Summary
A 58-year-old man with a history of diabetes mellitus and end-stage renal disease acquired pneumonia with acute respiratory failure during his stay in an intensive care unit (ICU). Empirical antimicrobial therapy with ceftazidime and vancomycin was initiated, and imipenem replaced ceftazidime 2 days later due to the patients pulmonary condition failed to improve. However, within 5 days, pulmonary consolidation rapidly progressed to necrotizing pneumonia complicated by lung abscess, empyema, pyopneumothorax, and tension pneumothorax, leading to the patient's death. After the patient had died, all bacterial isolates from cultures of pleural effusion, blood, and tracheal aspirate were identified as Enterobacter cloacae (E. cloacae), which was susceptible to imipenem but resistant to ceftazidime. E. cloacae should be considered in the differential diagnosis of complicated necrotizing pneumonia with lung abscess, empyema, pyopneumothorax, and tension pneumothorax. Carbapenem therapy should be immediately initiated until the pathogen in such rapidly progressive ICU-acquired pneumonia is confirmed. Increased awareness among physicians regarding E. cloacae-induced complicated necrotizing pneumonia acquired in ICUs could enable earlier detection and appropriate antimicrobial therapy for this invasive disease.
Keywords: Enterobacter cloacae, necrotizing pneumonia, lung abscess, pyopneumothorax, intensive care unit
1. Introduction
Enterobacter cloacae (E. cloacae) is one of the most frequently isolated and virulent human pathogens in its genus, and is increasingly significant as an intensive care unit (ICU)-acquired pathogen (1,2). This microorganism can cause a wide variety of infections, including bacteremia, soft tissue and ophthalmic infections (1), pneumonia (2), endocarditis (3), urinary tract infections (4), central nervous system infections (5), and osteomyelitis (6). Two cases of necrotizing pneumonia caused by E. cloacae were also reported (7,8). E. cloacae has emerged as a nosocomial pathogen from intensive care patients, especially who are on mechanical ventilation (9). However, no cases of E. cloacae-induced complicated necrotizing pneumonia with lung abscess, empyema, pyopneumothorax, and tension pneumothorax occurring simultaneously in an ICU has been reported. This paper reports an intractable case of ICU-acquired E. cloacae as the cause of fatal complicated necrotizing pneumonia. Ceftazidime or piperacillin-tazobactam is usually used empirically to treat ICU-acquired pneumonia. However, the increasing prevalence of extended-spectrum-beta-lactamase-producing E. cloacae is becoming a concern, not only for infection therapy and empirical use of antibiotics but also for infection control programs (10). This case also highlights the importance of choosing appropriate empirical antimicrobial therapy for E. cloacae-induced complicated necrotizing pneumonia in ICUs.
2. Case Report
A 58-year-old man with a history of diabetes mellitus and end-stage renal disease was admitted to an ICU of Tainan Municipal Hospital in March 2017 for hyperkalemia (serum potassium level: 7.06 mmol/L) resulting in bradycardia, shock, and acute respiratory failure. After cardiopulmonary cerebral resuscitation, endotracheal intubation with ventilator support, vasopressor therapy, and emergent hemodialysis, the patient's general condition gradually improved and he regained consciousness on day 3. He was successfully weaned from the ventilator and the endotracheal tube was removed on day 5.
