Summary
Hereditary haemorrhagic telangiectasia (HHT) results in arteriovenous malformations (AVMs), most commonly in the lungs, liver and brain. Discussion of cerebral vascular malformations is an important element of patient management. The current study objectives were to examine uptake and results of screening cerebral magnetic resonance (MR) scans, excluding symptomatic patients requiring neurological investigations. The remaining non-symptomatic individuals received formal pretest counselling that differed according to family history. For the 603 patients with no neurological symptoms of concern, screening scan uptake was higher after publication of the ARUBA trial. Patients with a family history of cerebral haemorrhage were 4 to 14-fold more likely to have a screening scan than patients with no such family history. For patients without neurological symptoms suggesting cerebral AVMs, none of the 59 screening scans performed at our institution demonstrated a cerebral AVM. Four scans (6.8%) demonstrated small aneurysms. The most common abnormality was cerebral infarction (20/59, 33.9%), predominantly identified in patients with pulmonary AVMs. Of 29 pulmonary AVM patients with no previous history of clinical stroke, 16 (55.2%) had between one and five silent infarcts. For HHT patients with pulmonary AVMs, the most frequently affected sites were the cerebellum (40%) and thalamus (14.3%), and the age-adjusted odds ratio for an infarct was 21.6 (95% confidence intervals 3.7, 126), p = 0.001. We concluded that for cerebral screening programmes in HHT, the findings support informed patient choice incorporating understanding that cerebral AVMs are rare in non-symptomatic HHT patients, but that screening scans commonly detect silent cerebral infarction due to pulmonary AVMs.
Keywords: Cerebral infarction, cardioembolic stroke, counselling, magnetic resonance imaging, paradoxical emboli, arteriovenous fistulas, stroke, pulmonary arteriovenous malformations
1. Introduction
Hereditary haemorrhagic telangiectasia (HHT) is a vascular dysplasia that is inherited as an autosomal trait (1). Epidemiological studies in a variety of countries suggest HHT affects approximately 1 in 6,000 individuals (2-4) although there are regional differences, and higher prevalences in isolated communities due to founder effects (3,5). HHT results in mucocutanous and visceral vascular malformations with nosebleeds (epistaxis) the hallmark of HHT. Arteriovenous malformations (AVMs) are most frequent in the lungs and liver, with pulmonary and hepatic AVMs each affecting approximately 50% of HHT patients (6,7). Latest data suggest cerebral AVMs affect between 7.7-12.8% of HHT patients (8-10). Cerebral AVMs and fistulae raise concern due to the possibility of cerebral haemorrhage, and it is the risk of undiagnosed cerebral AVMs that seems to cause most concern to HHT communities (11,12).
Risk benefit considerations strongly support the screening of asymptomatic individuals with HHT to identify pulmonary AVMs, and if identified, to treat in order to reduce the risks of paradoxical ischaemic stroke and cerebral abscess, and improve low blood oxygen levels (hypoxaemia) (13). Asymptomatic screening programmes have been in place for decades, with treatments (usually by embolization) demonstrated to reduce the risk of ischaemic stroke and brain abscess (14). Patients who have had pulmonary AVMs embolized are often left with smaller, untreatable AVMs (15,16) and remain at lower (16,17) but not negligible risks of complications.
Although the risk-benefit considerations for cerebral AVM screening have always been less clear cut than for pulmonary AVMs, HHT patients commonly undergo screening cerebral magnetic resonance imaging (MRI) scans for the purposes of identifying cerebral vascular malformations (8-10,18). We, like many other groups, initially considered the risk of cerebral haemorrhage to be sufficient to support asymptomatic screening (19). International Guidelines generated in 2006 (18) recommended screening of "adult patients with possible or definite HHT for cerebral vascular malformations" although at marginal levels of evidence and agreement (level of evidence III; strength of recommendation: weak; agreement: 77%). Taken together with the non-negligible risks of treatments, the practice of screening remained controversial in many countries, and was not universally adopted. Publication of the ARUBA trial (20), demonstrating that for unruptured cerebral AVMs in the general population, stroke risk was higher following treatments used at that time, and data that suggest haemorrhage risk may be lower for cerebral AVMs in patients with HHT than in the general population (21,22), have heightened the controversy (23).
Good clinical practice advocates involving patients in important decisions regarding their care, and this is particularly relevant where the question concerns an area for which evidence to support a particular investigation or treatment is less than compelling. For this reason, we offer pretest counselling prior to arranging any screening cerebral MR scan. Further, to support the screening pathway, in 2010 we adopted the formal categorisation proposed by the late Pr. Pierre Lasjaunias (24): Patients are assigned to Group 1 if they have neurological symptoms potentially of concern, and a cerebral MR scan is organised to investigate the symptoms, alongside formal neurological review. The remaining non-symptomatic group receive formal pretest counselling that differs according to their family history. Patients are assigned to Group 2 if they have no personal symptoms that raise concern regarding cerebrovascular malformations, but where at least one family member has had a cerebral haemorrhage (this was an adaptation of general population recommendations regarding familial Berry aneurysms (24)). For patients assigned to Group 2 due to their family history in the absence of personal symptoms of concern, we suggest they may wish to have a cerebral MR scan to rule out high haemorrhagic-risk lesions such as aneurysms or arteriovenous fistulae. Patients with no symptoms and no family history of cerebral haemorrhage are assigned to Group 3. We inform them that they are in our lowest risk category, do not recommend a scan, though say that a scan can be arranged if they wish.
