Introduction
Atherosclerosis is a chronic disease characterized by accumulation of fat and cholesterol in the arterial wall leading to restriction of blood flow1–3. Atherosclerotic plaques contain a number of cell types including smooth muscle cells (SMCs), macrophages, endothelial cells (ECs), B cells, T cells, and fibroblasts, among others4–8. Recent lineage tracing studies provide strong evidence that a substantial proportion of cells in the plaque are derived from the endothelium. This process, referred to as endothelial-to-mesenchymal transition (EndMT) is characterized by the loss of normal endothelial and gain of mesenchymal fate markers. Functional consequences include deposition of pro-inflammatory extracellular matrix, facilitation of recruitment of leukocytes and growth of unstable plaque. Here, we review the current understanding of molecular controls of EndMT and its contribution to the development of atherosclerosis.
Atherosclerosis
Atherosclerosis is best viewed as a chronic, progressive inflammatory disease. The slow evolution of atherosclerotic lesions combines endothelial dysfunction with recruitment of leukocytes, extensive lipid deposition, proliferation of medial SMCs, and remodeling of the extracellular matrix. Endothelial cells play a critical role in the development of atherosclerosis. The normal endothelium is involved in multiple aspects of vascular physiology including regulation of vascular barrier function, leukocyte trafficking, thrombosis prevention, and regulation of vascular tone. Endothelial dysfunction is an important step in the development and progression of atherosclerotic lesions and its occurrence has been considered a predictor of subsequent development of atherosclerosis. Most atherosclerosis triggers, including both mechanical and local inflammatory factors, can activate the endothelium, resulting in expression of chemokines and cytokines (e.g. IL-1, IL-6, IL-8, MCP-1) and adhesion receptors (e.g. ICAM-1, VCAM-1, E-selectin) that attract and facilitate immune cell extravasation9.
Endothelial to mesenchymal transition (EndMT)
Recent studies have demonstrated that endothelial cells exhibit a high degree of organ heterogeneity and plasticity both in physiologic and pathologic settings. Endothelial plasticity can range from a subtle shift from quiescent to activated state to a full-blown fate change characterized by a nearly complete loss of endothelial and acquisition of mesenchymal, fate markers. The latter process, termed EndMT, is not necessarily pathologic. Indeed, EndMT is critical to normal embryogenesis, most notably in developing cardiac valves. Here, EndMT results in conversion of endocardial cells of the atrio-ventricular canal into mesenchymal cells that populate A-V cushions and subsequently give rise to the mitral and tricuspid valves10. EndMT is also involved in generation of cardiac fibroblasts and formation of intracardiac septa11. While some degree of EndMT in adult tissues may be a normal component of wound healing/injury recovery response, prolonged and/or extensive EndMT is clearly pathologic12. Various studies demonstrated its occurrence in a number of disease settings including organ fibrosis13–15, restenosis16, transplant arteriopathy17, vein graft remodeling18, pulmonary hypertension19, and cancer20.
More recently, EndMT has been shown to be an important contributes to atherosclerosis, both in mouse models and in patients21–23. Of note, there is a strong correlation between the anatomically determined severity of human coronary artery disease and the extent of EndMT in luminal coronary endothelial cells22, 23. Indeed, this correlation may be causative: macrophage-derived foam cells impair endothelial barrier function by inducing EndMT24 while EndMT promotes deposition of fibronectin and expression of endothelial adhesion molecules ICAM-1 and VCAM-1, thereby promoting recruitment of circulating monocytes and leukocytes and accumulation of monocyte-derived macrophages22. One may hypothesize that this leads to establishment of a feed-forward loop that further aggravates endothelial cell dysfunction and drives progression of atherosclerosis12.
Abnormal shear stress occurring in certain segments of the arterial vasculature has long been known to be responsible for the preponderance of atherosclerotic lesions in these locations via its effect on luminal endothelial cells25. Low shear stress promotes, while high shear stress suppresses, vascular inflammation and thrombosis. More recently, this has also been linked to EndMT with uniform laminar shear stress inhibiting EndMT development via activation of ERK5 signaling, whereas disturbed flow promoted it21. Low shear stress-dependent stimulation of may involve upregulation of expression of TWIST and Snail transcription factors26,27.
The high-density (HDL) cholesterol fraction that has been associated with anti-atherosclerotic effects, reduces TGFβ1-induced EndMT via upregulation of the inhibitor Smad (Smad7) and decreases expression of EndMT transcription factor Slug and ZEB128. Furthermore, the lipoprotein ApoA-I/ABCA1 inhibits TGFβ1-induced EndMT through repression of the TGFβ1/Smad2/Smad3/Snail/Slug pathway in human coronary artery endothelial cells29.-Overall, all these data point to a potentially critical role played by EndMT in atherosclerosis.