However, the patient subsequently developed shortness of breath that progressed to respiratory distress on day 7. Arterial blood gas (ABG) analysis revealed a pH of 7.287, PaCO2 level of 58.5 mmHg, PaO2 level of 113.8 mmHg, and bicarbonate concentration of 27 mmol/L while the patient was breathing through a non-rebreathing mask. He underwent secondary endotracheal intubation with ventilator support for acute hypercapnia respiratory failure. Laboratory examinations yielded a white blood cell count of 14,950/μL with 84% neutrophils; a platelet count of 92,000/μL; and a C-reactive protein level of 6.28 mg/dL (reference range: < 0.3 mg/dL). A chest radiograph (Figure 1A) revealed bilateral consolidation, suggesting pneumonia. Empirical broad-spectrum antimicrobial therapy consisting of ceftazidime (2 g every 8 hours) and vancomycin (1 g every 12 hours) was immediately initiated. The patient's pulmonary condition failed to improve within the first 2 days (days 8 and 9) of antimicrobial therapy. Imipenem (500 mg every 6 hours) replaced ceftazidime on day 10. However, tachypnea, respiratory distress, decreased consciousness, and shock occurred on day 11. A physical examination revealed absent breath sounds on the right side, subcutaneous crepitus over the right chest wall, and a distended abdomen. Under the impression of tension pneumothorax, a needle was promptly inserted into the right upper intercostal space for chest decompression. A large amount of air immediately flowed from the needle, thereby increasing the patient's blood pressure. ABG analysis revealed a pH of 7.081, PaCO2 level of 83.4 mmHg, PaO2 level of 130.4 mmHg, and bicarbonate concentration of 24.2 mmol/L while the patient was breathing 100% FiO2. Laboratory examinations yielded a white blood cell count of 19,320/μL with 88% neutrophils; a platelet count of 79,000/μL; and a serum potassium level of 3.89 mmol/L. A chest radiograph (Figure 1B) revealed cavitation in the consolidation of the right lower and left upper lobes. Moreover, subcutaneous emphysema was observed. A right chest tube thoracostomy was promptly performed, and air and 500 mL of a foul-smelling reddish pleural effusion were drained. The patient's right pleural effusion was cloudy and contained 7 mg/dL glucose (serum glucose: 180 mg/dL), 4.6 mg/dL protein (serum protein: 5.4 mg/ dL), 18,720 U/L lactate dehydrogenase (serum lactate dehydrogenase: 510 U/L), 100,000/μL red blood cells, and 80,000/μL white blood cells with 87 % neutrophils. Pleural effusion, blood, and tracheal aspirate cultures were performed. Computed tomography (CT; Figure 2) revealed necrotizing pneumonia, lung abscess, pyopneumothorax, pneumoperitoneum, and subcutaneous emphysema. The patient's condition rapidly deteriorated and he died on day 12.
Figure 1.
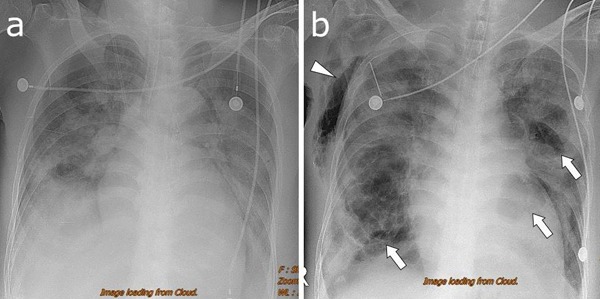
(A) Chest radiography on hospital day 7 reveals a diffuse consolidation of the left lung and patchy areas of the consolidation in the right lung. (B) Radiography on hospital day 12 shows multiple cavities of variable sizes within the consolidation of the bilateral lungs (arrows). Subcutaneous emphysema of the right chest can be observed (arrowhead). A needle is inserted in the right upper intercostal space.
Figure 2.
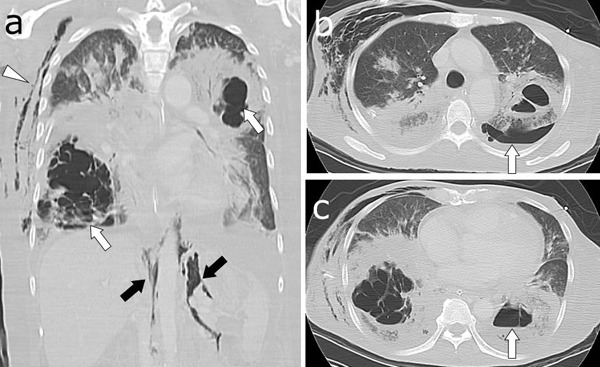
(A) Lung window of a CT shows necrotizing pneumonia in the right lower and left upper lobes (white arrows). Subcutaneous emphysema (arrowhead) and pneumoperitoneum (black arrows) can be observed. (B) An air-fluid level is present in the left pleural space (arrow), representing pyopneumothorax. (C) A cavity contains an air-fluid level within the left lower lobe consolidation (arrow), suggesting lung abscess. CT, computed tomography.
Bacterial isolates were identified using the BD Phoenix automated microbiology system (Becton Dickinson Diagnostic Systems, Sparks, MD, USA). Antimicrobial susceptibility was tested using the disc diffusion method. Interpretation was performed according to the criteria of the Clinical and Laboratory Standards Institute (11). After the patient had died, all bacterial isolates were identified as E. cloacae, and the results of antibiotic susceptibility testing indicated susceptibility to imipenem, levofloxacin, aminoglycosides, and tigecycline but resistance to ceftriaxone, ceftazidime, cefmetazole, trimethoprim-sulfamethoxazole (TMP-SMX), and ampicillin-sulbactam.
