Abstract
Acute severe ulcerative colitis is a medical emergency that requires prompt recognition, evaluation, and intervention. Patients require hospital admission with laboratory, radiographic, and endoscopic evaluation with initiation of corticosteroid treatment. Despite early intervention, many patients require salvage medical therapy, with some progressing to colectomy. Here we review important concepts and recent advances in the evaluation and medical management of adult and pediatric patients with acute severe ulcerative colitis.
Keywords: acute severe ulcerative colitis, salvage therapy, infliximab, medical management
INTRODUCTION
Acute severe ulcerative colitis (ASUC) is a life-threatening condition and medical emergency. Approximately 25% of adults and children with ulcerative colitis (UC) will develop ASUC requiring hospitalization.1, 2 Despite available medical salvage therapies, colectomy rates remain high for patients with ASUC, as 14%–20% will require a colectomy by 1 year.3, 4 This statistic highlights the need for improvements in medical management. Between 2010 and 2017, a number of comprehensive consensus statements and systematic reviews have been published that provide guidance for the management of ASUC.5–10 Our aim is to concisely review the practical aspects of the management of patients with ASUC and shed light on emerging data that may change our approach to these patients now and in the future.
DEFINITION
Acute severe ulcerative colitis was originally defined by Truelove and Witts as an exacerbation of UC with at least 6 daily bloody stools and 1 of the following: temperature > 37.8°C, anemia (hemoglobin < 10.5 g/dL), tachycardia (>90 beats per minute), or elevated erythrocyte sedimentation rate (ESR; >30 mm/h).5 For pediatric patients, it has been defined as a Pediatric Ulcerative Colitis Activity Index (PUCAI) score of at least 65 points.11 Once the diagnosis of ASUC is made, patients should be admitted to the hospital for monitoring, further evaluation, and management.
INITIAL MANAGEMENT
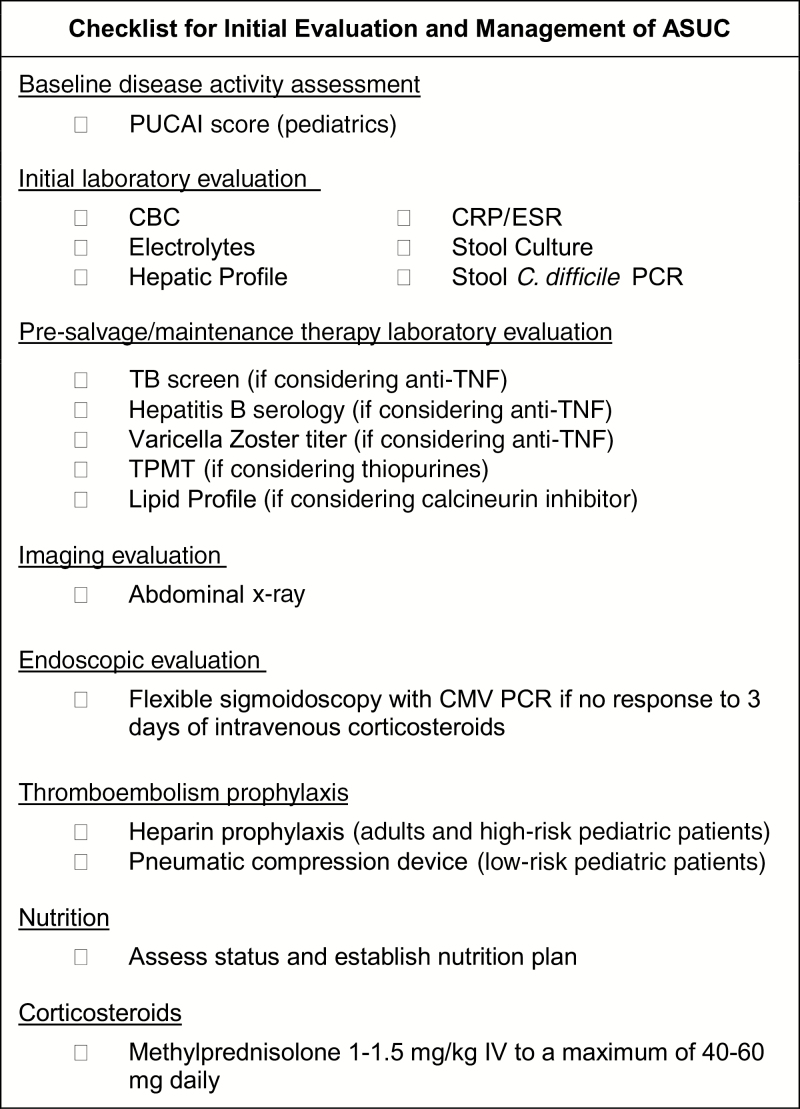
A practical checklist for the initial evaluation and management of patients with ASUC is provided in Figure 1.
FIGURE 1.
Admission checklist for initial evaluation and management of hospitalized patients with ASUC.
Laboratory, Radiographic, and Endoscopic Evaluation
Upon admission, patients with ASUC should undergo both laboratory and radiologic evaluations to assess the disease severity, risk of complications, and concomitant infections. The initial laboratory evaluation should include complete blood count, electrolytes including magnesium, C-reactive protein (CRP), ESR, liver function panel including albumin, stool culture, and Clostridium difficile testing. In addition, an abdominal radiograph should be obtained to assess for toxic megacolon.6 In anticipation of the potential need for salvage therapy with infliximab, also consider obtaining a tuberculosis interferon gamma release assay (which generally takes 48 hours for results), varicella zoster titer, and hepatitis B serologies at admission. If considering a calcineurin inhibitor, obtain a lipid profile and magnesium level, as there is an increased risk for neurologic adverse effects in patients with hypocholesterolemia or hypomagnesemia.12 Each patient should have their vital signs, electrolytes, albumin, complete blood count, and CRP (±ESR) assessed regularly throughout their hospitalization to assess for disease progression and complications.5
Endoscopic evaluation is important to assess for superimposed cytomegalovirus (CMV) as a driver of disease exacerbation in patients with ASUC not responding to intravenous corticosteroids. Full colonoscopy is not recommended in patients with ASUC due to risk of perforation. For pediatric patients, a joint consensus statement by the European Crohn’s and Colitis Organization (ECCO) and the European Society for Pediatric Gastroenterology, Hepatology, and Nutrition (ESPGHAN) specifically recommends an unprepped flexible sigmoidoscopy be performed on those without a clear clinical response to intravenous corticosteroids, as evidenced by a PUCAI score greater than 45 on day 3 of therapy.5 The latest ECCO consensus statement for adult patients similarly states that an unprepped flexible sigmoidoscopy should be considered in addition to intravenous corticosteroids.7 Diagnosis of CMV should be made through analysis of colon tissue, rather than peripheral blood, as peripheral CMV activation intermittently occurs in patients with UC without clear effects on disease course.13, 14 Although histopathologic features of enlarged cells with intranuclear inclusion bodies may be detected on routine hematoxylin and eosin (H&E) staining, immunohistochemistry or in situ hybridization on paraffin-embedded tissues, or tissue polymerase chain reaction (PCR) for CMV DNA—is a more sensitive mode of detection. The best tissue test for CMV complicating ASUC has not been determined, and the relative merits of various assays have been reviewed in depth by others.15–17 The ECCO consensus guidelines on opportunistic infections in IBD recommend assessment by either tissue PCR or immunohistochemistry.17
Toxic Megacolon
Toxic megacolon is a potentially life-threatening complication of ASUC. In the setting of acutely worsening pain, bleeding, fever, or new vital sign instability, an abdominal radiograph must be obtained to evaluate for toxic megacolon. In adults, a transverse colon dilated ≥55 mm and signs of systemic toxicity are indicative of toxic megacolon. In children younger than 10 years of age, 40 mm may be used as the criteria for colon diameter.5 The signs of toxemia include fever, tachycardia, leukocytosis, anemia, worsening pain, abdominal distention, hypotension, dehydration, altered mental status, or electrolyte abnormalities.18 An emergent surgical consultation should be obtained in the setting of toxic megacolon. Initial conservative treatment includes close monitoring, nil per os, intravenous fluids, correction of electrolyte abnormalities and anemia, and broad-spectrum antibiotics. Placement of a nasogastric tube for decompression should also be considered. If the patient is unable to be treated conservatively due to vital sign instability or toxicity, then emergent colectomy should be performed. Cyclosporine and anti–tumor necrosis factor (TNF) therapies should not be used in the setting of toxic megacolon.5
Thromboembolism Prophylaxis
ASUC is a pro-inflammatory state associated with increased risk of vascular thrombosis. Low–molecular weight heparin (LMWH) prophylaxis and graduated compression stockings are recommended for all adult patients hospitalized with ASUC without additional risk factors for bleeding.6 This is true especially for older patients as there is a linear correlation with venous thromboembolism and age.6, 19 In addition to heparin prophylaxis, it is important to avoid prolonged immobilization, prevent dehydration, minimize use of central venous catheters, and hold oral contraceptives and tobacco products.18, 19
According to the most recent pediatric consensus guidelines, there is not sufficient evidence to support the routine use of prophylactic LMWH for preventing thromboembolic complications in children with ASUC.5 Although increased over baseline, the absolute risk of thromboembolic complications in children hospitalized with UC is 1%–1.9%.20, 21 Additional risk factors for thrombosis in children with ASUC include presence of a central venous catheter, known hypercoagulable disorder, oral contraceptive drugs, and history of thromboembolism in a first-degree relative.20, 21 The practice of the authors caring for children is to encourage pediatric patients to get out of bed if stable, use intermittent pneumatic compression devices, and start LMWH prophylaxis in patients with any of the aforementioned risk factors.