Signaling pathways regulating EndMT
Fibroblast growth factor (FGF) signaling pathway
The twenty-two fibroblast growth factors family members signal via four plasma membrane tyrosine kinase receptors (FGFR1–4). In the vasculature, FGF signaling is involved in numerous biological processes including modulation of angiogenesis, maintenance of vascular integrity, and regulation of endothelial cell identity30, 31. Binding of an FGF ligand to an FGFR induces the latter to dimerize and trans-phosphorylate specific tyrosine residues in its cytoplasmic kinase domains thereby leading to its activation32. Subsequent phosphorylation events in receptors’ cytoplasmic domains and a constitutively docked adaptor protein fibroblast growth factor receptor substrate 2 alpha (FRS2α), generate docking sites for numerous cytoplasmic proteins. This, in turn, results in activation of downstream signaling cascades including Ras/mitogen-activating protein (MAP) kinase, phosphoinositide-3-kinase (PI3K)/Akt pathway, and phospholipase C gamma (PLCγ) pathways33.
All FGF receptors (FGFR1–4), the adaptor protein FRS2α, and their co-receptor Klotho and Syndecan-4 have been genetically knockout in mice34–41. However, these global knockout phenotypes are not very informative with regard to vascular development either due to very early embryonic lethality occurring prior to critical stages of blood vessel development (i.e. Fgfr1−/−) or the absence of a phenotype due to compensation by other FGFRs. More detailed insights in the role of FGF signaling in the endothelium emerged from cell-type specific knockouts of FGF receptors. A conditional endothelial-specific knockout of Fgfr1 and Fgfr2 showed that mice are viable with no vascular developmental defects42. However, there was a significant impairment of angiogenic response after eye injury and delayed skin wound healing in adult mice. At the same time, an endothelial-specific knockout of Fgfr1 in combination with a global Fgfr3 knockout resulted in impaired development of blood and lymphatic vessels43. Interestingly, an endothelial-specific knockout of either Fgfr1 or Frs2α increased neointima formation in transplant arteriopathy and atherosclerosis models due to induction of EndMT17, 22. Finally, FGF signaling also plays an important role in the maintenance of VEGFR2 expression and is required for the maintenance of both blood and lymphatic endothelial cells identity through FGF-Ras-MAPK signaling31, 44–46.
Transforming growth factor β (TGFβ) signaling pathway
TGFβ, a member of the TGF superfamily of growth factors, has been reported to be involved in a wide range of diverse and often contradictory functions. It plays important roles both in physiological (embryonic development, differentiation, cell growth, cell death, tissue homeostasis) and pathological processes (auto-immune, inflammation, fibrosis, angiogenesis, oncogenesis, and cardiovascular disease)47. The three TGFβ isoforms (TGFβ1, TGFβ2, and TGFβ3) signal through two types of serine/threonine kinase receptors, called types I and II TGFβ receptors48. Once dimerized by ligand binding. these protein complexes trigger signal transduction by phosphorylation of their specific receptor-regulated Smads (R-Smads2 and 3). Activated R-Smads interact with the common partner Smad, Smad4, and accumulate in the nucleus, where the Smad complex directly binds to defined elements on the DNA and regulates target gene expression together with other factors49, 50. In addition to activating the canonical Smad signaling pathway, TGFβ signaling can also activate non-canonical cascades including PI3K/AKT, MAPK and Rho-like GTPase signaling pathways51, 52. However, the relationship between TGFβ signaling and non-Smad activation has been poorly understood. The type III TGFβ receptors, betaglycan and endoglin, function as ligand reservoirs, holding ligands close to the cell surface in a manner analogous to the role played by heparan sulfate proteoglycans in FGF signaling53.
In vitro, TGFβ has been shown to inhibit endothelial cell proliferation and migration and to stimulate smooth muscle and mesenchymal cell differentiation and extracellular matrix accumulation54–56. TGFβ signaling in endothelial cells is essential for precise regulation of blood vessel assembly, normal embryonic development, postnatal angiogenesis, and homeostasis maintenance in the adult vasculature. When induced early, endothelial cell-specific knockouts of Tgfbr1 or Tgfbr2 result in embryonic lethality at ~E10.5 with extensive yolk sac vascular network defects57. Endothelial-specific excision activation of Tgfbr2 at E11.5 leads to abnormal ventricular septation and cerebral hemorrhage58. Finally, endothelial Tgfbr2 excision at the postnatal day 2 (P2) results in angiogenic defects in the retina59. Extensive studies also showed that TGFβ promotes SMC differentiation and stimulates SMC marker gene expression through Smad2/Smad3 activation60–62. Selective deletion of Tgfbr1 or Tgfbr2 in SMCs cause embryonic lethality due to vascular deformities57. Adult mice lacking Tgfbr2 in VSMCs also show vascular defects including increased VSMC proliferation, decreased smooth muscle contractile markers, and exhibiting aortic thickening, dilatation, and dissection52.
