Abstract
Objective.
Calcium pyrophosphate dihydrate (CPPD) and basic calcium phosphate (BCP) crystals occur in up to 60% of osteoarthritic joints and predict an increased severity of arthritis. Articular cartilage vesicles (ACVs) generate CPPD crystals in the presence of ATP and BCP crystals with added β-glycerophosphate. While ACVs are present in normal articular cartilage, they mineralize primarily in cartilage from osteoarthritic joints. The aim of this study was to explore the hypothesis that ACV mineralization is regulated by components of the surrounding extracellular matrix.
Methods.
Porcine ACVs were embedded in aga-rose gels containing type II and/or type I collagen and/or proteoglycans. Mineralization was measured as45Ca accumulation stimulated by ATP or β-glycerophosphate and reflects both nucleation and growth. Synthetic CPPD and BCP crystals were embedded in similar gels to isolate the effect of matrix components on crystal growth.
Results.
After establishing baseline responsiveness of ACVs to ATP and β-glycerophosphate in agarose gels, we examined the ability of ATP and β-glycerophosphate to stimulate mineral formation in gels containing various matrix components. Type II collagen suppressed the ability of ATP to stimulate mineralization, while a combination of type II plus type I collagen increased the effect of ATP and β -glycerophosphate on mineralization. Type I collagen affected ACV mineralization in a dose-responsive manner. Neither type of collagen significantly affected crystal growth or levels of mineralization-regulating enzymes. Proteoglycans suppressed mineral formation by ACVs in gels containing both type I and type II collagen.
Conclusion.
Cartilage matrix changes that occur with osteoarthritis, such as increased quantities of type I collagen and reduced proteoglycan levels, may promote ACV mineralization.
Osteoarthritis is the most common form of arthritis in adults and results in large personal and societal costs (1). Pathogenic calcium-containing crystals, including calcium pyrophosphate dihydrate (CPPD) and hydroxyapatite-like basic calcium phosphate (BCP) crystals, are found in up to 60% of osteoarthritic joints at the time of joint replacement (2,3). Ample evidence supports the important contributions of both types of calcium-containing crystals to articular damage in osteoarthritis (4); yet, how and why calcium-containing crystals form in normally unmineralized articular cartilage matrix remains unclear.
Small membrane-bound vesicles known as matrix vesicles play a key role in both normal and pathologic matrix mineralization in many tissues (5). In articular cartilage, these chondrocyte-derived extracellular organelles have been identified histologically (6), and concentrate enzymes, ions, and substrates necessary for crystal formation. Derfus et al (7,8) demonstrated that isolated articular cartilage vesicles (ACVs) generated both CPPD and BCP crystals in vitro. CPPD crystal formation was preferentially stimulated in the presence of ATP, while in its absence, BCP crystals were primarily generated (7). Extracellular calcium, phosphate, and pyrophosphate are necessary for mineral formation. These ions and their precursors are likely present in sufficient quantities in joints susceptible to calcium crystal deposition (9–11), and transient increases in ATP, the major source of pyrophosphate, may be induced in normal cartilage by trauma (12).
The temptation to assign an important role to ACVs during pathologic mineralization in articular cartilage has been tempered by 2 puzzling findings. The first is that ACVs exist in and are easily isolatable from normal healthy articular cartilage, but little matrix mineralization occurs in these tissues. The second is that ACVs isolated from osteoarthritic cartilage, a common setting for CPPD and BCP crystal formation in vivo, display no greater capacity for crystal formation than vesicles isolated from normal cartilage (8). These findings strongly suggest that the milieu of the vesicle (i.e., its surrounding extracellular matrix) strongly influences its ability to mineralize.
Indeed, in growth plate cartilage, there is ample precedent for an important interaction between matrix vesicles and components of their surrounding extracellular matrix, such as collagens and proteoglycans. Boskey et al (13) demonstrated that growth plate matrix vesicles generated hydroxyapatite crystals in gelatin gels. Type II collagen increased calcium uptake by growth plate matrix vesicles, by binding to and activating the calcium channel protein annexin V (14). Both type I and type II collagen fibrils also act as scaffolds along which the hydroxyapatite crystals of bone grow (15,16). In contrast, large proteoglycans typically inhibit crystal formation, probably by filling in the “hole zones” between collagen fibrils with highly anionic glycosaminoglycans and sterically hindering crystal nucleation and growth (17).
In articular cartilage, little is known about the relationship between extracellular matrix components and ACV mineralization. Indeed, regulatory factors for ACV-mediated mineralization remain largely unidentified. Osteoarthritic cartilage matrix contains altered type II collagen fibers, increased quantities of type I collagen, and fewer large proteoglycans (18,19). We hypothesized that interactions between ACVs and extra-cellular matrix components would influence their ability to generate pathologic crystals and could explain the strong clinical association between osteoarthritis and calcium-containing crystals.
MATERIALS AND METHODS
Materials.
Cartilage proteoglycans from human cartilage were purchased from MD Biosciences (St. Paul, MN). All other reagents were obtained from Sigma (St. Louis, MO), except where indicated otherwise.
ACV isolation.