Nutrition
Nutrition is an important aspect of treatment for all hospitalized patients to support healing. Patients should be rehydrated and, unless there are concerns for toxicity, started on a regular diet upon admission. Three randomized controlled trials in the 1980s and 1990s did not show any benefit of bowel reset in the management of ASUC, and parental nutrition and bowel rest are associated with increased risk of infectious complications in this setting.22–24 Only if patients are unable to tolerate a regular diet is enteral or parenteral nutrition recommended. The notable exception is in the setting of toxic megacolon, where the patient should be promptly made nil per os.5
Pain Control
Balancing patient comfort without exacerbating the disease process is a challenge in ASUC. Pain may be a symptom of the ongoing chronic inflammation or related to a more serious complication of ASUC.5, 25 Physicians should examine patients and assess for toxic megacolon or colonic perforation in the setting of acute severe exacerbations of abdominal pain in ASUC. Thromboembolic complications should be considered in the setting of new chest or extremity pain. Hot compresses, relaxation techniques, and acetaminophen are often sufficient for pain control in ASUC.5 Nonsteroidal anti-inflammatory drugs (NSAIDs) should be avoided due to risk of further exacerbating disease.26 Opioid pain medicines should be used sparingly and at a low dose because they are relatively ineffective for the intermittent colicky abdominal pain associated with UC, they decrease gut motility and increase risk of toxic megacolon with colonic dilatation, and they are associated with increased mortality in IBD.27, 28 Consultation with a pain specialist and/or psychologist may be helpful in patients with inadequately controlled pain in whom disease complications have been excluded.25
Anemia
Throughout their admission, patients will have their hemoglobin monitored closely for signs of anemia in the setting of chronic inflammation and acute blood loss. Blood transfusions may be needed to maintain adequate hemoglobin. In the acute setting, oral iron should be avoided as it may cause increased inflammation and potentially produce free radicals.29, 30
Pregnancy
Pregnancy does not change the management of ASUC for patients. Pregnancy with active inflammatory bowel disease (IBD) is associated with adverse pregnancy outcomes including preterm birth, small for gestational age, and increased rates of cesarean section, stillborn, and neonatal death.6 Therefore, it is recommended to treat pregnant patients effectively in an effort to prevent morbidity and mortality for neonates. Corticosteroids, calcineurin inhibitors, and infliximab may be used in the setting of ASUC exacerbation during pregnancy.31 Methotrexate should be avoided during pregnancy due to its teratogenicity.
OPPORTUNISTIC INFECTIONS
Cytomegalovirus
It remains unclear whether CMV reactivation is the cause or consequence of ASUC exacerbations. Although CMV is typically a mild self-limited illness in immunocompetent individuals, in an immunocompromised individual, CMV has the potential to progress to a severe systemic disease or end-organ damage such as colitis.32 In patients with UC, the catabolic state and impaired natural killer T-cell function also predisposes these patients to infection, especially when there is active inflammation.33 Furthermore, steroid treatment has been shown to pose an increased risk for CMV reactivation.34 The reported incidence of CMV detection in the tissue of patients with steroid-refractory UC by immunohistochemistry or PCR ranges between 25% and 57%.35 CMV-positive patients are more likely to have steroid resistance and to require salvage therapy and colectomy in the setting of ASUC.34 Although several observational studies have reported response rates of 60% to 80% (with and without continuation of immunosuppressive therapy depending on the study), meta-analyses report conflicting results. One meta-analysis reported an overall increased rate of colectomy in CMV-positive patients treated with antiviral therapy compared with those not treated, whereas another concluded that there was a reduced risk of colectomy with antiviral treatment only in those CMV-positive patients refractory to corticosteroids.36, 37 Both groups of authors agreed that the quality of evidence to support antiviral treatment for CMV in UC is low. The ECCO guidelines on opporunisitic infections in IBD recommend that patients with corticosteroid-refractory ASUC and a positive tissue diagnosis of CMV be treated with intravenous ganciclovir (2–3 weeks) in consultation with an infectious disease specialist.17 If patients respond to treatment with clinical improvement, transitioning to oral valganciclovir after 3–5 days may be considered.17, 34 Although the ECCO guidelines recommend considering discontinuation of immunosuppressive therapy during antiviral treatment for CMV, a recent large multicenter retrospective study observed no difference in colectomy rates between those treated with infliximab and cyclosporine in addition to antiviral therapy compared with those treated with antiviral therapy alone.38
Clostridium difficile
Patients admitted for ASUC should be evaluated for C. difficile stool PCR upon admission.5, 29 Patients with C. difficile in the setting of ASUC have a more severe disease course with increased morbidity and mortality, requiring longer hospitalization.39 Specifically, patients need to be monitored for toxic megacolon, colonic perforation, and venous thromboembolism, as there is increased risk in the setting of C. difficile.39 Patients with C. difficile–complicating ASUC by definition have severe disease and are often on immunosuppressive medical therapy, and therefore should be treated initially with oral vancomycin.17 A recent multicenter large retrospective cohort study, presented in abstract form, reported that initiation of corticosteroids or biologic medications after antibiotic treatment for C. difficile was not associated with an increased risk of C. difficile recurrence, and was actually associated with a reduced risk of death, sepsis, or colectomy.40 This preliminary work suggests that escalation of immunosuppressive treatment in the setting of treated C. difficile infection may not worsen clinical outcomes.
Pneumocystis jiroveci
The risk of pneumonia from P. Jiroveci in patients with IBD on corticosteroids and/or immunosuppressive therapy is quite low.41 ECCO guidelines recommend P. Jiroveci prophylactic treatment with trimethoprim-sulfamethoxazole in patients on triple immunosuppressive therapy, or dual immunosuppressive therapy that includes a calcineurin inhibitor.17
CORTICOSTEROIDS
Intravenous (IV) corticosteroids are firstline therapy for ASUC and should be initiated promptly upon admission to the hospital. Therapy should not be delayed pending completion of evaluation for superimposed infections. For pediatric patients, IV methylprednisolone 1–1.5 mg/kg/d to a maximum of 40–60 mg daily is the corticosteroid of choice due to fewer mineralocorticoid effects compared with hydrocortisone.5 For adults, either 100 mg 4 times daily of IV hydrocortisone or 60 mg daily of IV methylprednisolone has been recommended.42 Treatment with IV corticosteroids is not beneficial beyond 7–10 days of treatment.43 Timing and indications for initiation of rescue therapy are discussed below. If a patient with established corticosteroid-dependent UC is admitted, infliximab may be considered at admission.5, 44
Between 50% and 67% of adult patients and 63% and 74% of pediatric patients will exhibit an initial response to IV corticosteroids.3, 43, 45, 46 In fact, in the recent pediatric PROTECT trial, 21% of pediatric patients treated with IV steroids at diagnosis achieved week 12 corticosteroid-free remission on mesalamine maintenance therapy alone.46 These data highlight the continued utility of intravenous corticosteroids as induction therapy in ASUC, before introduction of second-line rescue therapy.
