Abstract
Objective:
Certain food additives may promote the pathogenesis of Crohn’s disease (CD), but thus far the evaluation of food additive exposures in humans has been limited. The objective of this study was to quantify food additive exposures in children with CD.
Methods:
In a trial for bone health in CD, children were followed over 24 months with evaluation of disease characteristics, dietary intake, and body composition. At baseline, participants completed three 24-hour dietary recalls. Foods were categorized and the ingredient list for each item was evaluated for the presence of select food additives: polysorbate-80, carboxymethylcellulose, xanthan gum, soy lecithin, titanium dioxide, carrageenan, maltodextrin, and aluminosilicates. The frequency of exposures to these food additives was described for study participants and for food categories.
Results:
At study baseline, 138 participants, mean age 14.2 ± 2.8 years, 95% having inactive or mild disease were enrolled and dietary recalls were collected. A total of 1,325 unique foods were recorded. Mean exposures per day for xanthan gum was 0.96 ± 0.72, carrageenan 0.58 ± 0.63, maltodextrin 0.95 ± 0.77, and soy lecithin 0.90 ± 0.74. The other additives had less than 0.1 exposures per day. For the 8 examined food additives, participants were exposed to a mean (SD) of 3.6 ± 2.1 total additives per recall day and a mean (SD) of 2.4 ± 1.0 different additives per day.
Conclusion:
Children with CD frequently consume food additives, and the impact on disease course needs further study.
Keywords: Food additives, Crohn’s disease, dietary recall, nutrition, methodology
Introduction
Diet is associated with the development of Crohn’s disease (CD) and diet can also be used as effective therapy [1]. The most common pharmacologic therapies for CD involve long-term suppression of the immune system, but food-exclusion diets have also demonstrated efficacy at inducing remission of active disease [2–5]. Exclusive enteral nutritional therapy, i.e., a formula-based diet with exclusion of foods, has been widely studied in children with CD and is able to effectively induce remission in 70–80% of children with active CD [6, 7]. Several food-based exclusion diets such as the specific carbohydrate diet, CD exclusion diet, and “anti-inflammatory” diets have additionally generated broad interest [4, 8, 9]. A common theme in these exclusion diets is the avoidance of “processed foods.” Potential mechanisms by which diet may impact gut inflammation include via the mechanical properties of food, repletion of essential nutrients, modulation of the gut microbiome and its metabolites, alteration in bile acid secretion, or exposure to or avoidance of deleterious substances [1].
The risk of developing CD is associated with “western” lifestyle exposures [10, 11]. Western dietary patterns include increased consumption of processed foods with additives; however, to date, epidemiologic studies have not evaluated the association between food additive exposure and the risk of CD relapse. Food additives are utilized for a variety of functions including promoting dispersion of phase, anti-caking, food brightening, and thickening. Ex vivo and animal studies have suggested the pro-inflammatory effect of several foods and food additives. Studies with preclinical models have demonstrated the potential harmful effect of emulsifiers/stabilizers (i.e., polysorbate-80, carboxymethylcellulose, xanthan gum, carrageenan), aluminosilicates, titanium dioxide, and maltodextrin [12–15]. Although the FDA has deemed the food additives under study here as safe, some of these additives have been shown in pre-clinical studies to be associated with alterations in the intestinal microbiome, decreased thickness of the mucus barrier protecting the intestinal epithelium, and intestinal inflammation [14, 16, 17]. Soy lecithin, an emulsifier, has been suggested to have a beneficial effect on human cell membranes and inflammatory pathways [18].
Current methods of dietary evaluation are limited in their ability to capture food additive intake. Food frequency questionnaires (FFQ) estimate dietary exposures over time, but lack the necessary detail to capture intake of specific food additives [19]. Food diaries and 24-hour dietary recalls capture detailed data on macro- and micro-nutrient intake, but the associated dietary databases do not provide information on food additive content [20]. Accurate capture of food additive exposures is an important step in evaluating the association with diseases that are increasing in prevalence, such as CD. Demonstrating associations with increased risk of disease, or lack thereof, is important for designing dietary intervention studies for the treatment of CD and guiding future therapeutic strategies.
