Abstract
Background
TNF-like cytokine 1A (TL1A) and its functional receptor, death-domain-receptor-3 (DR3), are multifunctional mediators of effector and regulatory immunity. We aimed to evaluate the functional role and therapeutic potential of TL1A/DR3 signaling in Crohn’s disease–like ileitis.
Methods
Ileitis-prone SAMP1/YitFc (SAMP) and TNFΔARE/+ mice were rendered deficient for DR3 or TL1A by microsatellite marker-assisted backcrossing. Pathological and immunological characteristics were compared between control and knockout mice, and mucosal immunophenotype was analyzed by Nanostring microarray assay. The therapeutic effect of pharmacological TL1A neutralization was also investigated.
Results
DR3 deficiency was associated with restoration of a homeostatic mucosal immunostat in SAMP mice through the regulation of several pro- and anti-inflammatory genes. This led to suppression of effector immunity, amelioration of ileitis severity, and compromised ability of either unfractionated CD4+ or CD4+CD45RBhi mucosal lymphocytes to transfer ileitis to severe combined immunodeficient mice recipients. TNF-driven ileitis was also prevented in TNFΔARE/+xDR3-/- mice, in association with decreased expression of the pro-inflammatory cytokines TNF and IFN-γ. In contrast to DR3, TL1A was dispensable for the development of ileitis although it affected the kinetics of inflammation, as TNFΔARE/+xTL1A-/- demonstrated delayed onset of inflammation, whereas administration of a neutralizing, anti-TL1A antibody ameliorated early but not late TNFΔARE/+ ileitis.
Conclusion
We found a prominent pro-inflammatory role of DR3 in chronic ileitis, which is only partially mediated via interaction with TL1A, raising the possibility for additional DR3 ligands. Death-domain-receptor-3 appears to be a master regulator of mucosal homeostasis and inflammation and may represent a candidate therapeutic target for chronic inflammatory conditions of the bowel.
INTRODUCTION
Inflammatory bowel disease (IBD) includes ulcerative colitis (UC) and Crohn’s disease (CD), which are immune-mediated, chronic inflammatory disorders involving dysregulated interaction between the gut mucosal immune system and luminal antigens in genetically susceptible individuals.1 Activated effector lymphocytes play a pivotal role in the pathogenesis of IBD because they heavily infiltrate the intestinal mucosa and mediate tissue damage through secretion of pro-inflammatory cytokines.2 Previously, strict diversity of effector pathways in IBD pathogenesis has been proposed leading to the “Th1/CD vs Th2/UC” paradigm.3 This model, however, has been challenged recently as accumulating evidence supports the presence of redundant and overlapping pro-inflammatory pathways in both CD and UC, spanning the whole spectrum of lymphocytic polarization.4, 5
The generation of effector T lymphocytes is initiated by the engagement of T cell receptors to their respective antigens. This process remains incomplete unless supported by “costimulatory” signals, which help to stabilize and amplify T cell activation. Although initial costimulation is typically dependent upon CD28-B7 interactions, subsequent signals are provided by members of the TNF/TNFR superfamily of proteins6. Among these, TNF-like cytokine 1A (TL1A, TNFSF15) and its functional receptor, death-receptor 3 (DR3, TNFRSF25), have recently emerged as powerful costimulatory molecules, with particular significance in mucosal immunity.7 DR3 is expressed primarily by activated lymphocytes, whereas TL1A has been detected in a variety of cell populations, including dendritic cells, macrophages, endothelial cells, and lymphocytes.8, 9 Engagement of TL1A on antigen-presenting cells to DR3 on lymphocytes provides proliferative signals for the latter and induces the secretion of cytokines.10 Initially thought to be a Th1-polarizing stimulus, TL1A/DR3 signaling has now been shown to function in a broader, nonspecific manner, leading to a generalized upregulation of effector pathways. In fact, TL1A/DR3 costimulation leads to amplification of Th1,11, 12 Th2,13 and/or Th1714, 15 responses. Moreover, these proteins may also participate in the regulation of proliferation and suppressive activity of T regulatory cells (Tregs).16 Altogether, these data strongly suggest that this cytokine ligand/receptor pair may represent a master regulator of chronic inflammation in auto-inflammatory diseases.
In recent years, evidence has accumulated to support a significant role of the TL1A/DR3 system in the pathogenesis of intestinal inflammation and IBD.17 First, activated peripheral blood and mucosal T cells and NK cells express surface DR3 and respond to stimulation with TL1A.12, 18 Interestingly, the most prominent stimulatory effect was seen for CC chemokine receptor 9–expressing lymphocytes—a small, intestinal-specific population of T lymphocytes that show an activated phenotype in CD.19 Second, mucosal expression of TL1A and DR3 is significantly elevated in inflamed intestinal areas with active IBD, and soluble TL1A is detected in the peripheral blood of patients.8, 20, 21 Third, polymorphisms in TL1A have been associated with the risk for developing IBD, independent of ethnicity, and may also have functional implications.22–25 Finally, studies in animal models of intestinal inflammation have shown that constitutive overexpression of TL1A leads to chronic small intestinal inflammation,26, 27 whereas the blockade of TL1A/DR3 suppresses chemically induced murine colitis.28
To date, the effects of TL1A and DR3 deficiency in the pathogenesis of chronic, small intestinal inflammation remain unknown. In the present study, we hypothesized that TL1A/DR3 signaling may be essential for the development and maintenance of chronic ileitis by amplifying pro-inflammatory pathways via costimulation of effector lymphocytes. To test our hypothesis, we studied the effects of DR3 or TL1A deletion in 2 models of CD-like ileitis, the spontaneous SAMP1/YitFc (SAMP), and the TNF-dependent TNFΔARE mouse strains. Our results show that DR3 is essential for the development of intestinal inflammation in both models because it critically affects the function of effector lymphocytes at the onset and during the progression of disease. Furthermore, we show that DR3 deletion normalizes inflammatory gene expression in small intestinal tissues. By comparison, TL1A-deficient mice are not protected from chronic ileitis, suggesting the possibility of TL1A-independent DR3 signaling in mucosal inflammation. Clinical trials with agents that specifically neutralize TL1A and DR3 are needed to determine the role of this cytokine/receptor pair as a novel therapeutic target for the treatment of IBD.