3. Discussion
We searched PubMed Advanced Search Builder for papers published in English until 30 September 2018 using the following terms: ("Enterobacter cloacae" [All Fields] AND ("necrotizing pneumonia" [All Fields]). Of the 5 results, 3 were not relevant. Of the rest 2 results, necrotizing pneumonia caused by E. cloacae was reported in an adult and a neonate, respectively (7,8). According to our review of relevant studies, this case report is the first to describe E. cloacae as the cause of life-threatening pulmonary complications including necrotizing pneumonia, lung abscess, empyema, pyopneumothorax, and tension pneumothorax occurring simultaneously in an ICU. In our case, the patient likely acquired an E. cloacae infection through the respiratory tract during his ICU stay because of his underlying immunocompromised status. Common organisms associated with necrotizing pneumonia include Streptococcus pneumoniae, Staphylococcus aureus (S. aureus), methicillin-resistant S. aureus, Klebsiella pneumoniae, and Pseudomonas aeruginosa (12). E. cloacae should be considered in the differential diagnosis of complicated necrotizing pneumonia with lung abscess, empyema, pyopneumothorax, and tension pneumothorax. E. cloacae is predictably resistant to ampicillin and cefoxitin; however, aminoglycosides and ciprofloxacin are active against most strains of E. cloacae, whereas TMP-SMX has variable activity (1). Although ceftazidime or piperacillin-tazobactam is usually used empirically to treat ICU-acquired pneumonia, these are not appropriate antimicrobial therapies for ICU-acquired complicated necrotizing pneumonia caused by E. cloacae. E. cloacae strains that possess chromosomally encoded ampC β-lactamases can develop resistance to broad-spectrum cephalosporins (13). In addition, E. cloacae strains with extended-spectrum β-lactamases are increasingly described worldwide (14), and the emergence of greater resistance to carbapenems has become a global concern (15). However, carbapenems remain among the most active β-lactam antibiotics used to fight E. cloacae (1). In our case, the patient's underlying immunocompromised status was the major cause of his death. Early initiation of carbapenems therapy in patients with underlying immunocompromised status could exert an appropriate therapeutic effect, thereby yielding an improved prognosis. Therefore, carbapenems therapy should be immediately initiated until the pathogen in such rapidly progressive ICU-acquired pneumonia is confirmed.
The true pathogenesis of E. cloacae-induced complicated necrotizing pneumonia remains unclear. E. cloacae is known to produce enterotoxins, α-hemolysin and thiol-activated pore-forming cytotoxins similar to the Panton-Valentine leukocidin produced by S. aureus, which causes leukocyte destruction and tissue necrosis (16,17). Thus, the potential pathogenesis of complicated necrotizing pneumonia is associated with pore-forming cytotoxins produced by E. cloacae.
In this case, although necrotizing pneumonia could be diagnosed using a plain radiograph, the degree of parenchymal destruction was underestimated. Moreover, detection of other pulmonary complications including empyema, lung abscess, and pyopneumothorax on a plain radiograph was difficult. Therefore, CT should be employed immediately after plain radiography if a patient's condition progressively deteriorates to confirm the diagnosis and assess parenchymal complications that are not evident on the plain radiograph.
In conclusion, E. cloacae should be considered in the differential diagnosis of complicated necrotizing pneumonia with lung abscess, empyema, pyopneumothorax, and tension pneumothorax acquired in an ICU. Empirical carbapenems therapy should be immediately initiated until the pathogen in rapidly progressive pneumonia is confirmed. Increased awareness among physicians regarding E. cloacae-induced complicated necrotizing pneumonia acquired in ICUs could enable earlier detection and appropriate antimicrobial therapy for this invasive disease.