There do not appear to have been any studies on pre-test counselling for HHT patients prior to cerebral AVM screening scans, or patient choices that result after such counselling. The aim of this study were to evaluate the uptake of screening opportunities, and the outcomes of the cerebral screening scans, in order to add to the information provided to patients considering screening for cerebral AVMs.
2. Materials and Methods
2.1. Ethical approvals
Ethics approval was from the Hammersmith and Queen Charlottes Local Research Ethics Committee (LREC 2000/5764: "Case Notes Review: Hammersmith Hospital patients with pulmonary arteriovenous malformations and hereditary haemorrhagic telangiectasia (HHT).'') The ethics committee approved the review of the case notes for research purposes without seeking individual consents.
2.2. Cerebral AVM screens: Informed choice
As part of routine management, at our HHT reference centre that receives HHT referrals from across the UK, all patients with HHT are informed of an approximate 10% risk of cerebral AVMs. Discussions have evolved, with 3 key boundaries to time intervals (Figure 1). Two are universally applicable - the 2008 acceptance of HHT International Guidelines (18) which recommended screening MR scans for all HHT patients (although at marginal levels of evidence and agreement (level of evidence III; strength of recommendation: weak; agreement: 77%)), and the 2014 publication of the ARUBA trial that demonstrated for unruptured AVMs, stroke risk was higher following current treatments (20). The third time boundary was specific to our unit-the local formal categorisation of individual patients according to symptoms and family history (24). This was instituted as a formal clinic guidance sheet in early 2010, in order to assist patients to make an informed choice as to whether to have a screening cerebral MRI scan or not (Supplementary Figure S1, http://www.irdrjournal.com/ action/getSupplementalData.php?ID=31). The ARUBA data have been referred to in pre-test counselling, but as they refer to general population data, have not been added to the HHT-specific cerebral AVM screening sheet (Supplementary Figure S1, http://www.irdrjournal.com/ action/getSupplementalData.php?ID=31).
Figure 1.
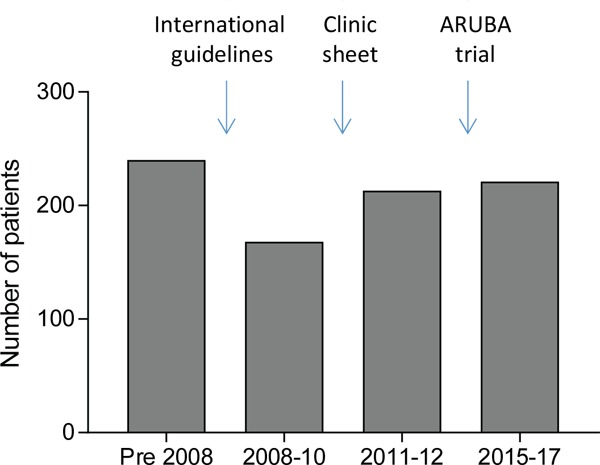
Audit time periods and boundaries. Number of patients refers to number of HHT cases audited across the 4 time periods. Bar charts indicate the % (mean) and 95% confidence intervals of the mean.
To evaluate the evolution of cerebral AVM screening recommendations, two audits of HHT patient notes were performed. The first in 2012 categorised patients into 3 periods of relatively comparable numbers: pre- 2008 (pre-international guidelines recommending scan); 2008-2009 (pre-formal categorisation sheet), and 2010- February 2012. A fourth group was added in 2018, of new HHT patients first reviewed consecutively in 2015-2017 for whom the ARUBA trial results (20) were consistently mentioned in clinic discussions.
To evaluate clinical features present at the time of the MR scans, the original handwritten patient paper notes including clinic letters were reviewed. Since reported scan results influenced subsequent management (e.g. re-review of symptoms knowing the site of an abnormality; referral to neurology etc), only features documented prior to the scan date were analysed, though note was made of subsequent clinical progress.
2.3. Cerebral MR Scans and evaluations
To optimise consistency of scan methodologies and comparisons, scan results focussed purely on those performed at our institution. For the purposes of this study, all MR screening scans were evaluated, including 12 performed between March 2012-2014 when the uptake rates were not formally audited because the advice to patients was inconsistent during this period. Two emergency scans performed early in the series (and of inferior quality to the remainder) were excluded.
The screening MR scans had been performed between 18/08/2000 and 08/03/2018, all using a 1.5 Tesla (T) device, though there were evolutions of scan protocols with time: A T2-weighted image was performed in all patients. 59 (98%) had T1-weighted imaging; 58 (98%) had fluid-attenuated inversion recovery imaging (FLAIR); 58 (98%) had diffusion weighted imaging (DWI); 14 (24%) had susceptibility weighted imaging (SWI) or gradient echo T2*; 50 (85%) had gadolinium (contrast) enhanced imaging; and 58 (98%) had time-of-flight angiography (TOF-MRA). From 2017 onwards, 67% of patients had SWI in keeping with an update to the standard imaging sequence protocol. All scans were analysed using visual diagnosis by two independent neuroradiologists, blinded to patient demographics and PAVM status. Standardisation was ensured with the addition of a further Radiologist opinion should there be a discrepancy between reports, after which a consensus agreement was reached.
2.4. Clinical corollaries
Scan data were supplied in Excel charts to a third researcher who unblinded the data, added clinical demographics and performed statistical analyses. All patients who had MRIs had already had at least one thoracic computerised tomography (CT) scan to screen for pulmonary AVMs.
