Abstract
Introduction:
P-gp/ABCB1 and BCRP/ABCG2 are highly expressed in the placenta and fetus throughout gestation and can modulate exposure and toxicity of drugs and xenobiotics to the vulnerable fetus during the sensitive times of growth and development. We aim to provide an update on current knowledge on placental and fetal expressions of the two transporters in different species, and to provide insight on interpreting transporter expression and fetal exposure relative to the concept of fraction of drug transported.
Areas covered:
Comprehensive literature review through PubMed (primarily from July 2010 to February 2018) on P-gp and BCRP expression and function in the placenta and fetus of primarily human, mouse, rat, and guinea pig.
Expert opinion:
While there are many commonalities in the expression and function of P-gp and BCRP in the placenta and fetal tissues across species, there are distinct differences in expression levels and temporal changes. Further studies are needed to quantify protein abundance of these transporters and functionally assess their activities at various gestational stages. Combining the knowledge of interspecies differences and the concept of fraction of drug transported, we may better predict the magnitude of impact these transporters have on fetal drug exposure.
Keywords: ABCB1, ABCG2, ABC transporter, ATP-binding cassette transporter, BCRP, Breast Cancer Resistance Protein, Fetus, MDR1, P-glycoprotein, P-gp, Placenta, Pregnancy
1. Introduction
ATP-binding cassette (ABC) efflux transporters encompass 49 members in humans that are primarily implicated in the movement of endogenous substances, drugs, xenobiotics and metabolites across cell membranes [1–4]. Two dominant transporters within this superfamily are P-glycoprotein (P-gp) and breast cancer resistance protein (BCRP). P-gp is encoded by the gene ABCB1, also known as the multidrug resistance 1 (MDR1). P-gp can transport a very broad range of substrates with different ionization states, acid/base properties, hydrophobicities or amphipathic properties. P-gp is widely expressed throughout human and animal bodies and is the most extensively studied drug transporter [5]. P-gp is highly expressed on the apical membrane of intestinal epithelium, liver hepatocytes, kidney proximal tubular epithelium, brain microvessel endothelial cells, and placental syncytiotrophoblasts [6,7] and therefore plays an important role in the absorption, biliary elimination, renal clearance, and brain distribution of drugs and xenobiotics [8]. P-gp can also restrict fetal exposure to drugs and xenobiotics due to its expression and activity in the placental barrier [9]. P-gp in rodents is encoded by two genes, namely Abcb1a (or Mdr1a) and Abcb1b (or Mdr1b), with over 80% homology to human P-gp [10,11]. The distribution and activity of the two Abcb1 proteins in rodents are similar to P-gp in humans. BCRP was first discovered independently by two laboratories in 1998 [12–14]. Doyle et al. cloned BCRP from MCF-7 breast cancer cell lines, and at the same time, Allikmets et al. discovered abundant expression of the same transporter in the placenta and originally named it ABCP (a placenta-specific ATP-binding cassette transporter) [12,14]. Human BCRP is encoded by the gene ABCG2. The pattern of tissue expression and cellular localization of BCRP assimilates to that of P-gp [15,16]. BCRP also transports a wide range of substrates, including conjugated organic anions (e.g., estrone-3-sulfate), anticancer drugs, chemical toxicants and nucleoside analogs [2]. There is only one Abcg2 gene in rodents which is 80–90% homologous to human ABCG2 [17].
Inhibition by xenobiotics and genetic polymorphisms of these transporters have been shown to clinically impact pharmacokinetics and pharmacodynamics of drugs [18]. This consideration would be especially important in special populations where drug efficacy and toxicity is not well defined; one such example is the pregnant woman and her fetus [19,20]. Due to ethical restrictions, it is not always possible to obtain placental and fetal tissue samples throughout gestation to fully characterize transporter expression and function. Thus, extensive research has been done in animal models to understand the roles of P-gp and BCRP in determining drug disposition during pregnancy including fetal drug exposure. In this review, we summarized the most recent findings on P-gp and BCRP expression and function in the placenta and fetus in humans and animal models such as mice, rats and guinea pig. Since the previous review by Myllynen et al. was published in 2010 [21], further progress in this area has been made. Therefore, we aim to provide an update of current knowledge on this topic. Although several excellent reviews on P-gp, BCRP and other placental transporters have been published [22–25], this review focuses on the most recent findings about P-gp and BCRP in the placenta and fetal tissues and relevant issues that have not been properly addressed.
2. Placental structures in different species
We begin with a brief overview on species differences of placental structures. The placenta is an organ that connects the fetal compartment and maternal circulation to allow nutrient uptake, waste elimination and gas exchange for the fetus during pregnancy. The shape and structure of placenta vary greatly from one species to another. In humans and macaques, the placentas are single and (bi)discoid in shape, with the villous internal structure and multi-villous blood flow pattern [26]; while in rodents such as mice, rats, rabbits and guinea-pigs, the placentas have labyrinthine structures and countercurrent blood flow patterns, which provide a more efficient exchange system compared to humans.
According to the Grosser’s concept of the degree of erosion of the maternal tissues, placentas can also be classified into four types: epitheliochorial (e.g. pig and horse); syndesmochorial (e.g. sheep, goat and cow); endotheliochorial (e.g. cat, dog and ferret); and hemochorial (e.g. human, macaque, bats, mice and rats) [27]. Hemochorial placentas are further subdivided based on the number of trophoblast cell layers between the fetal and maternal blood circulation. Human placentas in which the maternal and fetal blood are separated by a single syncytiotrophoblast cell layer are classified to be hemomonochorial (Figure 1) [28]. In contrast, mouse and rat placentas in which there are three cell layers (cytotrophoblast, syncytiotrophoblast I and II) between the fetal and maternal blood are called hemotrichorial placentas (Figure 2) [28]. The feto-maternal interface in guinea pig is similar to that in humans with a single layer syncytiotrophoblast (Figure 3), but the cytotrophoblasts do not persist into the second half of gestation [22]. At present, whether and how species differences in placental structure might affect in vivo function of P-gp and BCRP in determining fetal drug exposure is unknown.
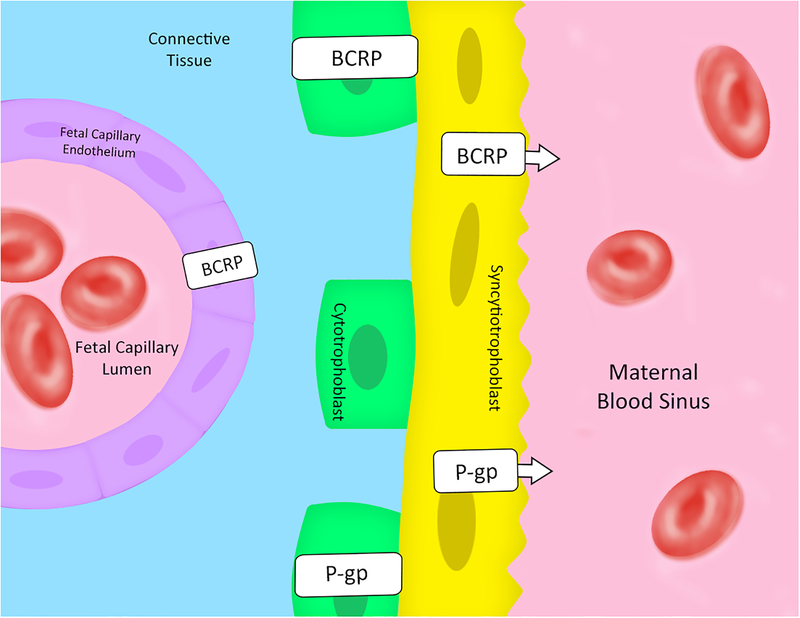
Figure 1: Schematic illustration of human placenta with cross-section through a chorionic villus.
P-gp and BCRP are localized on the microvillous membrane (apical membrane) of the syncytiotrophoblasts. BCRP is also expressed in fetal capillary endothelium. First trimester immunostaining also revealed BCRP and P-gp expression in the cytotrophoblasts. Arrows indicate the direction of transport. [29–32, 44, 62–67]
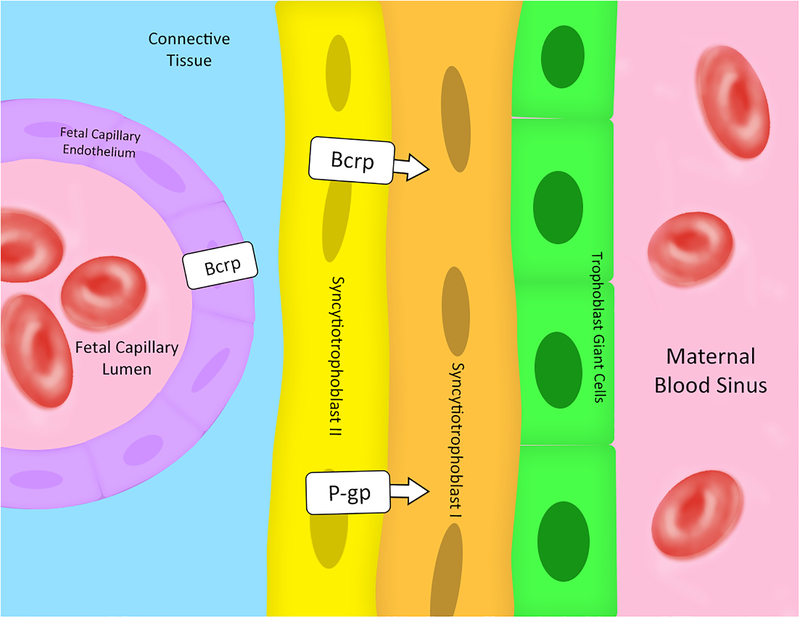
Figure 2: Schematic illustration of rodent placenta (mouse and rat) from the fetal to maternal interface.
P-gp and Bcrp are mainly expressed on the apical membrane of the syncytiotrophoblast layer II. Arrows indicate the direction of transport. Bcrp is also expressed in the fetal capillary endothelium; however, at present, no information is available about cellular localization and function of Bcrp in fetal capillary endothelium. Therefore, the direction of transport of Bcrp in fetal capillary endothelium is unknown. [32–37, 68–70]
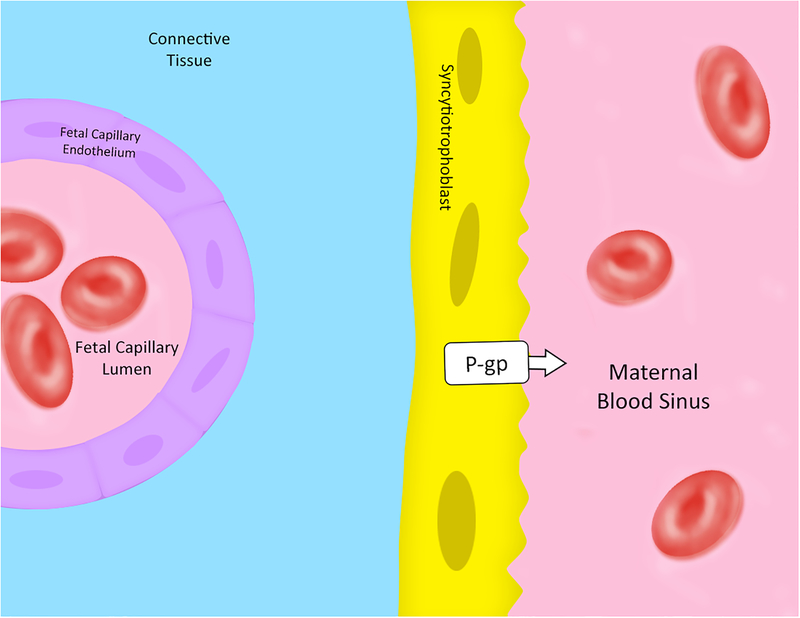
Figure 3: Schematic illustration of guinea pig placenta from the fetal to maternal interface.