Methods
Study participants
Dietary data for the current analysis were derived from a study of 138 children and young adults ages 8–21 years who were enrolled in a randomized controlled trial evaluating the effect of low magnitude mechanical stimuli on bone density and structure in pediatric CD [21]. As previously reported, the intervention resulted in a modest increase in spine trabecular bone mineral density over 12 months, with no other effect seen on muscle strength, measures of bone density, bone structure or body composition. Participants were eligible for study entry 6 months after the initial diagnosis of CD and if bone density Z-score was below the 25th percentile for age, sex and race. Exclusion criteria included other chronic diseases or medications known to influence growth, nutrition, or body composition. The protocol was approved by the Institutional Review Board at The Children’s Hospital of Philadelphia. Informed consent was obtained from study participants over 18 years of age and assent and informed consent from the parents or guardians of those less than 18 years.
Dietary assessment
Three 24-hour dietary recalls were obtained following each study visit: at baseline, 6 months, 12 months, and 24 months. For each visit, two recalls were obtained on weekdays and one recall on a weekend day. For our study, only data from the baseline visit were utilized. The 24-hour recalls were obtained by telephone using a validated multi-pass method with prompting from a trained bionutritionist [22]. The dietary recall data were processed using the University of Minnesota Nutrition Data Systems for Research (NDSR) 2012, with the University of Minnesota Nutrition Coordinating Center Food and Nutrition Database serving as the source of food and composition information. This database allows for evaluation of macronutrient and micronutrient composition of food, but not food additive content.
Ingredient Data Collection
All food items captured from the baseline 24-hour recalls were sorted by alphabetical order and a database of all unique foods was created. A spreadsheet was created with a column for the unique food items and subsequent columns to denote inclusion/exclusion of each of the eight food additives of interest: polysorbate-80, carboxymethylcellulose, xanthan gum, soy lecithin, titanium dioxide, carrageenan, maltodextrin, and aluminosilicates. The heterogeneity of ingredients within food categories necessitated detailed evaluation of individual food labels. Permission was obtained from a Safeway grocery store manager to obtain ingredient label information over multiple days. This grocery store was chosen because of its generalizability; it is a national chain belonging to one of the largest food and drug retailers in the U.S. (Albertsons--with over 2,200 stores across 33 states) [23]. For food items available in the grocery store, ingredient labels were evaluated and the presence of any of the eight food additives of interest. Food items not evaluated in the grocery store were evaluated using the www.walmart.com website which provides a complete list of ingredients for food products.
For food purchased in restaurants, websites for the specific restaurants were evaluated for ingredient information. The database of each unique food item with newly collected data on food additive content was merged back into the original 24-hour dietary recall dataset containing all dietary recall data. This protocol for the capture of food additives was established in collaboration with the Nutrition Assessment Shared Resource (NASR) at the Fred Hutchinson Cancer Research Center, Seattle, Washington.
Food Groupings
Foods from the dietary recalls were grouped based on the NDSR-assigned Nutrition Coordinating Center (NCC) Food Group IDs. The NCC distributes and supports the NDSR dietary analysis software and maintains a research-quality food and nutrient database. The NCC Food Group IDs specify 135 unique food groups under 16 broad food group headings. The 135 NCC food group IDs were re-combined to 31 more descriptive broad food groupings to facilitate further analysis (Supplemental Table).
Statistical Analysis
Analyses were conducted using STATA 12.1 (Stata Corporation, College Station, TX). Demographic, clinical, and food additive variables were summarized using frequencies and percentages for categorical variables and mean, standard deviation, median, range, and interquartile range (IQR) for continuous variables, as appropriate. Data from the 3 recall days were averaged for each study participant.
Results
Baseline participant characteristics
A total of 138 participants enrolled in the study, with mean age 14.2 ± 2.8 years, with males (48%) and females (52%) evenly represented (Table 1). Of the enrolled participants, 135 completed baseline dietary recalls. In general, participants were not newly diagnosed with CD, as mean ± SD disease duration was 3.2 ± 2.7 years. Clinical disease activity, as measured by the Pediatric Crohn’s Disease Activity Index (PCDAI) [24], demonstrated that the majority of the participants had inactive (62%) or mild disease activity (33%), with only a small percentage having moderate-to-severe disease (5%).