2.5. Data Analyses
For data analysis and to generate graphs, Excel chart data were uploaded to STATA IC v15 (Statacorp, Texas) and Graph Pad Prism 7.03. Summary statistics were generated. For comparison of groups, patients were categorised by time-period of screening, family history of haemorrhage (Group 2 present, versus Group 3, none), or by presence/absence of pulmonary AVMs. For three or more groups, p values were calculated using Kruskal Wallis with Dunn's post test correction used. For two groups, p values were calculated by Mann Whitney, or for categorical analyses, using Fisher's exact test. STATA IC v15 was also used to perform multiple regression analyses.
3. Results
3.1. Overview of screening scan uptake cohort
Over the study period, case records of 864 patients with HHT were audited. Data on cerebral screening were available for 842. 188/842 had already had scans performed externally, predominantly for investigation of acute cerebrovascular events. For these scans arranged by other hospitals, over the period of study, there was a trend for the overall number of HHT patients having a cerebral MR scan to increase, with the proportion relatively unchanged since 2008 (Figure 2A). A further 51 patients had neurological symptoms of potential concern (Group 1) and the identification of cerebral vascular malformations in their scans is to be reported elsewhere. The current manuscript presents uptake of screening scans in the remaining 603 "non-symptomatic" patients who were categorised by presence (Group 2) or absence (Group 3) of a family history of cerebral haemorrhage.
Figure 2.
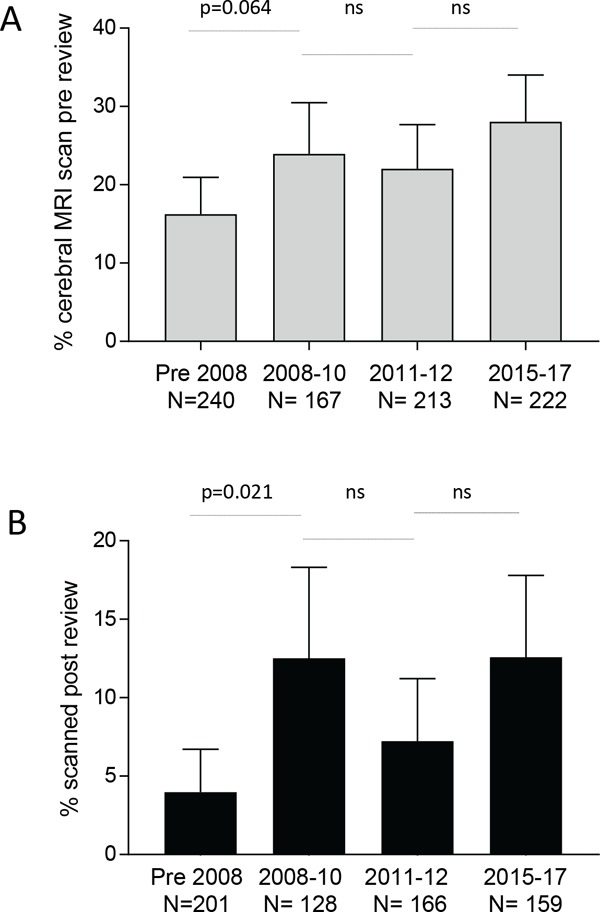
Overall MR scans rates. Percentage of 842 patients audited who had MR scans performed for any indication, across the 4 time periods as in Figure 1: (A) Prior to review by us (external scans), and (B) Following review by us (internal and external scans). Bar charts indicate the % (mean) and 95% confidence intervals of the mean. Displayed p-values calculated by Dunn's test after Kruskal Wallis (overall p-values A: p = 0.023; B: p = 0.0091).
3.2. Cerebral MR screening scans rates
Figure 2B demonstrates the screening scan rates in the 603 HHT patients who had no previous cerebral scan, and no neurological symptoms. Scan rates were initially very low, and increased over the audit period (p = 0.0002). There were trends for the scan rates to increase after dissemination and online publication of the international guidelines, and to fall with the introduction of our formal clinic sheet categorising patients. The most notable change was an increase in scan rates after publication of the ARUBA trial (Figure 2B).
3.3. Scan rates differed by family history of cerebral haemorrhage
To evaluate the influence of our screening sheet categorisations, we focused on scan uptake in the most recent period (2015-2017) when the results of the ARUBA trial were communicated. None of the patients had neurological symptoms of concern with respect to possible cerebral vascular malformations. (If such symptoms were present, patients were categorised into Group 1 and MR scans performed urgently, usually at their local institutions).
In this period there were 183 non symptomatic patients - 23 with a family history of cerebral haemorrhage (Group 2) and 160 with no family history of cerebral haemorrhage (Group 3). As shown in Figure 3, 10 (43.4%) Group 2 patients and 17 (10.6%) Group 3 patients had screening scans instituted prior to review by us. The overall screening rate was therefore 14.8%. This low rate in part reflected the lack of a formal HHT diagnosis prior to review, but notably was 4.1-fold higher for those with a family history of cerebral haemorrhage, compared to those with no such family history (Figure 3).
Figure 3.