Cartilage was obtained from 3–5-year-old pigs that had been slaughtered by Johnsonville Foods (Watertown, WI). Hyaline articular cartilage was removed from the patellar, tibial, and femoral surfaces of the knee joint and then minced, washed, and weighed. Cartilage pieces were incubated for 10 minutes in Dulbecco’s modified Eagle’s medium (DMEM; Mediatech, Herndon, VA) with 0.1% hyaluronidase (1 ml/gm of wet weight cartilage) to remove surface hyaluronate and for 10 minutes with 0.5% trypsin (1 ml/gm of cartilage). All incubations were done at 37°C, with stirring. Trypsin inhibitor (0.2% soybean trypsin inhibitor; 1 ml/gm of cartilage) was added to inactivate any remaining trypsin. After washing, cartilage pieces were incubated for 45 minutes with0.2% collagenase (2.5 ml/gm of cartilage). Additional medium was added so that the final collagenase concentration was0.05% in a total of 10 ml of medium per gram of cartilage, and this was incubated overnight with stirring. The mixture was filtered and centrifuged at 500g for 15 minutes to remove cells, then at 37,000g for 15 minutes to remove large cell fragments and organelles. The supernatant was then centrifuged at 120,000g for 60 minutes to pellet the ACV fraction. Protein concentrations were determined by the Lowry assay (20). The ACV-containing pellet was resuspended in DMEM to a protein concentration of 12–15 mg/ml.
Electron microscopy.
To ensure that membrane-bound vesicular structures were indeed present in the vesicle fraction, we performed transmission electron microscopy on randomly chosen vesicle fractions.
Alkaline phosphatase assay.
Activity of the phosphate-generating enzyme alkaline phosphatase was measured using p-nitrophenyl phosphate as a chromogenic substrate according to the manufacturer’s directions (Sigma kit 104-LS). Results were corrected for protein levels in the samples using the Lowry assay.
Nucleoside triphosphate pyrophosphohydrolase (NTPPPH) assay.
Activity of the pyrophosphate-generating enzyme NTPPPH was measured using 2 mM p-nitrophenyl thymidine monophosphate as a substrate at pH 7.4, as previously described (21). Results were corrected for protein levels in the samples using the Lowry assay.
The 5′-nucleotidase (5′-NT) assay.
Activity of the phosphate-generating enzyme 5′-NT was determined using a modification of the assay based on Sigma kit 265-UV (22). Results were corrected for protein levels in the samples using the Lowry assay.
Pyrophosphatase assay.
ACVs were incubated for 1 hour with32P-labeled sodium pyrophosphate (PerkinElmer, Waltham, MA) in the presence of MgCl2. The hydrolysis of pyrophosphate was measured by determining the quantity of radiolabel in the phosphomolybdate precipitate before and after exposure to ACVs (23).
Phosphate assay.
Phosphate was measured in collagen preparations using a commercial assay (QuantiChrom Phosphate Assay kit; Bioassay Systems, Hayward, CA).
Biomineralization assay.
ACVs were added to make a 1 mg/ml solution in 2% warm agarose dissolved in calcifying salt solution (CSS; 2.2 mM CaCl2, 1.6 mM KH2PO4, 1 mM MgCl2, 85 mM NaCl, 15 mM KCl, 10 mM NaHCO3, 2% penicillin/streptomycin/amphotericin B, and 50 mM N-tris(hydroxymethyl)methyl-2-aminoethanesulfonic acid, pH7.6). Two hundred microliters of the ACV/agarose mixture was added to each well of a 48-well tissue culture plate and solidified at room temperature. Five hundred microliters of CSS with 1 μCi/ml of45Ca were added to each well, with or without 1 mM ATP or 1 mM β-glycerophosphate, and ACVs were incubated for 3–7 days at 37°C. Every 48 hours, 10 μl of 5 mM ATP or β-glycerophosphate solution or 10 μl of CSS was added to maintain adequate levels of the original phosphate and pyrophosphate sources. Controls included identical agarose gels containing no ACVs. In some experiments, additional controls included agarose gels containing ACVs that had been inactivated by heat treatment. At the end of the experiment, overlying media were removed, and the gels were thoroughly washed with ice-cold CSS. After dissolving the agarose with6.5% (volume/volume) sodium hypochlorite (commercial bleach) at 100°C for 1 minute and thoroughly washing the ACVs, radioactivity in the ACV fraction was measured by liquid scintigraphy.
Crystal identification.
Mineralized gel-embedded ACVs were treated with hot bleach to melt adherent agarose. Pellets were washed with ice-cold CSS, centrifuged at 100,000g for 40 minutes, frozen, and then lyophilized. The lyophilized pellet was subjected to synchrotron Fourier transform infrared spectroscopy (FTIR) at the Synchrotron Radiation Center (Stoughton, WI), and the spectra were compared with standard spectra for CPPD and BCP crystals.
Western blotting.
Thirty micrograms of ACVs in sample buffer and positive control proteins were loaded and run on 10% Bis-Tris gels (NuPAGE; Invitrogen, Carlsbad, CA). Proteins were transferred to polyvinylidene difluoride and then exposed for 1.5 hours to antibodies against type I collagen (Abcam, Cambridge, MA), type II collagen (Chemicon, Temecula, CA), annexins V (Chemicon), link protein (R&D Systems, Minneapolis, MN), or CD44 (Abcam). After washing, the appropriate secondary antibody was added for 1 hour. Blots were developed with SuperSignal West Pico chemiluminescent substrate (Pierce, Rockford, IL).
Seeded crystal growth assay.
Agarose plugs containing type II collagen or type II plus type I collagen, with or without proteoglycans, were seeded with 200 μg/ml of synthetic CPPD or BCP crystals. Controls included crystals seeded in agarose containing no additives. CPPD crystals were generated in our laboratory using the method of Brown et al (24). BCP crystals were a kind gift from Dr. Neil Mandel (National VA Crystal Identification Center, Milwaukee, WI). Crystal-containing agarose plugs and control plugs with no crystals were incubated at 37°C for 5 days with CSS containing45Ca and either 16 mM Na4H2PO4 (for BCP crystals) or 16 mM NaP2O7 (for CPPD crystals). Plugs were processed in the same manner as for the ACV mineralization assay, and45Ca levels in the crystal fraction were quantified by liquid scintigraphy.