FGF-TGFβ cross-talk
As already outlined, FGF and TGFβ signaling pathways play important roles in regulating endothelial and smooth muscle cell behaviors. However, the two signaling systems often have diametrically opposite effects: for example, while FGFs induce SMC proliferation, TGFβ inhibits it56, 63. Even though the fact that these signaling cascades can influence, and counteract, each has been known for some time, until now little was known about molecular details of these interactions.
Early studies showed that in vitro FGF2 inhibits TGFβR1 expression64. It also antagonizes TGFβ-mediated induction of SMC markers expression in pericytes and smooth muscle cells65 and can revert TGFβ-induced epithelial-to-mesenchymal transition (EMT)66. More recent studies showed that inhibition of FGF signaling in the endothelium induced by Fgfr1 or Frs2α deletion results in increased TGFβ signaling leading to EndMT17, 67. The molecular basis of this cross-talk proved rather unusual. FGF signaling input is required for maintenance of let-7 miRNA expression. Both TGFβR1 and Smad2 3’UTR contain multiple let-7 binding sites: a decrease in FGF signaling input leads to a 20–120 decline in let-7 levels. This, in turn, markedly prolongs TGFβR1 (and Smad 2) mRNA half-lives, thus increasing their protein expression and allowing activation of TGFβ signaling17.
The effect of FGF signaling shutdown is not limited to TGFβR1: expression of all TGFβ receptors is increased as is expression of TGFβ ligands, with TGFβ2 demonstrating the biggest increase17. Whether all of these TGFβ family members’ expression is also directly regulated by let-7 or is due to something else, has not been established. While initially described in endothelial cells, the FGF-let-7-TGFβ cross-talk appears quite universal, having also been observed in smooth muscle cells and fibroblasts56.
FGF-TGFβ cross-talk in endothelial cells
While FGF regulation of TGFβ signaling appears pretty universal, functional consequences of this cross-talk are quite different for different cell types. It is particularly instructive to construct biological consequences of extinguishing FGF signaling and activating TGFβ signaling in endothelial vs. smooth muscle cells. In vitro, endothelial cells treated with TGFβ undergo EndMT as demonstrated by changes in cell shape, loss of endothelial cell markers and gain mesenchymal marker gene expression. A dominant-negative form of Smad4, the TGFβR1 kinase inhibitor SB431542, and the TGFβ antagonist BMP7, all block TGFβ-induced EndMT13. In agreement with these data, in vivo studies confirmed the central role played by activation of endothelial TGFβ signaling in EndMT induction including both in development68 and disease settings17, 22. Like these in vitro observations, EndMT in blood vessels vivo results in endothelial cells losing their highly differentiated state, becoming proliferative, and acquiring mesenchymal characteristics. This, in turn, promotes disease processes such as atherosclerosis22.
FGF-TGFβ cross-talk in smooth muscle cells
As in endothelial cells, there is strong evidence linking FGF signaling to suppression of TGFβ signaling and the loss of FGF signaling input to activation of TGFβ signaling output. Yet biological consequences are very different. FGF2 is a potent SMC mitogen and a powerful inducer of the SMC contractile-to-proliferative phenotype switch63. The induction of a proliferative phenotype is characterized by the loss of normal contractile protein expression, appearance of non-muscle isoforms of muscle proteins, secretion of extracellular matrix and increased proliferative and migratory behavior. In vivo, this is commonly observed in injury settings when local FGF release leads to contractile-to-proliferative phenotype transition. The end result is a marked increase in medial SMC proliferation and neointima formation, features of several common diseases including atherosclerosis and restenosis69, 70.