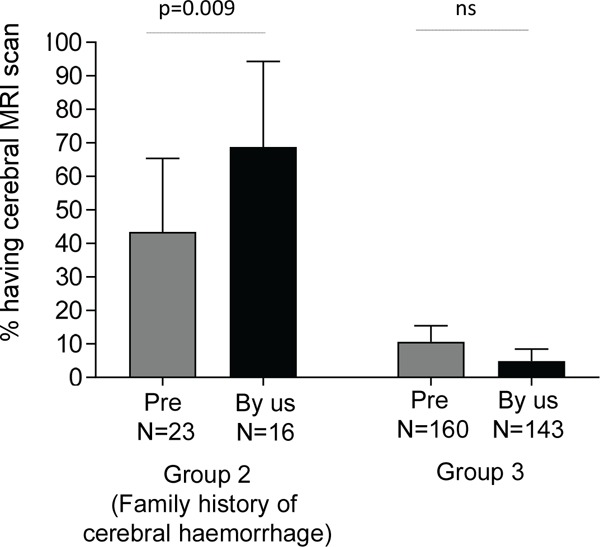
Proportion of non-symptomatic cases undergoing screening scans in 2015-2017. Patients are categorised by family history of haemorrhage (Group 2), and by whether the scans were performed by other institutions (grey bars, "Pre") or at our institution (black bars, "By us"). Bar charts indicate the % (mean) and 95% confidence intervals of the mean. Overall Kruskal-Wallis p < 0.0001, displayed p-values calculated by Dunn's test.
At our institution where all patients received a formal diagnosis of HHT and screening scans were only requested followed pre-test counselling and categorisation to Group 2 or Group 3, a more pronounced difference was observed in screening scan rates between the two categories (Figure 3). For patients with a family history of cerebral haemorrhage (Group 2), 11/16 (68.8%) of patients took up the opportunity to have a screening scan. In contrast only 7/143 (4.9%) of group 3 patients (no family history of haemorrhage), chose to have a scan (p < 0.0001).
3.4. Cerebral screening MRI scan results
Overall, at our institution between 20/04/2009 and 05/04/2018, 59 screening MR scans were performed in non-symptomatic patients with a clinical or molecular diagnosis of HHT, aged 16-74 (median 41) years. 26 (44.1%) of the cases were males. 23 (39.0%) were patients from Group 2 families where 1-4 (median 1) relatives had suffered a cerebral haemorrhage, between the ages of 3 months and mid 50s (median 29 years). 36 patients who had a scan (61.0%) gave no family history of cerebral haemorrhage.
None of the 59 screening MR scans performed at our institution demonstrated a cerebral AVM or cerebral arteriovenous fistula. One provided evidence of a prior cerebral microhaemorrhage. Four scans (6.8%) demonstrated changes compatible with small aneurysms, and two (3.4%) demonstrated possible telangiectatic changes. In this cohort therefore, the overall proportion of cerebrovascular abnormalities was 6/59 (10.2%, Table 1). There was no significant difference in any finding between Groups 2 and 3.
Table 1. Abnormalities identified by screening MR scans.
| Items | All patients (n = 59) | Group 2 (n = 24) | Group 3 (n = 35) | P value |
|---|---|---|---|---|
| Age, ys | 16-74, median 41 | 21-66(median 40.9) | 17-74 (median 41) | 0.58 |
| Sex, N (%) female) | 35 (59.3) | 17 (70.8) | 18 (51.4) | 0.14 |
| Arteriovenous malformation (AVM) | 0 | 0 | 0 | > 0.99 |
| Arteriovenous fistula | 0 | 0 | 0 | > 0.99 |
| Evidence of prior haemorrhage | 0 | 0 | 1 (2.7%) | 0.41 |
| Aneurysm† | 4 (6.8) | 1 (4.3) | 3 (8.3%) | 0.51 |
| Telangiectasia* | 2 (3.4) | 1 (4.3%) | 1 (2.7%) | > 0.99 |
| Infarcts | 20 (33.9) | 9 (39.1) | 11 (30.6) | 0.63 |
| Posterior circulation infarcts | 15 (25.4) | 6 (26.1) | 9 (25) | 0.95 |
| Anterior circulation infarcts | 9 (15.3) | 5 (21.7) | 4 (11.1) | 0.33 |
| Microangiopathic changes | 9 (15.3) | 3 (13.0) | 6 (16.7) | 0.45 |
| Contusion | 2 (3.4) | 1 (4.3) | 1 (2.7) | > 0.99 |
| Meningioma | 1 (1.7) | 0 | 1 (2.7) | > 0.99 |
†One at right ophthalmic/left middle cerebral artery junction, one at left internal carotid artery. *one had left cerebellum, left parietal, and left frontal telangiectasia, the second, contrast enhancement suggestive of telangiectasia. P-value calculations: age by Mann Whitney, remainder by Fisher exact test.
Only 32/59 (54.2%) of scans were reported as normal. As noted in Table 1, the most commonly identified abnormality was cerebral infarction which affected 20/59 (33.9%) of patients. Three of these cases had a prior neurological event many years earlier-one clinical stroke, one cerebral abscess and one prior cerebral haemorrhage with normal subsequent angiography. None of the 59 cases had epilepsy, and none had received a formal diagnosis of transient ischaemic attacks.
3.5. Clinical corollaries with cerebral infarcts
To examine further, potential associations between the infarcts and clinical characteristics were examined. There was no discernible association between infarcts and neurological symptoms described by many patients such as migraines (25-28) or pre-syncopal type dizzy spells (29) (Supplementary Table S1, http:// www.irdrjournal.com/action/getSupplementalData.php?ID=31). The most notable clinical association was with pulmonary AVMs which had been present in 30 (50.9%) of the patients scanned. 22 patients had been treated previously (by embolization or surgery), and 3 went on to have their first treatment (embolization) within the subsequent 3 months.
