Summary
Bronchial asthma (BA), atopic dermatitis (AD), and allergic rhinitis (AR) are well known atopic disorders with complex etiologies. This study was undertaken to investigate the role of filaggrin, eosinophil major basic protein (MBP) and leukotriene B4 (LTB4) in patients with BA, AD, and AR. Sera from 1,246 patients with different atopic disorders and 410 normal healthy controls were collected and were evaluated for filaggrin, MBP and LTB4 by specific sandwich ELISAs, whereas immunoglobulin E (IgE) was used as a positive control for atopic patients. Serum analysis showed that filaggrin levels were remarkably high in patients with AD and in patients with multiple (mixed) atopic disorders (p < 0.001), whereas its levels in BA and AR patients were low but much higher than in normal human sera (p < 0.01). MBP levels were also high in AR, BA and mixed atopic patients, whereas AD patients showed no increase of MBP (p > 0.05). In contrast, LTB4 level was found to be significantly low in all tested atopic patients groups as compared to the levels of LTB4 present in normal human sera (p < 0.001). In conclusion, these findings support an association between filaggrin, MBP or LTB4 and atopic disorders. Our data strongly suggest that filaggrin, MBP or LTB4 might be useful in elucidating the mechanisms involved in the pathogenesis of these atopic disorders.
Keywords: Bronchial asthma, atopic dermatitis, allergic rhinitis, filaggrin, LTB4, MBP
1. Introduction
Bronchial asthma (BA), atopic dermatitis (AD) and allergic rhinitis (AR) are common atopic disorders with complicated etiologies. The atopic march from early AD to BA, AR, or both later in life and the extensive comorbidity among them indicates, that these atopic disorders might share a common mechanism (1). Moreover, heritability of these atopic disorders is high, being 35-95% for BA, 71-84% for AD, 33- 91% for AR and 34-68% for allergen-specific serum immunoglobulin E (IgE) levels (1,2).
Filaggrin is now considered as a major predisposing gene for many atopic disorders, which result in a major paradigm in dermatology and allergy research (3). Many studies pointed out an association of the filaggrin gene with different atopic disorders. More specifically, loss-of-function mutations in the filaggrin gene have been reported to have an association with various atopic/allergic disorders (3). Batchelor et al., reported that there is a strong and consistent association between filaggrin mutations and development of AD (4), but an associations between filaggrin mutations with AR and BA are not pronounced (3,4). Currently, it is not fully known whether mutation in the filaggrin gene also effects its protein secretion in patients with BA, AD and AR, therefore, the present study was hypothesized to determine the role of production of filaggrin protein in hyperreactivity in these allergic patients. To prove this hypothesis, we determined the serum levels of filaggrin protein in patients with BA, AR, AD and also in those atopic patients, which were affected by multiple atopic disorders (mixed atopic patients). Not only have we measured these, we also determined the levels of IgE as a positive control for the allergic patients as studies have shown a well-defined association between serum IgE levels with allergic disorders (5,6). Bronchial hyperactivity has long been recognized as a hallmark of a number of allergic disorders but it's association with the dysfunctioning of mast cells and eosinophils is still not completely defined and remains controversial (7,8). We assumed that bronchial hyperactivity might have correlations with eosinophil's major basic protein (MBP) and leukotriene B4 (LTB4) in atopic disorders. Therefore, MBP and LTB4 were also estimated in these atopic patients to determine their roles in these allergic conditions.
2. Methods
2.1. Human subjects
This is a prospective case-control study, which enrolled AD, BA and AR individuals based on having a typical atopic picture according to recent guidelines described by Global Initiatives for Asthma (GINA) for BA (9), AR and its Impact on Asthma (ARIA) for AR (10) and SCORAD index for AD (11). The study was carried out in accordance with the Code of Ethics of the World Medical Association (Declaration of Helsinki as revised in Tokyo 2004) for humans and was approved by National Plan for Science, Technology and Innovation of KSA (NSTIP # 11-BIO1459-09) and was also approved by institutional review board (IRB) of College of Medicine, Qassim University, KSA. Informed consent from all studied subjects was taken before sample collection. All studied atopic individuals were consecutively recruited from Outpatient Clinics affiliated to Qassim University (pulmonology, pediatric and dermatology Clinics). Out of 1,246 atopic patients, BA (n = 445; age 38.1 ± 8.9), AR (n = 225; age 41.7 ± 13.9), AD (n = 216; age 25.6 ± 10.4), patients having mixed atopic disorders (n = 360; age 38.6 ± 11.4) were selected. Normal healthy humans (n = 410; age 39.13 ± 11.2) were selected and were used as controls. All selected control humans have no history of allergic disease. Venous blood samples from all studied subjects were taken and sera were stored at -80ºC until analyzed as described previously (12-14). Demographic details of all studied subjects are summarized in Table 1.