Statistical analysis.
All experiments were repeated 3–5 times. Student’s t-test was used to determine statistically siginificant differences between groups. P values less than 0.05 were considered significant.
RESULTS
ACV characterization.
A heterogeneous population of membrane-bound vesicles of 50–200 nm (data not shown) appeared identical to published pictures of ACVs (7). Functional matrix vesicles are often characterized by levels of phosphate-generating and pyrophosphate-generating enzymes, such as NTPPPH, alkaline phosphatase, and 5′-NT. ACV fractions (n =10) contained activity levels of mineralization-regulating enzymes (data not shown) similar to those previously reported (7).
Biomineralization behavior.
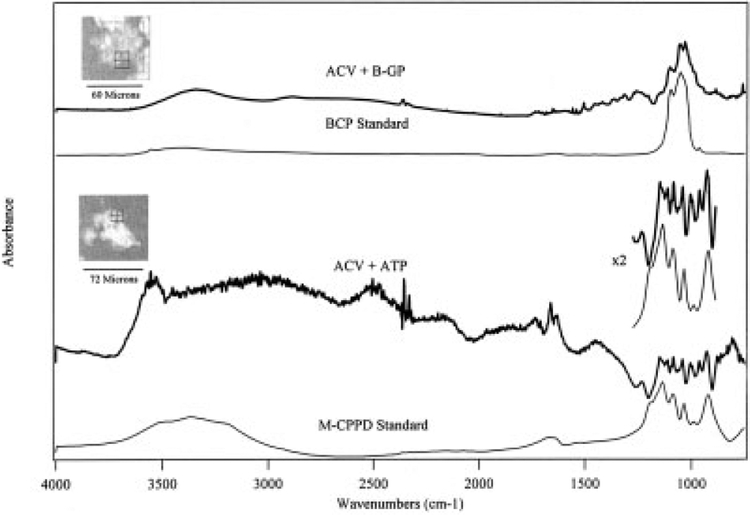
ACVs are typically mineralized in solution and, to our knowledge, have not previously been mineralized in a solid matrix. ACVs were embedded in agarose gels and incubated in CSS with or without 1 mM ATP or β-glycerophosphate. In the presence of ATP, ACVs generated FTIR-proven CPPD crystals (Figure 1). When β-glycerophosphate or the inorganic phosphate in CSS was used a phosphate source, BCP-like crystals were the predominant species generated (Figure 1).
Figure 1.
Generation of both calcium pyrophosphate dihydrate (CPPD) and basic calcium phosphate (BCP) crystals by articular cartilage vesicles (ACVs). ACVs were incubated for 5 days in agarose gels with either 1 mM ATP or 1 mM β-glycerophosphate (B-GP). The crystals were isolated and analyzed by synchrotron Fourier transform infrared spectroscopy (FTIR) microscopy. Representative results are shown. In the upper portion of the figure, the thick line represents the FTIR spectrum of the crystal shown in the inset at the upper left (ACVs incubated with β-glycerophosphate), as compared with a BCP crystal standard (thin line). In the lower portion of the figure, the thick line represents the FTIR spectrum of the crystal shown in the inset at the middle left (ACVs incubated with ATP), as compared with a monoclinic CPPD (M-CPPD) crystal standard (thin line). The area containing the characteristic CPPD peaks is enlarged and is shown at the middle right as ×2.
To confirm that we could accurately quantify crystal production in agarose gels, we incubated embedded ACVs with CSS containing45Ca with or without added ATP or β-glycerophosphate. As shown in Figure 2, both ATP and β-glycerophosphate markedly stimulated45Ca precipitation by ACVs as compared with CSS alone (P < 0.001). One millimolar ATP produced a 2.17 ± 0.56–fold increase in total mineral formation (mean ± SD), while 1 mM β-glycerophosphate elicited a 2.74 ± 0.08 fold increase in mineral formation.
Figure 2.
Effect of heat treatment on mineralization of articular cartilage vesicles (ACVs). ACVs were heat-treated (121°C with 15 pounds of pressure for 15 minutes) or were left untreated. Untreated and heat-treated ACVs were embedded in agarose gels and incubated for 5 days in calcifying salt solution that had been trace-labeled with45Ca alone (solid bars) or with 1 mM ATP (shaded bars) or 1 mM β-glycerophosphate (hatched bars). Levels of45Ca in ACVs were measured by liquid scintigraphy. Values are the mean and SD (n = 6 samples). Heat treatment decreased the total mineral formation and abolished the ability of ACVs to mineralize in the presence of ATP or β-glycerophosphate (P < 0.0001).
As an additional control and to ensure that we were not measuring passive trapping of45Ca by protein or lipids, we heat-treated ACVs until enzyme levels were undetectable. When these heat-treated ACVs were embedded in agarose, very little45Ca accumulated in the ACV fraction under any conditions (Figure 2). These findings suggest that we were measuring an active, enzyme-mediated process.
Effect of various matrix components on the ability of ATP and β-glycerophosphate to increase mineralization.