Ages did not differ between pulmonary AVM and non- pulmonary AVM cohorts (median 40.5 (range 16- 74) versus 42 (21-66) years respectively, p = 0.94). A cerebral infarct was present in only 3/29 (10.3%) of patients without pulmonary AVMs, when scanned aged 41, 59 and 65 years, compared to 17/30 (56.7%) patients who had at least one pulmonary AVM (p = 0.0002). There was also an excess of microangiopathic change in the pulmonary AVM cohort. As a result, while 22 (76%) of the 29 HHT patients without pulmonary AVMs had a normal scan, only 10 (33%) of the HHT cohort with pulmonary AVMs had a normal scan (p = 0.0012).
Nine of the 30 patients with pulmonary AVMs (30%) had between 2 and 5 infarcts at median age 53 (range 39-65) years - none had a previous history of stroke. Of the 8 patients with pulmonary AVMs and a single cerebral infarct, one had a prior clinical diagnosis of stroke in the corresponding territory. Table 2 provides further detail of the infarcts present in pulmonary AVM patients and indicates that the excess of pulmonary AVM-associated cerebral infarcts occurred in posterior circulation territories.
Table 2. Infarct distribution in 30 HHT patients known to have had PAVMs.
| Items | Patients affected, total (%) | Left | Right | Bilateral | Total sites | Sites per patient (n = 30 PAVM) |
Sites per patient (n = 17 infarct- affected) |
|---|---|---|---|---|---|---|---|
| All infarcts | 17 (56.7) | 8 | 4* | 7 | 31 | 1.13 | 2.13 |
| Posterior circulation | 14 (46.7) | 5 | 0 | 8 | 26 | 0.83 | 1.56 |
| Cerebellar | 12 (40.0) | 4 | 2 | 6 | 18 | 0.60 | 1.06 |
| Thalamus | 4 (16.7) | 3 | 1 | 4 | 0.13 | 0.24 | |
| Temporal | 1 (4.2) | 0 | 1 | 1 | 0.03 | 0.06 | |
| Pons | 1 (4.2) | 1 | 1 | 0.03 | 0.06 | ||
| Midbrain | 1 (4.2) | 1 | 1 | 0.03 | 0.06 | ||
| Occipital | 1 (4.2) | 1 | 1 | 0.03 | 0.06 | ||
| Anterior circulation | 7 (23.3) | 3 | 4 | 0 | 10 | 0.33 | 0.59 |
| Parietal | 2 (6.7) | 1 | 1 | 3 | 0.10 | 0.18 | |
| Frontal | 2 (8.3) | 1 | 1 | 2 | 0.07 | 0.12 | |
| Striatocapsular | 2 (6.7) | 2 | 2 | 0.07 | 0.12 | ||
| Caudate | 1 (4.2) | 1 | 1 | 0.03 | 0.06 |
*One corresponded to a known clinical stroke.
For HHT patients with pulmonary AVMs, the age-adjusted odds ratios for any infarct was 21.6 (95% confidence intervals 3.7, 126, p = 0.001). This reflected the higher number of posterior circulation territory infarcts (Table 3). The pulmonary AVM patients' age-adjusted odds ratios for any infarct or microangiopathic change (considered a marker of ischaemia) was 10.6 (95% confidence intervals 2.12, 53.0, p = 0.004). As in the univariate analyses above, there was no association between MR-confirmed infarcts and patient-described symptoms of migraines or dizzy spells, once adjusted for pulmonary AVMs (data not shown).
Table 3. Association of pulmonary AVMs with cerebral infarction by multivariate regression.
| Items | Age-adjusted* odds ratio (95% CI) | Pseudo R2 | p-value for PAVM |
|---|---|---|---|
| Any cerebral infarct | 21.6 (3.7, 126) | 0.38 | 0.001 |
| Anterior circulation infarct | 4.51 (0.70, 28.9) | 0.29 | 0.110 |
| Posterior circulation infarct | 28.0 (3.16, 249) | 0.32 | 0.003 |
| Any infarct or microangiopathic change | 10.6 (2.12, 53.0) | 0.38 | 0.004 |
*Similar findings in crude analyses without age-adjustment, data not shown.
4. Discussion
The aim of this study was to provide data that can add to the information provided as part of informed consent for screening for cerebral AVMs in non-symptomatic patients with HHT. The data indicate low scan uptake rates in patents with no family history of cerebral haemorrhage, and a low rate of cerebrovascular malformation detection where patients with potential neurological symptoms of concern have been separated out. However, the study demonstrated cerebral infarcts in more than 50% of "non-stroke" pulmonary AVM patients.
We are unaware of any previous evaluations of pre-test counselling for HHT patients prior to cerebral AVM screening scans, or patient choices that result after the counselling. Further, PubMed searches performed most recently on 27.08.2018 using the search terms ["HHT" "counselling" "cerebral" "screening"] or ["HHT" "choice" "cerebral" "screening"] did not identify any earlier studies examining HHT patient preference for cerebral MR screening scans. This study has clear limitations, particularly it was a single centre, retrospective study of two prospectively recorded datasets in adults only (patient preferences; MR scans). Study numbers for the MR scans were relatively small reflecting the "real-life" nature of ordering. Nevertheless, we think the information valuable for patients and our fellow practitioners who are struggling with how to weight patient perceptions (11,12) and older recommendations (18) in the light of emerging (20-23) and potential future evidence.