INDICATION FOR RESCUE THERAPY
Several predictive indices have been developed that may allow determination of need for salvage therapy after 3–5 days of corticosteroid treatment. In one series, 85% of adult patients with >8 bowel movements per day or 3–7 per day and CRP >45 mg/L on day 3 of admission ultimately required colectomy.47, 48 Another group determined that a score including stool frequency and CRP, calculated as bowel movements/d + (0.14*CRP mg/L) × 0.14, had a positive predictive value of 72% for colectomy.49 Other scoring systems, one including stool frequency, blood, nocturnal stools, pain, and activity level, and another including stool frequency, colonic dilatation, and serum albumin, have been proposed with similar predictive accuracy.45
For pediatric patients, the PUCAI is a simple weighted score that has been prospectively validated for predicting response to corticosteroids in pediatric ASUC (Table 1).11 A PUCAI score of greater than 45 points on day 3 of IV corticosteroids has a positive predictive value of 43% and negative predictive value of 94% for predicting need for rescue therapy. Therefore, patients with a PUCAI of ≤45 on day 3 are likely to respond to corticosteroids. Preparations for rescue therapy (required laboratory evaluation, flexible sigmoidoscopy with CMV testing) should be made for patients with a PUCAI >45 after 3 days of corticosteroids. On day 5, a PUCAI score greater than 65 has both a positive and negative predictive value of 82% for predicting need for rescue therapy. Therefore, it is reasonable to initiate rescue therapy in those patients with PUCAI scores >65 on day 5. A PUCAI between 60 and 35 on day 5 of corticosteroids warrants another 2–5 days of treatment to assess for delayed response. Finally, if a patient has a PUCAI of <35 on day 5 or later, they are unlikely to require salvage therapy before discharge.5, 50
Table 1.
Pediatric Ulcerative Colitis Activity Index
| Item | Points |
|---|---|
| 1. Abdominal pain | |
| No pain | 0 |
| Pain can be ignored | 5 |
| Pain cannot be ignored | 10 |
| 2. Rectal bleeding | |
| None | 0 |
| Small amount only, in <50% of stools | 10 |
| Small amount with most stools | 20 |
| Large amount (>50% of the stool content) | 30 |
| 3. Stool consistency of most stools | |
| Formed | 0 |
| Partially formed | 5 |
| Completely unformed | 10 |
| 4. No. stools per 24 hours | |
| 0–2 | 0 |
| 3–5 | 5 |
| 6–8 | 10 |
| >8 | 15 |
| 5. Nocturnal stools (any episode causing wakening) | |
| No | 0 |
| Yes | 10 |
| 6. Activity level | |
| No limitation of activity | 0 |
| Occasional limitation of activity | 5 |
| Severe restricted activity | 10 |
| Sum of PUCAI | (0–85) |
Turner et al.10
Endoscopic indices and histopathologic features may also prove useful for predicting response to corticosteroids. In a retrospective study of 89 patients, the Ulcerative Colitis Endoscopic Index of Severity (UCEIS) was identified as an independent predictor of rescue therapy or colectomy. Ninety-two percent of patients with a UCEIS greater than or equal to 7 required rescue therapy, colectomy, or subsequent readmission.51 In the recent large pediatric PROTECT UC study of standardized 5-ASA and corticosteroid therapy for newly diagnosed pediatric UC, among the 141 patients treated with IV corticosteroids, total Mayo score, rectal biopsy peak eosinophil count ≤32 eosinophils per high-power field, and rectal biopsy surface villiform changes were independently associated with rescue therapy or colectomy by 12 weeks.46
SALVAGE THERAPY OPTIONS
Options for salvage therapy include the anti-TNF biologic drug infliximab or calcineurin inhibitors such as cyclosporine and tacrolimus. Adalimumab, vedolizumab, and golimumab have proven efficacy in ambulatory patients with moderate to severe ulcerative colitis; however, there are insufficient data to support their use as rescue therapy for ASUC.52 Colectomy may also be considered after failure of intravenous corticosteroids depending on patient preference and comorbidities. Consultation with a surgeon at the time of salvage therapy initiation is recommended so that patients may start to become familiar and hopefully more comfortable with colectomy should the need arise.
In determining which salvage therapy to use, patient comorbidities, prior ineffective medications, and future maintenance therapy strategy should be considered. There is an increased risk of neurologic adverse effects with cyclosporine in patients with low magnesium or cholesterol; therefore, cyclosporine is not recommended in these patients.7, 12 Calcineurin inhibitors are generally only appropriate as induction therapy, and bridging to maintenance therapy with a thiopurine (or potentially vedolizumab as more data emerge) should be planned. Alternatively, infliximab is appropriate for both induction and maintenance therapy. Two randomized controlled trials have now demonstrated similar short- and long-term efficacy and colectomy rates after treatment with infliximab or cyclosporine for ASUC.53–55 Given its similar efficacy, fewer adverse effects (compared with calcineurin inhibitors), and suitability as maintenance therapy, infliximab has become the most commonly used salvage therapy.
Calcineurin Inhibitors
Before anti-TNF therapy, cyclosporine was firstline treatment for ASUC refractory to intravenous corticosteroids.56 Randomized controlled trials demonstrated an early response rate of 82%–85% within a median of 4 days of IV cyclosporine treatment.56–58 Long-term response rates after transition to maintenance therapy are 40%–66%.59 Cyclosporine is initially administered as a continuous intravenous infusion at 2–4 mg/kg/d until serum trough levels of 150–300 ng/mL are obtained. After 7 days of treatment and clinical response, patients may be converted to oral cyclosporine at 5–8 mg/kg/d for at least 3 months, with goal trough levels of 100–200 ng/mL.5, 60 Trough drug levels should be measured to improve treatment, optimize dosing, and minimize toxicity. Patients on cyclosporine must be monitored for potential serious adverse effects including neuropathy, hyperkalemia, infection, and hypertension.34 After 3 months of oral cyclosporine treatment, maintenance therapy with a thiopurine drug is generally attempted. Recently, the use of IV cyclosporine as a bridge to vedolizumab in patients with ASUC was reported in abstract form.61 In this retrospective study of 17 adult patients, those responding to intravenous cyclosporine after 8 days were started on standard induction and maintenance vedolizumab, and cyclosporine was discontinued after 8 weeks. Ten of 15 patients who responded to cyclosporine were in endoscopic remission at week 10, and 14 of 15 patients were in clincal remission at week 20. The future published report of this study and additional prospective studies are warranted before making broad recommendations about this approach.
Tacrolimus is a macrolide calcineurin inhibitor that has also been studied as salvage therapy for ASUC. Tacrolimus has better oral bioavailability, tolerability, and fewer serious adverse effects as compared with cyclosporine.18 In a randomized controlled trial, oral tacrolimus resulted in a clinical response of 50%, compared with 13% in the placebo group.62 In a small, multicenter, open-label pediatric trial, the short-term response rate to tacrolimus was 69%, but only 38% ultimately avoided colectomy after 1 year.63 Trough levels should be monitored, with an initial goal of 10–15 ng/mL, then 5–10 ng/mL once remission is obtained.5 In addition to trough drug levels, for all calcineurin inhibitors, magnesium, creatinine, and serum cholesterol need to be monitored closely to assess for toxicity. Intravenous cyclosporine may lead to nephrotoxicity and to a lowered seizure threshold in the setting of hypomagnesemia and hypocholesterolemia.5, 10
Infliximab
Anti–tumor necrosis factor therapy has become a mainstay of salvage therapy for ASUC in both pediatric and adult patients. Although the majority of patients will respond to anti-TNF therapy, 30% of patients are primary nonresponders and will not achieve remission with treatment.64 Another 20%–40% of patients will only demonstrate a partial response to anti-TNF treatment. Additionally, 30% of adults with ASUC will require a colectomy within 60 days and 24% of pediatric patients will require a colectomy before hospital discharge.65 These statistics are generated from mostly observational studies where patients received the standard infliximab induction regimen of 5 mg/kg at times 0, 2, and 6 weeks, followed by maintenance therapy every 8 weeks.
Emerging evidence suggests that patients with severe colitis exhibit rapid infliximab clearance related to disease severity and resulting in reduced drug exposure. We and others have previously reviewed the likely mechanisms for rapid infliximab clearance in ASUC in detail.65, 66 In brief, rapid clearance is likely mediated high-serum and mucosal TNF burden that saturates the therapeutic antibody, upregulation of reticuloendothelial system phagocytes with subsequent proteolytic degradation, accelerated reticuloendothelial clearance, direct leakage of infliximab through the diseased colonic mucosa, and degradation of infliximab by high levels of tissue matrix metalloproteinases.44, 65, 67 Dosing of infliximab guided by an understanding of the underlying pharmacokinetics is important because infliximab exposure, as measured by serum levels, is associated with improved outcomes.42, 44 Patients with more severe UC have detectable fecal loss of infliximab, which is associated with poor short-term outcomes.68, 69 Furthermore, adult UC patients with absent response to infliximab in the first 5 days exhibit significantly lower early drug exposure.70 Finally, infliximab levels during induction therapy are associated with short-term clinical remission, colectomy avoidance, and mucosal healing (Table 2).71–74 Patients with ASUC are often hypoalbuminemic, and low serum albumin concentration has been consistently identified as the strongest predictor of rapid infliximab clearance in UC pharmacokinetic studies.66, 70, 73, 75
Table 2.