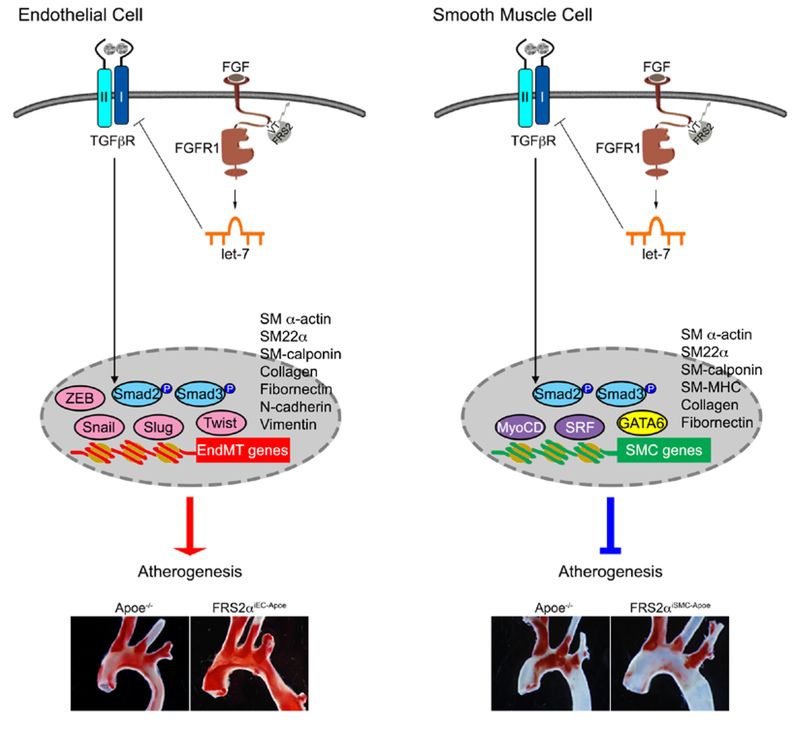
In contrast to FGF, TGFβ promotes SMC differentiation and converts proliferative SMCs back to contractile state63. In vivo activation of SMC TGFβ signaling induced by the loss of FGF signaling input engineered by an SMC-specific Frs2α deletion, results in markedly reduced neointima lesion development in both atherosclerosis and carotid artery ligation models56, 63. Thus, the loss of FGF signaling in both the endothelium and smooth muscle cells activates TGFβ signaling. In the endothelium this leads to the loss of cell differentiation, transition to an undifferentiated state and leads to promotion of diseases such as atherosclerosis. In contrast, in SMCs, the same process shifts undifferentiated cells to the differentiated phenotype and suppresses atherosclerotic progression (Figure 1). In summary, FGF-TGFβ cross-talk is a common biological occurrence that plays multifactorial roles both in development and disease. Importantly, while a decline in FGF signaling invariably leads to an increase in TGFβ signaling, the functional consequences of this interplay are highly cell type-dependent.
Figure 1: FGF-TGFβ signaling antagonizing in endothelial cells and smooth muscle cells.
In both endothelial cells and smooth muscle cells, inhibition of FGF signaling leads to upregulate TGFβ signaling activity, however, the biological outcome of this antagonizing effects is completely different. In endothelial cell (left panel), activation of TGFβ signaling cascade induces endothelial cell to mesenchymal transition (EndMT), increases inflammation, permeability, and extracellular matrix (ECM) deposition, and therefore, accelerates atherosclerosis. In smooth muscle cell (right panel), activation of TGFβ signaling cascade promotes differentiation/maturation, maintains vascular homeostasis, and increases extracellular matrix deposition, therefore inhibits the progression of atherosclerosis22, 56.
References:
- 1.Virmani R, Burke AP, Farb A, Kolodgie FD. Pathology of the vulnerable plaque. J Am Coll Cardiol. 2006;47:C13–18 [DOI] [PubMed] [Google Scholar]
- 2.Bentzon JF, Otsuka F, Virmani R, Falk E. Mechanisms of plaque formation and rupture. Circ Res. 2014;114:1852–1866 [DOI] [PubMed] [Google Scholar]
- 3.Yahagi K, Kolodgie FD, Otsuka F, Finn AV, Davis HR, Joner M, Virmani R. Pathophysiology of native coronary, vein graft, and in-stent atherosclerosis. Nat Rev Cardiol. 2016;13:79–98 [DOI] [PubMed] [Google Scholar]
- 4.Tabas I, Garcia-Cardena G, Owens GK. Recent insights into the cellular biology of atherosclerosis. J Cell Biol. 2015;209:13–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hedrick CC. Lymphocytes in atherosclerosis. Arterioscler Thromb Vasc Biol. 2015;35:253–257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mallat Z, Taleb S, Ait-Oufella H, Tedgui A. The role of adaptive t cell immunity in atherosclerosis. J Lipid Res. 2009;50 Suppl:S364–369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tsiantoulas D, Diehl CJ, Witztum JL, Binder CJ. B cells and humoral immunity in atherosclerosis. Circ Res. 2014;114:1743–1756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Morris-Rosenfeld S, Lipinski MJ, McNamara CA. Understanding the role of b cells in atherosclerosis: Potential clinical implications. Expert Rev Clin Immunol. 2014;10:77–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Davignon J, Ganz P. Role of endothelial dysfunction in atherosclerosis. Circulation. 2004;109:III27–32 [DOI] [PubMed] [Google Scholar]
- 10.Nakajima Y, Yamagishi T, Hokari S, Nakamura H. Mechanisms involved in valvuloseptal endocardial cushion formation in early cardiogenesis: Roles of transforming growth factor (tgf)-beta and bone morphogenetic protein (bmp). Anat Rec. 2000;258:119–127 [DOI] [PubMed] [Google Scholar]