For the past eight years we have counselled those HHT patients who do not have concerning neurological symptoms regarding additional investigation. This has given them the opportunity to embark on cerebral screening knowing the risk-benefits of treatment of the vascular abnormalities that may be detected, or to decline screening in an informed manner for what is a highly emotive issue in those with HHT. It is intriguing that our local screening scan rates rose after communication of the ARUBA trial results which may have been expected to reduce screening scan numbers. Our interpretation is that the extra reassurance offered in case an AVM was detected may have enabled some patients to take that risk when seeking a screening scan that could exclude more worrying intracerebral pathology. The pattern of enhanced uptake by patients with a family history of cerebral haemorrhage was also seen in patients reviewed by other clinicians, although the increase was more pronounced in our clinic using a formal clinic categorisation sheet (14-fold, compared to 4.1-fold).
Cerebral AVM detection rates were lower than reported in other HHT series (8-10) - none were identified in the 59 screening scans in this series. While there are a number of possible explanations, including chance given the study size, the most obvious is the removal of patients with potentially relevant symptoms to a higher risk group, and exclusion from the "non-symptomatic screening cohorts". It is important to note that Group 2 and 3 patients were not "asymptomatic" with respect to neurological symptoms. The majority of patients with pulmonary AVMs experience migraine with aura (25-28), and in addition, many have transient dizzy spells that cannot be assigned to postural haemodynamic changes (29). Neither symptom was considered suggestive of cerebral AVMs in the setting of pulmonary AVMs, and patients remained in Group 2 or 3. The absence of cerebral AVM detection supports this classification with respect to cerebrovascular malformation screening.
Four patients (6.8%) had findings compatible with very small cerebral aneurysms. Aneurysms have been described before in HHT, including 8/376 (2.1%) cases in (10), and 9/372 (2.4%) cases in (9). Cerebral aneurysms were the cause of cerebral haemorrhages in three HHT cohorts (8,19,24). However, a systematic meta-analysis in the general population estimated the prevalence of cerebral aneurysms as 3.2% (95% CI: 1.9-5.2) in a population without comorbidity, with a mean age of 50 years (30). The cerebral aneurysm rate in HHT is therefore comparable to the general population.
The study findings support the long-held clinical viewpoint that neurological manifestations of HHT are more commonly attributable to pulmonary AVMs than to cerebrovascular abnormalities (31). A seventeen year old study using computerised tomography demonstrated cerebral infarcts in 34/67 (51%) patients with pulmonary AVMs at median age 42 years (25), and a more recent study demonstrated silent brain infarcts in approximately 28% of patients with pulmonary AVMs and no clinical ischaemic history (32). Based on previous data (27,29), we had expected patients with migraines and dizzy spells would have more infarcts, but in crude, age, and pulmonary AVM-adjusted analyses, no relationships were identified. This could support the interpretation that a proportion of infarcts occurred at a prior symptomatic stage (e.g. prior to PAVM treatments). Further study is needed to address the extent to which cerebral infarction is ongoing after embolization of pulmonary AVMs.
The excess of posterior circulation territory infarcts in patients with pulmonary AVMs was unexpected. Since paradoxical emboli through PAVMs will traverse the left atrium and ventricle, the high rate of posterior circulation infarcts may simply be secondary to the natural consequence of cardioembolic sources of cerebral infarction, rather than any specific relation to pulmonary AVMs: In the wider population, approximately 20% of all infarcts are within the posterior circulation (33), which is a relatively small number. However, approximately 25% of all cerebral infarcts are caused by cardioembolic events (34) and 40% of posterior circulation infarcts are due to cardioembolic events (35) indicating a natural propensity for cardioembolic events to affect the posterior circulation. Importantly, PAVMs are currently not included in many lists of causes of cardioembolic infarcts (33-35), and they should be as they result in paradoxical emboli travelling through the left ventricle.
These data do not alter any guidance for management of children, symptomatic adults, or the management of a cerebral AVM once detected in HHT, but we think they can helpfully support decisions on screening of patients. For cerebral AVM screening in HHT, we suggest all patients are first categorized into whether or not they have neurological symptoms of concern (e.g. epilepsy, severe headaches that are non migrainous, focal neurological deficits), and that all patients with such symptoms are scanned. For the remainder, they may be advised that their risk of cerebral AVMs due to HHT is lower than previously thought, and additionally, advised that the ARUBA trial demonstrated that for unruptured cerebral AVMs, stroke risk was higher if cerebral AVMs were treated. It still seems appropriate to offer a screening scan in the setting of a family history of haemorrhage, as in the general population (24). But given the emotive and controversial nature of the discussions, we consider it appropriate to then let patients make a decision on whether or not to have a scan, recognizing that it may identify silent cerebral infarcts due to pulmonary AVMs, or cerebral aneurysms/other neurological pathologies at a comparable rate to the general population. These risks should be articulated prior to performing the screening scan, noting detection of an incidental pathology would then be managed as for detection in any other setting. Particular comment is required for patients who have had pulmonary AVMs, indicating that scans detect their lifetime of risks which would be different before and after treatment. An important question for the future is whether patients with pulmonary AVMs should have a cerebral MR following their final planned treatment, to use as a baseline against which to judge the occurrence of new infarcts, and whether such infarct-detection scans should be performed regularly, or only after symptomatic events (as would usually occur). This leads to a further difficult area, weighing risks and benefits of antiplatelet therapies for a patient population at higher risk of haemorrhage (13,18,23,36,37).
In conclusion, in non-symptomatic screened cohorts with HHT, there was a negligible detection of cerebral AVMs, and this may further modify rates of screening scan uptake in HHT populations offered informed choice. However, pulmonary AVMs place patients at greatly enhanced risk of cerebral infarction, and in our opinion, the possibility of detection of silent infarcts should be added to prescreening information.