As shown in Figure 2, embedded ACVs responded to added ATP or β-glycerophosphate by increasing mineralization. Across multiple experiments, this increase was ~2.2-fold for ATP and ~2.7-fold for β-glycerophosphate as compared with CSS controls (n = 35 experiments). We investigated the ability of various matrix components to alter this response to substrate by expressing levels of mineralization with ATP or β-glycerophosphate as the fold increase over the levels in the CSS control. This allowed us to adjust for some variability in baseline mineralization levels between various ACV fractions and to compare these responses across multiple experiments. Moreover, we thought that this analysis accurately modeled the response of ACVs in cartilage matrix to an increase in phosphate or pyrophosphate substrate, as might be seen with joint injury or stress (12).
We first explored the effect of type II collagen, the major fibrillar collagen of normal articular cartilage matrix, on ACV mineralization. Levels of phosphate in the collagen preparations were negligible (data not shown). As shown in Table 1, in the presence of 1.6 mg/ml of type II collagen, exogenous ATP was no longer able to stimulate increased mineral formation. Mineralization in the presence and absence of ATP in type II collagen–containing gels was essentially the same (mean ± SD ratio of ATP to CSS 0.99 ± 0.06). In contrast, in similar gels, β-glycerophosphate produced a1.96 ± 0.32–fold increase over CSS controls, compared with a 2.74 ± 0.08–fold increase in gels containing agarose alone. This suggests that type II collagen strongly inhibits CPPD crystal formation and exerts a more modest inhibitory effect on BCP crystal formation.
Table 1.
Effect of type II collagen, type II plus type I collagen, and proteoglycans on mineralization of ACVs*
| ATP treatment | BGP treatment | |
|---|---|---|
| Agarose | 2.17 ± 0.55 | 2.74 ± 0.08 |
| Type II collagen | 0.99 ± 0.06 | 1.96 ± 0.32 |
| Type II plus type I collagen | 3.74 ± 0.35 | 3.09 ± 0.29 |
| Type II collagen plus proteoglycans | 1.01 ± 0.03 | 1.32 ± 0.07 |
| Type II plus type I collagen plus proteoglycans | 0.57 ± 0.07 | 1.5 ± 0.09 |
Articular cartilage vesicles (ACVs) were embedded in agarose gels with type II collagen alone or with type II plus type I collagen (10:1 ratio), with or without cartilage proteoglycans. After incubation for 5 days with45Ca and calcifying salt solution (CSS) containing ATP or β-glycerophosphate (BGP),45Ca levels in the ACVs were measured by liquid scintigraphy. Values are the mean ± SD ratio of ATP to CSS and of BGP to CSS from 7 experiments. Type I collagen increased both calcium pyrophosphate dihydrate (CPPD) and basic calcium phosphate (BCP) crystal formation when added to type II collagen, as compared with type II collagen alone (P < 0.001). Proteoglycans suppressed both CPPD (P < 0.01) and BCP (P < 0.02) crystal formation in the presence of type I collagen.
ACVs were then embedded in gels containing a combination of 10% type I and 90% type II collagen to mimic conditions seen in osteoarthritic cartilage (19). As mentioned above, ATP did not stimulate45Ca accumulation compared with CSS alone in the presence of type II collagen. However, when ACVs were mineralized in gels containing both type I and type II collagen, ATP increased45Ca accumulation 3.74 ± 0.35–fold over CSS alone (Table 1). Similar effects were seen in the presence of β-glycerophosphate. There was a 1.96 ± 0.32–fold increase in the presence of type II collagen and β-glycerophosphate compared with CSS alone, and a3.09 ± 0.29–fold increase when ACVs were exposed to β-glycerophosphate and type I and type II collagen (P < 0.01). Thus, the addition of type I collagen resulted in a release of the potent inhibitory action of type II collagen on CPPD crystal formation and dramatically reversed the modest inhibitory effect of type II collagen on BCP crystal formation.
Proteoglycans account for 10–15% of the wet weight of hyaline articular cartilage. Large proteoglycans, such as aggrecan, are typically considered mineralization inhibitors (17), and loss of large proteoglycans is an early and pervasive finding in the osteoarthritic cartilage matrix. We embedded ACVs in agarose gels containing a ratio of 10% human cartilage proteoglycan to 90% collagen by weight. As shown in Table 1, proteoglycans had little effect on ATP-induced or β-glycerophosphate–induced mineralization in the presence of type II collagen. In contrast, proteoglycans significantly suppressed mineralization induced by both ATP and β-glycerophosphate when both type I and type II collagen were present. These findings suggest that proteoglycans in the presence of type II collagen, as would be seen in normal cartilage, exert little additional inhibition of either CPPD or BCP crystal formation. However, in the presence of both type I and type II collagen, proteoglycans have important inhibitory effects on mineralization.
Effect of collagen and proteoglycans on crystal growth.
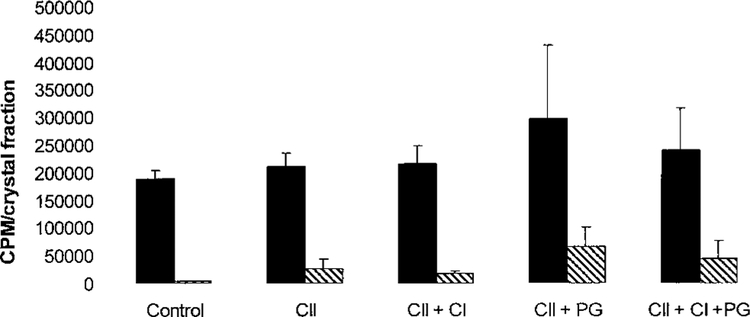
We separated the effects of these matrix components on crystal nucleation and crystal growth in further studies. In order to bypass the nucleation step, we seeded agarose gels with synthetic CPPD or BCP crystals, provided ample calcium and phosphate or pyrophosphate, and measured the amount of45Ca that accumulated in the crystal fraction after 5 days of incubation. As shown in Figure 3, BCP crystals accumulated significantly more 45Ca during the incubation period than did CPPD crystals. Neither type II collagen nor the combination of type II plus type I collagen significantly affected crystal growth. Surprisingly, in the presence of either type II collagen or type II plus type I collagen, proteoglycans had little effect on BCP or CPPD crystal growth. These findings suggest that the effects of collagens and proteoglycans on ACV mineralization at these concentrations and time points are not primarily due to their effects on crystal growth.