MATERIALS AND METHODS
Mice
Colonies of SAMP1/YitFc (SAMP, AKR/J background) and TNFΔARE/+ (C57BL/6 background) mice are maintained at the Animal Resource Center at Case Western Reserve University. AKR/J and severe combined immunodeficient (SCID) mice were purchased from Jackson Laboratories (Bar Harbor, ME). DR3-/- mice (Tnfrs25-/-, C57BL/6 background) were kindly provided by Dr. Eddie C.Y. Wang, Cardiff University, Wales, UK; TL1A-/- mice (Tnfsf15-/-, C57BL/6 background) were kindly provided by Dr. Linda Burkly from Biogen Idec Inc. (Cambridge, MA). The knockout strains of SAMPxDR3-/- and SAMPxTL1A-/- were generated by genotyping and microsatellite marker-assisted backcrossing of TL1A-/- or DR3-/- mice onto the SAMP background. SAMPxDR3+/+ and SAMPxTL1A+/+ WT littermates were used as controls. TNFΔARE/+xDR3-/-, and TNFΔARE/+xTL1A-/- mice were generated by crossing DR3-/- and TL1A-/- mice to TNFΔARE/+ mice. TNFΔARE/+xDR3+/+, and TNFΔARE/+xTL1A+/+ were used as controls. Scientific rigor, biological variables, and data reproducibility were addressed according to published guidelines.48 Mice were maintained under specific pathogen-free conditions in ventilated micro-isolator cages (Allentown Inc., NJ) with 1/8-inch corn bedding and cotton nestlets for environmental enrichment (Envigo, UK) and kept on 12-hour light/dark cycles. All mice had ad libitum access to water and standard laboratory rodent diet P3000 (Harlan Teklad) throughout the experiments. All experimental procedures were approved by the Institutional Animal Care and Use Committee and Animal Resource Center at Case Western Reserve University.
Anti-TL1A Treatment
For in vivo treatment studies (protocol A), groups of 16-week-old SAMP and TNFΔARE/+ mice (n = 6) were treated intraperitoneally twice per week for 4 weeks with a murine monoclonal antibody against TL1A (anti-TL1A mAb, kindly provided by Hailing Hsu, Amgen, Inc., Thousand Oaks, CA) at the dose of 100µg/mouse. Negative controls received an isotype immunoglobulin 1 (IgG1, Amgen, Inc., Thousand Oaks, CA) at the same dose, and positive controls were treated with Dexamethasone 3 times per week (2 mg/kg). For pretreatment studies (protocol B), groups of 4-week-old SAMP and TNFΔARE/+ mice (n = 6) were treated as in protocol A for 6 weeks. To validate the neutralizing activity of the anti-TL1A mAb, we tested the ability of increasing concentration of anti-TL1A mAb (0.1-to-1µg/mL) to block cytokine production induced by of recombinant mTL1A (100µg/mL, R&D System, Minneapolis, MN) in splenocytes and mesenteric lymph node (MLN) cells isolated from 20-week old SAMP mice. Cells, plated at the concentration of 106 cells, were incubated with 0.5 μg/mL of anti-TL1A mAb and 1.0μg/mL of anti-CD3e (clone 2C11, BD Biosciences, San Diego, CA) and soluble anti-CD28 (1 μg/mL, clone 37.51, BD Biosciences, San Diego, CA) for 48 hours, and supernatants were collected for cytokine measurement.
Adoptive Transfer
In the CD4+ adoptive transfer model, SCID mice received CD4+ donor lymphocytes from SAMP or SAMPxDR3-/- mice. In the CD45RBhi model, SCID mice received CD45RBhi donor cells from SAMP or SAMPxDR3-/- mice. Donor cells were sorted in a FACS Aria sorter (BD Biosciences, San Jose, CA) and ≥90%–95%purity was used as a cutoff for use in adoptive transfer experiments. A total of 3–10 × 104 cells per mouse were injected intraperitoneally.
Histologic Assessment of Intestinal Inflammation
Mice coli and distal ilea were removed upon sacrifice, placed in Bouin fixative solution (Fisher Scientific, Pittsburgh, PA), embedded in paraffin, and sectioned. Sections of 3 µm were stained with hematoxylin and eosin (H&E). Histological evaluation of inflammation in the colon and distal ileum was performed by a pathologist blinded to the experimental design, using a semiquantitative validated scoring system.49, 50
Myeloperoxidase Activity Assay
Ileal samples were assayed using a validated method of biochemical inflammation based on the enzymatic detection of MPO activity.51
Ileal Tissue Culture
Distal ilea were removed under sterile conditions and flushed with ice-cold PBS containing 100 U/mL of penicillin, 100 U/mL of streptomycin, and 10 μg/mL of gentamycin. Ilea was cut into 1-cm pieces, placed in 24-well plates, and incubated for 72 hours in 2-mL RPMI medium supplemented with 10% FBS and 1 × glutamine (37°C, 5% CO2). Supernatants were collected and stored at −80°C for later cytokine assays.
Isolation and Culture of CD4+ T Cells
Mesenteric lymph node cells were removed aseptically and gently pressed against a 100-μm cell strainer to obtain single-cell suspensions. Enriched CD4+ cells were obtained by magnetic sorting, using a CD4+ T cell isolation kit from Miltenyi Biotec Inc. (Auburn, CA). The procedure is reported in the publication,34 and supernatants were collected after incubation for 72 hours at 37°C and 5% CO2, and stored at −80°C until further use.
Cytokine Measurements
Cytokines (IFNγ, TNF, IL-2, IL-10, IL-4, IL-5, and IL-13) were measured by ELISA (eBioscience, San Diego, CA) or Q-Plex Array Mouse cytokine screen IR Quansys16-Plex kit (Quansys Biosciences, Logan, UT), according to the manufacturer’s instructions.
NanoString Assay
Total RNA was extracted from distal ilea of 20-week-old experimental mice using RNeasy Mini kits (Qiagen, Hilden, Germany). An inflammation panel containing 248 target genes and 6 housekeeping genes was used in the assay, which was conducted by NanoString Technologies (Seattle, Washington, USA). Raw data was analyzed by nSolver software. Data quality control (QC) was set up and validated for all samples by inclusion of 6 internal positive control probes and 8 negative control probes. In particular, the square of the Pearson correlation (R2) of positive control RNA target concentration vs counts was ≥0.98, whereas the positive control probe count (POS_E) was greater than the average negative control counts (background) in all assays. The normalization of raw data was conducted in nSolver in 2 separate steps: normalized by internal positive control genes and then normalized by reference (housekeeping) genes. The biological examination of variance between groups of mice after data normalization was followed. The fold change of gene expression between mice groups was analyzed in nSolver. Differences in gene expression were considered significant when there was a fold-change greater than 3 times compared with the control animal group and the P value for comparison between target and control group was ≤0.05.
Statistical Analysis
All data were analyzed using the 2-sided independent Student t test, with an α-level below 0.05 considered statistically significant. One- and 2-way ANOVA and Log-rank (Mantel-Cox) tests were also used as needed. All data were analyzed using GraphPad Prism 5 statistical software (La Jolla, CA).
RESULTS
DR3 Deletion Protects SAMP Mice from Developing Chronic Ileitis
We studied the effects of DR3 deficiency on ileitis development by comparing SAMPxDR3-/- and SAMP WT littermates before (5 weeks old) and after (10, 20, and 60 weeks old) the development of ileitis. Mesenteric lymph node cells from SAMPxDR3-/- mice were significantly smaller than those from SAMP mice (Fig. 1A); this observation was reflected in lower MLN weights (Fig. 1B) and total number of cells (Fig. 1C) as early as 10 weeks of age. Similarly, the terminal ilea of SAMPxDR3-/- mice did not appear enlarged or edematous (Fig. 1D), correlating to decreased disease severity compared with SAMP littermates (Fig. 1E). In addition, myeloperoxidase (MPO) activity was decreased in 10- and 20-week-old SAMPxDR3-/- ilea compared with DR3 competent SAMP (Fig. 1F). Our time-course study revealed no significant inflammation in SAMPxDR3-/- mice up to 60 weeks of age compared with SAMP littermates (Fig. 1G). All individual components of intestinal inflammation were significantly lower in SAMPxDR3-/- compared with WT littermates (Fig. 1H).