- 11.Armstrong EJ, Bischoff J. Heart valve development: Endothelial cell signaling and differentiation. Circ Res. 2004;95:459–470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schwartz MA, Vestweber D, Simons M. A unifying concept in vascular health and disease. Science. 2018;360:270–271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zeisberg EM, Tarnavski O, Zeisberg M, Dorfman AL, McMullen JR, Gustafsson E, Chandraker A, Yuan X, Pu WT, Roberts AB, Neilson EG, Sayegh MH, Izumo S, Kalluri R. Endothelial-to-mesenchymal transition contributes to cardiac fibrosis. Nat Med. 2007;13:952–961 [DOI] [PubMed] [Google Scholar]
- 14.Zeisberg EM, Potenta SE, Sugimoto H, Zeisberg M, Kalluri R. Fibroblasts in kidney fibrosis emerge via endothelial-to-mesenchymal transition. Journal of the American Society of Nephrology : JASN. 2008;19:2282–2287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Manetti M, Romano E, Rosa I, Guiducci S, Bellando-Randone S, De Paulis A, Ibba-Manneschi L, Matucci-Cerinic M. Endothelial-to-mesenchymal transition contributes to endothelial dysfunction and dermal fibrosis in systemic sclerosis. Ann Rheum Dis. 2017;76:924–934 [DOI] [PubMed] [Google Scholar]
- 16.Beranek JT. Vascular endothelium-derived cells containing smooth muscle actin are present in restenosis. Lab Invest. 1995;72:771. [PubMed] [Google Scholar]
- 17.Chen PY, Qin L, Barnes C, Charisse K, Yi T, Zhang X, Ali R, Medina PP, Yu J, Slack FJ, Anderson DG, Kotelianski V, Wang F, Tellides G, Simons M. Fgf regulates tgf-beta signaling and endothelial-to-mesenchymal transition via control of let-7 mirna expression. Cell reports. 2012;2:1684–1696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cooley BC, Nevado J, Mellad J, Yang D, St Hilaire C, Negro A, Fang F, Chen G, San H, Walts AD, Schwartzbeck RL, Taylor B, Lanzer JD, Wragg A, Elagha A, Beltran LE, Berry C, Feil R, Virmani R, Ladich E, Kovacic JC, Boehm M. Tgf-beta signaling mediates endothelial-to-mesenchymal transition (endmt) during vein graft remodeling. Science translational medicine. 2014;6:227ra234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ranchoux B, Antigny F, Rucker-Martin C, Hautefort A, Pechoux C, Bogaard HJ, Dorfmuller P, Remy S, Lecerf F, Plante S, Chat S, Fadel E, Houssaini A, Anegon I, Adnot S, Simonneau G, Humbert M, Cohen-Kaminsky S, Perros F. Endothelial-to-mesenchymal transition in pulmonary hypertension. Circulation. 2015;131:1006–1018 [DOI] [PubMed] [Google Scholar]
- 20.Zeisberg EM, Potenta S, Xie L, Zeisberg M, Kalluri R. Discovery of endothelial to mesenchymal transition as a source for carcinoma-associated fibroblasts. Cancer research. 2007;67:10123–10128 [DOI] [PubMed] [Google Scholar]
- 21.Moonen JR, Lee ES, Schmidt M, Maleszewska M, Koerts JA, Brouwer LA, van Kooten TG, van Luyn MJ, Zeebregts CJ, Krenning G, Harmsen MC. Endothelial-to-mesenchymal transition contributes to fibro-proliferative vascular disease and is modulated by fluid shear stress. Cardiovascular research. 2015;108:377–386 [DOI] [PubMed] [Google Scholar]
- 22.Chen PY, Qin L, Baeyens N, Li G, Afolabi T, Budatha M, Tellides G, Schwartz MA, Simons M. Endothelial-to-mesenchymal transition drives atherosclerosis progression. J Clin Invest. 2015;125:4514–4528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Evrard SM, Lecce L, Michelis KC, Nomura-Kitabayashi A, Pandey G, Purushothaman KR, d’Escamard V, Li JR, Hadri L, Fujitani K, Moreno PR, Benard L, Rimmele P, Cohain A, Mecham B, Randolph GJ, Nabel EG, Hajjar R, Fuster V, Boehm M, Kovacic JC. Endothelial to mesenchymal transition is common in atherosclerotic lesions and is associated with plaque instability. Nature communications. 