Figure 3.
Effect of type II collagen, type II plus type I collagen, and proteoglycans (PGs) on the growth of calcium pyrophosphate dihydrate (CPPD) and basic calcium phosphate (BCP) crystals. Synthetic CPPD (solid bars) or BCP (hatched bars) crystals were embedded in agarose gels (control) or gels containing type II collagen (CII), type II plus type I collagen (CII + CI), with or without proteoglycans. CPPD crystal–containing gels were incubated with calcifying salt solution (CSS) containing45Ca and pyrophosphate, while BCP crystal–containing gels were incubated with CSS containing45Ca and phosphate. After 5 days of incubation,45Ca in the crystal fraction was measured. Values are the mean and SD (n = 10 samples).
Effect of various concentrations of type I and type II collagen on total mineral formation.
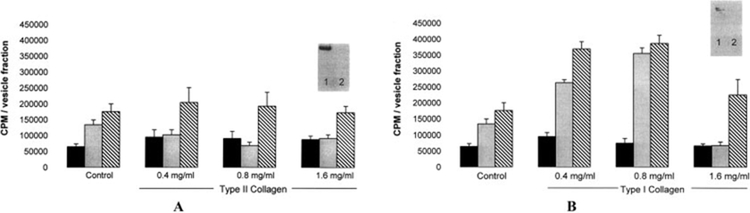
The finding that the effect of type II collagen on ACV mineralization was altered by the addition of small quantities of type I collagen, taken together with the minimal effects of collagens on crystal growth, led us to examine whether collagens had a dose-responsive effect on total mineral formation by ACVs. When ACVs were embedded in various concentrations of type II collagen and incubated with ATP, they produced significantly less mineral (P < 0.003) than when embedded in agarose gels without collagen at all doses tested (Figure 4A). Little or no type II collagen was seen in the freshly isolated ACV fraction by Western blotting (inset in Figure 4A), and this effect was not dose-responsive. In contrast, with β-glycerophosphate as the substrate source, type II collagen had no effect on mineralization. These findings support the inhibitory effect of type II collagen on CPPD crystal formation and confirm the findings in Table 1.
Figure 4.
Effect of various concentrations of type I and type II collagen on mineralization of articular cartilage vesicles (ACVs). ACVs were treated with various doses of type II collagen (A) or type I collagen (B) in45Ca-containing calcifying salt solution, either alone (solid bars), with ATP (shaded bars), or with β-glycerophosphate (hatched bars). Controls included agarose without added collagen.45Ca accumulation was measured by liquid scintigraphy. Values are the mean and SD (n = 6 samples). Type II collagen inhibited calcium pyrophosphate dihydrate (CPPD) crystal formation (P < 0.003), whereas type I collagen stimulated both CPPD and basic calcium phosphate crystal formation (P < 0.001). Levels of type II and type I collagen in ACVs were measured by Western blotting (insets). In the Western blots, lane 1 shows positive controls for type II collagen in A and for type I collagen in B; lane 2 shows ACVs.
Similar experiments were performed with type I collagen. Again, little or no type I collagen was present in ACVs by Western blotting (inset in Figure 4B). ACVs embedded in agarose gels containing type I collagen generated more mineral compared with those embedded in agarose alone (P < 0.001) (Figure 4B). This effect was similar in the presence of ATP or β-glycerophosphate and peaked at 0.8 mg/ml. With higher concentrations, type I collagen inhibited ATP-induced mineralization and had only a modest stimulatory effect on β-glycerophosphate–induced mineralization. These data suggest a significant dose response for type I collagen and a robust increase in total mineral formation of both CPPD and BCP crystals in the presence of low concentrations of type I collagen.
Effect of collagen on activity levels of mineralization-regulating enzymes.
We then investigated whether collagens affected the specific activity levels of mineralization-regulating enzymes present on ACVs. Neither type I nor type II collagen affected the levels of alkaline phosphatase, NTPPPH, pyrophosphatase, or 5′-NT (data not shown).
Characterization of putative collagen-binding and proteoglycan-binding proteins on ACVs.
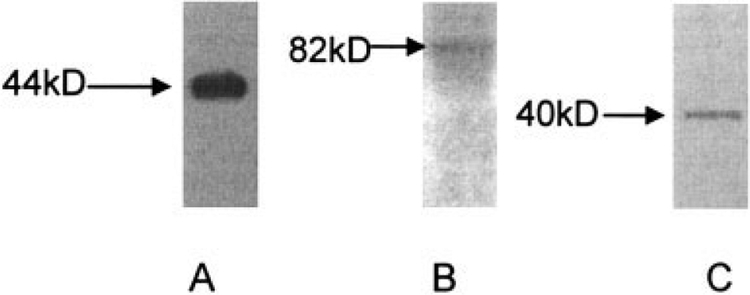
In growth plate matrix vesicles, collagens bind to annexin V, alkaline phosphatase, and link protein (25,26). As shown in Figure 5, Western blotting demonstrated the presence of annexin V, CD44, and link protein on ACVs. Thus, the repertoire of collagen- and proteoglycan-binding proteins in ACVs and growth plate matrix vesicles are quite similar, despite important differences in the actions of collagens and proteoglycans on ACV mineralization compared with growth plate vesicle mineralization.