FIGURE 1.
DR3 deletion ablates ileitis in SAMP mice. A, Macroscopic comparison of MLNs from 20-week-old SAMP and age-matched SAMPxDR3-/- mice. B, MLN weights of 5-, 10- and 20-week-old SAMP and SAMPxDR3-/- mice. C, MLN cellularity of 10- and 20-week-old SAMP and SAMPxDR3-/- mice. D, Macroscopic comparison of ilea from 20-week-old SAMP and age-matched SAMPxDR3-/- mouse. E, Photomicrographs of H&E–stained sections of 20-week-old SAMP and SAMPxDR3-/- mice. F, MPO levels in ilea from 5-, 10- and 20-week old SAMP and SAMPxDR3-/- mice. G, Total inflammatory index calculated as the sum of individual scores for (H) villous distortion, active inflammation, chronic inflammation, and mononuclear cell infiltration. Representative data of 3 independent experiments and presented as average ± SEM; unpaired 2-tailed t test, *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001, n ≥ 6.
DR3 Deletion Prevents the Generation of Pro-inflammatory Effector Lymphocytic Pathways
To examine whether DR3 deficiency affects the ability of SAMP to mount effector responses, we evaluated spontaneous cytokine secretion from cultured terminal ilea of 10-week-old SAMP and age-matched SAMPxDR3-/- mice. Significantly lower production of all major effector cytokines was observed in cell cultures from SAMPxDR3-/- vs SAMPxDR3+/+ littermates (Fig. 2A); these data were confirmed in TCR-stimulated MLN cells (Fig. 2B and Suppl. Fig. 1). These results demonstrate that DR3 deficiency prevents mucosal accumulation of Th1/Th2 pro-inflammatory cytokines. To further assess the effect of DR3 signaling on the pathogenic potential of mucosal effector lymphocytes, we used the previously described SAMP→SCID adoptive transfer model29, 30 to test the hypothesis that CD4+ cells lacking DR3 lose their capacity to induce ileitis upon adoptive transfer. Overall, our study clearly showed that CD4+ lymphocytes from SAMPxDR3-/- mice failed to induce ileitis and caused less severe colitis and body weight loss in SCID recipients compared with those from SAMPxDR3+/+ mice (Figs. 3A–C). Mesenteric lymph node cellularity, colon length, and MPO tissue content was reduced in recipients of SAMPxDR3-/- vs SAMPxDR3+/+ cells (Figs. 3B–D). In addition, histological assessment of both ileal and colonic inflammation was decreased in SAMPxDR3-/- compared with SAMP recipients (Figs. 3E and 3F). Finally, the secretion of pro-inflammatory cytokines, including TNF and IFN-γ, was lower in cultured tissue homogenates from SAMPxDR3-/- compared with SAMP- recipients (Fig. 3G).
FIGURE 2.
DR3 deletion mitigates secretion of inflammatory cytokines in SAMP mice. Cytokine levels from (A) ilea and (B) TCR-stimulated MLN CD4+ cells of SAMP and SAMPxDR3-/- mice. Representative data of 3 independent experiments and presented as average ± SEM. *P < 0.05, **P < 0.01, and ***P < 0.001, n ≥ 5.
FIGURE 3.
Adoptive transfer of mucosal lymphocytes from DR3-competent—but not DR-deficient—SAMP mice induces inflammation in SCID recipients. A, Time course of body weight change; SCID mice receiving PBS only served as controls. B, MLN cellularity, (C) colon length, (D) ileal MPO, and (E) disease severity in recipient mice. F, Photomicrographs of H&E–stained sections of ilea and colons from recipient mice. G, Cytokine levels from ileal tissues of recipient mice. Data correspond to 2 independent experiments and are presented as average ± SEM. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001; 1-way ANOVA and unpaired 2-tailed t test, n = 2–4 per experiment.
The development of colitis that follows adoptive transfer of lymphocytes into immune-deficient recipients is mediated by an effector population within the fraction of CD45RBhigh–naïve CD4+ lymphocytes.31 Therefore, we hypothesized that attenuated colitis in SAMPxDR3-/- recipients may be the result of compromised pro-inflammatory potential of this population. To test this hypothesis, we repeated our adoptive transfer experiments using CD4+CD45RBhi lymphocytes from either SAMP or SAMPxDR3-/- donors. As expected, donor cells from WT SAMP induced severe colitis in SCID recipients, resulting in weight loss, reduced colon length, and histological evidence of inflammation (Suppl. Fig. 2). In contrast, when CD4+CD45RBhi lymphocytes from SAMPxDR3-/- mice were transferred into SCID mice, no weight loss or colitis was observed (Suppl. Fig. 2). These experiments established the importance of lymphocyte-derived DR3 for the development of chronic SAMP ileitis. Taken together, these results show that DR3 deficiency critically affects the function of effector lymphocytes in SAMP mice by diminishing their ability to secrete pro-inflammatory cytokines. This defect is associated with a reduced pathogenic potential of these cells and protection from ileitis.