References
- 1. Sanders WE Jr, Sanders CC. Enterobacter spp.: Pathogens poised to flourish at the turn of the century. Clin Microbiol Rev. 1997; 10:220-241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hennigs JK, Baumann HJ, Schmiedel S, Tennstedt P, Sobottka I, Bokemeyer C, Kluge S, Klose H. Characterization of Enterobacter cloacae pneumonia: A single-center retrospective analysis. Lung. 2011; 189:475-483. [DOI] [PubMed] [Google Scholar]
- 3. Moon J, Smith T, Sahud AG, Bhanot N. An unusual etiology of infective endocarditis: Enterobacter cloacae. J Infect Chemother. 2012; 18:925-930. [DOI] [PubMed] [Google Scholar]
- 4. Lee CH, Lee YT, Kung CH, Ku WW, Kuo SC, Chen TL, Fung CP. Risk factors of community-onset urinary tract infections caused by plasmid-mediated AmpC β-lactamase-producing Enterobacteriaceae. J Microbiol Immunol Infect. 2015; 48:269-275. [DOI] [PubMed] [Google Scholar]
- 5. Cascio A, Mezzatesta ML, Odierna A, Di Bernardo F, Barberi G, Iaria C, Stefani S, Giordano S. Extended-spectrum beta-lactamase-producing and carbapenemase-producing Enterobacter cloacae ventriculitis successfully treated with intraventricular colistin. Int J Infect Dis. 2014; 20:66-67. [DOI] [PubMed] [Google Scholar]
- 6. Yuvaraj A, Mathew M, Nair S, Nagarajan P, Abraham A, Abraham G. Enterobacter cloacae osteomyelitis induced post-infectious glomerulonephritis on diabetic nephropathy with progressive renal failure. Clin Kidney J. 2013; 6:659-661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Broughton WA, Kirkpatrick MB. Acute necrotizing pneumonia caused by Enterobacter cloacae. South Med J. 1988; 81:1061-1062. [DOI] [PubMed] [Google Scholar]
- 8. Al Tassan RS, Al Alola S, Al Shaalan H, Al Saleh S. Necrotizing pneumonia following cardiac surgery in a neonate. J Infect Public Health. 2013; 6:154-157. [DOI] [PubMed] [Google Scholar]
- 9. Mezzatesta ML, Gona F, Stefani S. Enterobacter cloacae complex: Clinical impact and emerging antibiotic resistance. Future Microbiol. 2012; 7:887-902. [DOI] [PubMed] [Google Scholar]
- 10. Manzur A, Tubau F, Pujol M, Calatayud L, Dominguez MA, Peña C, Sora M, Gudiol F, Ariza J. Nosocomial outbreak due to extended-spectrum-beta-lactamase-producing Enterobacter cloacaein a cardiothoracic intensive care unit. J Clin Microbiol. 2007; 45:2365-2369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Clinical and Laboratory Standards Institute. Performance standards for antimicrobial susceptibility testing; 24th informational supplement M100-MS24. Wayne, PA: CLSI; 2014; pp. 50-57. [Google Scholar]
- 12. Chatha N, Fortin D, Bosma KJ. Management of necrotizing pneumonia and pulmonary gangrene: A case series and review of the literature. Can Respir J. 2014; 21:239-245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jacoby GA, Munoz-Price LS. The new beta-lactamases. N Engl J Med. 2005; 352:380-391. [DOI] [PubMed] [Google Scholar]
- 14. Lee CC, Lee NY, Yan JJ, Lee HC, Chen PL, Chang CM, Wu CJ, Ko NY, Wang LR, Chi CH, Ko WC. Bacteremia due to extended-spectrum-beta-lactamase-producing Enterobacter cloacae: Role of carbapenem therapy. Antimicrob Agents Chemother. 2010; 54:3551-3556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Yang FC, Yan JJ, Hung KH, Wu JJ. Characterization of Ertapenem-Resistant Enterobacter cloacae in a Taiwanese University Hospital. J Clin Microbiol. 2012; 50:223-226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mezzatesta ML, Gona F, Stefani S. Enterobacter cloacae complex: Clinical impact and emerging antibiotic resistance. Future Microbiol. 2012; 7:887-902. [DOI] [PubMed] [Google Scholar]
- 17. Genestier AL, Michalete MC, Prévoset G, Bellot G, Chalabreysse L, Peyrol S, Thivolet F, Etienne J, Lina G, Vallette FM, Vandenesch F, Genestier L. Staphylococcus aureus Panton-Valentine leukocidin directly targets mitochondria and induces Bax-independent apoptosis of human neutrophils. J Clin Invest. 2005; 115:3117-3127. [DOI] [PMC free article] [PubMed] [Google Scholar]