Figure 5.
Collagen and proteoglycan binding proteins on articular cartilage vesicles (ACVs). Proteins from ACVs were run on sodium dodecyl sulfate–polyacrylamide gel electrophoresis gels and transferred to polyvinylidene difluoride membranes. Western blots were performed with antibodies to annexin V (A), CD44 (B), and link protein (C). Expected molecular weights for these proteins are shown at the left of each blot.
DISCUSSION
Calcium-containing crystals, including CPPD and BCP crystals, are common components of osteoarthritic joints. They contribute to joint damage by directly eliciting catabolic mediators as well as by inducing inflammation (27,28). ACVs contain fully functional mineral-forming machinery capable of producing both CPPD and BCP crystals in vitro and have been postulated to participate in articular CPPD and BCP crystal formation (29). However, their presence in healthy, unmineralized articular cartilage matrix remains unexplained. While mechanisms of vesicle-mediated mineralization have been well studied in growth plate cartilage, very little is known about how and why ACVs mineralize (5).
We show here that extracellular matrix components present in normal and osteoarthritic cartilage modulate ACV mineralization. Specifically, type II collagen, the primary fibrillar collagen in normal articular cartilage matrix, strongly inhibits CPPD crystal formation, probably during the nucleation phase of CPPD crystal development. In contrast, type I collagen, which is found in increased quantities in osteoarthritic cartilage, promotes the formation of CPPD and BCP crystals in combination with type II collagen. Cartilage proteoglycans suppress the stimulatory effect of type II plus type I collagen. Taken together, these findings support the hypothesis that normal cartilage matrix, comprised of type II collagen and proteoglycans, suppresses CPPD crystal formation by ACVs. However, matrix changes in osteoarthritic cartilage, notably, increased levels of type I collagen and loss of proteoglycans, facilitate both CPPD and BCP crystal formation.
Several previous studies addressed the association between ACV mineralization and osteoarthritis. Derfus et al (30) showed that when ACVs were isolated from human osteoarthritic cartilage, their ability to mineralize was not significantly enhanced compared with ACVs isolated from normal cartilage. In an earlier study, Einhorn et al (31) demonstrated increased levels of mineralization-regulating enzymes on ACVs isolated from osteoarthritic cartilage compared with those from normal cartilage. This correlated with a modest increase in mineralization. However, small numbers of samples and significant intersample variability make the study findings difficult to interpret (31).
Little is known about the factors modulating ACV mineralization in cartilage. Growth factors, such as transforming growth factor β, have been shown to increase mineralization by ACVs, likely by stimulating vesicle production by chondrocytes or by altering the phenotype of the chondrocyte toward that of an osteo-blast (32). Our findings suggest that the extracellular matrix milieu is another key regulatory factor controlling ACV mineralization.
In growth plate cartilage, collagens are important regulators of mineralization. In our study, type II collagen suppressed CPPD crystal formation by ACVs. Kirsh and Wuthier (14) showed that type II collagen enhanced growth plate matrix vesicle mineralization in solution. In chicken growth plate, type II collagen was found to be tightly bound to matrix vesicles, and mineralization was reduced when type II collagen was removed. This effect was dependent upon annexin V acting as a collagen-stimulated calcium channel. While similar annexins are present on ACVs, type II collagen has no such effect on ACV mineralization, and little or no type II collagen was found in our ACV fractions. These findings underscore the important differences between growth plate matrix vesicles and vesicles from tissues that are normally unmineralized, such as ACVs.
Type I collagen is a potent stimulant of ACV mineralization in our experience, but it plays a somewhat controversial role in growth plate mineralization. It is not present in human growth plate in significant quantities, but early hydroxyapatite crystals preferentially form along type I collagen fibers in chickens (33). In chicken mesenchymal cell cultures, inclusion of type I collagen–blocking antibodies at certain times during the mineralization process inhibited mineralization (34). Al though it is often considered a template for mineral deposition in other tissues (16), type I collagen had minimal effects on crystal growth in our assay.
Large proteoglycans, such as aggrecan, constitute the bulk of cartilage proteoglycans and are generally considered to be steric inhibitors of hydroxyapatite formation in growth plate cartilage (17,35). Their role in articular cartilage mineralization is less clear, since they are often concentrated in areas of crystal formation (36,37). Indeed, proteoglycans inhibited the stimulatory effect of type I collagen on the formation of calcium-containing crystals, but had little effect in the presence of type II collagen. Few studies have directly examined their interactions with matrix vesicles, yet proteoglycan-associated proteins, such as link protein and hyaluronic acid–binding region, are present in growth plate matrix vesicles (26). We also noted the presence of CD44, an important receptor for hyaluronic acid, on ACVs. The cartilage proteoglycan fraction used in these experiments contained a heterogeneous group of proteoglycans, and further studies using individual proteoglycans are warranted.
The mechanisms of these effects are not clear. It is interesting to us that the inhibitory effect of type II collagen was much stronger for CPPD crystals, whereas type I collagen had similar effects on both BCP and CPPD crystal formation. This suggests a “nonmechanical” effect of fibrillar collagens on ACV mineralization. Receptor-mediated effects are often differentially induced by type I and type II collagen (38), and the dose responsiveness of at least some of these observations supports a possible receptor-mediated mechanism. While collagens may affect activities of mineralization-regulating enzymes, such as alkaline phosphatase, in growth plate matrix vesicles (26), they did not do so in our system. Similarly, type I and type II collagen had no effect on the growth of synthetic crystals under similar conditions and incubation times. It is possible that these matrix components alter the activity of calcium and phosphate transporters, and this potential mechanism warrants further exploration. It is also possible that they interact with other important mediators of matrix mineralization, such as matrix metalloproteases or transglutaminase enzymes.