Table 1:
Baseline Characteristics of Participants with Crohn’s Disease
| Variable | (n=138) |
|---|---|
| Age in years, mean (Range) | 14.2 ± 2.8 (8.0, 21.7) |
| Male sex, n (%) | 66 (48) |
| Race, % Black | 6 |
| Height Z score (range) | −0.76 ± 1.0 (−3.5, 1.4) |
| BMI Z score (range) | −0.25 ± 1.1 (−3.6, 2.2) |
| Duration since diagnosis (yr) | 3.2 ± 2.7 |
| PCDAI, mean | 11.0 ± 10.0 |
| PCDAI categories | |
| No active disease (≤10), n (%) | 85 (62) |
| Mild (11-30), n (%) | 46 (33) |
| Moderate to severe (>30), n (%) | 7 (5) |
| Albumin (g/dL), median (range) | 4.4 (2.7, 5.3) |
| ESR (mm/h), median (range) | 15 (0, 100) |
| Site of disease, n (%) | |
| Ileal | 13 (9) |
| Colonic | 22 (16) |
| Ileocolonic | 95 (69) |
| Any upper tract disease | 106 (77) |
| Perianal involvement | 8 (6) |
Mean ± standard deviation
Median (range)
Food additives by food group
A preliminary assessment of food categories for food additive content revealed that with the exception of most dry and hot cereal, soda and juice, the majority of packaged items contained one or more additive of interest. Subsequent detailed evaluation of the collected data demonstrated that many food groups contained several additives of interest (Table 2). A total of 4,965 food items were noted in the dietary recalls with 1,325 unique items. Of all food items consumed, 390 (8%) contained xanthan gum, 386 (8%) contained maltodextrin, 364 (7%) contained soy lecithin, 235 (5%) contained carrageenan, 37 (0.7%) contained titanium dioxide, 28 (0.6%) contained polysorbate-80, and 21 (0.4%) contained carboxymethylcellulose. Aluminosilicates were not observed on any ingredient lists.
Table 2.
Frequency of Food Additives In Food Groups
| Food Group | n | PS-80 n (%)* |
CMX | Xanthan gum |
Soy lecithin |
TD | CGN | MDX |
|---|---|---|---|---|---|---|---|---|
| Whole meat, fish, and poultry | 253 | 0 (0) | 0 (0) | 5 (2) | 2 (1) | 0 (0) | 2 (1) | 6 (2) |
| Processed meat | 141 | 1 (1) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 43 (31) | 0 (0) |
| Meat, poultry, fish recipes | 179 | 7 (4) | 3 (2) | 36 (20) | 15 (8) | 0 (0) | 6 (3) | 55 (31) |
| Frozen dessert | 98 | 7 (7) | 2 (2) | 44 (45) | 12 (12) | 0 (0) | 65 (66) | 9 (9) |
| Cocoa and milk-type beverages | 15 | 0 (0) | 0 (0) | 6 (40) | 4 (27) | 0 (0) | 3 (20) | 1 (7) |
| Milk and cheese recipes | 15 | 0 (0) | 0 (0) | 2 (13) | 0 (0) | 0 (0) | 2 (13) | 1 (7) |