2016;7:11853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang Y, Luo NS, Ying R, Xie Y, Chen JY, Wang XQ, Gu ZJ, Mai JT, Liu WH, Wu MX, Chen ZT, Fang YB, Zhang HF, Zuo ZY, Wang JF, Chen YX. Macrophage-derived foam cells impair endothelial barrier function by inducing endothelial-mesenchymal transition via ccl-4. Int J Mol Med. 2017;40:558–568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Baeyens N, Bandyopadhyay C, Coon BG, Yun S, Schwartz MA. Endothelial fluid shear stress sensing in vascular health and disease. The Journal of clinical investigation. 2016;126:821–828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mahmoud MM, Kim HR, Xing R, Hsiao S, Mammoto A, Chen J, Serbanovic-Canic J, Feng S, Bowden NP, Maguire R, Ariaans M, Francis SE, Weinberg PD, van der Heiden K, Jones EA, Chico TJ, Ridger V, Evans PC. Twist1 integrates endothelial responses to flow in vascular dysfunction and atherosclerosis. Circulation research. 2016;119:450–462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mahmoud MM, Serbanovic-Canic J, Feng S, Souilhol C, Xing R, Hsiao S, Mammoto A, Chen J, Ariaans M, Francis SE, Van der Heiden K, Ridger V, Evans PC. Shear stress induces endothelial-to-mesenchymal transition via the transcription factor snail. Sci Rep. 2017;7:3375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Spillmann F, Miteva K, Pieske B, Tschope C, Van Linthout S. High-density lipoproteins reduce endothelial-to-mesenchymal transition. Arterioscler Thromb Vasc Biol. 2015;35:1774–1777 [DOI] [PubMed] [Google Scholar]
- 29.Feng J, Zhang J, Jackson AO, Zhu X, Chen H, Chen W, Gui Q, Yin K. Apolipoprotein a1 inhibits the tgf-beta1-induced endothelial-to-mesenchymal transition of human coronary artery endothelial cells. Cardiology. 2017;137:179–187 [DOI] [PubMed] [Google Scholar]
- 30.Murakami M, Nguyen LT, Zhuang ZW, Moodie KL, Carmeliet P, Stan RV, Simons M. The fgf system has a key role in regulating vascular integrity. The Journal of clinical investigation. 2008;118:3355–3366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Murakami M, Nguyen LT, Hatanaka K, Schachterle W, Chen PY, Zhuang ZW, Black BL, Simons M. Fgf-dependent regulation of vegf receptor 2 expression in mice. The Journal of clinical investigation. 2011;121:2668–2678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Goetz R, Mohammadi M. Exploring mechanisms of fgf signalling through the lens of structural biology. Nat Rev Mol Cell Biol. 2013;14:166–180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Beenken A, Mohammadi M. The fgf family: Biology, pathophysiology and therapy. Nat Rev Drug Discov. 2009;8:235–253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Deng CX, Wynshaw-Boris A, Shen MM, Daugherty C, Ornitz DM, Leder P. Murine fgfr-1 is required for early postimplantation growth and axial organization. Genes Dev. 1994;8:3045–3057 [DOI] [PubMed] [Google Scholar]
- 35.Arman E, Haffner-Krausz R, Chen Y, Heath JK, Lonai P. Targeted disruption of fibroblast growth factor (fgf) receptor 2 suggests a role for fgf signaling in pregastrulation mammalian development. Proc Natl Acad Sci U S A. 1998;95:5082–5087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Deng C, Wynshaw-Boris A, Zhou F, Kuo A, Leder P. Fibroblast growth factor receptor 3 is a negative regulator of bone growth. Cell. 1996;84:911–921 [DOI] [PubMed] [Google Scholar]
- 37.Colvin JS, Bohne BA, Harding GW, McEwen DG, Ornitz DM. Skeletal overgrowth and deafness in mice lacking fibroblast growth factor receptor 3. Nat Genet. 1996;12:390–397 [DOI] [PubMed] [Google Scholar]
- 38.Weinstein M, Xu X, Ohyama K, Deng CX. Fgfr-3 and fgfr-4 function cooperatively to direct alveogenesis in the murine lung. Development. 1998;125:3615–3623 [DOI] [PubMed] [Google Scholar]