Induction and Maintenance Infliximab Levels Associated With UC Outcomes
| Study | Time Point, wk | Target IFX Level, μg/mL | Outcome | Sensitivity/Specificity, % |
|---|---|---|---|---|
| Kobayashi et al.101 | 2 | >21.3 | Clinical remission (wk14) | 61/69 |
| Papamichael et al.102 | 2 | <16.5 | Colectomy | 80/70 |
| Papamichael et al.71 | 2 | ≥28.3 | Mucosal healing (wk10–14) | NR |
| Adedokun et al.73 | 6 | >22 | Clinical response (wk8) | 60/62 |
| Papamichael et al.71 | 6 | ≥15 | Mucosal healing (wk10–14) | 60/74 |
| Brandse et al.70 | 6 | >6.6 | Endoscopic response (wk8) | 88/73 |
| Adedokun et al.73 | 8 | >41.2 | Clinical response (wk8) | 63/62 |
| Adedokun et al.74 | 8 | >41.1 | Mucosal healing | NR |
| Papamichael et al.71 | 14 | ≥2.1 | Mucosal healing (wk10–14) | 84/62 |
| Adedokun et al.73 | 14 | >5.1 | Clinical response (wk30) | 66/63 |
| Adedokun et al.73 | 14 | >3.5 | Clinical response (wk54) | 82/50 |
| Arias et al.103 | 14 | >2.5 | Relapse-free survival (mo6) | 81/75 |
| Van Stappen et al.104 | 14 | ≥2.1 | Mucosal healing (wk10–14) | 100/50 |
| Adedokun et al.73 | 30 | >3.7 | Clinical response (wk30) | 65/71 |
Abbreviations: ATI, antibodies to infliximab; IFX, infliximab; NR, not reported.
Given more rapid clearance of infliximab in severe disease, many patients with ASUC, especially those with hypoalbuminemia, will likely benefit from higher total infliximab dosing and subsequent drug level monitoring. Although randomized trials have proven the efficacy of the standard induction regimen and 5-mg/kg dosing for the average ambulatory patient with moderate to severe UC, patients hospitalized with ASUC were excluded from these studies.76, 77 In a retrospective cohort study of 50 adult patients hospitalized with ASUC, patients treated with an accelerated infliximab induction regimen (3 doses within an average 24 days) had improved short-term colectomy-free survival compared with those treated with the standard induction regimen.78 Although medium-term colectomy rates over 2 years were ultimately similar between the 2 groups, it should be noted that both groups received standard 5 mg/kg every-80-weeks maintenance infliximab dosing after the induction period.78 In our retrospective cohort of pediatric patients hospitalized with severe colitis and treated with infliximab, 65% of patients required dose escalation within the first year of therapy, and dose escalation was associated with low albumin and high erythrocyte sedimentation rate.79 A similar 70% incidence of dose escalation was observed in a retrospective national Irish pediatric cohort.80 These studies support that patients with ASUC may require alternative infliximab dosing regimens.
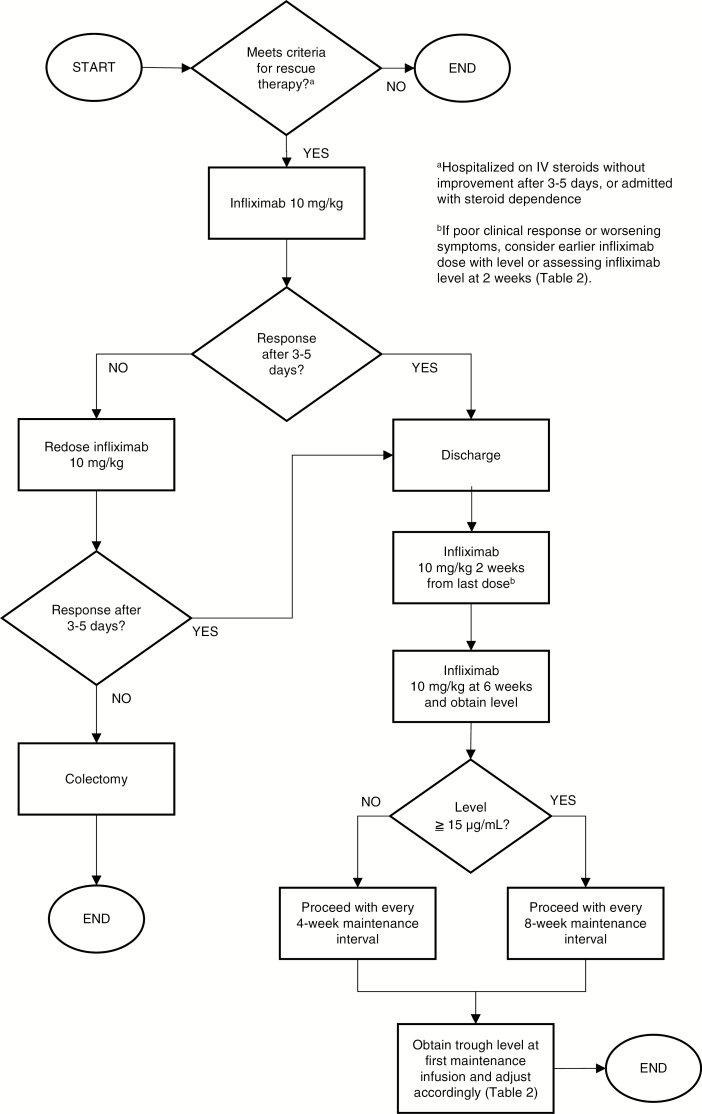
Screening for tuberculosis and hepatitis B should be performed before initiation of infliximab therapy. The clinical practice of the authors for the use of infliximab as salvage therapy for pediatric patients and young adults with ASUC is detailed in Figure 2. We administer an infliximab starting dose of 10 mg/kg and will redose in 3–5 days if there is no clinical response. Absent clinical improvement after a second dose, we recommend colectomy. After discharge, we administer the first outpatient infliximab dose 2 weeks after the last inpatient dose, and the next dose 4 weeks later with a concurrent trough level measurement. We determine whether to spread out the dosing interval to every 8 weeks based on this trough level measurement 6 weeks after the last inpatient dose. Reported target infliximab trough levels at various time points are summarized in Table 2.
FIGURE 2.
An algorithm for infliximab induction based on the authors’ clinical practice.
Sequential Therapy
Current guidelines for both adult and pediatric patients do not support treating with sequential rescue therapy after initial salvage therapy has failed.5, 6 This includes giving a calcineurin inhibitor first, followed by an anti-TNF treatment, and vice versa. In adults, reported responses to second salvage therapy ranged from 25% to 66% of patients.81–83 However, the potential benefits must be weighed against grave potential risks. Serious adverse events reported with sequential therapy include herpetic esophagitis and pneumonia, pancreatitis with bacteremia, and sepsis leading to death.81, 82
SURGERY
The surgical management of ASUC has been reviewed in detail by others, and an extensive discussion is beyond the scope of this review.6, 29, 67, 84, 85 Colectomy is recommended for those patients failed by medical salvage therapy and in the setting of toxic megacolon. Patients with ASUC often have risk factors for surgical complications, including severe disease, poor nutritional status, high-dose corticosteroid exposure, and hypoalbuminemia. Therefore, most patients will be best served by an initial abdominal (subtotal) colectomy and end ileostomy in preparation for future ileal pouch anal anastomosis.86 Surgery should not be delayed to enhance nutrition or taper steroids. Venous thromboembolism prophylaxis should be continued after surgery.5, 6
EMERGING TREATMENTS
Antibiotics
There is now ample evidence for dysregulation of the intestinal microbiome in UC. Although the routine use of empiric antibiotics is not currently recommended in ASUC, several small studies have suggested the potential benefit of oral antibiotics. Randomized controlled trials in adults showed no benefit of IV antibiotics administered intravenously over placebo in addition to IV corticosteroids.87–89 On the other hand, one controlled trial of oral vancomycin in addition to corticosteroids had a statistical trend toward reduction in colectomy in the vancomycin group.90 Similarly, a small pilot trial of rifaximin showed a nonsignificant 25% absolute increase in clinical response.91 In a recent retrospective uncontrolled study in pediatric patients with corticosteroid-refractory UC (two-thirds with ASUC, most also refractory to anti-TNF therapy), combination antibiotic therapy with oral metronidazole, amoxicillin, doxycycline, and vancomycin benefited half of patients.92 A randomized controlled trial of this combination antibiotic regimen is currently underway.