| Alternative dairy products | 15 | 0 (0) | 0 (0) | 1 (7) | 5 (33) | 0 (0) | 8 (53) | 0 (0) |
| Salad dressing | 54 | 0 (0) | 0 (0) | 19 (35) | 0 (0) | 0 (0) | 0 (0) | 6 (11) |
| Vegetable products and recipes | 159 | 0 (0) | 2 (1) | 26 (16) | 3 (2) | 0 (0) | 1 (1) | 5 (3) |
| Grains and breads | 475 | 0 (0) | 0 (0) | 102 (21) | 28 (6) | 19 (4) | 2 (0.4) | 41 (9) |
| Hot and cold cereals | 183 | 0 (0) | 0 (0) | 0 (0) | 18 (10) | 0 0) | 0 (0) | 18 (10) |
| Pasta, rice and grain recipes | 242 | 0 (0) | 5 (2) | 63 (26) | 8 (3) | 0 (0) | 0 (0) | 60 (25) |
| Savory snacks | 322 | 0 (0) | 7 (2) | 6 (2) | 74 (23) | 0 (0) | 12 (4) | 54 (17) |
| Soups, gravy and sauces | 106 | 0 (0) | 0 (0) | 23 (22) | 0 (0) | 0 (0) | 0 (0) | 6 (6) |
| Cookies | 140 | 3 (2) | 0 (0) | 0 (0) | 56 (40) | 6 (4) | 15 (11) | 3 (2) |
| Non-cookie desserts | 91 | 0 (0) | 0 (0) | 19 (21) | 12 (13) | 3 (3) | 7 (8) | 6 (7) |
| Candy, sugar, sweets | 252 | 1 (0.4) | 0 (0) | 12 (5) | 52 (21) | 8 (3) | 14 (6) | 17 (7) |
| Meal replacement beverages | 115 | 0 (0) | 0 (0) | 1 (1) | 67 (58) | 0 (0) | 35 (30) | 70 (61) |
| Commercial entrees | 17 | 1 (6) | 2 (12) | 6 (35) | 5 (29) | 1 (6) | 3 (18) | 8 (47) |
n (%): number of items in Food Group containing select food additive, and percentage of all foods in category containing select additive
Abbreviations: PS-80, polysorbate-80; CMX, carboxymethylcellulose; TD, titanium dioxide; CGN, Carrageenan; MDX, maltodextrin
Food groups not shown: dairy; eggs and egg recipes; fats, oils and nuts; fruits and fruit products, vegetables and legumes, non-alcoholic beverages; coffee and tea; miscellaneous; condiments, pickles, and olives; supplements and drugs.
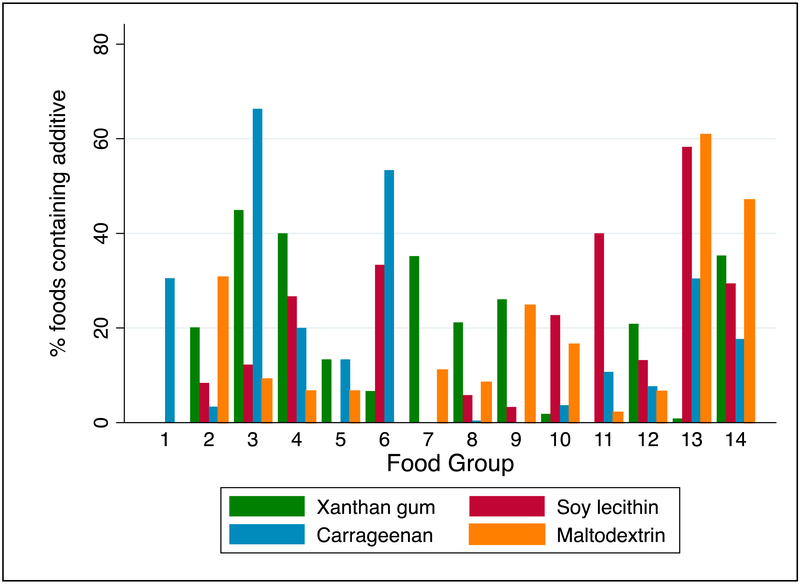
Of the 98 frozen dessert items consumed by participants, 66% contained carrageenan, 45% xanthan gum, and 12% soy lecithin (Table 2). While “Whole meat, fish, and poultry” (n=253) contained few additives of interest, in the “Meat, poultry, and fish recipes” category (n=179), 31% of food contained maltodextrin, 20% xanthan gum, and 8% soy lecithin. In the “Grains and Breads” category (n=475), 21% of items contained xanthan gum, 9% maltodextrin, and 6% soy lecithin. Soy lecithin was common in “Meal Replacement Beverages” (58%), “Cookies” (40%), “Savory Snacks” (23%), and “Non-Cookie Desserts” (13%). Maltodextrin was common in “Meal Replacement Beverages” (61%) and “Commercial Entrees” (47%). Numerous food groups contained multiple food additives of interest (Figure).