Table 1. Demographic details of all studied subjects.
| No. | Subjects | Number (n) | Age (mean ± SD) | Sex (F/M) |
|---|---|---|---|---|
| 1 | Bronchial Asthma | 445 | 38.1 ± 8.9 | 254 F/191 M |
| 2 | Atopic dermatitis | 216 | 25.6 ± 10.4 | 98 F/118 M |
| 3 | Allergic rhinitis | 225 | 41.7 ± 13.9 | 119 F/106 M |
| 4 | Mixed atopic disorders | 360 | 38.6 ± 11.4 | 178 F/182 M |
| 5 | Normal human controls | 410 | 39.1 ± 11.2 | 209 F/201 M |
SD, standard deviation; F, females; M, males; n, number.
2.2. Measurement of filaggrin, IgE, eosinophil's major basic protein, LTB4 in atopic patients
Levels of filaggrin, IgE, MBP and LTB4 were measured in serum samples of all selected atopic patients and their levels were compared with normal healthy controls' sera. Serum filaggrin levels were measured by specific human filaggrin sandwich ELISA according to the manufacturers' instructions (cat. # SEJ103Hu, Cloud- Clone Corp., Hubei, PRC.). Whereas, IgE serum levels were measured by human IgE specific sandwich ELISA (cat. # 20783-72876, GenWay Biotech, CA, USA). Serum MBP and serum LTB4 levels were measured by human MBP and LTB4 specific sandwich ELISAs, respectively (cat. # SEB650Hu; cat. # CEA562Ge) according to their manufacturers' instructions (Cloud- Clone Corp., Hubei, PRC.).
2.3. Statistical analysis
Results are expressed as the mean+SEM unless stated otherwise. One-way ANOVA of variance followed by Tukey-Kramer multiple comparisons test, or Two-way ANOVA of variance followed by Bonferroni comparisons test. p < 0.05 was considered significant. All statistical analysis was carried out by Graph Pad Prism version 5.0 (Graph Pad Software Inc., San Diego, CA, USA).
3. Results
3.1. Filaggrin in different atopic disorders
In this study, we determined the serum levels of filaggrin in patients with different atopic disorders and their levels were compared with healthy human controls. The data showed a significant increase in serum filaggrin levels (p < 0.001) in 1,246 different atopic disorders patients compared with 410 healthy controls of the same age group. The average filaggrin levels (± SEM) in all studied atopic subjects and controls humans were 7.13 ± 0.09 and 2.09 ± 0.04 ng/mL, respectively (Figure 1A). More specifically, the average filaggrin levels (± SEM) in the patients sera with AD (n = 216), AR (n = 225), BA (n = 445) and mixed atopic patients (n = 360) were 8.74 ± 0.81, 6.51 ± 0.36, 6.96 ± 0.12, and 8.29 ± 0.33 ng/mL, respectively (Figure 1B). These results showed that filaggrin levels were significantly increased in AD patients as compared with AR or BA patients (p < 0.05), whereas patients with multiple atopic disorders had almost similar levels of filaggrin as AD patients (p > 0.05). Data of all tested serum proteins including filaggrin in BA, AD, AR and in patients with mixed atopic disorders are summarized in Table 2.
Figure 1.
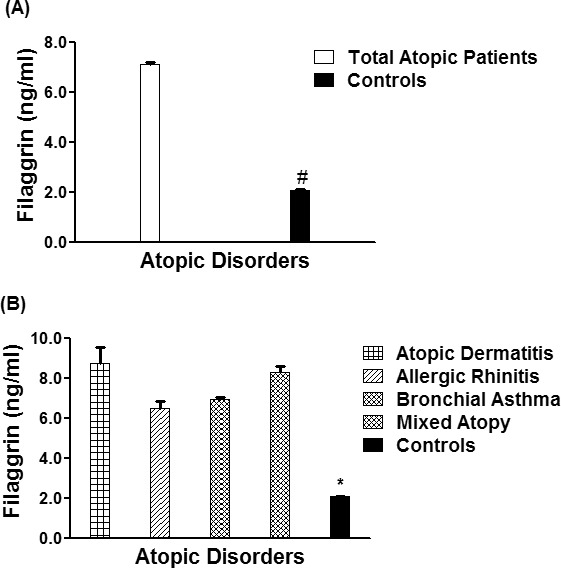
Filaggrin in different atopic disorders. (A) Levels of filaggrin in the sera of all studied atopic patients (n = 1,246) and controls (410). *p < 0.001 vs. all atopic patients. (B) Levels of filaggrin in the patients' sera of atopic dermatitis (n = 216), allergic rhinitis (n = 225), bronchial asthma (n = 445), mixed atopic patients (n = 360) and in controls' sera (n = 410). #p < 0.0001 vs. atopic dermatitis; #p < 0.001 vs. allergic rhinitis; #p < 0.001 vs. bronchial asthma; #p < 0.0001 versus mixed atopic patients. Each bar shows the mean ± SEM.