Biosimilars
The infliximab biosimilar CT-P13 is now approved in Europe, Canada, and the United States for the treatment of UC. In a prospective observational study of CT-P13 for UC, 48% of patients achieved corticosteroid-free clinical remission and mucosal healing at 14 weeks. The subset treated as rescue therapy for ASUC (24 patients, 38% of the study population) achieved similar outcomes.93 In another retrospective study, investigators compared the response of 55 patients rescued with CT-P13 for ASUC with 27 patients rescued with originator infliximab. Similar rates of clinical response, clinical remission, and mucosal healing were observed in both groups.94
Hyperbaric Oxygen
In patients with UC, underlying mucosal hypoxia triggers inflammation and edema. Hyperbaric oxygen therapy improves tissue oxygen delivery and therefore has been proposed as a potential treatment for UC.95, 96 In a recent randomized controlled trial investigating hyperbaric oxygen for hospitalized patients with moderate to severe UC, 18 patients were randomized to daily hyperbaric oxygen or sham control in addition to IV corticosteroids.97 Clinical remission at day 5 was achieved by 50% of patients in the hyperbaric oxygen group compared with no patients in the control group. There was also a trend toward less progression to second-line therapy in the hyperbaric oxygen group. Future larger studies need to be performed to confirm the efficacy of hyperbaric oxygen in this setting, but it appears to be relatively safe and well tolerated in IBD patients.96
Granulocyte/Monocyte Adsorbtive Apheresis
Granulocytes and monocytes infiltrate the colon mucosa in UC and are an important source of cytokines, reactive oxygen species, and proteases that induce tissue damage.97 Granulocyte and monocyte adsorbtive apheresis (GMAA) is an extracorporeal procedure that removes activated granulocytes and monocytes from peripheral blood and has been studied for the treatment of UC. An open-label randomized trial comparing GMAA with intravenous corticosteroids in patients with ASUC showed a numerically but not statistically significantly higher response rate in patients treated with GMAA, with fewer adverse effects.98 However, the results of this study must be interpreted with caution as randomization procedures were not reported, and there was no mention of investigator blinding. In fact, many studies of GMAA have similar methodologic limitations. In a systematic review of GMAA for UC, the authors did not undertake a formal meta-analysis because most of the elibible studies were deemed to be at high risk of bias due to study methodology.99 In a multicenter, multinational, double-blind, sham-controlled study of GMAA for active ulcerative colitis, GMAA was not more efficacious than sham procedure for inducing remission in moderate to severe UC.100 In a post hoc analysis of this study, a significantly higher response rate over sham procedure was observed in patients with the most severe histologic disease severity. Larger investigator-blinded randomized controlled trials in patients with ASUC with high-quality methodology are needed before the role of GMAA in the treatment of ASUC can be determined.
OPPORTUNITIES FOR FURTHER INVESTIGATION
Although there are a number of detailed, evidence-based ASUC guidelines available to guide patient mangement,6–8,10, 17 there are many areas where stronger evidence is needed to support definitive consensus recommendations. For instance, it is still not clear whether CMV reactivation drives disease refractoriness or is simply a consequence of severe inflammation and/or immunosuppression. Moreover, the optimal test for detecting CMV in the tissue (immunohistochemistry, in situ hybridization, tissue qualitative or quantitatve PCR) is now known. Large randomized studies are required to determine if antiviral treatment of colon CMV reactivation improves response to therapy. With regard to thromboembolism prophylaxis, current pediatric guidelines state that there is not sufficient evidence to support the routine use of prophylactic LMWH in pediatric patients as is recommended for adults.5 Large comparative effectiveness studies will be needed to determine whether the benefit of routine LMWH for reducing thromboembolic complications outweighs any risks in pediatric patients. In the area of anti-TNF salvage therapy, we need more research to determine whether early infliximab pharmacokinetics in ASUC affects outcomes, whether accelerated dosing strategies are more effective, and if so, what dosing strategy is most effective? Future research will also determine how alternative treatments such as antibiotic cocktails and hyperbaric oxygen, and newer therapies including vedolizumab and tofacitinib, may be incorporated into treatment algorithms for ASUC.
CONCLUSION
Although advances continue to be made in the medical management of ASUC, more research needs to be done to improve patient outcomes. ASUC is a medical emergency requiring hospital admission. The initial evaluation includes clinical, laboratory, radiologic, and endoscopic assessment to assess severity of disease, rule out toxic megacolon, and diagnosis superimposed infections. Treatment with IV corticosteroids should be initiated promptly, and various tools are available to determine which patients require salvage therapy after the first 3–5 days of corticosteroid treatment. Recent evidence supports that patients with ASUC have rapid clearance of infliximab. When infliximab is used as salvage therapy, higher total dose induction regimens with subsequent therapeutic drug monitoring may improve patient outcomes.
Supported by: This work was supported by the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health under award numbers T32DK007727 to K.G.W. and K23DK09483 to M.J.R.
Conflicts of interest: Michael Rosen, MD, MSCI, served on an advisory board for Celgene and receives research support from Prometheus Laboratories. Kaitlin Whaley has no financial relationships to disclose.
REFERENCES
- 1. Dinesen LC, Walsh AJ, Protic MN, et al. . The pattern and outcome of acute severe colitis. J Crohns Colitis. 2010;4:431–437. [DOI] [PubMed] [Google Scholar]
- 2. Turner D, Walsh CM, Benchimol EI, et al. . Severe paediatric ulcerative colitis: incidence, outcomes and optimal timing for second-line therapy. Gut. 2008;57:331–338. [DOI] [PubMed] [Google Scholar]
- 3. Turner D, Mack D, Leleiko N, et al. . Severe pediatric ulcerative colitis: a prospective multicenter study of outcomes and predictors of response. Gastroenterology. 2010;138:2282–2291. [DOI] [PubMed] [Google Scholar]
- 4. Aratari A, Papi C, Clemente V, et al. . Colectomy rate in acute severe ulcerative colitis in the infliximab era. Dig Liver Dis. 2008;40:821–826. [DOI] [PubMed] [Google Scholar]
- 5. Turner D, Travis SP, Griffiths AM, et al. . European Crohn’s and Colitis Organization; Porto IBD Working Group, European Society of Pediatric Gastroenterology, Hepatology, and Nutrition Consensus for managing acute severe ulcerative colitis in children: a systematic review and joint statement from ECCO, ESPGHAN, and the Porto IBD Working Group of ESPGHAN. Am J Gastroenterol. 2011;106:574–588. [DOI] [PubMed] [Google Scholar]
- 6. Chen JH, Andrews JM, Kariyawasam V, et al. . IBD Sydney Organisation and the Australian Inflammatory Bowel Diseases Consensus Working Group Review article: acute severe ulcerative colitis—evidence-based consensus statements. Aliment Pharmacol Ther. 2016;44:127–144. [DOI] [PubMed] [Google Scholar]
- 7. Harbord M, Eliakim R, Bettenworth D, et al. . Third European evidence-based consensus on diagnosis and management of ulcerative colitis. Part 2: current management. J Crohns Colitis. 2017;11:769–784. [DOI] [PubMed] [Google Scholar]