DR3 Deficiency Ameliorates the Severity of TNF-Driven Ileitis in TNFΔARE/+ Mice
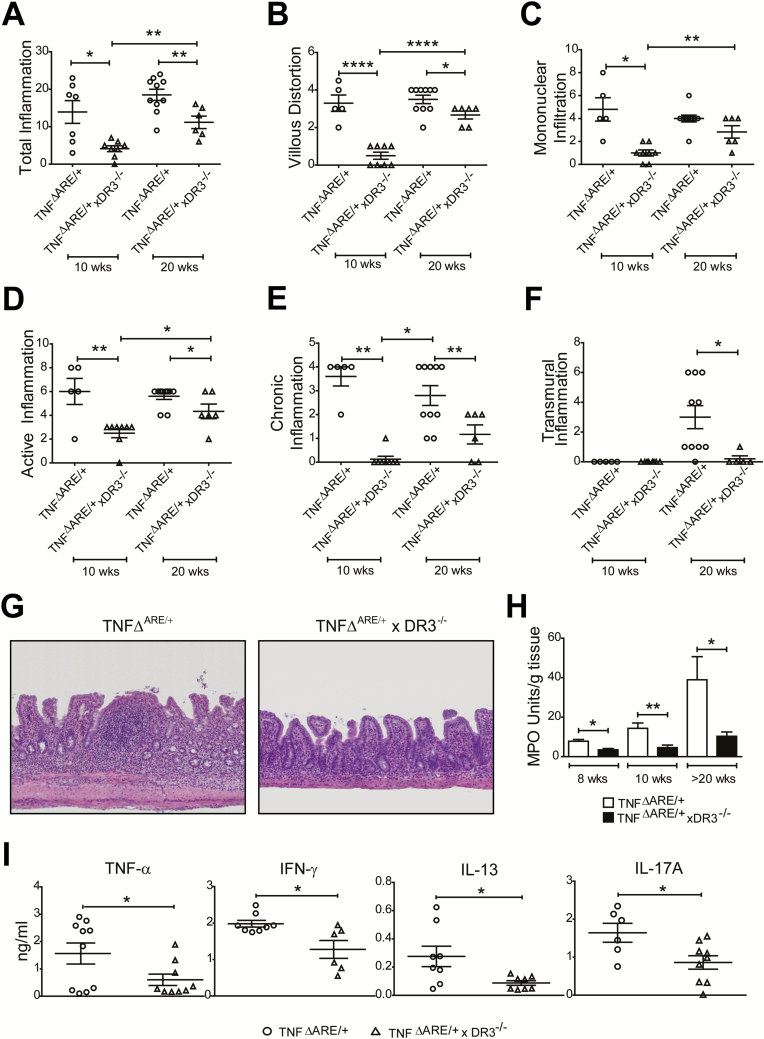
To further substantiate our findings, we tested the effects of DR3 deficiency in the TNFΔARE/+ mouse model of ileitis.32 As depicted in Fig. 4, DR3 deficiency almost completely prevented the development of small intestinal inflammation in TNFΔARE/+xDR3-/- mice at 10 weeks of age (Figs. 4A–H). Moreover, nearly all parameters of histological disease severity were decreased in TNFΔARE/+xDR3-/- versus control mice, at both 10 and 20 weeks of age (Figs. 4A–G). Similarly, ileal MPO content was lower in TNFΔARE/+xDR3-/- compared with TNFΔARE/+ mice by 8 weeks of age (Fig. 4H). Finally, the secretion of pro-inflammatory cytokines by TCR-stimulated MLN cells was suppressed in TNFΔARE/+xDR3-/- mice compared with controls (Fig. 4I).
FIGURE 4.
DR3 deletion reduces ileitis in TNFΔARE/+ mice. A, Total inflammatory index comprised of (B) villous distortion, (C) mononuclear cell infiltration, (D) active inflammation, (E) chronic inflammation, and (F) transmural infiltration in TNFΔARE/+ and TNFΔARE/+xDR3-/- mice. G, Photomicrographs of H&E–stained sections displaying severe inflammation in 20-week-old TNFΔARE/+ (right) compared with minimal inflammation in TNFΔARE/+xDR3-/- mice (left). H, MPO measured in ilea of 8-, 10- and 20-week-old TNFΔARE/+ and TNFΔARE/+xDR3-/- mice. I, Cytokine levels from TCR-stimulated MLN from 20-week-old TNFΔARE/+ and TNFΔARE/+xDR3-/- mice. Representative data of 3 independent experiments and presented as average ± SEM. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001; 1-way ANOVA and unpaired 2-tailed t test, n ≥ 4.
TL1A Is Dispensable for the Development of Chronic Ileitis
At present, the only known ligand for DR3 is TL1A. This implies that the protective effects seen with DR3 deficiency for the development of ileitis in both SAMP and TNFΔARE/+ mice are mediated through a defect in TL1A/DR3 signaling. As such, TL1A deficiency should lead to similar protective effects. Surprisingly, however, we observed that TL1A deficiency in TNFΔARE/+ mice did not prevent the development of ileitis but delayed its full establishment (Figs. 5A-C). Indeed, although ileitis persisted in TNFΔARE/+xTL1A-/- mice (Fig. 5B), they displayed lower MLN cellularity (Fig. 5A) and less severe ileitis at 8 weeks compared with TNFΔARE/+ mice (Fig. 5B); by week 20, no differences between TNFΔARE/+xTL1A-/- and TNFΔARE/+xTL1A+/+ mice were observed. Interestingly, TL1A deficiency did not appear to influence the onset or severity of ileitis in SAMP (Figs. 5D and 5E).
FIGURE 5.
TL1A deficiency ameliorates ileitis in young TNFΔARE but not in SAMP mice. A, MLN cellularity in 8- and 20-week-old TL1A-/-, TNFΔARE/+, and TNFΔARE/+xTL1A-/- mice. B, Severity of ileitis and (C) representative photomicrographs of H&E–stained sections in ilea of 8- and 20-week-old TL1A-/-, TNFΔARE/+, and TNFΔARE/+xTL1A-/- mice. D, Severity of ileitis and (E) representative photomicrographs of H&E–stained sections from 20-week-old SAMP and SAMPxTL1A-/- mice. F, Severity of ileitis in 12- and 20-week-old SAMP and TNFΔARE/+mice treated with either anti-TL1A or isotype control Ab. Representative data of 3 independent experiments and presented as average ± SEM. *P < 0.05, **P < 0.01, unpaired 2-tailed t test, n ≥ 6.
Of note, treatment of TNFΔARE/+ mice with an anti-TL1A monoclonal antibody (mAb) resulted in reduced ileitis severity at 12 weeks of age compared with those treated with isotype control (Fig. 5F); this protective effect was not seen in 20-week TNFΔARE/+ mice or in TL1A-competent SAMP mice administered anti-TL1A mAbs (Figs. 5F and 5G). We tested the ability of our anti-TL1A mAb to neutralize the effect of recombinant TL1A in splenocytes and MLN cells from SAMP mice and confirmed their ability to block secretion of multiple Th1- and Th2-type cytokines (Suppl. Fig. 3).
DR3 Deletion Restores the Mucosal Immune Balance in SAMP Mice
To further elucidate the mechanisms that underlie protection of SAMPxDR3-/- mice from ileitis, we used a multigene array to compare mRNA expression for several inflammatory mediators in SAMP vs SAMPxDR3-/- mice, using AKR to define baseline homeostatic expression (Figs. 6A–C) and observed differences for 22% of the genes studied (Table 1). Conversely, SAMPxDR3-/- mice showed a very similar pattern to AKR controls, with only 20 genes (8.06%) being significantly altered in comparison with baseline expression (Fig. 6B). Interestingly, 51 genes were significantly upregulated in inflamed SAMP and 3 were downregulated (Table 1). The highest upregulation was seen for Ccr3, Cxcr2, Alox15, and MMP3 (all >100-fold compared with AKR), whereas Defa-rs1 demonstrated a 45-fold decrease relatively to baseline AKR expression. Functional classification of the 53 genes that were different between SAMP and AKR mice showed that the majority belonged to families of immunological factors involved in adaptive and innate immune pathways (Fig. 6D). In particular, 20 genes encoding for chemokines and their receptors and 11 cytokine genes were identified. Furthermore, genes that encode for proteins of the complement-pathway (n = 5) and toll-like receptors (n = 4) were over-represented. Deletion of DR3 negatively affected the expression of all these genes and shifted expression towards baseline of AKR mice. Finally, we applied the same strict criteria of differential expression (>2-fold change and P < 0.05) to identify genes that were significantly altered between SAMP and SAMPxDR3-/- mice (Table 1). There were 14 such genes, including chemokines Ccl8, Ccl11, Ccl21a, and Cxcl3, chemokine receptor Cxcr2, the Th2 cytokines Il-4 and Il-5, toll-like receptor Tlr4, metalloproteinase MMP3, lipoxygenases Alox15, Alox5, prostaglandin D2 receptor 2, Gpr44, and Trem2. In addition, a significant increase was observed for the expression of the alpha defensin Defa-rs1.