Table 2. Serum levels of filaggrin, eosinophil's MBP, LTB4 and IgE in all studied subjects.
| No. | Subjects | Number (n) | Filaggrin (ng/mL) | eMBP (ng/mL) | LTB4 (ng/mL) | IgE (IU/mL) |
|---|---|---|---|---|---|---|
| 1 | Bronchial Asthma | 445 | 6.96 ± 0.12a | 11.08 ± 0.29f | 10.21 ± 0.14l | 69.53 ± 2.07q |
| 2 | Atopic dermatitis | 216 | 8.74 ± 0.81b | 5.47 ± 1.16g | 7.12 ± 0.36m | 48.65 ± 10.60r |
| 3 | Allergic rhinitis | 225 | 6.51 ± 0.36c | 14.39 ± 0.92h | 9.94 ± 0.69n | 82.17 ± 6.50s |
| 4 | Mixed atopic disorders | 360 | 8.29 ± 0.33d | 9.51 ± 0.78i | 8.45 ± 0.29o | 74.04 ± 6.24t |
| 5 | Normal human controls | 410 | 2.09 ± 0.04e | 5.12 ± 0.19k | 13.05 ± 0.18z | 45.40 ± 1.98u |
Statistical significance among studied groups for filaggrin: bp < 0.05 vs. a or b; dp < 0.05 vs. a or b; ep < 0.05 vs. a, b, c or d. Statistical significance among studied groups for eMBP: hp < 0.05 vs. g, h, f or i. kp < 0.05 vs. f, g, h or i. Statistical significance among studied groups for LTB4: lp < 0.05 versus z; mp < 0.05 vs. z; dp < 0.05 vs. z; op < 0.05 versus z. Statistical significance among studied groups for IgE: sp < 0.05 vs. q, r; tp < 0.05 vs. q or r; up < 0.05 versus q, r, s or t. Data represented as mean ± SEM. Abbreviations: eMBP, eosinophils major basic protein; LTB4, leukotriene B4; IgE, immunoglobulin E; n, number of samples tested.
3.2. Total IgE in different atopic disorders
The serum levels of total IgE in patients with different atopic disorders (n = 1,246) were found to be significantly higher as compared to healthy controls (n = 410) (p < 0.0001). Average IgE levels (± SEM) in all studied atopic subjects and human controls were 68.9 ± 2.06 and 45.4 ± 1.98 IU/mL, respectively (Figure 2A). Specifically, the average IgE levels (± SEM) in the patients sera with AD (n = 216), AR (n = 225), BA (n = 445) and mixed atopic patients (n = 360) were 48.65 ± 10.6, 82.17 ± 6.50, 69.53 ± 2.07, and 74.04 ± 6.24 IU/mL, respectively (Figure 2B). Results also pointed out that IgE levels were significantly increased in AR patients as compared to AD or BA patients (p < 0.05), whereas patients with multiple atopic disorders had almost similar levels of IgE as AR patients (p > 0.05).
Figure 2.
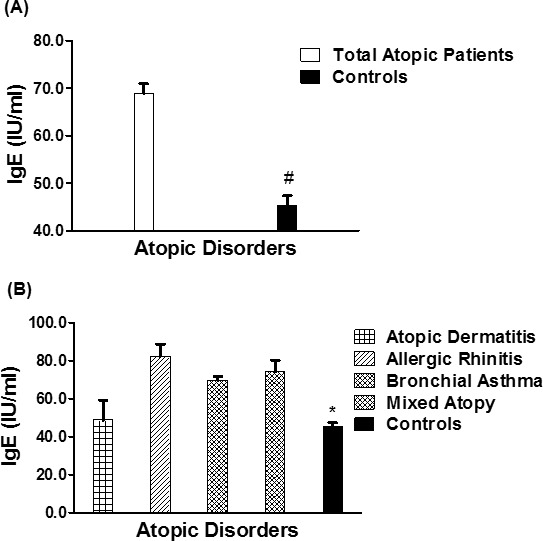
IgE in different atopic disorders. (A) Levels of IgE in the sera of all studied atopic patients (n = 1,246) and controls (410). *p < 0.001 vs. all atopic patients. (B) Levels of IgE in the patients' sera of atopic dermatitis (n = 216), allergic rhinitis (n = 225), bronchial asthma (n = 445), mixed atopic patients (n = 360) and in controls' sera (n = 410). #p <0.05 vs. atopic dermatitis; #p < 0.001 vs. allergic rhinitis; #p < 0.001 vs. bronchial asthma; #p < 0.0001 versus mixed atopic patients. Each bar shows the mean ± SEM. IgE, immunoglobulin E.