- 8. Kornbluth A, Sachar DB; Practice Parameters Committee of the American College of Gastroenterology Ulcerative colitis practice guidelines in adults: American College of Gastroenterology, Practice Parameters Committee. Am J Gastroenterol. 2010;105:501–533; quiz 524. [DOI] [PubMed] [Google Scholar]
- 9. Magro F, Gionchetti P, Eliakim R, et al. ; European Crohn’s and Colitis Organisation Third European evidence-based consensus on diagnosis and management of ulcerative colitis. Part 1: definitions, diagnosis, extra-intestinal manifestations, pregnancy, cancer surveillance, surgery, and ileo-anal pouch disorders. J Crohns Colitis. 2017;11:649–670. [DOI] [PubMed] [Google Scholar]
- 10. Mowat C, Cole A, Windsor A, et al. ; IBD Section of the British Society of Gastroenterology Guidelines for the management of inflammatory bowel disease in adults. Gut. 2011;60:571–607. [DOI] [PubMed] [Google Scholar]
- 11. Turner D, Otley AR, Mack D, et al. . Development, validation, and evaluation of a pediatric ulcerative colitis activity index: a prospective multicenter study. Gastroenterology. 2007;133:423–432. [DOI] [PubMed] [Google Scholar]
- 12. Bechstein WO. Neurotoxicity of calcineurin inhibitors: impact and clinical management. Transpl Int. 2000;13:313–326. [DOI] [PubMed] [Google Scholar]
- 13. Delvincourt M, Lopez A, Pillet S, et al. . The impact of cytomegalovirus reactivation and its treatment on the course of inflammatory bowel disease. Aliment Pharmacol Ther. 2014;39:712–720. [DOI] [PubMed] [Google Scholar]
- 14. Matsuoka K, Iwao Y, Mori T, et al. . Cytomegalovirus is frequently reactivated and disappears without antiviral agents in ulcerative colitis patients. Am J Gastroenterol. 2007;102:331–337. [DOI] [PubMed] [Google Scholar]
- 15. Kim JJ, Simpson N, Klipfel N, et al. . Cytomegalovirus infection in patients with active inflammatory bowel disease. Dig Dis Sci. 2010;55:1059–1065. [DOI] [PubMed] [Google Scholar]
- 16. Lawlor G, Moss AC. Cytomegalovirus in inflammatory bowel disease: pathogen or innocent bystander?Inflamm Bowel Dis. 2010;16:1620–1627. [DOI] [PubMed] [Google Scholar]
- 17. Rahier JF, Magro F, Abreu C, et al. ; European Crohn’s and Colitis Organisation (ECCO) Second European evidence-based consensus on the prevention, diagnosis and management of opportunistic infections in inflammatory bowel disease. J Crohns Colitis. 2014;8:443–468. [DOI] [PubMed] [Google Scholar]
- 18. Sobrado CW, Sobrado LF. Management of acute severe ulcerative colitis: a clinical update. Arq Bras Cir Dig. 2016;29:201–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Koutroumpakis EI, Tsiolakidou G, Koutroubakis IE. Risk of venous thromboembolism in patients with inflammatory bowel disease. Semin Thromb Hemost. 2013;39:461–468. [DOI] [PubMed] [Google Scholar]
- 20. Nylund CM, Goudie A, Garza JM, et al. . Venous thrombotic events in hospitalized children and adolescents with inflammatory bowel disease. J Pediatr Gastroenterol Nutr. 2013;56:485–491. [DOI] [PubMed] [Google Scholar]
- 21. Zitomersky NL, Levine AE, Atkinson BJ, et al. . Risk factors, morbidity, and treatment of thrombosis in children and young adults with active inflammatory bowel disease. J Pediatr Gastroenterol Nutr. 2013;57:343–347. [DOI] [PubMed] [Google Scholar]
- 22. González-Huix F, Fernández-Bañares F, Esteve-Comas M, et al. . Enteral versus parenteral nutrition as adjunct therapy in acute ulcerative colitis. Am J Gastroenterol. 1993;88:227–232. [PubMed] [Google Scholar]
- 23. Dickinson RJ, Ashton MG, Axon AT, et al. . Controlled trial of intravenous hyperalimentation and total bowel rest as an adjunct to the routine therapy of acute colitis. Gastroenterology. 1980;79:1199–1204. [PubMed] [Google Scholar]
- 24. McIntyre PB, Powell-Tuck J, Wood SR, et al. . Controlled trial of bowel rest in the treatment of severe acute colitis. Gut. 1986;27:481–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Morrison G, Van Langenberg DR, Gibson SJ, et al. . Chronic pain in inflammatory bowel disease: characteristics and associations of a hospital-based cohort. Inflamm Bowel Dis. 2013;19:1210–1217. [DOI] [PubMed] [Google Scholar]
- 26. Felder JB, Korelitz BI, Rajapakse R, et al. . Effects of nonsteroidal antiinflammatory drugs on inflammatory bowel disease: a case-control study. Am J Gastroenterol. 2000;95:1949–1954. [DOI] [PubMed] [Google Scholar]
- 27. Lichtenstein GR, Feagan BG, Cohen RD, et al. . Serious infection and mortality in patients with Crohn’s disease: more than 5 years of follow-up in the TREAT™ registry. Am J Gastroenterol. 2012;107:1409–1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gan SI, Beck PL. A new look at toxic megacolon: an update and review of incidence, etiology, pathogenesis, and management. Am J Gastroenterol. 2003;98:2363–2371. [DOI] [PubMed] [Google Scholar]
- 29. Fornaro R, Caratto M, Barbruni G, et al. . Surgical and medical treatment in patients with acute severe ulcerative colitis. J Dig Dis. 2015;16:558–567. [DOI] [PubMed] [Google Scholar]
- 30. Gasche C, Berstad A, Befrits R, et al. . Guidelines on the diagnosis and management of iron deficiency and anemia in inflammatory bowel diseases. Inflamm Bowel Dis. 2007;13:1545–1553. [DOI] [PubMed] [Google Scholar]
- 31. Schulze H, Esters P, Dignass A. Review article: the management of Crohn’s disease and ulcerative colitis during pregnancy and lactation. Aliment Pharmacol Ther. 2014;40:991–1008. [DOI] [PubMed] [Google Scholar]
- 32. Römkens TE, Bulte GJ, Nissen LH, et al. . Cytomegalovirus in inflammatory bowel disease: a systematic review. World J Gastroenterol. 2016;22:1321–1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Okahara K, Nagata N, Shimada T, et al. . Colonic cytomegalovirus detection by mucosal PCR and antiviral therapy in ulcerative colitis. PLoS One. 2017;12:e0183951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Cohen S, Martinez-Vinson C, Aloi M, et al. . CMV infection in pediatric severe ulcerative colitis—a multicenter study from the Pediatric IBD Porto Group of ESPGHAN. Pediatr Infect Dis J. 2017;106:574–588. [DOI] [PubMed] [Google Scholar]
- 35. Lawlor G, Moss AC. Cytomegalovirus in inflammatory bowel disease: pathogen or innocent bystander?Inflamm Bowel Dis. 2010;16:1620–1627. [DOI] [PubMed] [Google Scholar]
- 36. Kopylov U, Eliakim-Raz N, Szilagy A, et al. . Antiviral therapy in cytomegalovirus-positive ulcerative colitis: a systematic review and meta-analysis. World J Gastroenterol. 2014;20:2695–2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Shukla T, Singh S, Loftus EV Jr, et al. . Antiviral therapy in steroid-refractory ulcerative colitis with cytomegalovirus: systematic review and meta-analysis. Inflamm Bowel Dis. 2015;21:2718–2725. [DOI] [PubMed] [Google Scholar]
- 38. Kopylov U, Papamichael K, Katsanos K, et al. . Impact of infliximab and cyclosporine on the risk of colectomy in hospitalized patients with ulcerative colitis complicated by cytomegalovirus—a multicenter retrospective study. Inflamm Bowel Dis. 2017;23:1605–1613. [DOI] [PubMed] [Google Scholar]
- 39. Bhandari S, Mohammed Abdul MK, Dhakal B, et al. . Increased rate of venous thromboembolism in hospitalized inflammatory bowel disease patients with Clostridium difficile infection. Inflamm Bowel Dis. 2017;23:1847–1852. [DOI] [PubMed] [Google Scholar]
- 40. Lukin DJ, Lawlor G, Feathers A, et al. . Escalation of therapy in inflammatory bowel disease patients with Clostridium difficile infection is associated with better outcomes: an IBD remedy study. Gastroenterology. 2017;152:S575. [Google Scholar]