- 39.Gotoh N, Manova K, Tanaka S, Murohashi M, Hadari Y, Lee A, Hamada Y, Hiroe T, Ito M, Kurihara T, Nakazato H, Shibuya M, Lax I, Lacy E, Schlessinger J. The docking protein frs2alpha is an essential component of multiple fibroblast growth factor responses during early mouse development. Mol Cell Biol. 2005;25:4105–4116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kuro-o M, Matsumura Y, Aizawa H, Kawaguchi H, Suga T, Utsugi T, Ohyama Y, Kurabayashi M, Kaname T, Kume E, Iwasaki H, Iida A, Shiraki-Iida T, Nishikawa S, Nagai R, Nabeshima YI. Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature. 1997;390:45–51 [DOI] [PubMed] [Google Scholar]
- 41.Echtermeyer F, Streit M, Wilcox-Adelman S, Saoncella S, Denhez F, Detmar M, Goetinck P. Delayed wound repair and impaired angiogenesis in mice lacking syndecan-4. J Clin Invest. 2001;107:R9–R14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Oladipupo SS, Smith C, Santeford A, Park C, Sene A, Wiley LA, Osei-Owusu P, Hsu J, Zapata N, Liu F, Nakamura R, Lavine KJ, Blumer KJ, Choi K, Apte RS, Ornitz DM. Endothelial cell fgf signaling is required for injury response but not for vascular homeostasis. Proc Natl Acad Sci U S A. 2014;111:13379–13384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yu P, Wilhelm K, Dubrac A, Tung JK, Alves TC, Fang JS, Xie Y, Zhu J, Chen Z, De Smet F, Zhang J, Jin SW, Sun L, Sun H, Kibbey RG, Hirschi KK, Hay N, Carmeliet P, Chittenden TW, Eichmann A, Potente M, Simons M. Fgf-dependent metabolic control of vascular development. Nature. 2017;545:224–228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pepper MS, Mandriota SJ. Regulation of vascular endothelial growth factor receptor-2 (flk-1) expression in vascular endothelial cells. Exp Cell Res. 1998;241:414–425 [DOI] [PubMed] [Google Scholar]
- 45.Ichise T, Yoshida N, Ichise H. Fgf2-induced ras-mapk signalling maintains lymphatic endothelial cell identity by upregulating endothelial-cell-specific gene expression and suppressing tgfbeta signalling through smad2. J Cell Sci. 2014;127:845–857 [DOI] [PubMed] [Google Scholar]
- 46.Correia AC, Moonen JR, Brinker MG, Krenning G. Fgf2 inhibits endothelial-mesenchymal transition through microrna-20a-mediated repression of canonical tgf-beta signaling. J Cell Sci. 2016;129:569–579 [DOI] [PubMed] [Google Scholar]
- 47.Massague J Tgfbeta signalling in context. Nat Rev Mol Cell Biol. 2012;13:616–630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.ten Dijke P, Arthur HM. Extracellular control of tgfbeta signalling in vascular development and disease. Nat Rev Mol Cell Biol. 2007;8:857–869 [DOI] [PubMed] [Google Scholar]
- 49.Massague J How cells read tgf-beta signals. Nat Rev Mol Cell Biol. 2000;1:169–178 [DOI] [PubMed] [Google Scholar]
- 50.Shi Y, Massague J. Mechanisms of tgf-beta signaling from cell membrane to the nucleus. Cell. 2003;113:685–700 [DOI] [PubMed] [Google Scholar]
- 51.van Meeteren LA, ten Dijke P. Regulation of endothelial cell plasticity by tgf-beta. Cell and tissue research. 2012;347:177–186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Li W, Li Q, Jiao Y, Qin L, Ali R, Zhou J, Ferruzzi J, Kim RW, Geirsson A, Dietz HC, Offermanns S, Humphrey JD, Tellides G. Tgfbr2 disruption in postnatal smooth muscle impairs aortic wall homeostasis. The Journal of clinical investigation. 2014;124:755–767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bernabeu C, Lopez-Novoa JM, Quintanilla M. The emerging role of tgf-beta superfamily coreceptors in cancer. Biochim Biophys Acta. 2009;1792:954–973 [DOI] [PubMed] [Google Scholar]
- 54.Madri JA, Bell L, Merwin JR. Modulation of vascular cell behavior by transforming growth factors beta. Mol Reprod Dev. 1992;32:121–126 [DOI] [PubMed] [Google Scholar]