FIGURE 6.
Effect of TL1A or DR3 deletion on ileal gene expression in SAMP compared with AKR mice. A, Correlation of Log2 normalized gene expression data in the context of AKR gene expression variability. Note the effect of DR3 deletion on gene expression and high correlation/similarity with AKR (high R2 value; n = 3 per group). B, Ranked differential gene expression in SAMP WT mice with single DR3 or TL1A deletions compared with AKR mice. Ranking of genes based on P value for WT-SAMP and AKR mice comparison. Most significant genes (n = 73 with P < 0.05) are displayed to the left of the X-axis. The deletion of DR3 normalized several abnormal genes in SAMP (note the fold-change reduction in gene expression bars in DR3-SAMP plot). Inset, Myd88 gene, mRNA read abundance example of gained significance with the DR3 deletion in SAMP. C, “Housekeeping” gene expression patterns. Notice the similar expression of SAMP-TL1A compared with SAMP WT and that of SAMP DR3 compared with AKR. D, The pie chart represents groups of genes and functions significantly modified by the DR3 deletion in SAMP (see details in Table 1).
Table 1.
Mucosal mRNA Expression of Genes Encoding Inflammatory Mediators in AKR, SAMP, and SAMPxDR3-/- Mice
| Gene Name | Effect of Inflammation (SAMP vs AKR) | Effect of DR3 Deletion (SAMP vs SAMPxDR3-/-) | ||
|---|---|---|---|---|
| Fold Change* | P | Fold Change** | P | |
| Ccr3*** | –358.71 | 0.0033 | –63.85 | 0.0386 |
| Cxcr2 | –153.6 | 0.0074 | –21.14 | 0.0202 |
| Alox15 | –138.01 | 0.0045 | –19.33 | 0.0132 |
| MMP3 | –103.83 | 0.0026 | –8.39 | 0.0334 |
| Cxcl5 | –74.66 | 0.0071 | –14.25 | 0.1058 |
| Ccl8 | –58.67 | 0.0281 | –4.52 | 0.0195 |
| Alox5 | –57.46 | 0.0056 | –5.66 | 0.0423 |
| Il1rn | –37.79 | 0.0163 | –5.04 | 0.0859 |
| Il5 | –34.56 | 0.0272 | –30.05 | 0.0291 |
| Cxcl1 | –31.56 | 0.0076 | –3.44 | 0.2033 |
| Ccl24 | –19.88 | 0.0191 | –8.85 | 0.0738 |
| Gpr44 | –19.58 | 0.0174 | –9.28 | 0.0341 |
| Ccl11 | –19.37 | 0.0035 | –4.76 | 0.0269 |
| Il4 | –19.21 | 0.0275 | –16.7 | 0.0298 |
| Trem2 | –15.77 | 0.0344 | –4.59 | 0.1305 |
| Ccl7 | –15.27 | 0.0192 | –7.5 | 0.0625 |
| Ccl2 | –14.19 | 0.0288 | –3.53 | 0.1146 |
| Tnfsf14 | –12.48 | 0.0456 | –2.41 | 0.1538 |
| Cd55 | –12.12 | 0.0002 | –4.01 | 0.0818 |
| Il1b | –12.02 | 0.0205 | –2.59 | 0.1781 |
| Tnf | –10.44 | 0.0107 | –4.7 | 0.2029 |
| Cxcl3 | –10.1 | 0.0049 | –8.78 | 0.0043 |
| Tlr4 | –8.3 | 0.001 | –2.43 | 0.0259 |
| Areg | –7.68 | 0.0472 | 1.26 | 0.3977 |
| Mmp9 | –7.53 | 0.0158 | –2.33 | 0.1048 |
| C3 | –7.21 | 0.0387 | –1.76 | 0.3796 |
| Ccl3 | –6.72 | 0.021 | –2.84 | 0.4267 |
| Ccr2 | –6.47 | 0.0048 | –2.53 | 0.0561 |
| Ccr1 | –6.21 | 0.0071 | –2.59 | 0.052 |
| Il18rap | –5.55 | 0.0329 | –1.96 | 0.2402 |
| Ccl22 | –5.23 | 0.0452 | –1.83 | 0.2282 |
| Prkcb | –4.84 | 0.0113 | –1.57 | 0.3824 |
| Tlr8 | –4.59 | 0.0133 | –1.29 | 0.3715 |
| Tslp | –4.3 | 0.0024 | –1.64 | 0.1415 |
| Cysltr1 | –4.17 | 0.018 | –1.96 | 0.0893 |
| Cd163 | –4.05 | 0.0216 | 1.37 | 0.2601 |
| Hmgb2 | –3.8 | 0.0149 | –3.33 | 0.2878 |
| Il1a | –3.73 | 0.03 | –2.96 | 0.0747 |
| C1s | –3.5 | 0.0048 | –1.17 | 0.5317 |
| Hif1a | –3.36 | 0.0431 | –2.23 | 0.0856 |
| Tlr7 | –3.31 | 0.0071 | –1.4 | 0.1888 |
| Tgfb3 | –3.2 | 0.0079 | –1.33 | 0.2018 |
| Ccl17 | –3.04 | 0.0064 | –4.34 | 0.1475 |
| Mrc1 | –2.92 | 0.0026 | –1.08 | 0.7064 |
| Tyrobp | –2.9 | 0.0362 | –2.3 | 0.0697 |
| Cd40 | –2.76 | 0.0087 | –1.61 | 0.5253 |
| C4a | –2.66 | 0.0196 | –1.1 | 0.7457 |
| Tlr6 | –2.54 | 0.012 | –1.03 | 0.8668 |
| Itgb2 | –2.46 | 0.0377 | –1.44 | 0.2772 |
| C6 | –2.35 | 0.0437 | –1.44 | 0.3031 |
| Oas1a | –2.01 | 0.0122 | –1.01 | 0.9703 |
| Prkca | 2.75 | 0.0016 | 1.53 | 0.0236 |
| Defa–rs1 | 12.1 | 0.0134 | 6.54 | 0.0272 |
| Ccl21a | 45.41 | 0.0006 | 2.85 | 0.051 |
*Minus sign denotes increased expression in SAMP mice (vs AKR).
**Minus sign denotes decreased expression in SAMP/DR3–/- mice (vs SAMP).
***Bold denotes genes with significant differences in both comparisons.
Taken together, deletion of DR3 in SAMP mice decreases the expression of several pro-inflammatory genes and resets the mucosal immunostat to the homeostatic position, preventing the occurrence of chronic intestinal inflammation.