- 41. Cotter TG, Gathaiya N, Catania J, et al. . Low risk of pneumonia from pneumocystis jirovecii infection in patients with inflammatory bowel disease receiving immune suppression. Clin Gastroenterol Hepatol. 2017;15:850–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Seah D, De Cruz P. Review article: the practical management of acute severe ulcerative colitis. Aliment Pharmacol Ther. 2016;43:482–513. [DOI] [PubMed] [Google Scholar]
- 43. Turner D, Walsh CM, Steinhart AH, et al. . Response to corticosteroids in severe ulcerative colitis: a systematic review of the literature and a meta-regression. Clin Gastroenterol Hepatol. 2007;5:103–110. [DOI] [PubMed] [Google Scholar]
- 44. Hindryckx P, Novak G, Vande Casteele N, et al. . Review article: dose optimisation of infliximab for acute severe ulcerative colitis. Aliment Pharmacol Ther. 2017;45:617–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Ho GT, Mowat C, Goddard CJ, et al. . Predicting the outcome of severe ulcerative colitis: development of a novel risk score to aid early selection of patients for second-line medical therapy or surgery. Aliment Pharmacol Ther. 2004;19:1079–1087. [DOI] [PubMed] [Google Scholar]
- 46. Hyams JS, Davis S, Mack DR, et al. . Factors associated with early outcomes following standardised therapy in children with ulcerative colitis (PROTECT): a multicentre inception cohort study. Lancet Gastroenterol Hepatol. 2017;2:855–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Travis SP, Farrant JM, Ricketts C, et al. . Predicting outcome in severe ulcerative colitis. Gut. 1996;38:905–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Seo M, Okada M, Yao T, et al. . An index of disease activity in patients with ulcerative colitis. Am J Gastroenterol. 1992;87:971–976. [PubMed] [Google Scholar]
- 49. Lindgren SC, Flood LM, Kilander AF, et al. . Early predictors of glucocorticosteroid treatment failure in severe and moderately severe attacks of ulcerative colitis. Eur J Gastroenterol Hepatol. 1998;10:831–835. [DOI] [PubMed] [Google Scholar]
- 50. Turner D, Hyams J, Markowitz J, et al. . Pediatric IBD Collaborative Research Group Appraisal of the Pediatric Ulcerative Colitis Activity Index (PUCAI). Inflamm Bowel Dis. 2009;15:1218–1223. [DOI] [PubMed] [Google Scholar]
- 51. Corte C, Fernandopulle N, Catuneanu AM, et al. . Association between the Ulcerative Colitis Endoscopic Index of Severity (UCEIS) and outcomes in acute severe ulcerative colitis. J Crohns Colitis. 2015;9:376–381. [DOI] [PubMed] [Google Scholar]
- 52. Kokkinidis DG, Bosdelekidou EE, Iliopoulou SM, et al. . Emerging treatments for ulcerative colitis: a systematic review. Scand J Gastroenterol. 2017;52:923–931. [DOI] [PubMed] [Google Scholar]
- 53. Laharie D, Bourreille A, Branche J, et al. . Groupe d’Etudes Thérapeutiques des Affections Inflammatoires Digestives Ciclosporin versus infliximab in patients with severe ulcerative colitis refractory to intravenous steroids: a parallel, open-label randomised controlled trial. Lancet. 2012;380:1909–1915. [DOI] [PubMed] [Google Scholar]
- 54. Laharie D, Bourreille A, Branche J, et al. . Groupe d’Etudes Thérapeutiques des Affections Inflammatoires Digestives Long-term outcome of patients with steroid-refractory acute severe UC treated with ciclosporin or infliximab. Gut. 2018;67:237–243. [DOI] [PubMed] [Google Scholar]
- 55. Williams JG, Alam MF, Alrubaiy L, et al. . Infliximab versus ciclosporin for steroid-resistant acute severe ulcerative colitis (CONSTRUCT): a mixed methods, open-label, pragmatic randomised trial. Lancet Gastroenterol Hepatol. 2016;1:15–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Lichtiger S, Present DH, Kornbluth A, et al. . Cyclosporine in severe ulcerative colitis refractory to steroid therapy. N Engl J Med. 1994;330:1841–1845. [DOI] [PubMed] [Google Scholar]
- 57. Van Assche G, D’Haens G, Noman M, et al. . Randomized, double-blind comparison of 4 mg/kg versus 2 mg/kg intravenous cyclosporine in severe ulcerative colitis. Gastroenterology. 2003;125:1025–1031. [DOI] [PubMed] [Google Scholar]
- 58. Laharie D, Bourreille A, Branche J, et al. ; Groupe d’Etudes Thérapeutiques des Affections Inflammatoires Digestives Ciclosporin versus infliximab in patients with severe ulcerative colitis refractory to intravenous steroids: a parallel, open-label randomised controlled trial. Lancet. 2012;380:1909–1915. [DOI] [PubMed] [Google Scholar]
- 59. Cohen RD, Stein R, Hanauer SB. Intravenous cyclosporin in ulcerative colitis: a five-year experience. Am J Gastroenterol. 1999;94:1587–1592. [DOI] [PubMed] [Google Scholar]
- 60. Ordás I, Domènech E, Mañosa M, et al. . Long-term efficacy and safety of cyclosporine in a cohort of steroid-refractory acute severe ulcerative colitis patients from the ENEIDA registry (1989-2013): a nationwide multicenter study. Am J Gastroenterol. 2017;112:1709–1718. [DOI] [PubMed] [Google Scholar]
- 61. Tarabar D, El Jurdi K, Yvellez O, et al. . DOP049 combination therapy of cyclosporine and vedolizumab is effective and safe for severe, steroid-resistant ulcerative colitis patients: a prospective study. J Crohns Colitis. 2018;12:S065. [Google Scholar]
- 62. Ogata H, Kato J, Hirai F, et al. . Double-blind, placebo-controlled trial of oral tacrolimus (FK506) in the management of hospitalized patients with steroid-refractory ulcerative colitis. Inflamm Bowel Dis. 2012;18:803–808. [DOI] [PubMed] [Google Scholar]
- 63. Bousvaros A, Kirschner BS, Werlin SL, et al. . Oral tacrolimus treatment of severe colitis in children. J Pediatr. 2000;137:794–799. [DOI] [PubMed] [Google Scholar]
- 64. Papamichael K, Casteele NV, Ferrante M, et al. . Therapeutic drug monitoring during induction of anti-tumor necrosis factor therapy in inflammatory bowel disease: defining a therapeutic drug window. Inflamm Bowel Dis. 2017;23:1510–1515. [DOI] [PubMed] [Google Scholar]
- 65. Rosen MJ, Minar P, Vinks AA. Review article: applying pharmacokinetics to optimise dosing of anti-TNF biologics in acute severe ulcerative colitis. Aliment Pharmacol Ther. 2015;41:1094–1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Ungar B, Mazor Y, Weisshof R, et al. . Induction infliximab levels among patients with acute severe ulcerative colitis compared with patients with moderately severe ulcerative colitis. Aliment Pharmacol Ther. 2016;43:1293–1299. [DOI] [PubMed] [Google Scholar]
- 67. Hindryckx P, Jairath V, D’Haens G. Acute severe ulcerative colitis: from pathophysiology to clinical management. Nat Rev Gastroenterol Hepatol. 2016;13:654–664. [DOI] [PubMed] [Google Scholar]
- 68. Brandse JF, van den Brink GR, Wildenberg ME, et al. . Loss of infliximab into feces is associated with lack of response to therapy in patients with severe ulcerative colitis. Gastroenterology. 2015;149:350–355.e352 [DOI] [PubMed] [Google Scholar]
- 69. Beswick L, Rosella O, Rosella G, et al. . Exploration of predictive biomarkers of early infliximab response in acute severe colitis: a prospective pilot study. J Crohns Colitis. 2018;12:289–297. [DOI] [PubMed] [Google Scholar]
- 70. Brandse JF, Mathôt RA, van der Kleij D, et al. . Pharmacokinetic features and presence of antidrug antibodies associate with response to infliximab induction therapy in patients with moderate to severe ulcerative colitis. Clin Gastroenterol Hepatol. 2016;14:251–258.e1. [DOI] [PubMed] [Google Scholar]
- 71. Papamichael K, Van Stappen T, Vande Casteele N, et al. . Infliximab concentration thresholds during induction therapy are associated with short-term mucosal healing in patients with ulcerative colitis. Clin Gastroenterol Hepatol. 2016;14:543–549. [DOI] [PubMed] [Google Scholar]
- 72. Beswick L, Rosella O, Rosella G, et al. . Exploration of predictive biomarkers of early infliximab response in acute severe colitis: a prospective pilot study. J Crohns Colitis. 2018;12:289–297. [DOI] [PubMed] [Google Scholar]