Figure: Frequency of Food Additive Inclusion In Select Food Groups.
KEY:
1. Processed meat (n=141)
2. Meat/poultry/fish recipes (n=179)
3. Frozen desserts (n=98)
4. Cocoa/milk beverages (n=15)
5. Milk/cheese recipes (n=15)
6. Alternative dairy products (n=15)
7. Salad dressings (n=54)
8. Grains and breads (n=475)
9. Pasta/rice/grain recipes (n=242)
10. Savory snacks (n=322)
11. Cookies (n=140)
12. Non-cookie desserts (n=91)
13. Meal replacement beverages (n=115)
14. Commercial entrees (n=17)
Participant food additive exposures
In evaluating the eight food additives of interest, study participants were exposed to a mean of 3.6 ± 2.1 total additive per recall day and exposed to a mean of 2.4 ± 1.0 different additives per recall day. The food additive with the highest frequency of consumption in our study participants was xanthan gum with a mean of 0.96 ± 0.72 exposures per day (Table 3). Maltodextrin (0.95 ± 0.77 exposures per day), soy lecithin (0.90 ± 0.74 exposures per day), and carrageenan (0.58 ± 0.63 exposures per day) were also commonly consumed. The additives that appeared to be infrequently consumed included titanium dioxide, polysorbate-80 and carboxymethylcellulose. No differences in food additive exposures were seen when comparing inactive disease vs. mild to severe clinical disease activity.
Table 3.
Summary of Mean Daily Food Additive Exposures
| Additive | Mean exposures/day |
SD | Range |
|---|---|---|---|
| Polysorbate-80 | 0.07 | 0.16 | [0, 1] |
| Carboxymethylcellulose | 0.05 | 0.13 | [0, 0.67] |
| Xanthan gum | 0.96 | 0.72 | [0, 5.3] |
| Soy Lecithin | 0.90 | 0.74 | [0, 4] |
| Titanium Dioxide | 0.09 | 0.21 | [0, 1] |
| Carrageenan | 0.58 | 0.63 | [0, 3.67] |
| Maltodextrin | 0.95 | 0.77 | [0, 5] |
| Aluminosilicates | NF | NF | NF |
These values are derived from calculated mean exposures for each participant; NF – Not found in foods consumed.
Discussion
We detected frequent consumption of select food additives by children with CD. Although the quantification of food additive exposures required detailed, time-intensive evaluation of 1,325 unique food products, our study demonstrates that children with CD frequently consume a variety of food additives that have been associated with intestinal inflammation. The evaluation of individual food groups suggests a pattern of food additive presence in certain foods, and many food groups frequently contain multiple different food additives.
To our knowledge, to date, exposures have not been described for polysorbate-80, carboxymethylcellulose, xanthan gum, soy lecithin, titanium dioxide, carrageenan, maltodextrin, and aluminosilicates in children, despite the interest in food additives on the part of clinical gastroenterologists and investigators. As such, the comparative frequency of exposures in our study participants was difficult to assess. In adults, a US study utilized a questionnaire based upon foods containing carrageenan and incorporated food industry standards for carrageenan content and estimated exposures ranging from 0 to 7.7 g per day [25]. Other studies have evaluated different food additive exposures using a variety of methodologies ranging from population-based methods to individual assessment [26]. A study utilizing the Irish National Food Ingredient Database (INFID) evaluated the association of hyperactive behavior in children with the intake of seven food additives: sunset yellow, carmoisine, tartrazine, ponceau 4R, allura red, quinoline yellow, and sodium benzoate [27]. The INFID compiles ingredient label data by collecting packaging for food items consumed during a 7-day food diary. Another study conducted in France, Italy, the UK and Ireland evaluated the intake of 13 food additives deemed “priority additives” and utilized 4–7 day food diaries, a scheme to code the individual components in foods, and data from the food industry to estimate concentrations for each food additive in each food category [28]. Although the majority of data on food additives are descriptive, some data from clinical trials exist, including a small randomize controlled trial in children suggesting that artificial coloring and sodium benzoate increased hyperactivity in children [29].
