Summary
Shanghai has always attached importance to the prevention and treatment of rare diseases and has been at the forefront in China. The Shanghai Rare Diseases Diagnosis and Treatment Center, Shanghai Children's Rare Diseases Diagnosis and Treatment Center, and Shanghai Rare Diseases Specialist Clinic were established in February 2018. Moreover, with the development of clinical pathways for rare diseases and the provision of related services such as diagnosis, treatment, screening, information and training, the service system for diagnosis and treatment of rare diseases in Shanghai has formed, which greatly improves the accessibility of medical services for patients with rare diseases in Shanghai and surrounding areas, and is of great significance in reducing the burden on patients with rare diseases. Meanwhile, it also gives an important reference for other regions of China for providing rare disease diagnosis and treatment services.
Keywords: Rare disease, diagnosis and treatment, service system
The establishment of national or regional rare disease clinical medical centers and their network construction is an effective means to promote the availability of appropriate health services for patients with rare diseases (1). As an advanced area for the prevention and treatment of rare diseases in China, Shanghai has initially established a clinical diagnosis and treatment service system for rare diseases. This paper intends to introduce the systemfrom the aspects of clinical diagnosis and treatment centers, clinical pathways, diagnosis, screening, information and training.
1. Clinical diagnosis and treatment centers of rare diseasess
The clinical diagnosis and treatment center is a guarantee for patients with rare diseases to receive good quality medical services. As early as in 2011, in order to guarantee the diagnosis and treatment of children with Pompe disease, Gaucher's disease, Mucopolysaccharidosis, and Fabry disease, the Shanghai Children's Hospitalization Mutual Fund Management Office appointed three hospitals for this responsibility (2). With increasing attention to prevention and treatment of rare diseases in recent years, the former Shanghai Municipal Health and Family Planning Commission (SMHFPC) officially established Shanghai Rare Diseases Diagnosis and Treatment Center, Shanghai Children's Rare Diseases Diagnosis and Treatment Center, and the first batch of 5 Shanghai Rare Diseases Specialist Clinics (Figure 1) in February 2018 (3,4). Identification of the above centers and outpatient clinics mainly follow the principles of early research, more case accumulation, rich clinical experience, greater social impact, and higher academic status. It also requires them to benchmark the highest and best standards internationally to build a domesticallyleading and internationally first-class rare disease diagnosis and treatment institution. Additionally, in order to speed up the construction of a first-class medical center city and implement the national strategy of the Yangtze River Delta integration, Shanghai has proposed to build a laboratory diagnostic center for rare diseases in the Yangtze River Delta (5).
Figure 1.

The clinical diagnosis and treatment system of rare diseases in Shanghai.
2. Clinical pathways of diagnosis and treatment of rare disease
A clinical pathway is an important guide for the diagnosis and treatment of rare diseases. In 2011, Shanghai Municipal Science and Technology Commission listed research for prevention and treatment for 10 rare diseases such as maple syrup urine disease, tyrosinemia, methylmalonicacidemia, multiple carboxylase deficiency, hereditary tyrosinemia, multiple carboxylase deficiency, Gaucher's disease, Fabry disease, Pompe disease and mucopolysaccharidoses in a major scientific and technological project called Science and Technology Innovation Action Plan (6). Based on this project, relevant diagnosis and treatment standards for the above rare diseases were formulated. In addition, Shanghai Foundation for Rare Disease (SFRD) organized the compilation of Treatable Rare Diseases, which was published in 2017 (7). The book provided recommendations for diagnosis and treatment of 117 rare diseases, including 56 rare diseases listed in the List of Major RareDiseases in Shanghai (2016 Edition) (8) and another 61 rare diseases with clear diagnosis and viable treatment.
3. Diagnosis and treatment procedure for rare diseases
Timely and reasonable diagnosis and treatment is one of the main demands of patients with rare diseases. At present, the rare disease diagnosis and treatment center or outpatient clinic in Shanghai has established a group of rare disease experts, which is participated in by many clinical specialties and medical technology departments and is organized by experts from the hospital, the provincial level and even the national level. The multiple disciplinary team (MDT) is adopted to diagnose and treat rare diseases (Figure 2). Meanwhile, organizations have also vigorously strengthened the construction of various laboratory diagnostic platforms, including molecular genetics, cytogenetics, and genome-wide platforms.