P-gp is expressed on the apical membrane of the syncytiotrophoblasts. No information is available about Bcrp expression in the placenta and fetus of this species. Arrow indicates the direction of transport. [38, 39]
3. P-gp and BCRP in the placenta
3.1. P-gp in the placenta
P-gp in the placenta plays a defensive role to protect the fetus from exposure to drugs and xenobiotics present in the maternal circulation. In humans, immunostaining analyses [29–31] have clearly shown that P-gp is primarily localized on the apical membrane of the syncytiotrophoblasts and has direct contact with maternal blood (Figure 1). P-gp is also expressed in cytotrophoblasts isolated from human placenta [32]. The function of P-gp in cytotrophoblasts has not been fully understood, but may exert yet undescribed roles in placental biology and placental barrier development such as protection of the placental cells against endogenous and exogenous toxicants.
In rats and mice, although both of Abcb1a and Abcb1b have been shown to be expressed at term placenta [33,34], Abcb1a and Abcb1b are dominantly expressed in the brain and placenta, respectively [34]. Immunoblotting analysis revealed the presence of P-gp in the developing labyrinth zone of the rat placenta on gestation day 13; from gestation day 15 to the term, P-gp was seen as a dot-like continuous line in the syncytiotrophoblast layers [35]. P-gp was not observed in the cells of fetal capillaries in the labyrinth zone, spongiotrophoblasts and secondary giant trophoblasts of the junctional zone during rat pregnancy [35]. Contradicting data exist regarding the localization of P-gp in the syncytiotrophoblast bilayers of rodents. Earlier studies showed that P-gp was predominantly expressed on the apical membrane of the syncytiotrophoblast I layer in rat and mouse placentas [33,34]. However, recent studies [36,37] found that P-gp was mainly localized on the apical membrane of the syncytiotrophoblast II layer (Figure 2), but not the syncytiotrophoblast I layer. These studies suggest that both the syncytiotrophoblast I and II layers could play supportive and defensive roles.
Like humans, guinea pig genome contains only one gene encoding for P-gp, that is, Abcb1. This gene in guinea pigs, with at least three isoforms via alternative splicing and alternate exon usage, was found to be more closely related to human ABCB1 than to the rat or mouse isoforms [38]. In guinea pig placenta, P-gp is also mainly expressed on the apical membrane of syncytiotrophoblasts (Figure 3), likely protecting the fetus against drug/xenobiotic exposure [39].
At different stages of pregnancy across all the species, the levels of P-gp expression in the placenta change with gestational age. In humans, one earlier study revealed that P-gp was expressed in the placenta from the first trimester to term, and its levels of protein expression determined by immunoblotting decreased with advancing gestation [31]. Consistently, another study showed that P-gp expression in early gestational age human placenta (60–90 days) was significantly higher than that in term placenta (44.8 times higher at the protein levels and 6.5 times higher at mRNA levels) [40]. It was suggested that P-gp in human placenta was upregulated in early pregnancy to protect the fetus during the developmentally sensitive period from xenobiotic toxicity and this upregulation appeared to be related to hCG-β expression [40]. P-gp expression in the placenta also can be affected by other physiological factors. For example, inflammation such as chorioamnionitis may alter P-gp expression in the placenta. Several studies showed up-regulation of ABCB1 mRNA expression in preterm placenta with inflammation [41,42], but no change was observed from term placentas [43], suggesting that the effects of inflammation on P-gp expression may be dynamic. Lye et al. and Javam et al. showed increased expression of P-gp (both mRNA and protein) in cytotrophoblasts isolated from the first trimester placentas under hypoxic conditions [44,45]. ABCB1 mRNA expression was also shown to be affected by maternal emotional distress, with lean women (n = 43) having decreased ABCB1 mRNA expression in term placentas, but increased ABCB1 mRNA expressions in term placentas of severely obese women (n = 50) [46].
Similar to humans, a mouse study showed that the levels of P-gp protein in mouse placenta were the highest in early gestation and decreased with the progression of pregnancy. At near term (gestation day 19), placental P-gp protein expression was about one third of that on gestation day 10 [47]. In rats, the expression of placental P-gp could be detected as early as gestation day 11; however, unlike the patterns in humans and mice, placental P-gp expression in rats increased with advancing gestation as demonstrated by real-time PCR and immunoblotting analyses [35]. Similar to humans and mice, placental P-gp expression in guinea-pigs also decreases from mid to late gestation. The highest levels of mRNA and protein expression was observed on gestation day 40, followed by a progressive and significant decrease on gestation days 50 and 60 [39]. It is unclear how P-gp expression in the placenta of non-human primates changes with gestation. One study showed that the cyclosporine A-induced changes in fetal exposure to antihypertensive drug verapamil (likely due to inhibition of placental P-gp) increased from mid to late gestation in Macaca nemestrina, suggesting that P-gp activity at the placental barrier in the macaque increased with gestational age [48]. However, a follow-up study by the same group indicated that this increase became insignificant when taking into account variations in tissue blood flow [49].
Little is known about the mechanisms underlying the gestational age-dependent changes of P-gp expression in the placenta. Several potential contributing factors have been investigated. Pregnane X Receptor (PXR) may repress the basal expression of P-gp in the placenta [50]. A recent study analyzing PXR+/+, PXR+/− and PXR−/− placentas from the same dams obtained by mating PXR+/− female mice with PXR+/− male mice demonstrated that the expression of Abcb1a mRNA in PXR−/− placentas was about two-fold higher than that in PXR+/+ placentas; and fetal/maternal concentration ratios of antiviral lopinavir (a substrate of P-gp) were two-fold lower in PXR−/− fetuses as compared to PXR+/+ fetuses [51]. Histone deacetylase (HDCA)-2 was reported to regulate P-gp expression in placental cells. Inhibition of HDCA-2 significantly elevated P-gp expression and reduced intracellular accumulation of P-gp substrates in BeWo and JAR cells, and Hdac2 silencing in pregnant mice elevated placental P-gp expression and decreased the transfer rate of digoxin (antiarrhythmic) across the placental barrier from the maternal circulation to the fetal compartment [52]. Effects of glucocorticoids on placental P-gp expression are controversial and seem to vary from species to species. In a mouse study, glucocorticoid exposure was shown to up-regulate Abcb1a mRNA and Abcb1a protein expression at the transcriptional and translational levels, particularly in late gestation [53]. In guinea pigs, administration of betamethasone (steroid) on gestation days 40/41 and 50/51 resulted in a significant decrease in placental Abcb1 mRNA and Abcb1 protein expression [54]. In humans, no changes were identified in placental P-gp expression following antenatal glucocorticoids in pregnant women presenting in threatened preterm labor [55].
The protective role of P-gp in the placental barrier for the fetus has been primarily illustrated in animal studies using animal models with genetic [56,57] or chemical [58,59] knockout of P-gp. For example, the pivotal study done by Lankas et al. demonstrated the importance of placental P-gp in protecting the fetus for the first time. They used a strain of CF-1 mice that had natural P-gp knockout and exposed the dams to a known teratogenic compound during gestation [57]. Due to a lack of expression and protective activities of P-gp in the placental barrier, 100% of fetuses that were deficient in P-gp were born with cleft palate abnormalities as a result of teratogenic exposure, whereas fetuses with functional P-gp showed no birth defect from the exposure [57]. In humans, in vivo placental P-gp activity was inferred by the low fetal (cord)-to-maternal plasma concentration ratios (< 0.3) at the time of C-section in women receiving anti-HIV protease inhibitors (P-gp substrates) [60]. Consistent with the clinical data, the ex vivo perfusion study with human term placenta showed a low maternal-to-fetal transfer of antiviral saquinavir (a P-gp substrate), which was increased 6 – 8-fold after pre-perfusion with a P-gp inhibitor PSC833 or GG918 [9]. The most recent study indicated whether P-gp in the placenta exerts a protective role for the fetus also depends on the drug used. Liao et al. found that after retro-orbital injection of norbuprenorphine (metabolite of narcotic buprenorphine), an excellent P-gp substrate, the fetal/maternal plasma AUC ratios of norbuprenorphine remained unchanged in Abcb1a−/−/1b−/− and wild-type mice, while the maternal brain/maternal plasma AUC ratio in Abcb1a−/−/1b−/− mice was increased ~30-fold compared to that in wild-type mice [61]. Since fetal AUC of norbuprenorphine accounted for 60% of maternal plasma AUC in wild-type mice, it appears that passive diffusion of the drug across the placenta is quite extensive so that the activity of P-gp in the placenta is masked.
3.2. BCRP in the placenta
Like P-gp, BCRP has been found to be highly expressed in the placenta of humans and rodents studied thus far. In human placenta, immunostaining analyses showed that BCRP was primarily localized on the apical side of the syncytiotrophoblasts and fetal capillary endothelial cells (Figure 1) [62–65], and the expression of BCRP did not change across the placental disc [66]. Similar to P-gp, BCRP was also detected via immunostaining in cytotrophoblasts isolated from human placentas [44,67]. Likewise, the physiological function of BCRP in cytotrophoblasts is unknown. Subcellular analysis revealed that BCRP was co-localized with lipid raft proteins in detergent-resistant, lipid raft-containing fractions from the placental micro-villous membranes [62]. In mouse [68,69] or rat [68,70] placenta, Bcrp is also mainly expressed in the syncytiotrophoblast layers in the labyrinth and some vascular structures within the myometrium. Like P-gp, immunostaining analyses showed that Bcrp was on the apical membrane of the placental syncytiotrophoblast II layer, but not the syncytiotrophoblast I layer in rodents [36,37].
In human placenta, the pattern of BCRP expression over gestation has been found to be inconsistent among various studies. An analysis of syncytial micro-villous plasma membranes isolated from human placentas of various gestational ages (60–90 days, 90–120 days, and full-term) illustrated that BCRP expression (both protein and mRNA) did not significantly change with gestational age [40]. Likewise, another study with real-time PCR analysis showed that the mRNA levels of ABCG2 in human placenta did not significantly change as gestation progressed [64]. However, immunoblotting analyses revealed that the protein levels of BCRP in human placenta increased [71] or decreased [72] towards term.
In rats, the expression of Abcg2 mRNA in placenta peaks on gestation day 15 and declines significantly to one-third up to term [73]. Consistently, another study showed that the mRNA and protein levels of Bcrp in the rat placenta on gestation day 20 were lower than those on gestation day 14 [74]. Similar to rats, the mRNA and protein expression of Bcrp in mouse placenta peaks on gestation day 15 compared to gestation days 10 and 19 [75]. Another study did not show statistically significant gestational age-dependent changes in Bcrp protein levels in mouse placenta; however, the levels of Abcg2 mRNA peaked on gestation day 9.5 and then significantly decreased on gestation days 12.5 and 15.5 [76].