Current methods to quantify dietary exposures include FFQ, 24-hour dietary recall, food diary, and direct evaluation by a dietitian. FFQ is a validated methodology designed to evaluate exposures over time, but unlike the other methods does not capture granular detail [30]. Food diaries may impart observation bias, and direct dietitian evaluation can be highly variable. Multi-pass 24-hour dietary recall is subject to some recall bias [31]; however, it can be utilized to accurately estimate exposures to macronutrients, vitamins, and minerals. This approach is ideally suited for the evaluation of food additives, and the future development of a database including food additives may facilitate the broader study of the association between food additives and disease.
Our study has several strengths. It is the first to our knowledge to quantify frequency of exposure to specific food additives in a cohort of children and adolescents with CD. The cohort was well defined and dietary intake assessment was rigorously conducted. At the same time, as a retrospective study, the study is limited by the lack of necessary detail in the dietary data. A large portion of food items in the recall data contained only generic information and without specific information on brands. Further, insufficient detail was present for homemade foods (i.e. hamburgers or cookies). As such, our findings on the frequency of exposure to select food additives likely underestimates exposures. Exposures to food additives in non-food substances, such as titanium dioxide in toothpaste, should also be considered in future studies. While adults and older children do not swallow toothpaste, it is known that younger children often do ingest toothpaste, and this exposure may contribute to risk of disease [32]. Another limitation to this study, and currently others like it, is the inability to capture the quantity of food additives consumed. Capturing frequency of exposures is an important first step; however, future studies may be able to utilize more detailed food product data or biomarkers of food additive exposures to more precisely estimate the amount of exposure, which will allow for a better understanding of the potential dose effect of specific food additives [33]. Another limitation is the generalizability of our findings given the study population; however, because of the concerns regarding dietary exposures in pediatric CD patients it is a relevant population. Building upon the described methodology of evaluating exposure to food additives, it will be important for future studies to also evaluate food additive exposures in healthy individuals and also individuals with other disease conditions. Although 24-hour dietary recall is able to capture high quality dietary intake data, the potential for recall bias exists and this method of data collection may also be burdensome for children and their families.
Further studies, perhaps building upon our approach, are needed to better understand the association between food additives and disease. Modifications to conventional 24-hour dietary recall may help facilitate greater capture of details, including ingredients used in home-prepared meals and also the specific brands of foods. In the future, the development of a database of foods and their constituent food additives may be a practical resource for investigators who are using existing 24-hour recall techniques. Finally, work to better quantify amounts of food additive intake will be important but require more information from food manufacturers or the development of biomarkers to characterize exposure.
Conclusion
Diet plays an important role in pediatric CD. Among a population of children and adolescents with CD we found frequent exposures to xanthan gum, maltodextrin, soy lecithin, and carrageenan. Less frequent exposures to titanium dioxide, polysorbate-80, and carboxymethylcellulose occurred as well. This suggests that further studies in humans are needed to elucidate the role of these food additives in CD.
Supplementary Material
Acknowledgments
Funding sources: NIH R01-DK073946 (MBL), K24-DK076808 (MBL), T32-DK007740 (DL), the University of Pennsylvania Clinical and Translational Science Award (UL1-RR-024134 and UL1-TR-000003); P30 CA015704 (Cancer Core Grant funding Nutrition Assessment Shared Resource); and the Clinical Research Scholar’s Program at Seattle Children’s Hospital (DL).
Footnotes
Conflicts of interest: none
Contributor Information
Dale Lee, Seattle Children’s Hospital, 4800 Sand Point Way, Seattle WA 98105., Tel: (206) 987-7339. Fax: (206) 987-2721., Dale.Lee@seattlechildrens.org.
C. Kaiulani Swan, Nutrition Sciences, University of Washington, Seattle, Washington, 4800 Sand Point Way, Seattle WA 98105., Tel: n/a Fax: n/a, kaiswan@gmail.com.
David Suskind, Seattle Children’s Hospital, 4800 Sand Point Way, Seattle WA 98105., Tel: (206) 987-2521. Fax: (206) 987-2721., David.Suskind@seattlechildrens.org.
Ghassan Wahbeh, Seattle Children’s Hospital, 4800 Sand Point Way, Seattle WA 98105., Tel: (206) 987-2521. Fax: (206) 987-2721., Ghassan.Wahbeh@seattlechildrens.org.