- 55.Hirschi KK, Rohovsky SA, D’Amore PA. Pdgf, tgf-beta, and heterotypic cell-cell interactions mediate endothelial cell-induced recruitment of 10t1/2 cells and their differentiation to a smooth muscle fate. J Cell Biol. 1998;141:805–814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chen PY, Qin L, Li G, Tellides G, Simons M. Smooth muscle fgf/tgfbeta cross talk regulates atherosclerosis progression. EMBO Mol Med. 2016;8:712–728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Carvalho RL, Itoh F, Goumans MJ, Lebrin F, Kato M, Takahashi S, Ema M, Itoh S, van Rooijen M, Bertolino P, Ten Dijke P, Mummery CL. Compensatory signalling induced in the yolk sac vasculature by deletion of tgfbeta receptors in mice. Journal of cell science. 2007;120:4269–4277 [DOI] [PubMed] [Google Scholar]
- 58.Robson A, Allinson KR, Anderson RH, Henderson DJ, Arthur HM. The tgfbeta type ii receptor plays a critical role in the endothelial cells during cardiac development. Dev Dyn. 2010;239:2435–2442 [DOI] [PubMed] [Google Scholar]
- 59.Allinson KR, Lee HS, Fruttiger M, McCarty JH, Arthur HM. Endothelial expression of tgfbeta type ii receptor is required to maintain vascular integrity during postnatal development of the central nervous system. PLoS One. 2012;7:e39336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hautmann MB, Madsen CS, Owens GK. A transforming growth factor beta (tgfbeta) control element drives tgfbeta-induced stimulation of smooth muscle alpha-actin gene expression in concert with two carg elements. J Biol Chem. 1997;272:10948–10956 [DOI] [PubMed] [Google Scholar]
- 61.Tang Y, Urs S, Boucher J, Bernaiche T, Venkatesh D, Spicer DB, Vary CP, Liaw L. Notch and transforming growth factor-beta (tgfbeta) signaling pathways cooperatively regulate vascular smooth muscle cell differentiation. The Journal of biological chemistry. 2010;285:17556–17563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mack CP. Signaling mechanisms that regulate smooth muscle cell differentiation. Arterioscler Thromb Vasc Biol. 2011;31:1495–1505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chen PY, Qin L, Li G, Tellides G, Simons M. Fibroblast growth factor (fgf) signaling regulates transforming growth factor beta (tgf)-dependent smooth muscle cell phenotype modulation. Scientific Reports. 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fafeur V, Terman BI, Blum J, Bohlen P. Basic fgf treatment of endothelial cells down-regulates the 85-kda tgf beta receptor subtype and decreases the growth inhibitory response to tgf-beta 1. Growth Factors. 1990;3:237–245 [DOI] [PubMed] [Google Scholar]
- 65.Papetti M, Shujath J, Riley KN, Herman IM. Fgf-2 antagonizes the tgf-beta1-mediated induction of pericyte alpha-smooth muscle actin expression: A role for myf-5 and smad-mediated signaling pathways. Investigative ophthalmology & visual science. 2003;44:4994–5005 [DOI] [PubMed] [Google Scholar]
- 66.Ramos C, Becerril C, Montano M, Garcia-De-Alba C, Ramirez R, Checa M, Pardo A, Selman M. Fgf-1 reverts epithelial-mesenchymal transition induced by tgf-{beta}1 through mapk/erk kinase pathway. Am J Physiol Lung Cell Mol Physiol. 2010;299:L222–231 [DOI] [PubMed] [Google Scholar]
- 67.Chen PY, Qin L, Tellides G, Simons M. Fibroblast growth factor receptor 1 is a key inhibitor of tgfbeta signaling in the endothelium. Science signaling. 2014;7:ra90. [DOI] [PubMed] [Google Scholar]
- 68.Dickson MC, Slager HG, Duffie E, Mummery CL, Akhurst RJ. Rna and protein localisations of tgf beta 2 in the early mouse embryo suggest an involvement in cardiac development. Development. 1993;117:625–639 [DOI] [PubMed] [Google Scholar]
- 69.Lindner V, Lappi DA, Baird A, Majack RA, Reidy MA. Role of basic fibroblast growth factor in vascular lesion formation. Circ Res. 1991;68:106–113 [DOI] [PubMed] [Google Scholar]
- 70.Lindner V, Reidy MA. Proliferation of smooth muscle cells after vascular injury is inhibited by an antibody against basic fibroblast growth factor. Proc Natl Acad Sci U S A. 1991;88:3739–3743 [DOI] [PMC free article] [PubMed] [Google Scholar]