There were substantial inter-individual variations in the levels of ABCG2 mRNA and protein expression in human term placenta up to 47-fold and 14-fold, respectively; and the variations appeared not to be correlated with single nucleotide polymorphisms in the ABCG2 non-coding regions, while the coding region polymorphism C421A/Q141K was significantly associated with a 40–50% decrease in BCRP protein levels in 421C/A and 421A/A placentas compared to wild-type placentas (421C/C) [77,78]. Another study found that the inter-individual differences in the BCRP protein levels in human placenta were likely associated with the methylation patterns in the miR-328 5’-flanking region [79].
Numerous studies have investigated the mechanisms by which BCRP expression in placental cells is regulated and the factors that can affect BCRP expression. The pregnancy-specific hormones progesterone and 17β-estradiol have been shown to induce and down-regulate BCRP expression in BeWo cells, respectively [80,81]. Prostaglandin E2 (PGE2) was shown to induce BCRP expression in human placental cells via EP1 and EP3 receptor signaling cascades [82]. Peroxisome proliferator-activated receptor gamma (PPARγ) was found to be involved in up-regulation of BCRP expression in the placenta [83]. Hypoxia signaling could down-regulate BCRP expression and efflux activity in BeWo cells, and women who gave birth at a high altitude exhibited signs of chronic placental hypoxia and had reduced BCRP expression in placental micro-villous membranes compared to women at a moderate altitude [84]. Studies have also shown that environmental/dietary substances can significantly affect placental BCRP expression and consequently the placental protection. The toxic heavy metal cadmium was showed to down-regulate the expression of Bcrp in rat placenta and directly inhibit BCRP function in BeWo and MCF-7 cells [85,86]. Xenoestrogens, including bisphenol A and para-nonylphenol, decreased the expression of BCRP protein in human term placental explant cultures [87]. Continuous exposure of BeWo cells to genistein (a dietary isoflavone) for 48 hours reduced the expression of ABCG2 mRNA and protein by up to 40%, and the down-regulation could be attenuated by pharmacological inhibition of the estrogen receptor, suggesting that phytoestrogens may reduce BCRP expression through this hormone receptor pathway in BeWo cells [88]. Buprenorphine, norbuprenorphine, and methadone (narcotic), which are potent AhR agonists, can induce BCRP in human placental trophoblasts by activating AhR [89]. Acetaminophen (analgesic) was found to induce oxidative stress and down-regulate BCRP/Bcrp in human JEG-3 cells or rat placenta [90]. Inflammation of the placenta during gestation was found to alter ABCG2 mRNA expression, though conflicting data exists regarding up- or down-regulation of expression [41–43]. Maternal emotional distress was shown to increase ABCG2 mRNA expression in term placenta of severely obese women, but functional consequences have not been studied [46]. Exposure to lipopolysaccharide (a bacterial antigen) and polyinosinic-polycytidylic acid (a viral antigen) decreased ABCG2 mRNA and protein expression in human placental explants from the first and third trimesters, respectively, suggesting that bacterial infection may alter exposure of the conceptus to toxins and drugs in early pregnancy, whereas viral infection may disrupt fetal protection in later gestation [82]. Maternal body weight and fetal gender also seem to be associated with BCRP expression in the placenta [46].
The function of BCRP in the placenta has been investigated using ex vivo placental perfusion and in vivo animal models. A dual human placental perfusion study showed that nicardipine, a BCRP inhibitor, partially blocked the transfer of glyburide across the whole placenta presumably by inhibition of BCRP [91]. A dually rat placenta perfusion study revealed that etravirine (antiviral) reduced trans-placental passage of tenofovir disoproxil fumarate (antiviral) likely through inhibition of Bcrp [92]. The role of BCRP as a bile acid transporter in the placenta has been characterized. When rat dams were dosed with fumitremorgin C, a highly selective BCRP/Bcrp inhibitor, significantly decreased fetal-to-maternal transfer of glycocholic acid was observed possibly due to inhibition of Bcrp in the placenta [93]. Likewise, in pregnant Abcg2−/− rats with obstructive cholestasis, increased accumulation of bile acids in the placenta, fetal serum, and fetal livers were observed compared to wild-type rats, but the levels of bile acids in maternal serum were not changed between the knockout and wild-type rats [93]. In pregnant mice, the fetus/maternal plasma concentration ratio of topotecan was increased 2-fold by co-administration of the Bcrp inhibitor GF120918 as compared with control mice with no Bcrp inhibitor administered [94]. Likewise, the fetal/maternal plasma AUC ratios of nitrofurantoin (antibiotic) and glyburide (antidiabetic), drugs that are often prescribed to pregnant women, were found to be approximately 4-fold and 2-fold higher, respectively, in Abcg2−/− mice compared to wild-type mice [95,96].
4. P-gp and BCRP in the fetus
Previous studies have shown that P-gp and BCRP are also expressed in fetal tissues as summarized in Table 1. In addition, BCRP has been shown to be expressed in fetal capillary endothelium in humans and rodents [63,70]. We provide an overview of P-gp and BCRP in fetal tissues across species including the fetal brain, liver, kidney and intestine as follows. We would like to point out that, while the expression of P-gp and BCRP in fetal tissues has been reported, few studies have characterized the function of P-gp and BCRP in the fetal tissues and, therefore the roles of P-gp and BCRP in the fetus remain largely unclear.
Table 1.
P-gp and BCRP expression in fetal tissues and temporal changes over gestation
| Human | Mice | Rat | ||||
|---|---|---|---|---|---|---|
| P-gp | BCRP | P-gp | Bcrp | P-gp | Bcrp | |
| Brain | ↑ | ↔ | ↑ | + | ↑ | ↓ |
| Liver | ↑ | + | + | ↓ | ↔ | ↓ |
| Kidney | + | + | + | + | + | + |
| Intestine | ↔ | ↔ | ↑ | n/a | ↔ | + |
4.1. The fetal brain
The brain is a very vulnerable organ during the fetal development. Many studies have suggested that xenobiotic exposure to the brain during pregnancy may inhibit or slow the fetal neural development [5,97]. The blood-brain barrier (BBB) is a critical protective tissue barrier between the systemic circulation and the fetal central nervous system. The BBB consists of brain capillary endothelial cells that form tight junctions, restrict the paracellular movement of compounds into and out of the brain, and has high trans-endothelial electrical resistance. One of the key mechanisms that modulate drug exposure to the brain is the activity of ABC efflux transporters present at the BBB. P-gp and BCRP are known to be broadly expressed on the apical side of the brain micro-vessel endothelial cells and are responsible for reducing maternally administered xenobiotics to the fetal brain [98].
P-gp expression in human fetal brain was noted as early as 8 weeks of gestation and increased with gestational maturation [97,99,100]. Earlier studies reported low levels of P-gp expression in human fetal brain (n = 3) where P-gp gene expression was generally lower compared to the kidney, liver and intestine [101,102]. A recent study showed via immunostaining of postmortem cortex samples of human fetal brain from various gestational ages (n = 12) that P-gp intensity increased non-significantly from 20 to 40 weeks, but nearly doubled when comparing the P-gp intensity from week 20 to that of a newborn (n = 14) [103]. Nevertheless, P-gp expression in the newborn brain was still 2-fold lower than that of adults (n = 8) [103]. All these findings suggest that P-gp expression in human fetal brain begins early in gestation, but does not reach maturation until after birth. BCRP, on the other hand, has fairly consistent expression in the fetal brain from week 22 of gestation (n = 28) till birth [99]. One of the key concerns with low P-gp expression in the early fetal brain is the potential maternal opioid exposure. Opioids are well known to have high BBB penetration and P-gp and BCRP are often credited to influence the CNS exposure levels of opioids. During the early fetal development, as P-gp and BCRP expressions are low, most of the protective task is likely burdened on the placental syncytiotrophoblasts. Studies have shown that newborns exposed to morphine are more sensitive to drug-induced respiratory depression at the same doses or require less drug to achieve analgesic effects compared to adults [104–106], suggesting higher drug penetration to the fetal brain and less activity of ABC transporters at the BBB as one would expect in adults. Thus, the use of opioid drugs during early pregnancy is of concern due to immature and low expression of P-gp in the fetal brain.
The expression of Abcg2 mRNA in mouse fetal brain was shown to be significant on gestation day 15.5, but decreased on gestation day 18.5. This decrease in Abcg2 mRNA did not result in protein expression decline; instead, the Bcrp activity increased with gestational age [107]. This disagreement could possibly be due to posttranscriptional modification, mRNA stability or protein synthesis delay [107]. The expression of Abcb1a/b mRNA was detected in the mouse fetal brain and appeared to increase over gestation [108]. Viral infection significantly increased the fetal brain exposure and accumulation of digoxin in pregnant mice on gestation day 15 possibly due to disruption of P-gp function in the fetal brain [108].
In rats, one study found that the expression of Abcb1a mRNA in the Sprague-Dawley fetal rat cerebellum around birth to be about the same as the adult level, but Abcb1b mRNA expression in fetal cerebellum around birth was 17–20 times higher than the adult level [109]. Another study showed that while the expression of Abcb1b mRNA was higher in the Wistar fetal rat brain on gestation day 22 compared to the maternal brain, it was only by ~1-fold higher and the difference was not statistically significant [110]. However, Abcb1a mRNA expression in the Wistar fetal rat brain was almost 10-fold lower than that of the maternal brain [110]. Inconsistent with these findings, it has also been reported that Abcb1a mRNA expression in the Wistar rat fetal brain on gestation day 20 is ~5-fold higher than the maternal brain [111]. The expression of Abcg2 mRNA in the Wistar rat fetal brain was ~5 times higher than the maternal brain on gestation day 20, and decreased to nearly the same levels as the dam upon birth [109,111,112]. In a recent study with pregnant Sprague-Dawley rats, chronic administration of the antiviral drug zidovudine over gestation significantly induced Bcrp protein expression in the placenta and fetal brain and this induction of Bcrp was accompanied with a significant reduction in fetal brain concentration of zidovudine after a single dose intravenous administration, and the activity of Bcrp was inhibited by a Bcrp inhibitor [113]. These results suggest that Bcrp can limit drug exposure to the fetal brain, and the degree of toxicity may be affected by inhibition of Bcrp at both the BBB of the fetus and the placental barrier.
Overall, the universal expression of P-gp and BCRP in the fetal brain across species from early stages of gestation suggest the importance of the two transporters in limiting xenobiotic and drug penetration into the fetal brain and protecting the developing fetus.