Jairam Vanamala, Penn State University, 326 Rodney A. Erickson Food Science Building, University Park, PA 16802, Tel: 814-865-6842, juv4@psu.edu.
Robert N. Baldassano, The Children’s Hospital of Philadelphia, 324 S. 34th Street, Philadelphia, PA 19194, Tel: (267) 426-5123. Fax: (215) 590-3606, baldassano@email.chop.edu.
Mary B. Leonard, Chairman of Pediatrics Stanford University, 770 Welch Rd Ste 300, Palo Alto, CA 94304, Tel: (650) 723-5104. Fax: (650) 49806714, Leonard5@stanford.edu.
Johanna W. Lampe, Division of Public Health Sciences, Fred Hutchinson Cancer Research Center, 1100 Fairview Ave N, Seattle, WA 98109., Tel: (206) 667-6580. Fax: (206) 667-7850, JLampe@FredHutch.org.
References
- 1.Lee D, Albenberg L, Compher C, et al. Diet in the Pathogenesis and Treatment of Inflammatory Bowel Diseases. Gastroenterology 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Day AS, Whitten KE, Sidler M, et al. Systematic review: nutritional therapy in paediatric Crohn’s disease. Aliment Pharmacol Ther 2008;27(4):293–307. [DOI] [PubMed] [Google Scholar]
- 3.Obih C, Wahbeh G, Lee D, et al. Specific carbohydrate diet for pediatric inflammatory bowel disease in clinical practice within an academic IBD center. Nutrition 2016;32(4):418–25. [DOI] [PubMed] [Google Scholar]
- 4.Sigall-Boneh R, Pfeffer-Gik T, Segal I, et al. Partial enteral nutrition with a Crohn’s disease exclusion diet is effective for induction of remission in children and young adults with Crohn’s disease. Inflamm Bowel Dis 2014;20(8):1353–60. [DOI] [PubMed] [Google Scholar]
- 5.Suskind DL, Cohen SA, Brittnacher MJ, et al. Clinical and Fecal Microbial Changes With Diet Therapy in Active Inflammatory Bowel Disease. J Clin Gastroenterol 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Heuschkel RB, Menache CC, Megerian JT, et al. Enteral nutrition and corticosteroids in the treatment of acute Crohn’s disease in children. J Pediatr Gastroenterol Nutr 2000;31(1):8–15. [DOI] [PubMed] [Google Scholar]
- 7.Day AS, Lopez RN. Exclusive enteral nutrition in children with Crohn’s disease. World J Gastroenterol 2015;21(22):6809–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Suskind DL, Wahbeh G, Gregory N, et al. Nutritional therapy in pediatric Crohn disease: the specific carbohydrate diet. J Pediatr Gastroenterol Nutr 2014;58(1):87–91. [DOI] [PubMed] [Google Scholar]
- 9.Olendzki BC, Silverstein TD, Persuitte GM, et al. An anti-inflammatory diet as treatment for inflammatory bowel disease: a case series report. Nutr J 2014;13(5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Molodecky NA, Soon IS, Rabi DM, et al. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology 2012;142(1):46–54 e42; quiz e30. [DOI] [PubMed] [Google Scholar]
- 11.Hou JK, Abraham B, El-Serag H. Dietary intake and risk of developing inflammatory bowel disease: a systematic review of the literature. Am J Gastroenterol 2011;106(4):563–73. [DOI] [PubMed] [Google Scholar]
- 12.Roberts CL, Keita AV, Duncan SH, et al. Translocation of Crohn’s disease Escherichia coli across M-cells: contrasting effects of soluble plant fibres and emulsifiers. Gut 2010;59(10):1331–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Butler M, Boyle JJ, Powell JJ, et al. Dietary microparticles implicated in Crohn’s disease can impair macrophage phagocytic activity and act as adjuvants in the presence of bacterial stimuli. Inflamm Res 2007;56(9):353–61. [DOI] [PubMed] [Google Scholar]