Acknowledgements
We thank Dr. Harri Jenkins and Professor Richard Wise for clinical reviews of patients.
References
- 1. Shovlin CL. Hereditary haemorrhagic telangiectasia: Pathophysiology, diagnosis and treatment. Blood Rev. 2010; 24:203-219. [DOI] [PubMed] [Google Scholar]
- 2. Kjeldsen AD, Vase P, Green A. Hereditary haemorrhagic telangiectasia: A population-based study of prevalence and mortality in Danish patients. J Intern Med. 1999; 245:31-39. [DOI] [PubMed] [Google Scholar]
- 3. Bideau A, Brunet G, Heyer E, Plauchu H, Robert JM. An abnormal concentration of cases of Rendu-Osler disease in the Valserine valley of the French Jura: A geneological and demographic study. Ann of Hum Biol. 1992; 19:233-247. [DOI] [PubMed] [Google Scholar]
- 4. Dakeishi M, Shioya T, Wada Y, Shindo T, Otaka K, Manabe M, Nozaki J, Inoue S, Koizumi A. Genetic epidemiology of hereditary hemorrhagic telangiectasia in a local community in the northern part of Japan. Hum Mutat. 2002; 19:140-148. [DOI] [PubMed] [Google Scholar]
- 5. Westermann CJ, Rosina AF, De Vries V, de Coteau PA. The prevalence and manifestations of hereditary hemorrhagic telangiectasia in the Afro-Caribbean population of the Netherlands Antilles: A family screening. Am J Med Genet A. 2003; 116A:324-328. [DOI] [PubMed] [Google Scholar]
- 6. Cottin V, Plauchu H, Bayle JY, Barthelet M, Revel D, Cordier JF. Pulmonary arteriovenous malformations in patients with hereditary hemorrhagic telangiectasia. Am J Respir Crit Care Med. 2004; 169:994-1000. [DOI] [PubMed] [Google Scholar]
- 7. Buscarini E, Leandro G, Conte D, et al. Natural history and outcome of hepatic vascular malformations in a large cohort of patients with hereditary hemorrhagic teleangiectasia. Dig Dis Sci. 2011; 56:2166-2178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Krings T, Ozanne A, Chng SM, Alvarez H, Rodesch G, Lasjaunias PL. Neurovascular phenotypes in hereditary haemorrhagic telangiectasia patients according to age. Review of 50 consecutive patients aged 1 day-60 years. Neuroradiology. 2005; 47:711-720. [DOI] [PubMed] [Google Scholar]
- 9. Woodall MN, McGettigan M, Figueroa R, Gossage JR, Alleyne CH Jr. Cerebral vascular malformations in hereditary hemorrhagic telangiectasia. J Neurosurg. 2014; 120:87-92. [DOI] [PubMed] [Google Scholar]
- 10. Brinjikji W, Iyer VN, Wood CP, Lanzino G. Prevalence and characteristics of brain arteriovenous malformations in hereditary hemorrhagic telangiectasia: A systematic review and meta-analysis. J Neurosurg. 2017; 127:302-310. [DOI] [PubMed] [Google Scholar]
- 11. Cure HHT. HHT and Stroke. https://curehht.org/wp-content/uploads/2017/11/FACT-SHEET-HHT-and-Stroke.pdf (accessed August 27, 2018).
- 12. HHT Ireland. HHT-The Facts. https://hhtireland.org/the-facts (accessed August 27, 2018).
- 13. Shovlin CL, Condliffe R, Donaldson JW, Kiely DG, Wort SJ; British Thoracic Society. British thoracic society clinical statement on pulmonary arteriovenous malformations. Thorax. 2017; 72:1154-1163. [DOI] [PubMed] [Google Scholar]
- 14. Shovlin CL, Jackson JE, Bamford KB, Jenkins IH, Benjamin AR, Ramadan H, Kulinskaya E. Primary determinants of ischaemic stroke/brain abscess risks are independent of severity of pulmonary arteriovenous malformations in hereditary haemorrhagic telangiectasia. Thorax. 2008; 63:259-266. [DOI] [PubMed] [Google Scholar]
- 15. Gill SS, Roddie ME, Shovlin CL, Jackson JE. Pulmonary arteriovenous malformations and their mimics. Clin Radiol. 2015; 70:96-110. [DOI] [PubMed] [Google Scholar]
- 16. Shovlin CL, Chamali B, Santhirapala V, Livesey JA, Angus G, Manning R, Laffan MA, Meek J, Tighe HC, Jackson JE. Ischaemic strokes in patients with pulmonary arteriovenous malformations and hereditary hemorrhagic telangiectasia: Associations with iron deficiency and platelets. PLoS One. 2014; 9:e88812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Boother EJ, Brownlow S, Tighe HC, Bamford KB, Jackson JE, Shovlin CL. Cerebral abscess associated with odontogenic bacteremias, hypoxemia, and iron loading in immunocompetent patients with right-to-left shunting through pulmonary arteriovenous malformations. Clin Infect Dis. 2017; 65:595-603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Faughnan ME, Palda VA, Garcia-Tsao G, et al. International guidelines for the diagnosis and management of hereditary haemorrhagic telangiectasia. J Med Genet. 2011; 48:73-87. [DOI] [PubMed] [Google Scholar]