3.3. Major basic protein in different atopic disorders
The serum levels of MBP in patients with different atopic disorders (n = 1,246) were found to be significantly higher as compared with healthy controls (n = 410) (p < 0.01). The average MBP levels (± SEM) in all studied atopic subjects and human controls were 10.90 ± 0.27 and 5.12 ± 0.19 ng/mL, respectively (Figure 3A). Importantly, the average MBP levels (± SEM) in the patients sera with AD (n = 216), AR (n = 225), BA (n = 445) and mixed atopic patients (n = 360) were 5.47 ± 1.16, 14.39 ± 0.92, 11.08 ± 0.29, and 9.51 ± 0.78 ng/mL, respectively (Figure 3B). These results showed that MBP levels were significantly increased in AR patients as compared with AD, BA, or mixed atopic patients (p < 0.05). Moreover, results also indicated that MBP levels were not increased in AD patients as compared with the levels found in controls' sera (p > 0.05).
Figure 3.
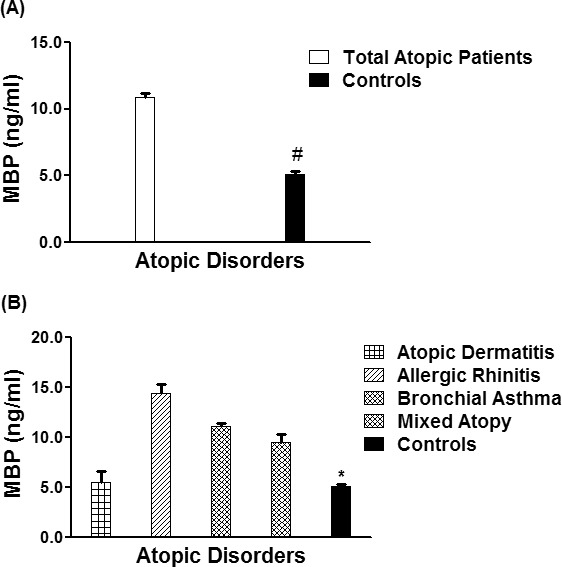
Eosinophil's major basic protein different atopic disorders. (A) Levels of major basic protein (MBP) in the sera of all studied atopic patients (n = 1,246) and controls (410). *p < 0.001 vs. all atopic patients. (B) Levels of MBP in the patients' sera of atopic dermatitis (n = 216), allergic rhinitis (n = 225), bronchial asthma (n = 445), mixed atopic patients (n = 360) and in controls' sera (n = 410). #p < 0.05 vs. atopic dermatitis; #p < 0.0001 vs. allergic rhinitis; #p < 0.001 vs. bronchial asthma; #p < 0.001 vs. mixed atopic patients. Each bar shows the mean ± SEM.
3.4. LTB4 in different atopic disorders
Serum levels of LTB4 in patients with different atopic disorders (n = 1,246) were found to be significantly low as compared with normal healthy controls (n = 410) (p < 0.001). The average LTB4 levels (± SEM) in all studied atopic subjects and human controls were 9.92 ± 0.13 and 13.05 ± 0.18 ng/mL, respectively (Figure 4A). Specifically, the average LTB4 levels (± SEM) in the patients sera with AD (n = 216), AR (n = 225), BA (n = 445) and mixed atopic patients (n = 360) were 7.12 ± 0.36, 9.94 ± 0.69, 10.21 ± 0.14, and 8.45 ± 0.29 ng/ mL, respectively (Figure 4B). These results showed that LTB4 levels were almost similar in all tested atopic patients groups including AD, AR, BA and also mixed atopic patients, but were significantly low as compared with their respective controls (p < 0.05).
Figure 4.
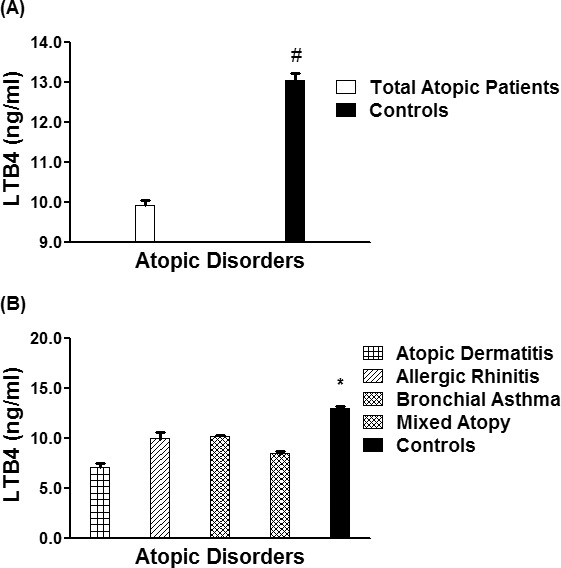
LTB4 in different atopic disorders. (A) Levels of LTB4 in the sera of all studied atopic patients (n = 1,246) and controls (410). *p < 0.001 vs. all atopic patients. (B) Levels of filaggrin in the patients' sera of atopic dermatitis (n = 216), allergic rhinitis (n = 225), bronchial asthma (n = 445), mixed atopic patients (n = 360) and in controls' sera (n = 410). #p < 0.0001 vs. atopic dermatitis; #p < 0.001 vs. allergic rhinitis; #p < 0.001 vs. bronchial asthma; #p < 0.0001 vs. mixed atopic patients. Each bar shows the mean ± SEM. LTB4, leukotriene B4.