- 14.Chassaing B, Koren O, Goodrich JK, et al. Dietary emulsifiers impact the mouse gut microbiota promoting colitis and metabolic syndrome. Nature 2015;519(7541):92–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tobacman JK. Review of harmful gastrointestinal effects of carrageenan in animal experiments. Environ Health Perspect 2001;109(10):983–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Swidsinski A, Ung V, Sydora BC, et al. Bacterial overgrowth and inflammation of small intestine after carboxymethylcellulose ingestion in genetically susceptible mice. Inflamm Bowel Dis 2009;15(3):359–64. [DOI] [PubMed] [Google Scholar]
- 17.Nickerson KP, Chanin R, McDonald C. Deregulation of intestinal anti-microbial defense by the dietary additive, maltodextrin. Gut Microbes 2015;6(1):78–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kullenberg D, Taylor LA, Schneider M, et al. Health effects of dietary phospholipids. Lipids Health Dis 2012;11(3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Willett W Nutritional epidemiology. New York: Oxford University Press; 1998. [Google Scholar]
- 20.Johnson RK. Dietary intake--how do we measure what people are really eating? Obes Res 2002;10 Suppl 1(63S–68S. [DOI] [PubMed] [Google Scholar]
- 21.Leonard MB, Shults J, Long J, et al. Effect of Low Magnitude Mechanical Stimuli on Bone Density and Structure in Pediatric Crohn’s Disease: A Randomized Placebo Controlled Trial. J Bone Miner Res 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Posner BM, Borman CL, Morgan JL, et al. The validity of a telephone-administered 24-hour dietary recall methodology. Am J Clin Nutr 1982;36(3):546–53. [DOI] [PubMed] [Google Scholar]
- 23.Albertsons. http://www.albertsons.com/our-company/traditions-history/. Accessed 11/21/16. [Google Scholar]
- 24.Hyams JS, Ferry GD, Mandel FS, et al. Development and validation of a pediatric Crohn’s disease activity index. J Pediatr Gastroenterol Nutr 1991;12(4):439–47. [PubMed] [Google Scholar]
- 25.Shah ZH FG. Current Availability and Consumption of Carrageenan-Containing Foods. Ecology of Food and Nutrition 2002;42(357–71. [Google Scholar]
- 26.Gibney MJ. Dietary intake methods for estimating food additive intake. Regul Toxicol Pharmacol 1999;30(2 Pt 2):S31–3. [DOI] [PubMed] [Google Scholar]
- 27.Connolly A, Hearty A, Nugent A, et al. Pattern of intake of food additives associated with hyperactivity in Irish children and teenagers. Food Addit Contam Part A Chem Anal Control Expo Risk Assess 2010;27(4):447–56. [DOI] [PubMed] [Google Scholar]
- 28.Vin K, Connolly A, McCaffrey T, et al. Estimation of the dietary intake of 13 priority additives in France, Italy, the UK and Ireland as part of the FACET project. Food Addit Contam Part A Chem Anal Control Expo Risk Assess 2013;30(12):2050–80. [DOI] [PubMed] [Google Scholar]
- 29.McCann D, Barrett A, Cooper A, et al. Food additives and hyperactive behaviour in 3-year-old and 8/9-year-old children in the community: a randomised, double-blinded, placebo-controlled trial. Lancet 2007;370(9598):1560–7. [DOI] [PubMed] [Google Scholar]
- 30.Willett WC, Sampson L, Stampfer MJ, et al. Reproducibility and validity of a semiquantitative food frequency questionnaire. Am J Epidemiol 1985;122(1):51–65. [DOI] [PubMed] [Google Scholar]
- 31.Ma Y, Olendzki BC, Pagoto SL, et al. Number of 24-hour diet recalls needed to estimate energy intake. Ann Epidemiol 2009;19(8):553–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rompelberg C, Heringa MB, van Donkersgoed G, et al. Oral intake of added titanium dioxide and its nanofraction from food products, food supplements and toothpaste by the Dutch population. Nanotoxicology 2016;10(10):1404–14. [DOI] [PubMed] [Google Scholar]
- 33.Scalbert A, Brennan L, Manach C, et al. The food metabolome: a window over dietary exposure. Am J Clin Nutr 2014;99(6):1286–308. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.