4.2. The fetal liver
The liver is a key organ for biotransformation and elimination of drugs [114]. During early organogenesis, the primary blood supply to fetal liver comes from the umbilical veins [115]. It is worth noting that the fetal liver has a quite different anatomical/histological structure and function compared to those of the adult liver. The sinusoids are formed as long cords early in hepatogenesis by invasion of hepatoblasts into the septum transversum [115,116]. The hepatic acinus and functional differentiation of the three hepatic zones are not fully developed until late gestation [116]. Furthermore, the unique composition of epithelial, mesenchymal, and hematopoetic cells within the fetal liver allows for a microenvironment that facilitates blood cell production functions during fetal development [114,117]. A grown fetal liver after hepatogenesis is complete primarily consists of hepatocytes where most drug detoxification processes occur. Hepatocytes form tight junctions, creating the bile ducts in between the cells that allow passage of compounds into the bile. P-gp and BCRP are both expressed on the canalicular membrane of hepatocytes and facilitate elimination of drugs, metabolites and endogenous substrates into the bile [2].
Numerous studies have reported ABCB1 mRNA expression in human fetal liver at varying gestational ages [102,118–120]. The consensus is that P-gp protein expression on the bile canaliculi appears as early as 5.5 weeks of gestation, but the expression levels of ABCB1 mRNA in the human fetal liver are lower than those in the adult liver [119,121]. One study quantified ABCB1 mRNA from the post-mortem fetal liver and reported that the levels of ABCB1 mRNA were 20–30-fold lower than the adults [118]. Another study showed that the levels of ABCB1 mRNA in the human fetal liver significantly increased between 15 and 27 weeks of gestation and then declined after 42 weeks [102,120]. BCRP expression in the human fetal liver was reportedly very low at 10 and 11 weeks of gestation, and there is currently no information on temporal changes in BCRP expression or activity in the human fetal liver throughout pregnancy [119].
In rodents, contradictory data exist regarding P-gp expression in the fetal liver. Several studies have reported that the expression of Abcb1a and Abcb1b mRNA in the rat fetal liver is very low to virtually nonexistent before birth [109,110,120,122,123]. However, one study found that Abcb1a mRNA expression in the rat fetal liver was about the same as that in the maternal liver [111]. Likewise, the expression of Abcb1a and Abcb1b mRNA was shown to be not significantly expressed in the mouse fetal liver [124]. On the other hand, Bcrp is abundantly expressed in the mouse fetal liver. On gestation day 17.5, Bcrp accounts for ~25% of total transporters expressed in the mouse fetal liver, but its levels decline shortly after birth [109]. Similarly, the expression of Abcg2 mRNA was reportedly at the highest level in the rat fetal liver prior to birth which was nearly 15 times more than the adult level, and then declined after birth [109]. Another study also demonstrated that Abcg2 mRNA expression in the rat fetal liver on gestation day 22 was at the similar levels as in the maternal liver [110].
Up to date, there have been no studies to report the function of P-gp and BCRP in the fetal liver. Based on the location and expression levels of P-gp in the human fetal liver, P-gp may exert biliary elimination of drugs from the fetus as early as 15 weeks of gestation when their expression in the fetal liver begins to significantly increase [102,120].
4.3. The fetal kidney
The kidney is an essential organ for elimination of endogenous and exogenous substances. P-gp and BCRP are primarily expressed on the apical membrane of the proximal tubular cells across species including humans and rodents [125,126], suggesting that they may play important roles in facilitating renal clearance of xenobiotics.
In human fetal kidney, P-gp protein could be detected on the apical membrane of the proximal tubules as early as 5.5 weeks of gestation, and BCRP protein expression was low compared to P-gp at the same gestational age [119]. Currently, there are no data regarding temporal changes of P-gp and BCRP expression in the human fetal kidney throughout gestation.
The expression of Abcb1a and Abcb1b mRNA in the mouse fetal kidney at the time of birth was low, at ~19% and ~11% of the adult level, respectively [127]. This is congruent with the reported inability of newborn mice to excrete digoxin, a drug exclusively excreted by P-gp, via tubular secretion before birth [127]. Abundant Bcrp protein expression on gestation day 18.5 in the fetal kidney tubules has been reported [76], but the levels were low compared to those in the kidney of adult mice [128].
In rats, the expression of Abcb1a mRNA in the fetal kidney was not detectable before birth, whereas Abcb1b mRNA was expressed in the fetal kidney at ~50% of the adult level in female fetus and 2 – 3 times that of the adult level in male fetus prior to birth [109]. Other studies reported that the levels of Abcb1a mRNA in the rat fetal kidney on gestation 20 were ~82 times less than those in the maternal kidney [111] or Abcb1 protein expression was undetectable in the rat fetal kidney [129]. Abcg2 mRNA expression was detectable in the rat fetal kidney on gestation day 22 at levels comparable to those in the maternal kidney [110]. However, another study reported that Abcg2 mRNA expression in the rat fetal kidney was much lower than that in the adult kidney [129]. Overall, P-gp and BCRP expression appears to be low in the rat kidney prior to birth and increases significantly with aging after birth.
Up to date, there have been no studies to characterize the function of P-gp and BCRP in the fetal kidney. Based on the location and expression levels of P-gp in the human fetal kidney, P-gp may facilitate renal clearance of drugs from the fetus as early as 5.5 weeks of gestation [119].
4.4. The fetal intestine
In the intestine, P-gp and BCRP are localized on the apical membrane of the intestinal epithelium [2,5] where they actively transport drugs that have entered the enterocytes back to the gut lumen, thus reducing the absorption and bioavailability of orally administered drugs.
Previous studies demonstrated that the expression of ABCB1 mRNA in human fetal intestine was detectable, but low at ~20 weeks of gestation [119,130]. A more recent study showed that P-gp protein expression could be detected in the enterocytes at 12 weeks of gestation and the levels remained consistent toward adulthood [119]. Another study reported concurring data that at the time of birth, the intestinal expression of ABCB1 mRNA in neonates was equivalent to that of the adults [118]. BCRP protein expression is also detectable as early as 5.5 weeks of gestation in the intestinal epithelium at levels comparable to adults [119].
In mice, P-gp protein expression in the fetal intestine at the time of birth was shown to be ~20% of the adults and increased until adulthood [131]. In rats, one study found that Abcb1a mRNA expression in the fetal intestine on gestation day 20 was 55 times higher than that in the maternal intestine [111]. Another study showed that Abcb1a mRNA expression in the rat fetal intestine on gestation day 22 was lower than that in the maternal intestine, whereas the mRNA levels of Abcb1b and Abcg2 in the fetal intestine were higher than those in the maternal intestine, though the differences were not statistically significant [110]. Up to date, very little is known about Bcrp expression in the rodent fetal intestine.
Up to date, there have been no studies to characterize the function of P-gp and BCRP in the fetal intestine. Based on the location and expression levels of P-gp in the human intestine, P-gp may play a role in determining the oral absorption and bioavailability of drugs in the fetus as early as 12 weeks of gestation [119].
5. Use of the concept of fraction transported to estimate the magnitude of impact of P-gp or BCRP on fetal drug exposure
There have been convincing evidence that P-gp and BCRP in the placenta play pivotal roles in limiting fetal exposure to drugs and xenobiotics [56,57,95,96,132]. However, our recent study showed that after normalization to maternal plasma exposure, fetal exposure to norbuprenorphine in P-gp knockout mice did not significantly differ from that in wild-type mice [61], indicating that P-gp knockout mice had no effect on fetal exposure to norbuprenorphine even though we know that norbuprenorphine is an excellent substrate of P-gp [133]. On the other hand, P-gp did strongly restrict norbuprenorphine exposure to the maternal brain of the same dams [61]. We have hypothesized that the differential impact of P-gp on brain and fetal exposure to norbuprenorphine could be due to lower P-gp expression in the placental barrier and more extensive passive diffusion across the placental barrier, compared to the maternal blood-brain barrier [61]. To fully understand and analyze the magnitude of the impact of P-gp or BCRP in restricting drug transfer across the placental barrier, we utilize the concept of fraction of drug transported (Ft) that has previously been applied to the blood-brain barrier, in which the term Ft was coined as an analog to Fm, the fraction of drug metabolized by an enzyme [134].
We assume that the placenta and fetus form a lumped compartment and that only the bidirectional unbound passive diffusion clearance (CLdiff) and unbound efflux clearance (CLefflux) mediated by P-gp or BCRP across the placental barrier contribute to fetal drug exposure. At steady-state or after a single dose, Kp, the ratio of unbound fetal drug concentration (Cssu,f) to the unbound maternal plasma drug concentration (Cssu,p) is defined by Equations 1 and 2.
| (1) |
or
| (2) |
Then, Ft can be estimated based on P-gp or Bcrp knockout (KO) and wild-type (WT) mouse studies using Equation 3.
| (3) |
Ft may also be estimated with the placental perfusion data in the presence and absence of a P-gp or BCRP inhibitor using Equation 4.
| (4) |
Ft values of several P-gp or Bcrp substrates across the placental barrier were estimated and are shown in Table 2. Notably, the Ft of P-gp for norbuprenorphine is zero, and Ft values of P-gp or BCRP for other compounds vary between 0.33 and 0.94. Thus, caution should be taken when we evaluate the impact of placental P-gp or BCRP on fetal drug exposure. Interestingly, the estimated Ft values predicted a similar role of human BCRP and mouse Bcrp in fetal exposure to glyburide (0.43 in humans vs. 0.50 in mice). Therefore, the estimation of Ft values based upon in vivo animal data or ex vivo placental perfusion results may help predict the magnitude of potential impact of P-gp or BCRP on fetal drug exposure. Nevertheless, we recognize that this analysis is based on a simplified compartment model in which many factors that may affect fetal drug exposure are not considered, such as other transporters in the placental barrier as well as drug metabolism and other elimination pathways in the placental and fetal unit. Factors other than placental transporters and passive diffusion that potentially affect fetal drug exposure have recently been evaluated by simulations and sensitivity analyses of a maternal-fetal physiologically based pharmacokinetic (PBPK) model [135].
Table 2.
A few examples of the estimated fractions of drug transported by P-gp or BCRP across the placenta
| Compound | Species | Transporter | Ft | Reference |
|---|---|---|---|---|
| Glyburide | Human | BCRP | 0.43 | [91] |
| D-luciferin | Mouse | Bcrp | 0.67 | [137] |
| Digoxin | Mouse | P-gp | 0.58 | [56] |
| Saquinavir | Mouse | P-gp | 0.86 | [56] |
| Paclitaxel | Mouse | P-gp | 0.94 | [56] |
| L-652,280 | Mouse | P-gp | 0.87 | [57] |
| Lopinavir | Mouse | P-gp | 0.5 | [51] |
| Norbuprenorphine | Mouse | P-gp | 0 | [61] |
| Nitrofurantoin | Mouse | Bcrp | 0.75 | [95] |
| Glyburide | Mouse | Bcrp | 0.50 | [96] |
| Cyclosporine | Rat | P-gp | 0.41 | [138] |
| Tenofovir | Rat | Bcrp | 0.33 | [92] |
Ft values of drugs in mice were estimated using Equation 3 with data in P-gp or Bcrp KO and WT mice. Ft values of drugs in humans or rats were estimated using Equation 4 with placental perfusion data in the presence and absence of an inhibitor. In all the calculations, total plasma or perfusate concentrations or AUCs were used, assuming that that the plasma protein binding of drugs remains the same across the placental barrier.