4. Discussion
This study demonstrated the role of filaggrin, IgE, eosinophil major basic protein, and LTB4 in patients with BA, AD, AR and in those patients, which had multiple atopic disorders. Filaggrin is a key structural protein required for the normal biogenesis and physiology of the stratum corneum (15). The findings of genetic variants in the gene encoding filaggrin in up to 50% of AD patients enhanced our understanding of the role of filaggrin in skin barrier defect, AD pathogenesis, and the subsequent progression along the atopic march (16,17). The atopic march concept describes the progression of atopic disorders from AD in infancy to AR and BA in childhood (17). It is now well documented that the mutations in the filaggrin gene are major risk factors for AD (18,19). Not only in patients with AD, mutations in the filaggrin gene also had significant association with BA and AR (20,21). In this study, we determined the protein levels of filaggrin in patients BA, AD, AR and patients with mixed atopic disorders and their levels were compared with normal healthy controls. The data showed a significant increase in serum filaggrin levels in 1,246 different atopic disorders patients compared with 410 healthy controls of the same age group. More specifically, filaggrin levels were significantly increased in AD patients as compared with AR or BA patients, whereas patients with multiple atopic disorders had almost similar levels of filaggrin as AD patients. These results indicated that filaggrin protein is clearly associated with almost all atopic disorders particularly with AD patients and with those patients, which have multiple disorders.
Atopy is defined as a personal or familial propensity to produce IgE antibodies and sensitization in response to many factors particularly environmental triggers (22). The IgE sensitization and severity of many atopic disorders were well studied and connected with each other particularly for AD progression and BA persistence (23,24). Previously we also concluded that IgE is useful in evaluating the progression of AD and in elucidating the mechanisms of disease pathogenesis (6). In this study, we found that the serum levels of total IgE in patients with different atopic disorders were significantly higher as compared with healthy controls. Not only have we measured these, our data also pointed out that IgE levels were significantly increased in AR patients as compared with AD or BA patients, whereas patients with multiple atopic disorders had almost similar levels of IgE as AR patients, indicating that diagnostic values of IgE in serum are more important for AR patients and patients with mixed atopy as compared to AD or BA patients.
Eosinophils have a vital role in allergic inflammatory processes and MBP is present in the secretory granules of the eosinophil (25). Evidence implicates that the eosinophil and its granule proteins are assumed to mediate hypersensitivity disorders, as MBP-1 levels are elevated in sputum and bronchoalveolar lavage of BA patients (25). Studies have also shown that MBP-1 has a role in tissue damage as tissue damage is directly associated with eosinophil infiltration in BA (25). Morital et al. demonstrated that serum major basic protein is elevated in patients with AD (26). In addition, activated eosinophils and depositions of eosinophil granule proteins have also found in AD skin biopsies (26). Serum eosinophil cationic protein and eosinophil peroxidase is a sensitive indicator of the disease activity in AR (27). Serum eosinophil cationic protein and eosinophil peroxidase in patients with seasonal rhinitis demonstrated a high predictive ability for later development of BA (28). In view of these, it is important to know the protein level of MBP in patients with various atopic disorders, therefore in this study we determined the levels of MBP in the serum samples of AD, AR, BA and in those patients which have multiple atopic disorders. The serum levels of MBP in patients with different atopic disorders were found to be significantly increased and high as compared with healthy controls. Moreover, our results also pointed out that MBP levels were remarkably high in AR patients as compared with AD, BA, or mixed atopic patients. However, MBP levels were not increased in AD patients. These data indicate that MBP serum level has more value in the diagnosis of AR rather than AD.
