicolas-Chanoine MH, Bertrand X, Madec JY. Escherichia coli ST131, an intriguing clonal group. Clin Microbiol Rev 2014; 27:573–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ferrer R, Martin-Loeches I, Phillips G, et al. Empiric antibiotic treatment reduces mortality in severe sepsis and septic shock from the first hour: results from a guideline-based performance improvement program. Crit Care Med 2014; 42:1749–55. [DOI] [PubMed] [Google Scholar]
- 14. Ortega M, Marco F, Soriano A, et al. Epidemiology and prognostic determinants of bacteraemic biliary tract infection. J Antimicrob Chemother 2012; 67:1508–13. [DOI] [PubMed] [Google Scholar]
- 15. Monogue ML, Nicolau DP. In vitro-in vivo discordance with humanized piperacillin-tazobactam exposures against piperacillin-tazobactam-resistant/pan-β-lactam-susceptible Escherichia coli. Antimicrob Agents Chemother 2016; 60:7527–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Stainton SM, Monogue ML, Nicolau DP. In vitro-in vivo discordance with humanized piperacillin-tazobactam exposures against piperacillin-tazobactam-resistant/pan-β-lactam-susceptible Klebsiella pneumoniae strains. Antimicrob Agents Chemother. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Waltner-Toews RI, Paterson DL, Qureshi ZA, et al. Clinical characteristics of bloodstream infections due to ampicillin-sulbactam-resistant, non-extended- spectrum-beta-lactamase-producing Escherichia coli and the role of TEM-1 hyperproduction. Antimicrob Agents Chemother 2011; 55:495–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Schechter LM, Creely DP, Garner CD, et al. Extensive gene amplification as a mechanism for piperacillin-tazobactam resistance in Escherichia coli. MBio. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hayashi Y, Roberts JA, Paterson DL, Lipman J. Pharmacokinetic evaluation of piperacillin-tazobactam. Expert Opin Drug Metab Toxicol 2010; 6:1017–31. [DOI] [PubMed] [Google Scholar]
- 20. Mediavilla JR, Schneider Z, Nwaigwe C, et al. Molecular characterization of piperacillin-tazobactam-resistant Escherichia coli susceptible to cephalosporins, monobactams, and carbapenems. Abstract 1181. Paper presented at: IDWeek 2015; October 7–11, 2015; San Diego, CA. [Google Scholar]
- 21. Knopp M, Andersson DI. Amelioration of the fitness costs of antibiotic resistance due to reduced outer membrane permeability by upregulation of alternative porins. Mol Biol Evol 2015; 32:3252–63. [DOI] [PubMed] [Google Scholar]
- 22. Tamma PD, Han JH, Rock C, et al. ; Antibacterial Resistance Leadership Group Carbapenem therapy is associated with improved survival compared with piperacillin-tazobactam for patients with extended-spectrum β-lactamase bacteremia. Clin Infect Dis 2015; 60:1319–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Jacoby GA. AmpC beta-lactamases. Clin Microbiol Rev 2009; 22:161–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Harris PNA, Tambyah PA, Lye DC, et al. ; MERINO Trial Investigators and the Australasian Society for Infectious Disease Clinical Research Network (ASID-CRN) Effect of piperacillin-tazobactam vs meropenem on 30-day mortality for patients with E coli or Klebsiella pneumoniae bloodstream infection and ceftriaxone resistance: a randomized clinical trial. JAMA 2018; 320:984–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.