6. Conclusion
It is now generally recognized that P-gp and BCRP in the placenta across species play important protective roles for the fetus from exposure to drugs and xenobiotics. Although the placental structure varies from species to species, P-gp and BCRP are all primarily localized on the apical membrane of syncytiotrophoblasts facing the maternal blood (Figures 1–3) [16,22,35–37]. The expression levels of P-gp and BCRP in the placenta change with gestational age in all species; however, the patterns of changes differ from species to species [22,31,35,58]. While there is a consensus that P-gp levels in human placenta decline with gestational age, controversial results exist regarding the changes of BCRP expression in human placenta over gestation [40,71]. P-gp and BCRP expression in the placenta could be regulated at the transcriptional, translational and post-translational levels [79,83,89,136]. Recent studies indicate that the roles of P-gp (likely BCRP as well) in determining fetal drug exposure depend on drugs administered even if the drugs are their substrates, a phenomenon that could be interpreted by the concept of fraction of drug transported [51,56,57,61,80,91,92,95,137,138]. In addition, since many transporters overlap in substrate specificity due to their promiscuous nature, transport of substrates by P-gp or BCRP in the placenta could be influenced by other transporters.
P-gp and BCRP are also broadly expressed in fetal tissues across species, particularly the fetal brain and liver [110,111,119,121]. Like in the placenta, there are temporal changes in P-gp and BCRP expression in fetal tissues with gestational age, but the patterns of changes vary from species to species [31,35,40,47,48,73,74,139]. Further studies are needed to characterize functions and the roles of P-gp and BCRP in fetal tissues in determining fetal exposure to drugs and xenobiotics.
7. Expert opinion
As reviewed in this article, due to their substantial and strategical expression in the placenta, P-gp and BCRP have been shown to limit fetal exposure to drugs and xenobiotics in vivo primarily in animal models. However, up to date, only a few studies have directly linked P-gp or BCRP in the placenta with fetal toxicity of drugs and xenobiotics [57,113,140,141]. Future studies should be more focused on understanding how P-gp and BCRP influence the toxicity of drugs and xenobiotics to the developing fetus. Since we have shown that fetal exposure to drugs and xenobiotics is not necessarily limited by placental P-gp or BCRP as the case of norbuprenorphine [61], future studies should be done to investigate other factors such as passive diffusion, placental and fetal metabolism, other transporters in the placenta, and transporters in fetal tissues that could also affect fetal exposure [135]. Such studies will further enhance our understanding of the concept of fraction transported by P-gp and BCRP in the maternal-fetal interface and their overall roles in determining fetal exposure to drugs and xenobiotics.
A significant challenge to investigate in vivo function of placental P-gp and BCRP to protect the fetus is how to translate the knowledge from animal studies (primarily in rodents) to humans. Since the expression levels of P-gp and BCRP and the patterns of temporal changes in their expression in the placenta in rodents (e.g., mice and rats) over gestation are not always the same as those in humans, the data obtained from animal studies could possibly not be directly translatable to humans. To address this concern, more future studies for characterization of placental P-gp and BCRP in animal species other than rodents that are similar to humans such as macaques are needed. On the other hand, pharmacokinetic modeling to predict fetal exposure to drugs in humans would be an alternative approach to address this concern. At present, the umbilical cord/maternal plasma (U/M) ratio at a single time point at or after delivery is routinely used as a measure of fetal drug exposure in the clinic. However, the values of U/M ratios can be dependent on timing of sample collection and therefore do not necessarily truly reflect fetal drug exposure. Therefore, using U/M ratios to quantitatively interpret in vivo function of placental P-gp or BCRP is often not reliable. Due to the fact that complete pharmacokinetic measurements in both the mother and her fetus after drug administration to the mother are not feasible due to ethical and logistic reasons, currently there are growing interests in developing maternal-fetal-physiologically based pharmacokinetic (m-f-PBPK) models to predict drug exposure in the pregnant mother and fetal organs [135,142,143]. In theory, placental transporters such as P-gp and BCRP can be integrated into the PBPK models and then their roles in determining fetal drug exposure evaluated by PBPK modeling and simulation. Thus far, no such studies have been done to incorporate placental transporters into the PBPK models. In order to do PBPK modeling, absolute levels of P-gp and BCRP protein in human placenta tissues would have to be quantified, which may be achieved by using LC/MS-based quantitative proteomics [144,145]. PBPK modeling and simulation would also allow prediction of gestational age-dependent changes in fetal drug exposure particularly during early gestational stages because gestational age-dependent physiological parameters and P-gp and/or BCRP protein levels in human placenta could be incorporated into the PBPK models. To achieve this, it is necessary to quantify the absolute protein abundance of P-gp and BCRP in human placenta from early gestation towards term.
Beside gestational age-dependent expression, recent studies indicate that there are substantial inter-individual variations in P-gp and BCRP expression in human placenta even at the similar gestational stages [77,146,147]. Such variations may contribute to inter-individual variations in fetal drug exposure and toxicity. Further studies are needed to understand the mechanisms by which P-gp and BCRP expression in the placenta are regulated in a gestational age-dependent manner and what factors contribute to the inter-individual variations of P-gp and BCRP expression in human placenta (e.g., single nucleotide polymorphisms, epigenetic factors, and disease conditions). Addressing the knowledge gap in these areas will help understand and predict in vivo activity of P-gp and BCRP in human placenta and individual fetal risk during pregnancy.
P-gp and BCRP have also been shown to be widely expressed in fetal tissues particularly the fetal brain and liver across species. P-gp and BCRP may provide additional protection against exposure to drugs and xenobiotics beyond their roles in the placental barrier. Few studies have examined P-gp and BCRP activities in fetal tissues. A recent study showed that chronic administration of zidovudine to pregnant rats markedly induced Bcrp in the fetal brain and placenta, which was accompanied with no significant effects on mitochondrial functionality of the fetal brain [113]. Thus, Bcrp induction appears to protect the fetal brain from zidovudine toxicity. Whether Bcrp in the fetal brain coordinately works with Bcrp in the placenta to reduce fetal exposure to zidovudine is unknown. With this regard, at present, very little is known about the roles of P-gp and BCRP in fetal tissues, particularly the fetal liver, kidney and intestine, in determining fetal exposure to drugs. More studies in the future should be done to characterize the expression and function of P-gp and BCRP in the fetal tissues of both humans and animal models.
In summary, P-gp and BCRP are widely expressed in the placenta and fetal tissues across species and play pivotal roles in protecting the fetus from insults of drugs and xenobiotics. An interspecies comparison will enhance mechanistic understanding of in vivo function of P-gp and BCRP in the placenta and fetus and help interspecies extrapolation. Such studies are important for improving drug treatment, efficacy and safety for the mother and her fetus.
Article highlights.
Literature review update of P-gp/ABCB1 and BCRP/ABCG2 expression and activity in the placenta and fetal tissues in different species.
P-gp expression in human, mouse, and guinea pig placentas decreases with gestation; however, P-gp expression in rat placenta is higher at term than in early gestation.
Conflicting data exist regarding the temporal changes in BCRP expression in human placenta over gestation; however, Bcrp expression in rodent placentas peaks at mid-gestation and then declines towards term.
P-gp is expressed in the fetal brain of humans, mice and rats where its levels of expression increase with gestation, whereas the temporal changes of BCRP expression in the fetal brain vary across species. Both transporters are also expressed in the fetal liver, kidney, and intestine across species throughout gestation. At present, little is known about the function of these transporters in fetal tissues during fetal development.
Genetic polymorphisms of P-gp and BCRP and drug-drug interactions at the placental barrier through inhibition of P-gp and BCRP may impact xenobiotic exposure to the fetus during pregnancy.
The impact of P-gp and BCRP on fetal exposure to drugs and xenobiotics depends on the fraction of drug transported across the placental barrier.
Acknowledgments
Funding
This work was supported by the National Institutes of Health [Grant DA032507].
Footnotes
Declaration of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
References
Papers of special note have been highlighted as:
• of interest
•• of considerable interest
- 1.Vasiliou V, Vasiliou K, Nebert DW. Human ATP-binding cassette (ABC) transporter family. Hum. Genomics 2009;3:281–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mao Q, Unadkat JD. Role of the Breast Cancer Resistance Protein (BCRP/ABCG2) in Drug Transport—an Update. AAPS J. 2015;17:65–82.• Comprehensive review on BCRP
- 3.Mao Q, Unadkat JD. Role of the breast cancer resistance protein (ABCG2) in drug transport. AAPS J. 2005;7:E118–E133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Choi YH, Yu A-M. ABC transporters in multidrug resistance and pharmacokinetics, and strategies for drug development. Curr. Pharm. Des 2014;20:793–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wolking S, Schaeffeler E, Lerche H, et al. Impact of Genetic Polymorphisms of ABCB1 (MDR1, P-Glycoprotein) on Drug Disposition and Potential Clinical Implications: Update of the Literature. Clin. Pharmacokinet 2015;54:709–735.• Comprehensive review on P-gp