LTB4 is a well-known mediator of leukocyte pathways involved in chemotactic properties of neutrophils, macrophages, monocytes, eosinophils, and dendritic cells (29). Studies have shown that LTB4 plays important roles in inflammatory and immune responses by activating phagocytic cells, differentiated T-cells, and dendritic cells (29,30). Dysfunction of LTB4 has been reported in various allergic disorders (29,30), therefore in this study we determined serum levels of LTB4 in patients with different atopic disorders and they were found to be significantly lower as compared with normal healthy controls. Specifically, our results also showed that LTB4 levels were almost similar in all tested atopic patients groups including AD, AR, BA and also mixed atopic patients, but were significantly lower as compared with their respective controls (p < 0.05). These data indicated that LTB4 might play a role in the pathogenesis of BA, AD and AR. As a whole, the present findings clearly suggest the roles of multiple proteins in the pathogenesis of BA, AR and AD. Our results are fully supported by numerous studies performed in different disorders including allergic disorders (31-34). In our previous studies we have also reported pathogenic effects of multiple proteins in patients with systemic lupus erythematosus (35-37). Moreover, studies have also shown dysfunction of multiple proteins in diabetes patients (38-40). Not only have these, inhibition of a wide array of enzyme activities have also been reported in the same group of patients (41-43). All these reports further strengthen our findings that the pathogenic effects can be generated by the abnormal behavior of multiple proteins rather than the involvement of a single protein. With the support of these studies, the findings from the present study in various atopic disorders strongly support an association between protein levels of filaggrin, MBP or LTB4 and AD, AR or BA. Our results suggest that filaggrin, IgE, MBP and LTB4 may be useful in elucidating the mechanisms of pathogenesis of these atopic disorders. In conclusion, our data clearly show that the levels of filaggrin, MBP and LTB4 were abnormal in patients with BA, AD, AR, and in those patients, which had multiple atopic disorders. These data clearly conclude that serum levels of filaggrin, MBP and LTB4 might be useful in the diagnosis of BA, AD and AR.
Acknowledgements
This work was supported by a grant from the National Science, Technology and Innovation Plan of KSA (NSTIP/KACST # 11-BIO1459-09). The authors thank Mr. Casimero A. Victoria (senior laboratory technologist) for help in experimentation.
Author's contribution: GBS, RS, TS, AA, KZ, AM, ME, AAR carried out the experimental work, data collection and interpretation. ZR, AAZ conceived of the study design, coordinated the studies, data interpretation and manuscript preparation. All authors have read and approved the final manuscript.
References
- 1. Gupta J, Johansson E, Bernstein JA, Chakraborty R, Khurana Hershey GK, Rothenberg ME, Mersha TB. Resolving the etiology of atopic disorders by using genetic analysis of racial ancestry. J Allergy Clin Immunol. 2016; 138:676-699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Thyssen JP, Linneberg A, Johansen JD, Carlsen BC, Zachariae C, Meldgaard M, Szecsi PB, Stender S, Menné T. Atopic diseases by filaggrin mutations and birth year. Allergy. 2012; 67:705-708. [DOI] [PubMed] [Google Scholar]
- 3. Sandilands A, Sutherland C, Irvine AD, McLean WH. Filaggrin in the frontline: Role in skin barrier function and disease. J Cell Sci. 2009; 122:1285-1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Batchelor JM, Grindlay DJ, Williams HC. What's new in atopic eczema? An analysis of systematic reviews published in 2008 and 2009. Clin Exp Dermatol. 2010; 35:823-827. [DOI] [PubMed] [Google Scholar]
- 5. Laske N, Bunikowski R, Niggemann B. Extraordinarily high serum IgE levels and consequences for atopic phenotypes. Ann Allergy Asthma Immunol. 2003; 91:202-204. [DOI] [PubMed] [Google Scholar]