DISCUSSION
In the present study, we report that DR3 deletion prevents the onset and progression of chronic ileitis in SAMP and TNFΔARE/+ mice by downregulating inflammatory genes and creating an immune-phenotype similar to control (AKR) mice. Death-receptor 3 deletion primarily affects the function of effector lymphocytes, which lose the capability to secrete pro-inflammatory cytokines and adoptively transfer ileitis. Finally, we show that the functional effects of DR3 deficiency are not identical to those resulting from deletion of its ligand, TL1A, raising the possibility of additional DR3-binding proteins.
The pathological similarities between SAMP ileitis and CD suggest that they may also share common immunological dysregulation. By NanoString analysis, we identified 73 inflammatory genes with significantly altered mucosal expression in SAMP vs AKR controls. Impressively, a 360- and 150-fold increase in chemokine receptors Ccr3 and Cxcr2, respectively, was noted in inflamed mucosa. C-C motif chemokine receptor 3 (CCR3) is expressed on several Th2-type immune cells, including eosinophils, mast cells, basophils, and a subset of Th2 lymphocytes.33 Thus, CCR3 may be implicated in the pathogenesis of SAMP ileitis, as the latter is characterized by pathogenic Th2-type responses, increased IL-33-dependent expression of CCR3 receptors Ccl11 and Ccl24, and therapeutic response to either depletion of eosinophils,or blockade of IL-33 signaling17, 34 Similarly, CXCR2 is a critical mediator for the recruitment of neutrophils, which play a significant role during acute flares of IBD but also heavily infiltrate ileal mucosa of SAMP mice.35 Thus, CXCR2 represents an attractive therapeutic target36 in line with the recent shift towards biological agents with anti-adhesion properties. We observed high upregulation of arachidonic acid metabolizers (ie, lipoxygenase 5 and 15, 57- and 138-fold, respectively), in SAMP mice, which further emphasizes the role of innate immunity. Recent studies have shown that lipoxygenase-deficient mice were protected from acute chemical colitis via prevention of neutrophil recruitment, whereas administration of a lipoxygenase inhibitor suppressed pro-inflammatory responses and prevented polyposis in mice.37, 38 Similarly to patients with CD, ileal expression of the α-defensin related gene defa-rs1 was markedly decreased in SAMP mice, supporting a pathogenetic role of deficient mucosal antimicrobial barrier in both clinical and experimental inflammation. Finally, we cannot exclude a potential role of microbiome alterations caused by DR3 activation or deletion during chronic intestinal inflammation. Our laboratory is actively involved in identifying the contribution of DR3 signaling to compositional and functional changes of the intestinal microbiota during experimental CD.
Our study clearly shows that deletion of DR3 restores the mucosal immunostat from inflammation to homeostasis. First, the mucosal transcriptome of SAMP/DR3-/- mice was similar to that of noninflamed AKR and clearly distinct from SAMP mice with ileitis. In fact, expression of all ileitis-inducible genes was restored to baseline levels upon abortion of DR3 signaling. Second, a clear downregulation was observed in the major effector pathways previously shown to participate in the pathogenesis of SAMP ileitis, including Th2-associated IL-4, IL-5, TSLP, and CCR3. These results are in line with earlier studies showing that SAMP ileitis is associated with mucosal abundance of Th2-related cells and mediators and that blockade of either IL-4 or IL-33 leads to significant amelioration of established disease.34, 39 In addition, other studies have also reported protection from Th2-mediated inflammation following blockade of DR3 signaling.40 The deletion of DR3 also ameliorated Th1 and Th17 responses, resulting in a mucosal milieu that was depleted from over-reactive effector adaptive responses. At the same time, however, innate cytokines were also downregulated, with significantly suppressed expression of IL-1 family members (IL1a, IL1b, IL1rn, and IL18rap). This important pro-inflammatory function of TL1A/DR3 is further supported by our recent finding that genetic deletion of DR3 is associated with a decrease in a nonregulatory FoxP3+ population with effector phenotype and expansion of IL-10–producing Tregs.41
The effects of DR3 on TNF regulation are of particular importance because TNF is considered a pivotal pathogenetic molecule in clinical and experimental IBD and a successful therapeutic target of the first generation of biological agents.42, 43 Recently, it was shown that TL1A acts upstream of TNF and regulates its expression.44 Herein, we provide mechanistic confirmation of such an effect by showing that, even in the presence of deregulated TNF over-expression in TNFΔARE/+ mice, DR3 deletion is sufficient to suppress pro-inflammatory pathways and prevent development of ileitis. This also includes a significant downregulation of TNF itself, indicating that endogenous programming for TNF overexpression is derailed in the presence of DR3 deficiency.
In our NanoString analysis, MMP3 was among the most highly upregulated genes in SAMP mice (>100-fold vs AKR), which is identical to patients with IBD (67-fold increase over baseline).45 In addition, downregulation of MMP3 efficiently discriminated responders from nonresponders to infliximab.45 In our study, MMP3 was significantly downregulated in SAMPxDR3-/- mice. As DR3 deficiency also suppressed the mucosal expression of TNF, a DR3-induced, TNF-dependent regulation of MMP3 may also exist in SAMP ileitis.
TNF-like cytokine 1A is the only known ligand for DR3 in humans. Thus, one would expect that TL1A and DR3 deletion should lead to identical immunological and pathological phenotypes. Our present study, however, clearly shows that this is not the case. In both SAMP and TNFΔARE/+ mice, deletion of TL1A led to delayed onset but not abrogation of intestinal inflammation relative to DR3-deficient mice. This raises the possibility that so far unidentified alternative ligands for DR3 may exist. Such ligands may compensate for the loss of TL1A during the late phase of ileitis, whereas DR3 deletion would eliminate signaling induced by all ligands. This hypothesis is further supported by our finding that neutralization of TL1A via mAb administration prevented the initial development of ileitis but did not suppress established disease in either model. In fact, previous reports exist of alternative DR3 binding. In one study, DR3 that was ectopically expressed on HT29 colon carcinoma cells was able to interact with endothelial E-selectin, leading to increased survival of cells.46 Moreover, DR3 was also shown to directly bind to Atsttrin, a pro-granulin derivative with the ability to bind to TNF receptors. Interestingly, Atsttrin demonstrated therapeutic properties against inflammatory arthritis.47 We are currently examining the possibility for a role of these alternative DR3 ligands during mucosal inflammation. An alternative explanation for this TL1A/DR3 functional diversity may relate to a self-stimulatory reaction of DR3 after an initial response to TL1A binding. In this case, TL1A may be necessary for initiating inflammation but not for maintaining chronic immunological responses. These data may have important translational implications, mainly regarding the therapeutic potential of TL1A and/or DR3 neutralization. Currently, anti-TL1A approaches are actively pursued, and therapeutic trials are imminent. Nevertheless, many patients with IBD usually enter clinical trials at advanced stages of disease, at which point blocking TL1A may not be effective, as shown by the inability of our anti-TL1A antibody to reverse established ileitis in SAMP mice. In support of this, both TL1A deletion and use of neutralizing anti-TL1A mAb had identical effects in early and late phases of experimental ileitis. In contrast, our results indicate that DR3 blockade may be a more attractive option, given its universal anti-inflammatory effect in either early or late stages of chronic intestinal inflammation, which is seen in our and other studies.