- 6.Jeannesson E, Siest G, Bastien B, et al. Association of ABCB1 gene polymorphisms with plasma lipid and apolipoprotein concentrations in the STANISLAS cohort. Clin. Chim. Acta 2009;403:198–202. [DOI] [PubMed] [Google Scholar]
- 7.Hodges LM, Markova SM, Chinn LW, et al. Very important pharmacogene summary: ABCB1 (MDR1, P-glycoprotein). Pharmacogenet. Genomics 2011;21:152–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bartels AL, de Klerk OL, Kortekaas R, et al. 11C-verapamil to assess P-gp function in human brain during aging, depression and neurodegenerative disease. Curr. Top. Med. Chem 2010;10:1775–1784. [DOI] [PubMed] [Google Scholar]
- 9.Mölsä M, Heikkinen T, Hakkola J, et al. Functional role of P-glycoprotein in the human blood-placental barrier. Clin. Pharmacol. Ther 2005;78:123–131.• Information on P-gp function on human placenta
- 10.van der Bliek AM, Kooiman PM, Schneider C, et al. Sequence of mdr3 cDNA encoding a human P-glycoprotein. Gene. 1988;71:401–411. [DOI] [PubMed] [Google Scholar]
- 11.Booth-Genthe CL, Louie SW, Carlini EJ, et al. Development and characterization of LLC-PK1 cells containing Sprague–Dawley rat Abcb1a (Mdr1a): Comparison of rat P-glycoprotein transport to human and mouse. J. Pharmacol. Toxicol. Methods 2006;54:78–89. [DOI] [PubMed] [Google Scholar]
- 12.Doyle LA, Yang W, Abruzzo LV, et al. A multidrug resistance transporter from human MCF-7 breast cancer cells. Proc. Natl. Acad. Sci. U. S. A 1998;95:15665–15670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nakanishi T, Ross DD. Breast cancer resistance protein (BCRP/ABCG2): its role in multidrug resistance and regulation of its gene expression. Chin. J. Cancer 2012;31:73–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Allikmets R, Schriml LM, Hutchinson A, et al. A human placenta-specific ATP-binding cassette gene (ABCP) on chromosome 4q22 that is involved in multidrug resistance. Cancer Res. 1998;58:5337–5339. [PubMed] [Google Scholar]
- 15.Rocchi E, Khodjakov A, Volk EL, et al. The product of the ABC half-transporter gene ABCG2 (BCRP/MXR/ABCP) is expressed in the plasma membrane. Biochem. Biophys. Res. Commun 2000;271:42–46. [DOI] [PubMed] [Google Scholar]
- 16.Maliepaard M, Scheffer GL, Faneyte IF, et al. Subcellular localization and distribution of the breast cancer resistance protein transporter in normal human tissues. Cancer Res. 2001;61:3458–3464. [PubMed] [Google Scholar]
- 17.Allen JD, Brinkhuis RF, Wijnholds J, et al. The mouse Bcrp1/Mxr/Abcp gene: amplification and overexpression in cell lines selected for resistance to topotecan, mitoxantrone, or doxorubicin. Cancer Res. 1999;59:4237–4241. [PubMed] [Google Scholar]
- 18.Ieiri I Functional significance of genetic polymorphisms in P-glycoprotein (MDR1, ABCB1) and breast cancer resistance protein (BCRP, ABCG2). Drug Metab. Pharmacokinet 2012;27:85–105. [DOI] [PubMed] [Google Scholar]
- 19.Feghali M, Venkataramanan R, Caritis S. Pharmacokinetics of drugs in pregnancy. Semin. Perinatol 2015;39:512–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Loebstein R, Lalkin A, Koren G. Pharmacokinetic Changes During Pregnancy and Their Clinical Relevance. Clin. Pharmacokinet 1997;33:328–343. [DOI] [PubMed] [Google Scholar]
- 21.Myllynen P, Kummu M, Sieppi E. ABCB1 and ABCG2 expression in the placenta and fetus: an interspecies comparison. Expert Opin. Drug Metab. Toxicol 2010;6:1385–1398. [DOI] [PubMed] [Google Scholar]
- 22.Walker N, Filis P, Soffientini U, et al. Placental transporter localization and expression in the Human: the importance of species, sex, and gestational age differences†. Biol. Reprod 2017;96:733–742.• Comprehensive review of transporters on human placenta
- 23.Joshi AA, Vaidya SS, St-Pierre MV., et al. Placental ABC Transporters: Biological Impact and Pharmaceutical Significance. Pharm. Res 2016;33:2847–2878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bloise E, Ortiga-Carvalho TM, Reis FM, et al. ATP-binding cassette transporters in reproduction: a new frontier. Hum. Reprod. Update 2015;22:dmv049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Koren G, Ornoy A. The role of the placenta in drug transport and fetal drug exposure. Expert Rev. Clin. Pharmacol 2018;11:373–385. [DOI] [PubMed] [Google Scholar]
- 26.De Rijk EPCT, Van Esch E. The Macaque Placenta - A Mini-Review. Toxicol. Pathol 2008;36:108S–118S. [Google Scholar]
- 27.Amoroso EC. Histology of the Placenta. Br. Med. Bull 1961;17:81–90. [DOI] [PubMed] [Google Scholar]
- 28.Enders, Blankenship. Comparative placental structure. Adv. Drug Deliv. Rev 1999;38:3–15.• Comprehensive overview of placenta structure and species differences
- 29.Atkinson DE, Sibley CP, Fairbairn LJ, et al. MDR1 P-gp Expression and Activity in Intact Human Placental Tissue; Upregulation by Retroviral Transduction. Placenta. 2006;27:707–714. [DOI] [PubMed] [Google Scholar]
- 30.Atkinson DE, Greenwood SL, Sibley CP, et al. Role of MDR1 and MRP1 in trophoblast cells, elucidated using retroviral gene transfer. Am. J. Physiol. Physiol 2003;285:C584–C591. [DOI] [PubMed] [Google Scholar]
- 31.Sun M, Kingdom J, Baczyk D, et al. Expression of the Multidrug Resistance P-Glycoprotein, (ABCB1 glycoprotein) in the Human Placenta Decreases with Advancing Gestation. Placenta. 2006;27:602–609. [DOI] [PubMed] [Google Scholar]
- 32.Lye P, Bloise E, Dunk C, et al. Effect of oxygen on multidrug resistance in the first trimester human placenta. Placenta. 2013;34:817–823. [DOI] [PubMed] [Google Scholar]
- 33.Pavek P, Staud F, Fendrich Z, et al. Examination of the Functional Activity of P-glycoprotein in the Rat Placental Barrier Using Rhodamine 123. J. Pharmacol. Exp. Ther 2003;305:1239–1250. [DOI] [PubMed] [Google Scholar]
- 34.Kalabis GM, Kostaki A, Andrews MH, et al. Multidrug Resistance Phosphoglycoprotein (ABCB1) in the Mouse Placenta: Fetal Protection1. Biol. Reprod 2005;73:591–597. [DOI] [PubMed] [Google Scholar]
- 35.Novotna M, Libra A, Kopecky M, et al. P-glycoprotein expression and distribution in the rat placenta during pregnancy. Reprod. Toxicol 2004;18:785–792. [DOI] [PubMed] [Google Scholar]
- 36.Tomi M, Akashi T, Takagi Y, et al. Subcellular localizations of efflux pumps, MDR1 and BCRP, in syncytiotrophoblast layers of rodent placenta. Placenta. 2016;46:111. [Google Scholar]
- 37.Akashi T, Nishimura T, Takaki Y, et al. Layer II of placental syncytiotrophoblasts expresses MDR1 and BCRP at the apical membrane in rodents. Reprod. Toxicol 2016;65:375–381. [DOI] [PubMed] [Google Scholar]
- 38.Pappas JJ, Petropoulos S, Suderman M, et al. The multidrug resistance 1 gene Abcb1 in brain and placenta: comparative analysis in human and guinea pig. PLoS One. 2014;9:e111135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kalabis GM, Petropoulos S, Gibb W, et al. Multidrug resistance phosphoglycoprotein (ABCB1) expression in the guinea pig placenta: developmental changes and regulation by betamethasone. Can. J. Physiol. Pharmacol 2009;87:973–978. [DOI] [PubMed] [Google Scholar]
- 40.Mathias AA, Hitti J, Unadkat JD. P-glycoprotein and breast cancer resistance protein expression in human placentae of various gestational ages. Am. J. Physiol. Integr. Comp. Physiol 2005;289:R963–R969.•• Ontogeny information on P-gp and BCRP in human placenta
- 41.Mason CW, Buhimschi IA, Buhimschi CS, et al. ATP-Binding Cassette Transporter Expression in Human Placenta as a Function of Pregnancy Condition. Drug Metab. Dispos 2011;39:1000–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.do Imperio GE, Bloise E, Javam M, et al. Chorioamnionitis Induces a Specific Signature of Placental ABC Transporters Associated with an Increase of miR-331–5p in the Human Preterm Placenta. Cell. Physiol. Biochem 2018;45:591–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Petrovic V, Kojovic D, Cressman A, et al. Maternal bacterial infections impact expression of drug transporters in human placenta. Int. Immunopharmacol 2015;26:349–356. [DOI] [PubMed] [Google Scholar]
- 44.Lye P, Bloise E, Dunk C, et al. Effect of oxygen on multidrug resistance in the first trimester human placenta. Placenta. 2013;34:817–823.• Information on P-gp and BCRP mRNA expression on cytotrophoblast in human
- 45.Javam M, Audette MC, Iqbal M, et al. Effect of oxygen on multidrug resistance in term human placenta. Placenta. 2014;35:324–330. [DOI] [PubMed] [Google Scholar]
- 46.Mina TH, Räikkönen K, Riley SC, et al. Maternal distress associates with placental genes regulating fetal glucocorticoid exposure and IGF2: Role of obesity and sex. Psychoneuroendocrinology. 2015;59:112–122. [DOI] [PubMed] [Google Scholar]
- 47.Zhang H, Wu X, Wang H, et al. Effect of pregnancy on cytochrome P450 3a and P-glycoprotein expression and activity in the mouse: mechanisms, tissue specificity, and time course. Mol. Pharmacol 2008;74:714–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chung F, Eyal S, Muzi M, et al. Positron emission tomography imaging of tissue P-glycoprotein activity during pregnancy in the non-human primate. Br. J. Pharmacol 2010;159:394–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ke AB, Eyal S, Chung FS, et al. Modeling cyclosporine A inhibition of the distribution of a P-glycoprotein PET ligand, 11C-verapamil, into the maternal brain and fetal liver of the pregnant nonhuman primate: impact of tissue blood flow and site of inhibition. J. Nucl. Med 2013;54:437–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gahir SS, Piquette-Miller M. Gestational and Pregnane X Receptor-Mediated Regulation of Placental ATP-Binding Cassette Drug Transporters in Mice. Drug Metab. Dispos 2011;39:465–471. [DOI] [PubMed] [Google Scholar]
- 51.Gahir S, Piquette-Miller M. The Role of PXR Genotype and Transporter Expression in the Placental Transport of Lopinavir in Mice. Pharmaceutics. 2017;9:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Duan H, Zhou K, Zhang Y, et al. HDAC2 was involved in placental P-glycoprotein regulation both in vitro and vivo. Placenta. 2017;58:105–114. [DOI] [PubMed] [Google Scholar]
- 53.Petropoulos S, Gibb W, Matthews SG. Effect of glucocorticoids on regulation of placental multidrug resistance phosphoglycoprotein (P-gp) in the mouse. Placenta. 2010;31:803–810. [DOI] [PubMed] [Google Scholar]