- 6. Zedan K, Rasheed Z, Farouk Y, Alzolibani AA, Bin Saif G, Ismail HA, Al Robaee AA. Immunoglobulin E, interleukin-18 and interleukin-12 in patients with atopic dermatitis: Correlation with disease activity. J Clin Diagn Res. 2015; 9:WC01-WC05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bystrom J, Garcia RC, Hakansson L, Karawajczyk M, Moberg L, Soukka J, Venge P. Eosinophil cationic protein is stored in, but not produced by, peripheral blood neutrophils. Clin Exp Allergy. 2002; 32:1082-1091. [DOI] [PubMed] [Google Scholar]
- 8. Hjern A, Haglund B, Hedlin G. Ethnicity, childhood environment and atopic disorder. Clin Exp Allergy. 2000; 30:521-528. [DOI] [PubMed] [Google Scholar]
- 9. Global Initiative for Asthma (GINA), National Heart, Lung and Blood Institute (NHLBI) Global strategy for asthma management and prevention. Global Initiative for Asthma (GINA), National Heart, Lung and Blood Institute (NHLBI): Bethesda (MD); 2006. 339. https:// ginasthma. org/ (accessed November 21, 2018). [Google Scholar]
- 10. Bousquet PJ, Combescure C, Neukirch F, Klossek JM, Mechin H, Daures JP. Visual analog scales can assess the severity of rhinitis graded according to ARIA guidelines. Allergy. 2007; 62:367-372. [DOI] [PubMed] [Google Scholar]
- 11. Oranje AP, Glazenburg EJ, Wolkerstorfer A, de Waard-van der Spek FB. Practical issues on interpretation of scoring atopic dermatitis: The SCORAD index, objective SCORAD and the three-item severity score. Br J Dermatol. 2007; 157:645-648. [DOI] [PubMed] [Google Scholar]
- 12. Rasheed Z, Ahmad R, Rasheed N, Ali R. Reactive oxygen species damaged human serum albumin in patients with hepatocellular carcinoma. J Exp Clin Cancer Res. 2007; 26:515-524. [PubMed] [Google Scholar]
- 13. Rasheed Z. Hydroxyl radical damaged Immunoglobulin G in patients with rheumatoid arthritis: Biochemical and immunological studies. Clinical Biochem. 2008; 41:663-669. [DOI] [PubMed] [Google Scholar]
- 14. Rasheed Z, Ahmad R, Ali R. The structure and immunological function of oxidized albumin in lung cancer patients: Its role in elevated oxidative stress. British J Biomed Sci. 2009; 66:1-7. [DOI] [PubMed] [Google Scholar]
- 15. McAleer MA, Irvine AD. The multifunctional role of filaggrin in allergic skin disease. J Allergy Clin Immunol. 2013; 131:280-291. [DOI] [PubMed] [Google Scholar]
- 16. ORegan GM, Sandilands A, McLean WH, Irvine AD. Filaggrin in atopic dermatitis. J Allergy Clin Immunol. 2009; 124:R2-R6. [DOI] [PubMed] [Google Scholar]
- 17. Spergel JM, Paller AS. Atopic dermatitis and the atopic march. J Allergy Clin Immunol. 2003; 112:S118-S127. [DOI] [PubMed] [Google Scholar]
- 18. Stemmler S, Parwez Q, Petrasch-Parwez E, Epplen JT, Hoffjan S. Two common loss-of-function mutations within the filaggrin gene predispose for early onset of atopic dermatitis. J Invest Dermatol. 2007; 127:722-724. [DOI] [PubMed] [Google Scholar]
- 19. Margolis DJ, Apter AJ, Gupta J, Hoffstad O, Papadopoulos M, Campbell LE, Sandilands A, McLean WH, Rebbeck TR, Mitra N. The persistence of atopic dermatitis and filaggrin (FLG) mutations in a US longitudinal cohort. J Allergy Clin Immunol. 2012; 130:912-917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Marenholz I, Kerscher T, Bauerfeind A, Esparza-Gordillo J, Nickel R, Keil T, Lau S, Rohde K, Wahn U, Lee YA. An interaction between filaggrin mutations and early food sensitization improves the prediction of childhood asthma. J Allergy Clin Immunol. 2009; 123:911-916. [DOI] [PubMed] [Google Scholar]
- 21. Ying S, Meng Q, Corrigan CJ, Lee TH. Lack of filaggrin expression in the human bronchial mucosa. J Allergy Clin Immunol. 2006; 118:1386-1388. [DOI] [PubMed] [Google Scholar]
- 22. Johansson SG, Bieber T, Dahl R, Friedmann PS, Lanier BQ, Lockey RF, Motala C, Ortega Martell JA, Platts- Mills TA, Ring J, Thien F, Van Cauwenberge P, Williams HC. Revised nomenclature for allergy for global use: Report of the Nomenclature Review Committee of the World Allergy Organization, October 2003. J Allergy Clin Immunol. 2004; 113:832-836. [DOI] [PubMed] [Google Scholar]
- 23. Novembre E, Cianferoni A, Lombardi E, Bernardini R, Pucci N, Vierucci A. Natural history of "intrinsic" atopic dermatitis. Allergy. 2001; 56:452-453. [DOI] [PubMed] [Google Scholar]
- 24. Wuthrich B, Schmid-Grendelmeier P. Natural course of AEDS. Allergy. 2002; 57:267-268. [DOI] [PubMed] [Google Scholar]