In conclusion, DR3 signaling appears to be a critical component of chronic ileitis, both for the initial induction and for perpetuating the maintenance phase. These effects show diversity in regard to ligand involvement because initiation of inflammation is mediated via TL1A/DR3 interaction, whereas the latter phase is TL1A-independent. This functional diversity in chronic ileitis combined with our recent report of a protective role for TL1A/DR3 during acute mucosa injury44 support the concept that these proteins are pivotal factors in mucosal immunology and merit further exploration of their pathogenic and therapeutic applications.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank Dennis Gruszka and Joshua Webster for assistance with animal husbandry and acknowledge Dr. Wei Xin for histological evaluation/interpretation. We also are grateful for the Core services provided by the Cleveland Digestive Research Core Center.
Supported by: This work was supported by National Institutes of Health Grants DK055812 to FC, DK042191 and DK091222 to FC/TTP, DK056762 to TTP, and the Crohn’s & Colitis Foundation RFA326877 to CDS. ZL is a research fellow supported by training grant T32DK083251. We also acknowledge the Mouse Models Core and the Histology/Imaging Core of the NIH P30 Silvio O. Conte Cleveland Digestive Disease Research Core Center (DK097948).
Author contributions: LFB and L-GJ contributed to the acquisition, analysis and interpretation of data, drafting of manuscript, and statistical analysis; KOA and AR-P contributed to the important intellectual content, drafting of manuscript, acquisition, analysis and interpretation of data, and statistical analysis; HT, DG, ZL, and CDS contributed to the acquisition of data; TTP contributed to the drafting and critical revision of manuscript for important intellectual content, obtained funding, important intellectual content, and study supervision; GB and FC contributed to the study concept and design, analysis and interpretation of data, drafting and critical revision of manuscript for important intellectual content, attainment of funding, and study supervision.
REFERENCES
- 1. Cader MZ, Kaser A. Recent advances in inflammatory bowel disease: mucosal immune cells in intestinal inflammation. Gut. 2013;62:1653–1664. [DOI] [PubMed] [Google Scholar]
- 2. Maynard CL, Weaver CT. Intestinal effector T cells in health and disease. Immunity. 2009;31:389–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bamias G, Cominelli F. Immunopathogenesis of inflammatory bowel disease: current concepts. Curr Opin Gastroenterol. 2007;23:365–369. [DOI] [PubMed] [Google Scholar]
- 4. Neurath MF. Cytokines in inflammatory bowel disease. Nat Rev Immunol. 2014;14:329–342. [DOI] [PubMed] [Google Scholar]
- 5. Bamias G, Nyce MR, De La Rue SA, et al. ; American College of Physicians; American Physiological Society New concepts in the pathophysiology of inflammatory bowel disease. Ann Intern Med. 2005;143:895–904. [DOI] [PubMed] [Google Scholar]
- 6. Watts TH. TNF/TNFR family members in costimulation of T cell responses. Annu Rev Immunol. 2005;23:23–68. [DOI] [PubMed] [Google Scholar]
- 7. Bamias G, Jia LG, Cominelli F. The tumor necrosis factor-like cytokine 1A/death receptor 3 cytokine system in intestinal inflammation. Curr Opin Gastroenterol. 2013;29:597–602. [DOI] [PubMed] [Google Scholar]
- 8. Bamias G, Martin C 3rd, Marini M, et al. . Expression, localization, and functional activity of TL1A, a novel Th1-polarizing cytokine in inflammatory bowel disease. J Immunol. 2003;171:4868–4874. [DOI] [PubMed] [Google Scholar]
- 9. Bamias G, Mishina M, Nyce M, et al. . Role of TL1A and its receptor DR3 in 2 models of chronic murine ileitis. Proc Natl Acad Sci U S A. 2006;103:8441–8446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Migone TS, Zhang J, Luo X, et al. . TL1A is a TNF-like ligand for DR3 and TR6/DcR3 and functions as a T cell costimulator. Immunity. 2002;16:479–492. [DOI] [PubMed] [Google Scholar]
- 11. Biener-Ramanujan E, Gonsky R, Ko B, et al. . Functional signaling of membrane-bound TL1A induces IFN-gamma expression. FEBS Lett. 2010;584:2376–2380. [DOI] [PubMed] [Google Scholar]
- 12. Papadakis KA, Zhu D, Prehn JL, et al. . Dominant role for TL1A/DR3 pathway in IL-12 plus IL-18-induced IFN-gamma production by peripheral blood and mucosal CCR9+ T lymphocytes. J Immunol. 2005;174:4985–4990. [DOI] [PubMed] [Google Scholar]
- 13. Fang L, Adkins B, Deyev V, et al. . Essential role of TNF receptor superfamily 25 (TNFRSF25) in the development of allergic lung inflammation. J Exp Med. 2008;205:1037–1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zhou M, Liu R, Su D, et al. . TL1A increased the differentiation of peripheral Th17 in rheumatoid arthritis. Cytokine. 2014;69:125–130. [DOI] [PubMed] [Google Scholar]
- 15. Pappu BP, Borodovsky A, Zheng TS, et al. . TL1A-DR3 interaction regulates th17 cell function and th17-mediated autoimmune disease. J Exp Med. 2008;205:1049–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Schreiber TH, Wolf D, Tsai MS, et al. . Therapeutic Treg expansion in mice by TNFRSF25 prevents allergic lung inflammation. J Clin Invest. 2010;120:3629–3640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. De Salvo C, Wang XM, Pastorelli L, et al. . IL-33 drives eosinophil infiltration and pathogenic type 2 helper T-cell immune responses leading to chronic experimental ileitis. Am J Pathol. 2016;186:885–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Prehn JL, Mehdizadeh S, Landers CJ, et al. . Potential role for TL1A, the new TNF-family member and potent costimulator of IFN-gamma, in mucosal inflammation. Clin Immunol. 2004;112:66–77. [DOI] [PubMed] [Google Scholar]
- 19. Saruta M, Yu QT, Avanesyan A, et al. . Phenotype and effector function of CC chemokine receptor 9-expressing lymphocytes in small intestinal Crohn’s disease. J Immunol. 2007;178:3293–3300. [DOI] [PubMed] [Google Scholar]
- 20. Bamias G, Kaltsa G, Siakavellas SI, et al. . High intestinal and systemic levels of decoy receptor 3 (DcR3) and its ligand TL1A in active ulcerative colitis. Clin Immunol. 2010;137:242–249. [DOI] [PubMed] [Google Scholar]
- 21. Bamias G, Kaltsa G, Siakavellas SI, et al. . Differential expression of the TL1A/DcR3 system of TNF/TNFR-like proteins in large vs small intestinal Crohn’s disease. Dig Liver Dis. 2012;44:30–36. [DOI] [PubMed] [Google Scholar]