- 54.Kalabis GM, Petropoulos S, Gibb W, et al. Multidrug resistance phosphoglycoprotein (ABCB1) expression in the guinea pig placenta: developmental changes and regulation by betamethasone. Can. J. Physiol. Pharmacol 2009;87:973–978. [DOI] [PubMed] [Google Scholar]
- 55.Hodyl NA, Stark MJ, Butler M, et al. Placental P-glycoprotein is unaffected by timing of antenatal glucocorticoid therapy but reduced in SGA preterm infants. Placenta. 2013;34:325–330. [DOI] [PubMed] [Google Scholar]
- 56.Smit JW, Huisman MT, van Tellingen O, et al. Absence or pharmacological blocking of placental P-glycoprotein profoundly increases fetal drug exposure. J. Clin. Invest 1999;104:1441–1447.• Functional evidence of importance of P-gp in fetal protection of xenobiotic exposure
- 57.Lankas GR, Wise LD, Cartwright ME, et al. Placental P-glycoprotein deficiency enhances susceptibility to chemically induced birth defects in mice. Reprod. Toxicol 12:457–463. [DOI] [PubMed] [Google Scholar]
- 58.Eyal S, Chung FS, Muzi M, et al. Simultaneous PET Imaging of P-Glycoprotein Inhibition in Multiple Tissues in the Pregnant Nonhuman Primate. J. Nucl. Med 2009;50:798–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bhuiyan M, Petropoulos S, Gibb W, et al. Sertraline Alters Multidrug Resistance Phosphoglycoprotein Activity in the Mouse Placenta and Fetal Blood–Brain Barrier. Reprod. Sci 2012;19:407–415. [DOI] [PubMed] [Google Scholar]
- 60.Marzolini C, Rudin C, Decosterd LA, et al. Transplacental passage of protease inhibitors at delivery. AIDS. 2002;16:889–893. [DOI] [PubMed] [Google Scholar]
- 61.Liao MZ, Gao C, Shireman LM, et al. P-gp/ABCB1 exerts differential impacts on brain and fetal exposure to norbuprenorphine. Pharmacol. Res 2017;119:61–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Szilagyi JT, Vetrano AM, Laskin JD, et al. Localization of the placental BCRP/ABCG2 transporter to lipid rafts: Role for cholesterol in mediating efflux activity. Placenta. 2017;55:29–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Evseenko DA, Murthi P, Paxton JW, et al. The ABC transporter BCRP/ABCG2 is a placental survival factor, and its expression is reduced in idiopathic human fetal growth restriction. FASEB J. 2007;21:3592–3605. [DOI] [PubMed] [Google Scholar]
- 64.Yeboah D, Sun M, Kingdom J, et al. Expression of breast cancer resistance protein (BCRP/ABCG2) in human placenta throughout gestation and at term before and after labor. Can. J. Physiol. Pharmacol 2006;84:1251–1258. [DOI] [PubMed] [Google Scholar]
- 65.Kozłowska-Rup D, Czekaj P, Plewka D, et al. Immunolocalization of ABC drug transporters in human placenta from normal and gestational diabetic pregnancies. Ginekol. Pol 2014;85:410–419. [DOI] [PubMed] [Google Scholar]
- 66.Memon N, Bircsak KM, Archer F, et al. Regional expression of the BCRP/ABCG2 transporter in term human placentas. Reprod. Toxicol 2014;43:72–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lye P, Bloise E, Nadeem L, et al. Glucocorticoids modulate multidrug resistance transporters in the first trimester human placenta. J. Cell. Mol. Med 2018; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Staud F, Vackova Z, Pospechova K, et al. Expression and Transport Activity of Breast Cancer Resistance Protein (Bcrp/Abcg2) in Dually Perfused Rat Placenta and HRP-1 Cell Line. J. Pharmacol. Exp. Ther 2006;319:53–62. [DOI] [PubMed] [Google Scholar]
- 69.Jonker JW, Buitelaar M, Wagenaar E, et al. The breast cancer resistance protein protects against a major chlorophyll-derived dietary phototoxin and protoporphyria. Proc. Natl. Acad. Sci. U. S. A 2002;99:15649–15654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Petrovic V, Wang J-H, Piquette-Miller M. Effect of Endotoxin on the Expression of Placental Drug Transporters and Glyburide Disposition in Pregnant Rats. Drug Metab. Dispos 2008;36:1944–1950. [DOI] [PubMed] [Google Scholar]
- 71.Yeboah D, Sun M, Kingdom J, et al. Expression of breast cancer resistance protein (BCRP/ABCG2) in human placenta throughout gestation and at term before and after labor. Can. J. Physiol. Pharmacol 2006;84:1251–1258. [DOI] [PubMed] [Google Scholar]
- 72.Meyer zu Schwabedissen HE, Grube M, Dreisbach A, et al. Epidermal growth factor-mediated activation of the map kinase cascade results in altered expression and function of ABCG2 (BCRP). Drug Metab. Dispos 2006;34:524–533. [DOI] [PubMed] [Google Scholar]
- 73.Cygalova L, Ceckova M, Pavek P, et al. Role of breast cancer resistance protein (Bcrp/Abcg2) in fetal protection during gestation in rat. Toxicol. Lett 2008;178:176–180. [DOI] [PubMed] [Google Scholar]
- 74.Yasuda S, Itagaki S, Hirano T, et al. Expression level of ABCG2 in the placenta decreases from the mid stage to the end of gestation. Biosci. Biotechnol. Biochem 2005;69:1871–1876. [DOI] [PubMed] [Google Scholar]
- 75.Wang H, Wu X, Hudkins K, et al. Expression of the breast cancer resistance protein (Bcrp1/Abcg2) in tissues from pregnant mice: effects of pregnancy and correlations with nuclear receptors. [DOI] [PubMed]
- 76.Kalabis GM, Petropoulos S, Gibb W, et al. Breast Cancer Resistance Protein (Bcrp1/Abcg2) in Mouse Placenta and Yolk Sac: Ontogeny and its Regulation by Progesterone. Placenta. 2007;28:1073–1081.• Ontogeny of Bcrp in mouse placenta
- 77.Bircsak KM, Moscovitz JE, Wen X, et al. Interindividual regulation of the BCRP/ABCG2 transporter in term human placentas. Drug Metab. Dispos 2018;dmd.117.079228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kobayashi D, Ieiri I, Hirota T, et al. Functional assessment of ABCG2 (BCRP) gene polymorphisms to protein expression in human placenta. Drug Metab. Dispos 2005;33:94–101. [DOI] [PubMed] [Google Scholar]
- 79.Saito J, Hirota T, Furuta S, et al. Association between DNA Methylation in the miR-328 5’-Flanking Region and Inter-individual Differences in miR-328 and BCRP Expression in Human Placenta. Navarro A, editor. PLoS One. 2013;8:e72906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wang H, Lee E-W, Zhou L, et al. Progesterone receptor (PR) isoforms PRA and PRB differentially regulate expression of the breast cancer resistance protein in human placental choriocarcinoma BeWo cells. Mol. Pharmacol 2008;73:845–854. [DOI] [PubMed] [Google Scholar]
- 81.Wang H, Zhou L, Gupta A, et al. Regulation of BCRP/ABCG2 expression by progesterone and 17beta-estradiol in human placental BeWo cells. Am. J. Physiol. Endocrinol. Metab 2006;290:E798–807. [DOI] [PubMed] [Google Scholar]
- 82.Lye P, Bloise E, Javam M, et al. Impact of Bacterial and Viral Challenge on Multidrug Resistance in First- and Third-Trimester Human Placenta. Am. J. Pathol 2015;185:1666–1675. [DOI] [PubMed] [Google Scholar]
- 83.Lin Y, Bircsak KM, Gorczyca L, et al. Regulation of the placental BCRP transporter by PPAR gamma. J. Biochem. Mol. Toxicol 2017;31:e21880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Francois LN, Gorczyca L, Du J, et al. Down-regulation of the placental BCRP/ABCG2 transporter in response to hypoxia signaling. Placenta. 2017;51:57–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Liu L, Zhou L, Hu S, et al. Down-regulation of ABCG2 and ABCB4 transporters in the placenta of rats exposed to cadmium. Oncotarget. 2016;7:38154–38163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kummu M, Sieppi E, Wallin K, et al. Cadmium inhibits ABCG2 transporter function in BeWo choriocarcinoma cells and MCF-7 cells overexpressing ABCG2. Placenta. 2012;33:859–865. [DOI] [PubMed] [Google Scholar]
- 87.Sieppi E, Vähäkangas K, Rautio A, et al. The xenoestrogens, bisphenol A and para-nonylphenol, decrease the expression of the ABCG2 transporter protein in human term placental explant cultures. Mol. Cell. Endocrinol 2016;429:41–49. [DOI] [PubMed] [Google Scholar]
- 88.Bircsak KM, Gupta V, Yuen PYS, et al. Genetic and Dietary Regulation of Glyburide Efflux by the Human Placental Breast Cancer Resistance Protein Transporter. J. Pharmacol. Exp. Ther 2016;357:103–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Neradugomma NK, Liao MZ, Mao Q. Buprenorphine, Norbuprenorphine, R-Methadone, and S-Methadone Upregulate BCRP/ABCG2 Expression by Activating Aryl Hydrocarbon Receptor in Human Placental Trophoblasts. Mol. Pharmacol 2017;91:237–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Blazquez AG, Briz O, Gonzalez-Sanchez E, et al. The effect of acetaminophen on the expression of BCRP in trophoblast cells impairs the placental barrier to bile acids during maternal cholestasis. Toxicol. Appl. Pharmacol 2014;277:77–85. [DOI] [PubMed] [Google Scholar]
- 91.Pollex E, Lubetsky A, Koren G. The Role of Placental Breast Cancer Resistance Protein in the Efflux of Glyburide across the Human Placenta. Placenta. 2008;29:743–747.• Activity evidence of BCRP in human placental transport
- 92.Reznicek J, Ceckova M, Tupova L, et al. Etravirine inhibits ABCG2 drug transporter and affects transplacental passage of tenofovir disoproxil fumarate. Placenta. 2016;47:124–129. [DOI] [PubMed] [Google Scholar]
- 93.Blazquez AG, Briz O, Romero MR, et al. Characterization of the Role of ABCG2 as a Bile Acid Transporter in Liver and Placenta. Mol. Pharmacol 2012;81:273–283. [DOI] [PubMed] [Google Scholar]
- 94.Jonker JW, Smit JW, Brinkhuis RF, et al. Role of Breast Cancer Resistance Protein in the Bioavailability and Fetal Penetration of Topotecan. J. Natl. Cancer Inst 2000;92:1651–1656. [DOI] [PubMed] [Google Scholar]
- 95.Zhang Y, Wang H, Unadkat JD, et al. Breast Cancer Resistance Protein 1 Limits Fetal Distribution of Nitrofurantoin in the Pregnant Mouse. Drug Metab. Dispos 2007;35:2154–2158. [DOI] [PubMed] [Google Scholar]
- 96.Zhou L, Naraharisetti SB, Wang H, et al. The Breast Cancer Resistance Protein (Bcrp1/Abcg2) Limits Fetal Distribution of Glyburide in the Pregnant Mouse: An Obstetric-Fetal Pharmacology Research Unit Network and University of Washington Specialized Center of Research Study. Mol. Pharmacol 2007;73:949–959. [DOI] [PubMed] [Google Scholar]
- 97.Lam J, Koren G. P-glycoprotein in the Developing Human Brain. Ther. Drug Monit. 2014;36:699–705. [DOI] [PubMed] [Google Scholar]
- 98.Saidijam M, Karimi Dermani F, Sohrabi S, et al. Efflux proteins at the blood–brain barrier: review and bioinformatics analysis. Xenobiotica. 2017;1–27. [DOI] [PubMed] [Google Scholar]
- 99.Daood M, Tsai C, Ahdab-Barmada M, et al. ABC transporter (P-gp/ABCB1, MRP1/ABCC1, BCRP/ABCG2) expression in the developing human CNS. Neuropediatrics. 2008;39:211–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Virgintino D, Errede M, Girolamo F, et al. Fetal Blood-Brain Barrier P-Glycoprotein Contributes to Brain Protection During Human Development. J. Neuropathol. Exp. Neurol 2008;67:50–61. [DOI] [PubMed] [Google Scholar]