- 25. Acharya KR, Ackerman SJ. Eosinophil granule proteins: Form and function. J Biol Chem. 2014; 289:17406-17415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Morita H, Yamamoto K, Kitano Y. Elevation of serum major basic protein in patients with atopic dermatitis. J Dermatol Sci. 1995; 9:165-168. [DOI] [PubMed] [Google Scholar]
- 27. Nielsen LP, Bjerke T, Christensen MB, Pedersen B, Rasmussen TR, Dahl R. Assessment of the allergic reaction in seasonal rhinitis: Acoustic rhinometry is a sensitive and objective method. Clin Exp Allergy. 1996; 26:1268-1275. [PubMed] [Google Scholar]
- 28. Nielsen LP, Peterson CG, Dahl R. Serum eosinophil granule proteins predict asthma risk in allergic rhinitis. Allergy. 2009; 64:733-737. [DOI] [PubMed] [Google Scholar]
- 29. Ohnishi H, Miyahara N, Gelfand EW. The role of leukotriene B(4) in allergic diseases. Allergol Int. 2008; 57:291-298. [DOI] [PubMed] [Google Scholar]
- 30. Liu M, Yokomizo T. The role of leukotrienes in allergic diseases. Allergol Int. 2015; 64:17-26. [DOI] [PubMed] [Google Scholar]
- 31. Mahmoud KH, Alzolibani AA, Rasheed Z, Farouk Y, Saif GB, Al Robaee AA. Interleukin-4 and Interferon-γ are possible allergic markers in pediatric patients with β-lactam hypersensitivity. Int J Appl Basic Med Res. 2016; 6:276-281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Rasheed Z, Zedan K, Saif GB, Salama RH, Salem T, Ahmed AA, El-Moniem AA, Elkholy M, Al Robaee AA, Alzolibani AA. Markers of atopic dermatitis, allergic rhinitis and bronchial asthma in pediatric patients: Correlation with filaggrin, eosinophil major basic protein and immunoglobulin E. Clin Mol Allergy. 2018; 16:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zhang H, Sternberger NH, Rubinstein LJ, Herman MM, Binder LI, Sternberger LA. Abnormal processing of multiple proteins in Alzheimer disease. Proc Natl Acad Sci U S A. 1989; 86:8045-8049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Farhan J, Alghasham A, Zafar U, Meki A-RMA, Rasheed Z. Impact of anti-glutamic acid decarboxylase-65, anti-insulin and anti-tyrosine phosphatase autoantibodies on disease activity in type 1 diabetes patients. J Diabetes Res Clin Met. 2013; 2:24. [Google Scholar]
- 35. Alzolibani A, AlRobaee AA, Al-Shobaili HA, Rasheed Z. 4-Hydroxy-2-nonenal modified histone-H2A: A possible antigenic stimulus for systemic lupus erythematosus autoantibodies. Cellular Immunology. 2013; 284:154-162. [DOI] [PubMed] [Google Scholar]
- 36. Al-Shobaili HA, AlRobaee AA, Alzolibani A, Khan MI, Rasheed Z. Hydroxyl radical modification of immunoglobulin G generated cross-reactive antibodies: Its potential role in systemic lupus erythematosus. Clin Med Insights Arthritis Musculoskelet Disord. 2011; 4:11-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Rasheed Z, Ahmad R, Rasheed N, Ali R. Enhanced recognition of reactive oxygen species damaged human serum albumin by circulating systemic lupus erythematosus autoantibodies. Autoimmunity. 2007; 40:512-520. [DOI] [PubMed] [Google Scholar]
- 38. Rasheed Z, Al-Shobaili HA, Alzolibani AA, Ismail Khan M, Tariq Ayub M, Khan MI, Rasheed N. Immunological functions of oxidized human immunoglobulin G in type 1~diabetes mellitus: Its potential role in diabetic smokers as a biomarker of elevated oxidative stress. Disease Markers. 2011; 31:47-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Rasheed Z, Ahmad R, Rasheed N, Ali R. Reactive oxygen species damaged hemoglobin presents unique epitopes for type 1 diabetes autoantibodies. Int J Biol Chem. 2008; 2:1-13. [Google Scholar]
- 40. Rasheed Z, Ali R. Reactive oxygen species damaged human serum albumin in patients with type 1 diabetes mellitus: Biochemical and immunological studies. Life Sci. 2006; 79:2320-2328. [DOI] [PubMed] [Google Scholar]
- 41. Al-Shobaili HA, Rasheed Z. Immunological studies of oxidized superoxide dismutase in patients with systemic lupus erythematosus: Correlation with disease induction and progression. Saudi Med J. 2012; 33:179-186. [PubMed] [Google Scholar]
- 42. Al-Shobaili HA, Alzolibani A, AlRobaee AA, Rasheed Z. Reactive oxygen species damaged catalase in patients with systemic lupus erythematosus: Correlation with disease activity. Immunol Invest. 2013; 42:191-203. [DOI] [PubMed] [Google Scholar]
- 43. Her M, Lee Y, Jung E, Kim T, Kim D. Liver enzyme abnormalities in systemic lupus erythematosus: A focus on toxic hepatitis. Rheumatol Int. 2011; 31:79-84. [DOI] [PubMed] [Google Scholar]