- 22. Michelsen KS, Thomas LS, Taylor KD, et al. . IBD-associated TL1A gene (TNFSF15) haplotypes determine increased expression of TL1A protein. Plos One. 2009;4:e4719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Haritunians T, Taylor KD, Targan SR, et al. . Genetic predictors of medically refractory ulcerative colitis. Inflamm Bowel Dis. 2010;16:1830–1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Yamazaki K, McGovern D, Ragoussis J, et al. . Single nucleotide polymorphisms in TNFSF15 confer susceptibility to Crohn’s disease. Hum Mol Genet. 2005;14:3499–3506. [DOI] [PubMed] [Google Scholar]
- 25. Thiébaut R, Kotti S, Jung C, et al. . TNFSF15 polymorphisms are associated with susceptibility to inflammatory bowel disease in a new European cohort. Am J Gastroenterol. 2009;104:384–391. [DOI] [PubMed] [Google Scholar]
- 26. Shih DQ, Barrett R, Zhang X, et al. . Constitutive TL1A (TNFSF15) expression on lymphoid or myeloid cells leads to mild intestinal inflammation and fibrosis. Plos One. 2011;6:e16090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Meylan F, Song YJ, Fuss I, et al. . The TNF-family cytokine TL1A drives IL-13-dependent small intestinal inflammation. Mucosal Immunol. 2011;4:172–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Takedatsu H, Michelsen KS, Wei B, et al. . TL1A (TNFSF15) regulates the development of chronic colitis by modulating both T-helper 1 and T-helper 17 activation. Gastroenterology. 2008;135:552–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Pizarro TT, Pastorelli L, Bamias G, et al. . SAMP1/YitFc mouse strain: a spontaneous model of Crohn’s disease-like ileitis. Inflamm Bowel Dis. 2011;17:2566–2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kosiewicz MM, Nast CC, Krishnan A, et al. . Th1-type responses mediate spontaneous ileitis in a novel murine model of Crohn’s disease. J Clin Invest. 2001;107:695–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Powrie F, Leach MW, Mauze S, et al. . Inhibition of th1 responses prevents inflammatory bowel disease in scid mice reconstituted with CD45RBHI CD4+ T cells. Immunity. 1994;1:553–562. [DOI] [PubMed] [Google Scholar]
- 32. Kontoyiannis D, Pasparakis M, Pizarro TT, et al. . Impaired on/off regulation of TNF biosynthesis in mice lacking TNF AU-rich elements: implications for joint and gut-associated immunopathologies. Immunity. 1999;10:387–398. [DOI] [PubMed] [Google Scholar]
- 33. Annunziato F, Galli G, Cosmi L, et al. . Molecules associated with human Th1 or Th2 cells. Eur Cytokine Netw. 1998;9:12–16. [PubMed] [Google Scholar]
- 34. Bamias G, Martin C, Mishina M, et al. . Pro-inflammatory effects of TH2 cytokines in a murine model of chronic small intestinal inflammation. Gastroenterology. 2005;128:654–666. [DOI] [PubMed] [Google Scholar]
- 35. Muthas D, Reznichenko A, Balendran CA, et al. . Neutrophils in ulcerative colitis: a review of selected biomarkers and their potential therapeutic implications. Scand J Gastroenterol. 2017;52:125–135. [DOI] [PubMed] [Google Scholar]
- 36. Boppana NB, Devarajan A, Gopal K, et al. . Blockade of CXCR2 signalling: a potential therapeutic target for preventing neutrophil-mediated inflammatory diseases. Exp Biol Med (Maywood). 2014;239:509–518. [DOI] [PubMed] [Google Scholar]
- 37. Gounaris E, Heiferman MJ, Heiferman JR, et al. . Zileuton, 5-lipoxygenase inhibitor, acts as a chemopreventive agent in intestinal polyposis, by modulating polyp and systemic inflammation. Plos One. 2015;10:e0121402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Cuzzocrea S, Rossi A, Mazzon E, et al. . 5-Lipoxygenase modulates colitis through the regulation of adhesion molecule expression and neutrophil migration. Lab Invest. 2005;85:808–822. [DOI] [PubMed] [Google Scholar]
- 39. Bamias G, Okazawa A, Rivera-Nieves J, et al. . Commensal bacteria exacerbate intestinal inflammation but are not essential for the development of murine ileitis. J Immunol. 2007;178:1809–1818. [DOI] [PubMed] [Google Scholar]
- 40. Yu X, Pappu R, Ramirez-Carrozzi V, et al. . TNF superfamily member TL1A elicits type 2 innate lymphoid cells at mucosal barriers. Mucosal Immunol. 2014;7:730–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Li Z, Buttó LF, Buela KA, et al. . Death receptor 3 signaling controls the balance between regulatory and effector lymphocytes in SAMP1/YitFc mice with Crohn’s disease-like ileitis. Front Immunol. 2018;9:362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Bamias G, Dahman MI, Arseneau KO, et al. . Intestinal-specific tnfα overexpression induces Crohn’s-like ileitis in mice. Plos One. 2013;8:e72594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Marini M, Bamias G, Rivera-Nieves J, et al. . TNF-alpha neutralization ameliorates the severity of murine Crohn’s-like ileitis by abrogation of intestinal epithelial cell apoptosis. Proc Natl Acad Sci U S A. 2003;100:8366–8371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Jia LG, Bamias G, Arseneau KO, et al. . A novel role for TL1A/DR3 in protection against intestinal injury and infection. J Immunol. 2016;197:377–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. de Bruyn M, Machiels K, Vandooren J, et al. . Infliximab restores the dysfunctional matrix remodeling protein and growth factor gene expression in patients with inflammatory bowel disease. Inflamm Bowel Dis. 2014;20:339–352. [DOI] [PubMed] [Google Scholar]
- 46. Porquet N, Poirier A, Houle F, et al. . Survival advantages conferred to colon cancer cells by E-selectin-induced activation of the PI3K-NFκB survival axis downstream of death receptor-3. BMC Cancer. 2011;11:285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Liu C, Li XX, Gao W, et al. . Progranulin-derived Atsttrin directly binds to TNFRSF25 (DR3) and inhibits TNF-like ligand 1A (TL1A) activity. Plos One. 2014;9:e92743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Arseneau KKO, Cominelli F. Improving the reproducibility and quality of reporting for animal studies in inflammatory bowel disease. Inflamm Bowel Dis. 2017;23:2069–2071. [DOI] [PubMed] [Google Scholar]
- 49. Rivera-Nieves J, Bamias G, Vidrich A, et al. . Emergence of perianal fistulizing disease in the SAMP1/YitFc mouse, a spontaneous model of chronic ileitis. Gastroenterology. 2003;124:972–982. [DOI] [PubMed] [Google Scholar]
- 50. Pastorelli L, Garg RR, Hoang SB, et al. . Epithelial-derived IL-33 and its receptor ST2 are dysregulated in ulcerative colitis and in experimental Th1/Th2 driven enteritis. Proc Natl Acad Sci U S A. 2010;107:8017–8022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Corridoni D, Kodani T, Rodriguez-Palacios A, et al. . Dysregulated NOD2 predisposes SAMP1/YitFc mice to chronic intestinal inflammation. Proc Natl Acad Sci U S A. 2013;110:16999–17004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.