- 19. Easey AJ, Wallace GM, Hughes JM, Jackson JE, Taylor WJ, Shovlin CL. Should asymptomatic patients with hereditary haemorrhagic telangiectasia (HHT) be screened for cerebral vascular malformations? Data from 22,061 years of HHT patient life. J Neurol Neurosurg Psychiatry. 2003; 74:743-748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mohr JP, Parides MK, Stapf C, et al. Medical management with or without interventional therapy for unruptured brain arteriovenous malformations (ARUBA): A multicentre, non-blinded, randomised trial. Lancet. 2014; 383:614-621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Willemse RB, Mager JJ, Westermann CJ, Overtoom TT, Mauser H, Wolbers JG. Bleeding risk of cerebrovascular malformations in hereditary hemorrhagic telangiectasia. J Neurosurg. 2000; 92:779-784. [DOI] [PubMed] [Google Scholar]
- 22. Yang W, Liu A, Hung AL, Braileanu M, Wang JY, Caplan JM, Colby GP, Coon AL, Tamargo RJ, Ahn ES, Huang J. Lower risk of intracranial arteriovenous malformation hemorrhage in patients with hereditary hemorrhagic telangiectasia. Neurosurgery. 2016; 78:684-693. [DOI] [PubMed] [Google Scholar]
- 23. Shovlin CL, Buscarini E, Kjeldsen AD, Mager HJ, Sabba C, Droege F, Geisthoff U, Ugolini S, Dupuis- Girod S. European Reference Network For Rare Vascular Diseases (VASCERN) outcome measures for hereditary haemorrhagic telangiectasia (HHT). Orphanet J Rare Dis. 2018; 13:136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Shovlin CL, Sodhi V, McCarthy A, Lasjaunias P, Jackson JE, Sheppard MN. Estimates of maternal risks of pregnancy for women with hereditary haemorrhagic telangiectasia (Osler-Weber-Rendu syndrome): Suggested approach for obstetric services. BJOG. 2008; 115:1108-1115. [DOI] [PubMed] [Google Scholar]
- 25. Moussouttas M, Fayad P, Rosenblatt M, Hashimoto M, Pollak J, Henderson K, Ma TY, White RI. Pulmonary arteriovenous malformations: Cerebral ischemia and neurologic manifestations. Neurology. 2000; 55:959-964. [DOI] [PubMed] [Google Scholar]
- 26. Elphick A, Shovlin CL. Relationships between epistaxis, migraines, and triggers in hereditary hemorrhagic telangiectasia. Laryngoscope. 2014; 124:1521-1528. [DOI] [PubMed] [Google Scholar]
- 27. Patel T, Elphick A, Jackson JE, Shovlin CL. Injections of intravenous contrast for computerized tomography scans precipitate migraines in hereditary hemorrhagic telangiectasia subjects at risk of paradoxical emboli: Implications for right-to-left shunt risks. Headache. 2016; 56:1659-1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Post MC, Thijs V, Schonewille WJ, Budts W, Snijder RJ, Plokker HW, Westermann CJ. Embolization of pulmonary arteriovenous malformations and decrease in prevalence of migraine. Neurology. 2006; 66:202-205. [DOI] [PubMed] [Google Scholar]
- 29. Santhirapala V, Chamali B, McKernan H, Tighe HC, Williams LC, Springett JT, Bellenberg HR, Whitaker AJ, Shovlin CL. Orthodeoxia and postural orthostatic tachycardia in patients with pulmonary arteriovenous malformations: A prospective 8-year series. Thorax. 2014; 69:1046-1047. [DOI] [PubMed] [Google Scholar]
- 30. Vlak MH, Algra A, Brandenburg R, Rinkel GJ. Prevalence of unruptured intracranial aneurysms, with emphasis on sex, age, comorbidity, country, and time period: A systematic review and meta-analysis. Lancet Neurol. 2011; 10:626-636. [DOI] [PubMed] [Google Scholar]
- 31. Román G, Fisher M, Perl DP, Poser CM. Neurological manifestations of hereditary hemorrhagic telangiectasia (Rendu-Osler-Weber disease): Report of 2 cases and review of the literature. Ann Neurol. 1978; 4:130-144. [DOI] [PubMed] [Google Scholar]
- 32. Brinjikji W, Nasr DM, Wood CP, Iyer VN. Pulmonary arteriovenous malformations are associated with silent brain infarcts in hereditary hemorrhagic telangiectasia patients. Cerebrovasc Dis. 2017; 44:179-185. [DOI] [PubMed] [Google Scholar]
- 33. Savitz SI, Caplan LR. Vertebrobasilar disease. N Engl J Med. 2005; 352:2618-2626. [DOI] [PubMed] [Google Scholar]
- 34. Arboix A, Alio J. Acute cardioembolic cerebral infarction: Answers to clinical questions. Curr Cardiol Rev. 2012; 8:54-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Caplan LR, Wityk RJ, Glass TA, et al. New England Medical Center Posterior Circulation registry. Ann Neurol. 2004; 56:389-398. [DOI] [PubMed] [Google Scholar]
- 36. Devlin HL, Hosman AE, Shovlin CL. Antiplatelet and anticoagulant agents in hereditary hemorrhagic telangiectasia. N Engl J Med. 2013; 368:876-878. [DOI] [PubMed] [Google Scholar]
- 37. VASCERN Do's And Don'ts Factsheets For Hereditary Haemorrhagic Telangiectasia Patients Facing Frequent Situations. https://vascern.eu/wp-content/uploads/2018/09/Fiches_Hereditary-Haemorrhagic-Telangiectasia_FINAL-web.pdf (Accessed November 7th .2018)