Figure 2.
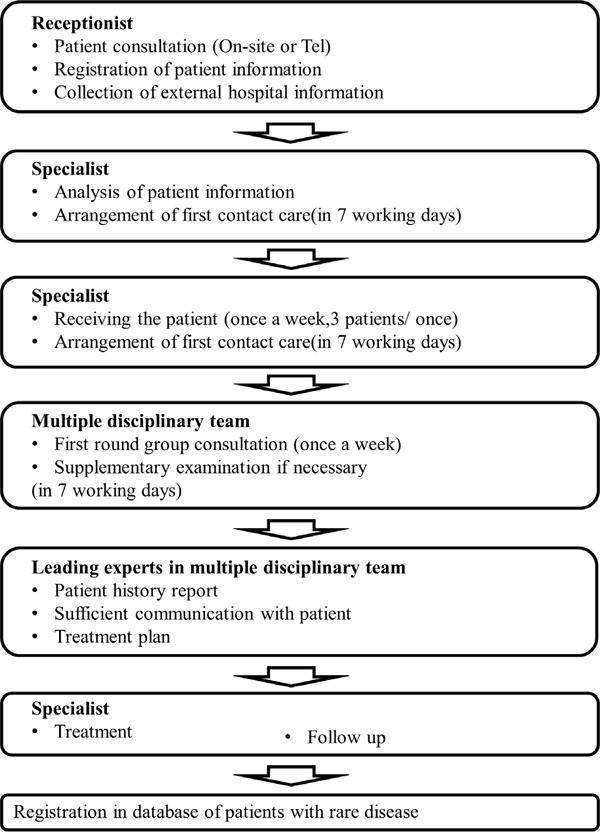
The diagnosis and treatment process of rare diseases in Shanghai Rare Diseases Diagnosis and Treatment Center.
4. Newborn screening for early detection of rare diseases
At present, newborn screening plays a major role in screening of rare diseases. In 1981, screening of phenylketonuria among all newborns was first achieved nationwide in Shanghai (9). In 2007, screening for congenital adrenal cortical hyperplasia and glucose-6- phosphate dehydrogenase deficiencywas available for all newborns. In addition, tandem mass spectrometry can be independently selected to detect a variety of rare diseases.
5. Referral information service for patients with rare diseases
Some rare disease diagnosis and treatment institutions have begun to implement patient registry, and established a patient's clinical information database and biobank. SFRD is also organizing construction of a registration platform for rare diseases patients in Shanghai. The first batch of registered units includes two centers and five specialist units (10). In addition, Shanghai has also actively organized Rare Disease Day activities (three sessions so far) and academic annual conferences (nine sessions so far) to convey important information such as rare disease research, diagnosis, treatment, services, policies, etc., and to raise social concerns and awareness.
6. Knowledge training for increasing the awareness of rare diseases
Increasing the awareness of medical staff about rare diseases is of great significance in reducing the rate of misdiagnosis and missed diagnosis of rare diseases. In March 2017, directed by the former SMHFPC, SFRD and the Shanghai Medical Association Rare Diseases Specialist Branch launched the Shanghai Rare Disease Prevention and Control Training Project for Medical Staff (11). The project aimed to gradually improve awareness and capacity of diagnosis and treatment for rare diseases among medical staff. The project would last for three years and would be available to medical staff in upper secondary and above medical institutions. Up to now, 10 sessions of training courses have been carried out.
7. Perspectives
It can be said that the Shanghai rare disease diagnosis and treatment service system has taken initial shape. This is not only a gospel for rare disease patients in Shanghai, but also for patients in the surrounding areas and even the whole country. This is of great significance for improving accessibility of diagnosis and treatment services for patients and reducing the burden on them. As a highlight of domestic medical education and technology, Shanghai should continue to strengthen the research, teaching and training of rare diseases, and make the current medical staff training programs long-term and institutionalized, and further build regional and national rare disease diagnosis and treatment centers. Moreover, it is necessary to speed up establishment and improvement of the rare disease registration system and research collaboration network based on the clinical system, and comprehensively collect important data such as physiological, psychological and economic burdens of patients with rare diseases, and provide an important basis for the formulation of relevant research, diagnosis, treatment, policies and laws (12). In addition, with the introduction of China's First National List of Rare Diseases (13,14) and the prioritized evaluation and approval system for orphan drugs policy, Shanghai should actively seek relevant drugs to be used in the above-mentioned medical institutions as soon as possible, and further expand the scope of rare diseases to be diagnosed and treated.