- 73. Adedokun OJ, Sandborn WJ, Feagan BG, et al. . Association between serum concentration of infliximab and efficacy in adult patients with ulcerative colitis. Gastroenterology. 2014;147:1296–1307.e5. [DOI] [PubMed] [Google Scholar]
- 74. Adedokun OJ, Xu Z, Padgett L, et al. . Pharmacokinetics of infliximab in children with moderate-to-severe ulcerative colitis: results from a randomized, multicenter, open-label, phase 3 study. Inflamm Bowel Dis. 2013;19:2753–2762. [DOI] [PubMed] [Google Scholar]
- 75. Fasanmade AA, Adedokun OJ, Olson A, et al. . Serum albumin concentration: a predictive factor of infliximab pharmacokinetics and clinical response in patients with ulcerative colitis. Int J Clin Pharmacol Ther. 2010;48:297–308. [DOI] [PubMed] [Google Scholar]
- 76. Hyams J, Damaraju L, Blank M, et al. ; T72 Study Group Induction and maintenance therapy with infliximab for children with moderate to severe ulcerative colitis. Clin Gastroenterol Hepatol. 2012;10:391–399.e1. [DOI] [PubMed] [Google Scholar]
- 77. Rutgeerts P, Sandborn WJ, Feagan BG, et al. . Infliximab for induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2005;353:2462–2476. [DOI] [PubMed] [Google Scholar]
- 78. Gibson DJ, Heetun ZS, Redmond CE, et al. . An accelerated infliximab induction regimen reduces the need for early colectomy in patients with acute severe ulcerative colitis. Clin Gastroenterol Hepatol. 2015;13:330–335.e1. [DOI] [PubMed] [Google Scholar]
- 79. Falaiye TO, Mitchell KR, Lu Z, et al. . Outcomes following infliximab therapy for pediatric patients hospitalized with refractory colitis-predominant IBD. J Pediatr Gastroenterol Nutr. 2014;58:213–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Akintimehin AO, O’Neill RS, Ring C, et al. . Outcomes of a national cohort of children with acute severe ulcerative colitis. Front Pediatr. 2018;6:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Herrlinger KR, Barthel DN, Schmidt KJ, et al. . Infliximab as rescue medication for patients with severe ulcerative/indeterminate colitis refractory to tacrolimus. Aliment Pharmacol Ther. 2010;31:1036–1041. [DOI] [PubMed] [Google Scholar]
- 82. Maser EA, Deconda D, Lichtiger S, et al. . Cyclosporine and infliximab as rescue therapy for each other in patients with steroid-refractory ulcerative colitis. Clin Gastroenterol Hepatol. 2008;6:1112–1116. [DOI] [PubMed] [Google Scholar]
- 83. Protic M, Seibold F, Schoepfer A, et al. . The effectiveness and safety of rescue treatments in 108 patients with steroid-refractory ulcerative colitis with sequential rescue therapies in a subgroup of patients. J Crohns Colitis. 2014;8:1427–1437. [DOI] [PubMed] [Google Scholar]
- 84. Ross H, Steele SR, Varma M, et al. . Practice parameters for the surgical treatment of ulcerative colitis. Dis Colon Rectum. 2014;57:5–22. [DOI] [PubMed] [Google Scholar]
- 85. Gu J, Stocchi L, Ashburn J, Remzi FH. Total abdominal colectomy vs. restorative total proctocolectomy as the initial approach to medically refractory ulcerative colitis. Int J Colorectal Dis. 2017;32:1215–1222. [DOI] [PubMed] [Google Scholar]
- 86. Zittan E, Wong-Chong N, Ma GW, et al. . Modified two-stage ileal pouch-anal anastomosis results in lower rate of anastomotic leak compared with traditional two-stage surgery for ulcerative colitis. J Crohns Colitis. 2016;10:766–772. [DOI] [PubMed] [Google Scholar]
- 87. Mantzaris GJ, Petraki K, Archavlis E, et al. . A prospective randomized controlled trial of intravenous ciprofloxacin as an adjunct to corticosteroids in acute, severe ulcerative colitis. Scand J Gastroenterol. 2001;36:971–974. [DOI] [PubMed] [Google Scholar]
- 88. Mantzaris GJ, Hatzis A, Kontogiannis P, et al. . Intravenous tobramycin and metronidazole as an adjunct to corticosteroids in acute, severe ulcerative colitis. Am J Gastroenterol. 1994;89:43–46. [PubMed] [Google Scholar]
- 89. Chapman RW, Selby WS, Jewell DP. Controlled trial of intravenous metronidazole as an adjunct to corticosteroids in severe ulcerative colitis. Gut. 1986;27:1210–1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Dickinson RJ, O’Connor HJ, Pinder I, et al. . Double blind controlled trial of oral vancomycin as adjunctive treatment in acute exacerbations of idiopathic colitis. Gut. 1985;26:1380–1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Gionchetti P, Rizzello F, Ferrieri A, et al. . Rifaximin in patients with moderate or severe ulcerative colitis refractory to steroid-treatment: a double-blind, placebo-controlled trial. Dig Dis Sci. 1999;44:1220–1221. [DOI] [PubMed] [Google Scholar]
- 92. Turner D, Levine A, Kolho KL, et al. . Combination of oral antibiotics may be effective in severe pediatric ulcerative colitis: a preliminary report. J Crohns Colitis. 2014;8:1464–1470. [DOI] [PubMed] [Google Scholar]
- 93. Farkas K, Rutka M, Golovics PA, et al. . Efficacy of infliximab biosimilar CT-P13 induction therapy on mucosal healing in ulcerative colitis. J Crohns Colitis. 2016;10:1273–1278. [DOI] [PubMed] [Google Scholar]
- 94. Kaniewska M, Moniuszko A, Rydzewska G. The efficacy and safety of the biosimilar product (Inflectra®) compared to the reference drug (Remicade®) in rescue therapy in adult patients with ulcerative colitis. Prz Gastroenterol. 2017;12:169–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Bekheit M, Baddour N, Katri K, et al. . Hyperbaric oxygen therapy stimulates colonic stem cells and induces mucosal healing in patients with refractory ulcerative colitis: a prospective case series. BMJ Open Gastroenterol. 2016;3:e000082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Dulai PS, Gleeson MW, Taylor D, et al. . Systematic review: the safety and efficacy of hyperbaric oxygen therapy for inflammatory bowel disease. Aliment Pharmacol Ther. 2014;39:1266–1275. [DOI] [PubMed] [Google Scholar]
- 97. Dulai PS, Buckey JC, Raffals LE, et al. . Hyperbaric oxygen therapy is well tolerated and effective for ulcerative colitis patients hospitalized for moderate–severe flares: a phase 2A pilot multi-center, randomized, double-blind, sham-controlled trial. Am J Gastroenterol. In press. [DOI] [PubMed] [Google Scholar]
- 98. Bresci G, Parisi G, Mazzoni A, et al. . Granulocytapheresis versus methylprednisolone in patients with acute ulcerative colitis: 12-month follow up. J Gastroenterol Hepatol. 2008;23:1678–1682. [DOI] [PubMed] [Google Scholar]
- 99. Thanaraj S, Hamlin PJ, Ford AC. Systematic review: granulocyte/monocyte adsorptive apheresis for ulcerative colitis. Aliment Pharmacol Ther. 2010;32:1297–1306. [DOI] [PubMed] [Google Scholar]
- 100. Sands BE, Sandborn WJ, Feagan B, et al. ; Adacolumn Study Group A randomized, double-blind, sham-controlled study of granulocyte/monocyte apheresis for active ulcerative colitis. Gastroenterology. 2008;135:400–409. [DOI] [PubMed] [Google Scholar]
- 101. Kobayashi T, Suzuki Y, Motoya S, et al. . First trough level of infliximab at week 2 predicts future outcomes of induction therapy in ulcerative colitis- results from a multicenter prospective randomized controlled trial and its post hoc analysis. J Gastroenterol. 2016;51:241–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Papamichael K, Rivals-Lerebours O, Billiet T, et al. . long-term outcome of patients with ulcerative colitis and primary non-response to infliximab. J Crohns Colitis. 2016;10:1015–1023. [DOI] [PubMed] [Google Scholar]
- 103. Arias MT, Vande Casteele N, Vermeire S, et al. . A panel to predict long- term outcome of infliximab therapy for patients with ulcerative colitis. Clin Gastroenterol Hepatol. 2015;13:531–538. [DOI] [PubMed] [Google Scholar]
- 104. Van Stappen T, Bollen L, Vande Casteele N, et al. . Rapid test for inflix- imab drug concentration allows immediate dose adaptation. Clin Transl Gastroenterol. 2016;7:e206. [DOI] [PMC free article] [PubMed] [Google Scholar]