These studies are not without limitations. The agarose gel system is not a perfect model for the highly structured matrix of cartilage. If these effects were nonspecific, however, then type I and type II collagen would act similarly, and that was not the case. The concentration of collagen or proteoglycan encountered by an individual vesicle is also difficult to estimate.
Clearly, further work on the molecular mechanisms of ACV mineralization is required in order to fully understand how matrix components alter these processes. Furthermore, while few differences in human and porcine ACVs have been noted (8), studies of ACVs from normal human and osteoarthritic cartilage using a similar model are currently under way in our laboratory.
In summary, we show here that extracellular matrix components, such as collagen and proteoglycans, regulate the ability of ACVs to mineralize and most likely affect the nucleation of crystals rather than their growth. Changes in the composition of cartilage extra-cellular matrix with osteoarthritis may promote CPPD and BCP crystal formation by ACVs and contribute to the strong association between pathologic cartilage mineralization and osteoarthritis.
ACKNOWLEDGMENTS
We thank Lawrence M. Ryan, MD, for expert editorial and scientific advice. We appreciate the kind gift of BCP crystals from Dr. Neil Mandel, PhD. We also appreciate the use of the facilities at the Synchrotron Radiation Center and the University of Wisconsin–Madison.
Dr. Rosenthal’s work was supported by NIH grant R01-AR-052615. The facilities at the Synchrotron Radiation Center were funded by National Science Foundation award DMR-0537588.
REFERENCES
- 1.Yelin E The economics of osteoarthritis In: Brandt K, Doherty M, Lohmander L, editors. Osteoarthritis. 2nd ed Oxford: Oxford University Press; 2003. p. 17–21. [Google Scholar]
- 2.Derfus BA, Kurian JB, Butler JJ, Daft LJ, Carrera GF, Ryan LM, et al. The high prevalence of pathologic calcium crystals in pre-operative knees. J Rheumatol 2002;29:570–4. [PubMed] [Google Scholar]
- 3.Nalbant S, Martinez JA, Kitumnuaypong T, Clayburne G, Sieck M, Schumacher H Jr. Synovial fluid features and their relations to osteoarthritis severity: new findings from sequential studies. Osteoarthritis Cartilage 2003;11:50–4. [DOI] [PubMed] [Google Scholar]
- 4.Rosenthal A, Ryan L. Crystals and osteoarthritis In: Brandt K, Doherty M, Lohmander L, editors. Osteoarthritis. 2nd ed Oxford: Oxford University Press; 2003. p. 120–5. [Google Scholar]
- 5.Anderson HC. Matrix vesicles and calcification. Curr Rheumatol Rep 2003;5:222–6. [DOI] [PubMed] [Google Scholar]
- 6.Ali S, Griffiths S. Formation of calcium phosphate crystals in normal and osteoarthritic cartilage. Ann Rheum Dis 1983;42 Suppl 1:45–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Derfus BA, Rachow JW, Mandel NS, Boskey AL, Buday M, Kushnaryov VM, et al. Articular cartilage vesicles generate calcium pyrophosphate dihydrate–like crystals in vitro. Arthritis Rheum 1992;35:231–40. [DOI] [PubMed] [Google Scholar]
- 8.Derfus BA, Kurtin SM, Camacho NP, Kurup I, Ryan LM. Comparison of matrix vesicles derived from normal and osteoarthritic human articular cartilage. Connect Tissue Res 1996;35: 391–6. [DOI] [PubMed] [Google Scholar]
- 9.Russell R, Bisaz S, Fleish H. Inorganic pyrophosphate in plasma, urine and synovial fluid of patients with pyrophosphate arthropathy (chondrocalcinosis or pseudogout). Lancet 1970;296: 895–902. [DOI] [PubMed] [Google Scholar]
- 10.Ryan L, Rachow J, McCarty D. Synovial fluid ATP: a potential substrate for the production of inorganic pyrophosphate. J Rheumatol 1991;18:716–20. [PubMed] [Google Scholar]
- 11.Pritzker M, Chateauvert J, Gympas M. Osteoarthritic cartilage contains increased calcium, magnesium, and phosphorus. J Rheumatol 1987;14:806–10. [PubMed] [Google Scholar]
- 12.Graff RD, Lazarowksi ER, Banes AJ, Lee GM. ATP release by mechanically loaded porcine chondrons in pellet culture. Arthritis Rheum 2000;43:1571–9. [DOI] [PubMed] [Google Scholar]
- 13.Boskey A, Boyan B, Schwartz Z. Matrix vesicles promote mineralization in a gelatin gel. Calcif Tissue Int 1997;60:309–15. [DOI] [PubMed] [Google Scholar]
- 14.Kirsch T, Wuthier RE. Stimulation of calcification of growth plate cartilage matrix vesicles by binding to type II and X collagens. J Biol Chem 1994;269:11462–9. [PubMed] [Google Scholar]
- 15.Anderson H Vesicles associated with calcification in the matrix of epiphyseal cartilage. J Cell Biol 1969;41:59–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Glimcher M Mechanisms of calcification in bone: role of collagen fibrils and collagen-phosphoprotein complexes in vitro and in vivo. Anat Rec 1989;224:139–53. [DOI] [PubMed] [Google Scholar]