Acknowledgements
This project was supported by Scientific Research General Project of Shanghai Municipal Health and Family Planning Commission: Study on security mechanism of rare diseases in Shanghai (Project number: 20134060), and Special Research Project on Health Development Policies of Shanghai Municipal Health and Family Planning Commission: Study on disease burden and security system of vulnerable population in Shanghai (Project number: 18Y08009).
References
- 1. European Project for Rare Diseases National Plans Development (EUROPLAN). Recommendations for the development of national plans for rare diseases guidance document. https://download2.eurordis.org/europlan/2_EUROPLAN_Guidance_Documents_for_the_National_Conference/2_EUROPLAN_Recommendations_for_Rare_Disease_National_Plans_Final.pdf (accessed November 15, 2018).
- 2. Shanghai Branch of the Red Cross Society of China, Notice on specific drugs for rare diseases that will be covered by the Children's Hospitalization Fund. http://www.redcross-sha.org/2015/View.aspx (accessed November 15, 2018).
- 3. Shanghai Municipal Health and Family Planning Commission. Notice on establishing Shanghai Rare Diseases Diagnosis and Treatment Center. http://www.wsjsw.gov.cn/fybj2/20180815/58246.html (accessed November 16, 2018).
- 4. Shanghai Municipal Health and Family Planning Commission. Notice on establishing the First Batch of Shanghai Rare Diseases Specialized Clinics. http://www.wsjsw.gov.cn/fybj2/20180815/58249.html (accessed November 16, 2018).
- 5. Shanghai Municipal People's Government. Several opinions of the Shanghai Municipal People's Government on promoting the quality development of the city's health service industry and accelerating the construction of a first-class dedicated center city. http://www.shanghai.gov.cn/nw2/nw2314/nw2319/nw12344/u26aw56500.html (accessed November 16, 2018).
- 6. Shanghai Municipal Science and Technology Commission. Guidance for Science and Technology Innovation Action Plan major scientific and technological project of Shanghai in 2011. http://www.stcsm.gov.cn/gk/kxjgxm/ktsbzn/148489.htm (accessed November 16, 2018).
- 7. Chen Jing. Treatable Rare Diseases. Shanghai: Shanghai Jiaotong University Press, 2017. (in Chinese) [Google Scholar]
- 8. Shanghai Municipal Health and Family Planning Commission. Notice on issuance of The List of Major Rare Diseasesin Shanghai (2016 Edition). http://www.wsjsw.gov.cn/wsj/n429/n432/n1487/n1511/u1ai136938.html (accessed November 16, 2018).
- 9. Mei L, Song PP, Xu LZ. Newborn screening and related policy against Phenylketonuria in China. Intractable Rare Dis Res. 2013; 2:72-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Shanghai Foundation for Rare Disease. Shanghai Foundation for Rare Disease held anexpert meeting on the registration ofpatients with rare disease in Shanghai and a bidding meeting with software companies. https://mp.weixin.qq.com/s/wDTwuZHfnREpT5AxL3Rm6w (accessed November 16, 2018).
- 11. Shanghai Foundation for Rare Disease. Shanghai Foundation for Rare Disease held a conference on the training of rare diseases prevention and treatment knowledge for medical staff in Shanghai. http://gobest1.w226.mc-test.com/Reception/GuidanceInfo.aspx?id=562&mid=6 (accessed November 16, 2018).
- 12. Song PP, He JJ, Li F, Jin CL. Innovative measures to combat rare diseases in China: The national rare diseases registry system, larger-scale clinical cohort studies, and studies in combination with precision medicine research. Intractable Rare Dis Res. 2017; 6:1-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. National Health Commission Ministry of Science and Technology Ministry of Industry and Information Technology National Medical Products Administration State Administration of Traditional Chinese Medicine. Notice on the publication of the first rare disease list. http://www.gov.cn/gongbao/content/2018/content_5338244.htm (accessed November 17, 2018).
- 14. He JJ, Kang Q, Hu JH, Song PP, Jin CL. China has officially released its first national list of rare diseases. Intractable Rare Dis Res. 2018; 7:145-147. [DOI] [PMC free article] [PubMed] [Google Scholar]