- 101.van Kalken CK, Giaccone G, van der Valk P, et al. Multidrug resistance gene (P-glycoprotein) expression in the human fetus. Am. J. Pathol 1992;141:1063–1072.• Information on human fetal P-gp expression
- 102.Fakhoury M, de Beaumais T, Guimiot F, et al. mRNA expression of MDR1 and major metabolising enzymes in human fetal tissues. Drug Metab. Pharmacokinet 2009;24:529–536. [DOI] [PubMed] [Google Scholar]
- 103.Lam J, Baello S, Iqbal M, et al. The ontogeny of P-glycoprotein in the developing human blood-brain barrier: implication for opioid toxicity in neonates. Pediatr. Res 2015;78:417–421. [DOI] [PubMed] [Google Scholar]
- 104.Bouwmeester NJ, Hop WCJ, van Dijk M, et al. Postoperative pain in the neonate: age-related differences in morphine requirements and metabolism. Intensive Care Med. 2003;29:2009–2015. [DOI] [PubMed] [Google Scholar]
- 105.Way WL, Costley EC, Leong Way E. Respiratory sensitivity of the newborn infant to meperidine and morphine. Clin. Pharmacol. Ther 1965;6:454–461. [DOI] [PubMed] [Google Scholar]
- 106.Koren G, Butt W, Chinyanga H, et al. Postoperative morphine infusion in newborn infants: assessment of disposition characteristics and safety. J. Pediatr 1985;107:963–967. [DOI] [PubMed] [Google Scholar]
- 107.Petropoulos S, Gibb W, Matthews SG. Breast Cancer-Resistance Protein (BCRP1) in the Fetal Mouse Brain: Development and Glucocorticoid Regulation. Biol. Reprod 2011;84:783–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Bloise E, Petropoulos S, Iqbal M, et al. Acute Effects of Viral Exposure on P-Glycoprotein Function in the Mouse Fetal Blood-Brain Barrier. Cell. Physiol. Biochem 2017;41:1044–1050. [DOI] [PubMed] [Google Scholar]
- 109.de Zwart L, Scholten M, Monbaliu JG, et al. The ontogeny of drug metabolizing enzymes and transporters in the rat. Reprod. Toxicol 2008;26:220–230. [DOI] [PubMed] [Google Scholar]
- 110.Cerveny L, Neumanova Z, Karbanova S, et al. Long-term administration of tenofovir or emtricitabine to pregnant rats; effect on Abcb1a, Abcb1b and Abcg2 expression in the placenta and in maternal and fetal organs. J. Pharm. Pharmacol 2016;68:84–92.• Information on Bcrp and P-gp expression in rat placenta and rat fetus
- 111.Saljé K, Lederer K, Oswald S, et al. Effects of Rifampicin, Dexamethasone, St. John’s Wort and Thyroxine on Maternal and Foetal Expression of Abcb1 and Organ Distribution of Talinolol in Pregnant Rats. Basic Clin. Pharmacol. Toxicol 2012;111:99–105.• Information on rat fetal expression of P-gp
- 112.Ek CJ, Wong A, Liddelow SA, et al. Efflux mechanisms at the developing brain barriers: ABC-transporters in the fetal and postnatal rat. Toxicol. Lett 2010;197:51–59. [DOI] [PubMed] [Google Scholar]
- 113.Filia MF, Marchini T, Minoia JM, et al. Induction of ABCG2/BCRP restricts the distribution of zidovudine to the fetal brain in rats. Toxicol. Appl. Pharmacol 2017;330:74–83. [DOI] [PubMed] [Google Scholar]
- 114.Si-Tayeb K, Lemaigre FP, Duncan SA. Organogenesis and Development of the Liver. Dev. Cell 2010;18:175–189. [DOI] [PubMed] [Google Scholar]
- 115.Collardeau-Frachon S, Scoazec J-Y. Vascular Development and Differentiation During Human Liver Organogenesis. Anat. Rec. Adv. Integr. Anat. Evol. Biol 2008;291:614–627. [DOI] [PubMed] [Google Scholar]
- 116.Grijalva J, Vakili K. Neonatal liver physiology. Semin. Pediatr. Surg 2013;22:185–189. [DOI] [PubMed] [Google Scholar]
- 117.Payushina OV Hematopoietic Microenvironment in the Fetal Liver: Roles of Different Cell Populations. ISRN Cell Biol. 2012;2012:1–7. [Google Scholar]
- 118.Mooij MG, Schwarz UI, de Koning BAE, et al. Ontogeny of Human Hepatic and Intestinal Transporter Gene Expression during Childhood: Age Matters. Drug Metab. Dispos 2014;42:1268–1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Konieczna A, Erdösová B, Lichnovská R, et al. Differential expression of ABC transporters (MDR1, MRP1, BCRP) in developing human embryos. J. Mol. Histol 2011;42:567–574.•• Information on human fetal tissue protein expression of P-gp and BCRP
- 120.Moscovitz JE, Nahar MS, Shalat SL, et al. Correlation between Conjugated Bisphenol A Concentrations and Efflux Transporter Expression in Human Fetal Livers. Drug Metab. Dispos 2016;44:1061–1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Elmorsi Y, Barber J, Rostami-Hodjegan A. Ontogeny of Hepatic Drug Transporters and Relevance to Drugs Used in Pediatrics. Drug Metab. Dispos 2016;44:992–998. [DOI] [PubMed] [Google Scholar]
- 122.Rosati A, Maniori S, Decorti G, et al. Physiological regulation of P-glycoprotein, MRP1, MRP2 and cytochrome P450 3A2 during rat ontogeny. Dev. Growth Differ 2003;45:377–387. [DOI] [PubMed] [Google Scholar]
- 123.Ceckova M, Reznicek J, Ptackova Z, et al. Role of ABC and Solute Carrier Transporters in the Placental Transport of Lamivudine. Antimicrob. Agents Chemother 2016;60:5563–5572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Cui JY, Gunewardena SS, Yoo B, et al. RNA-Seq Reveals Different mRNA Abundance of Transporters and Their Alternative Transcript Isoforms During Liver Development. Toxicol. Sci 2012;127:592–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Huls M, Brown CDA, Windass AS, et al. The breast cancer resistance protein transporter ABCG2 is expressed in the human kidney proximal tubule apical membrane. Kidney Int. 2008;73:220–225. [DOI] [PubMed] [Google Scholar]
- 126.Thiebaut F, Tsuruo T, Hamada H, et al. Cellular localization of the multidrug-resistance gene product P-glycoprotein in normal human tissues. Proc. Natl. Acad. Sci. U. S. A 1987;84:7735–7738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Pinto N, Halachmi N, Verjee Z, et al. Ontogeny of Renal P-glycoprotein Expression in Mice: Correlation with Digoxin Renal Clearance. Pediatr. Res 2005;58:1284–1289. [DOI] [PubMed] [Google Scholar]
- 128.Cheng X, Klaassen CD. Tissue Distribution, Ontogeny, and Hormonal Regulation of Xenobiotic Transporters in Mouse Kidneys. Drug Metab. Dispos 2009;37:2178–2185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Xu Y, Wang Y, Lu Y, et al. Age-associated differences in transporter gene expression in kidneys of male rats. Mol. Med. Rep 2016;15:474–482. [DOI] [PubMed] [Google Scholar]
- 130.Miki Y, Suzuki T, Tazawa C, et al. Steroid and xenobiotic receptor (SXR), cytochrome P450 3A4 and multidrug resistance gene 1 in human adult and fetal tissues. Mol. Cell. Endocrinol 2005;231:75–85. [DOI] [PubMed] [Google Scholar]
- 131.Mahmood B, Daood MJ, Hart C, et al. Ontogeny of P-glycoprotein in mouse intestine, liver, and kidney. J. Investig. Med 2001;49:250–257. [DOI] [PubMed] [Google Scholar]
- 132.Vlaming MLH, Lagas JS, Schinkel AH. Physiological and pharmacological roles of ABCG2 (BCRP): Recent findings in Abcg2 knockout mice. Adv. Drug Deliv. Rev 2009;61:14–25. [DOI] [PubMed] [Google Scholar]
- 133.Tournier N, Chevillard L, Megarbane B, et al. Interaction of drugs of abuse and maintenance treatments with human P-glycoprotein (ABCB1) and breast cancer resistance protein (ABCG2). Int. J. Neuropsychopharmacol 2010;13:905–915. [DOI] [PubMed] [Google Scholar]
- 134.Prasad B, Unadkat JD. The concept of fraction of drug transported (ft) with special emphasis on BBB efflux of CNS and antiretroviral drugs. Clin. Pharmacol. Ther 2015;97:320–323.•• Information on the concept of fraction of drug transported
- 135.Zhang Z, Imperial MZ, Patilea-Vrana GI, et al. Development of a Novel Maternal-Fetal Physiologically Based Pharmacokinetic Model I: Insights into Factors that Determine Fetal Drug Exposure through Simulations and Sensitivity Analyses. Drug Metab. Dispos 2017;45:920–938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Mason CW, Lee GT, Dong Y, et al. Effect of Prostaglandin E2 on Multidrug Resistance Transporters In Human Placental Cells. Drug Metab. Dispos 2014;42:2077–2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Kumar JS, Wei B-R, Madigan JP, et al. Bioluminescent imaging of ABCG2 efflux activity at the blood-placenta barrier. Sci. Rep 2016;6:20418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Pávek P, Fendrich Z, Staud F, et al. Influence of P-glycoprotein on the transplacental passage of cyclosporine. J. Pharm. Sci 2001;90:1583–1592. [DOI] [PubMed] [Google Scholar]
- 139.Rodrigues RF, Carter AM, Ambrosio CE, et al. The subplacenta of the red-rumped agouti (Dasyprocta leporina L). Reprod. Biol. Endocrinol 2006;4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Rawles LA, Acuna D, Erickson RP. The role of multiple drug resistance proteins in fetal and/or placental protection against teratogen-induced orofacial clefting. Mol. Reprod. Dev 2007;74:1483–1489. [DOI] [PubMed] [Google Scholar]
- 141.Bliek BJB, van Schaik RHN, van der Heiden IP, et al. Maternal medication use, carriership of the ABCB1 3435C > T polymorphism and the risk of a child with cleft lip with or without cleft palate. Am. J. Med. Genet. A 2009;149A:2088–2092. [DOI] [PubMed] [Google Scholar]
- 142.De Sousa Mendes M, Lui G, Zheng Y, et al. A Physiologically-Based Pharmacokinetic Model to Predict Human Fetal Exposure for a Drug Metabolized by Several CYP450 Pathways. Clin. Pharmacokinet 2017;56:537–550. [DOI] [PubMed] [Google Scholar]
- 143.De Sousa Mendes M, Hirt D, Vinot C, et al. Prediction of human fetal pharmacokinetics using ex vivo human placenta perfusion studies and physiologically based models. Br. J. Clin. Pharmacol 2016;81:646–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Heikkinen AT, Lignet F, Cutler P, et al. The role of quantitative ADME proteomics to support construction of physiologically based pharmacokinetic models for use in small molecule drug development. PROTEOMICS - Clin. Appl 2015;9:732–744. [DOI] [PubMed] [Google Scholar]
- 145.Vrana M, Whittington D, Nautiyal V, et al. Database of Optimized Proteomic Quantitative Methods for Human Drug Disposition-Related Proteins for Applications in Physiologically Based Pharmacokinetic Modeling. CPT Pharmacometrics Syst. Pharmacol 2017;6:267–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Cascorbi I Role of pharmacogenetics of ATP-binding cassette transporters in the pharmacokinetics of drugs. Pharmacol. Ther 2006;112:457–473. [DOI] [PubMed] [Google Scholar]
- 147.Iqbal M, Audette MC, Petropoulos S, et al. Placental drug transporters and their role in fetal protection. Placenta. 2012;33:137–142. [DOI] [PubMed] [Google Scholar]