- 17.Chen CC, Boskey A, Rosenberg L. The inhibitory effect of cartilage proteoglycans on hydroxyapatite growth. Calcif Tissue Int 1984;36:285–90. [DOI] [PubMed] [Google Scholar]
- 18.Heinegard D, Bayliss B, Lorenzo P. Articular cartilage: biochemistry and metabolism of normal and osteoarthritic cartilage In: Brandt K, Doherty M, Lohmander L, editors. Osteoarthritis. 2nd ed Oxford: Oxford University Press; 2003. p. 73–82. [Google Scholar]
- 19.Shakabei M, Abour-Rebyeh H, Merker HJ. Integrins in aging cartilage tissue in vitro. Histol Histopathol 1993;8:715–23. [PubMed] [Google Scholar]
- 20.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with Folin phenol reagent. J Biol Chem 1951;193: 265–75. [PubMed] [Google Scholar]
- 21.Rosenthal AK, Cheung HS, Ryan LM. Transforming growth factor [H9252]1 stimulates inorganic pyrophosphate elaboration by porcine cartilage. Arthritis Rheum 1991;34:904–11. [DOI] [PubMed] [Google Scholar]
- 22.Arkesteijn C A kinetic method for serum 5[H11032]-nucleotidase using stabilised glutamate dehydrogenase. J Clin Chem Clin Biochem 1976;14:155–8. [DOI] [PubMed] [Google Scholar]
- 23.Sugino Y, Miyoshi Y. The specific precipitation of orthophosphate and some biochemical applications. J Biol Chem 1964;239: 2360–72. [PubMed] [Google Scholar]
- 24.Brown E, Lehr J, Smith A. Preparation and characterization of some calcium pyrophosphates. J Agric Food Chem 1963;11: 214–22. [Google Scholar]
- 25.Wu L, Genge B, Wuthier R. Association between proteoglycans and matrix vesicles in the extracellular matrix of growth plate cartilage. J Biol Chem 1991;266:1187–94. [PubMed] [Google Scholar]
- 26.Wu L, Genge B, Lloyd G, Wuthier R. Collagen-binding proteins in collagenase-released matrix vesicles from cartilage. J Biol Chem 1991;266:1195–203. [PubMed] [Google Scholar]
- 27.McCarthy G, Augustine J, Baldwin A, Christopherson P, Cheung H, Westfall P, et al. Molecular mechanisms of basic calcium phosphate crystal-induced activation of human fibroblasts: role of nuclear factor [H9252], activator protein 1, and protein kinase C. J Biol Chem 1998;273:35161–9. [DOI] [PubMed] [Google Scholar]
- 28.Terkeltaub R and Pathogenesis and treatment of crystal-induced inflammation In: Koopman WJ, Moreland LW, editors. Arthritis and allied conditions: a textbook of rheumatology. 15th ed Philadelphia: Lippincott Williams & Wilkins; 2005. p. 2357–72. [Google Scholar]
- 29.Ali S Apatite-type crystal deposition in arthritic cartilage. Scanning Electron Microsc 1985;4:1555–66. [PubMed] [Google Scholar]
- 30.Derfus B, Kranendonk S, Camacho N, Mandel N, Kushnaryov V, Lynch K, et al. Human osteoarthritic cartilage matrix vesicles generate both calcium pyrophosphate dihydrate and apatite in vitro. Calcif Tissue Int 1998;63:258–62. [DOI] [PubMed] [Google Scholar]
- 31.Einhorn TA, Gordon SL, Siegel SA, Hummel CF, Avitable MJ, Carty RP. Matrix vesicle enzymes in human osteoarthritis. J Orthop Res 1985;3:160–9. [DOI] [PubMed] [Google Scholar]
- 32.Derfus BA, Camacho NP, Olmez U, Kushnaryov VM, Westfall PR, Ryan LM, et al. Transforming growth factor [H9252]-1 stimulates articular chondrocyte elaboration of matrix vesicles capable of greater calcium pyrophosphate precipitation. Osteoarthritis Cartilage 2001;9:189–94. [DOI] [PubMed] [Google Scholar]
- 33.Boskey A, Camacho N, Mendelsohn R, Doty S, Binderman I. FT-IR microscopic mappings of early mineralization in chick limb bud mesenchymal cell cultures. Calcif Tissue Int 1992;51:443–8. [DOI] [PubMed] [Google Scholar]
- 34.Boskey A, Stiner D, Binderman I, Doty S. Type I collagen influences cartilage calcification: an immunoblocking study in differentiating chick limb-bud mesenchymal cell cultures. J Cell Biochem 2000;79:89–102. [DOI] [PubMed] [Google Scholar]
- 35.Chen C, Boskey A. Mechanisms of proteoglycan inhibition of hydroxyapatite crystal growth. Calcif Tissue Int 1985;37:395–400. [DOI] [PubMed] [Google Scholar]
- 36.Boskey A, Bullough P. Cartilage calcification: normal and aberrant. Scanning Electron Microsc 1984;11:943–52. [PubMed] [Google Scholar]
- 37.Ohira T, Ishikawa K. Hydroxyapatite deposition in articular cartilage by intra-articular injections of methylprednisolone: a histological, ultrastructural, and x-ray-microprobe analysis in rabbits. J Bone Joint Surg Am 1986;68:509–19. [PubMed] [Google Scholar]
- 38.Tulla M, Pentikainen O, Viitasalo T, Kapyla J, Imola U, Nykvist P, et al. Selective binding of collagen subtypes by integrin α1I, α2I, and α10I domains. J Biol Chem 2001;276:48206–12. [DOI] [PubMed] [Google Scholar]