Abstract
PIK3CA-related overgrowth spectrum (PROS) refers to a group of disorders of segmental overgrowth of a wide variety of tissues as well as venous and lymphatic malformations. Clinical and molecular diagnosis can be challenging due to phenotypic heterogeneity and difficulties detecting low-level mosaicism using standard methods. Here, we report a patient with a severe presentation of PIK3CA-related overgrowth with analysis of 27 posthumously collected tissues by droplet digital PCR at autopsy. This patient had a complicated medical course, with coagulopathy, ischemic brain injury, and sepsis resulting in multi-organ failure and death at age 2 months despite sirolimus therapy. Five of the 27 tissues analyzed possesed a mosaic PIK3CA mutation (p.E545K), with mutation levels ranging from 3–20% across affected tissues. We found no correlation between tissue-specific disease severity and mutation levels, likely reflecting sampling limitations. We also tested a series of 22 individuals with somatic overgrowth and/or vascular-lymphatic malformations using a targeted next generation sequencing panel and found PIK3CA mutations in 9 individuals, identifying three novel PIK3CA variants. This report expands the clinical and molecular spectrum of PROS, emphasizes that different molecular methods can be complimentary in the diagnosis of these disorders, and highlights the risk of coagulopathy in a subset of patients with PIK3CA-related overgrowth.
Keywords: PIK3CA, ddPCR, Mosaicism, Overgrowth, Vascular Malformation
INTRODUCTION
PIK3CA-related overgrowth spectrum (PROS) refers to a group of disorders of segmental overgrowth caused by activating mutations in phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit alpha (PIK3CA). (Keppler-Noreuil et al., 2014; Riviere et al., 2012) PROS is an umbrella term that includes several diagnostic entities, including Congenital Lipomatous Overgrowth, Vascular malformations, Epidermal nevi, Scoliosis/skeletal and spinal (CLOVES) syndrome (OMIM #612918), Klippel-Trenaunay syndrome (KTS) (OMIM %149000), and the megalencephaly-capillary malformation syndrome (MCAP) (OMIM #602501). Several of these entities were independently described prior to recognition that PIK3CA mutations were common to all. (Keppler-Noreuil et al., 2014; Mirzaa et al., 2016) The mutations that cause PROS are typically, but not always, post-zygotic, meaning that the mutation is not present in every cell in the body. The resulting mosaicism produces some of the phenotypic variation, and makes molecular diagnosis challenging due to difficulties obtaining affected tissues, and detecting mutations with very low alternate allele frequencies (AAFs). Methods such as ultra-deep sequencing and unique molecular identifiers (UMI) have been used to detect low AAF variants, but these methods are more expensive and technically challenging. (Kuentz et al., 2017)
Recently, we and others have used droplet digitial PCR (ddPCR), an allele-specific method for detection of PIK3CA variants in patients with suspected PROS. (Luks et al., 2015) This method can be particularly effective for PROS because disease-causing mutations are concentrated among 3 residues, known as mutational “hot spots,” namely residues p.E542, p.E545, and p.H1047. Mutations in one of these 3 residues have been detected in tissues from >90% of patients with CLOVES syndrome, (Keppler-Noreuil et al., 2014) and 80–90% of patients with isolated lymphatic malformations, (Luks et al., 2015) making targeted analysis of these specific hotspots an efficient method for screening PROS-causing mutations. However, pathogenic PIK3CA mutations outside of the three hotspot residues have been identified, particulalry in patients with brain overgrowth (i.e. megalencephaly as seen with MCAP syndrome), in which only 10–15% of patients with disease causing muations have hotspot mutations. (Kuentz et al., 2017; Mirzaa et al., 2016) This observation has led to the hypothesis that the three hotspot mutations, which activate PI3K kinase activity to the highest levels of any activating PIK3CA mutations, (Gymnopoulos, Elsliger, & Vogt, 2007) produce fetal lethality when present more diffusely in the brain and other somatic tissues. Supporting this hypothesis is the observation that, of the hundreds of reported patients with PROS, very few patients with megalencephaly and hotspot PIK3CA mutations have been reported. (Keppler-Noreuil et al., 2014; Kuentz et al., 2017; Mirzaa et al., 2016)
Here, we report a severely affected PROS patient with widespread overgrowth due to a hotspot (p.E545K) PIK3CA mutation. Levels of PIK3CA mutation were determined by ddPCR analysis in 27 tissues taken at autopsy, providing a global picture of the mutation distribution across the entire body. Interestingly there was no evidence for a correlation between tissue-specific disease severity and mutation levels within tissues. In addition to detailed analysis of this patient, we also report clinical diagnostic results of PIK3CA sequencing in 22 patients with vascular malformations and somatic overgrowth. Pathogenic PIK3CA mutations were identified in 9 patients and include three novel variants. Several non-hotspot mutations were identified, emphasizing the importance of full gene sequencing in the diagnostic workup of PROS patients.
MATERIALS AND METHODS
Human subjects.
Patient LR16–397 was recruited to the Developmental Brain Disorders Research Program at Seattle Children’s Research Institute with informed consent. Individuals in the vascular malformation cohort were identified via retrospective review of all patients referred to the University of Washington’s Department of Laboratory Medicine over a two year period (May 2015-May 2017) for Megaplex™ gene panel sequencing. Only patients with testing indication was overgrowth or vascular malformation were included. Patients who had testing during this period for megalencephaly or primary brain overgrowth were specifically excluded from the cohort. Clinical information about each patient was obtained from the referring provider. Efforts were made to obtain “affected” tissue, when available. DNA tested was derived from fresh affected tissues, fibroblast culture of affected biopsies, blood, and formalin fixed paraffin embedded samples.
Sample processing and ddPCR testing.
Genomic DNA was extracted from frozen tissue samples taken at autopsy using standard protocols. 10 ng of DNA was used per ddPCR reaction, using previously described methods. (Hindson et al., 2011; Luks et al., 2015) Four independent replicates were peformed from each tissue except for brain tissue, which was done in triplicate. Analysis was performed using the QuantaSoft software. Wells with <14,000 total droplets were excluded from analysis. Mutant (FAM) and wild-type (HEX) droplet thresholds were established using positive and negative controls included in each run: wild-type DNA (0% AAF) and mutation positive dilution series to produce 25% AAF and 1.5% AAF, as well as a no-template control. Alternate-allele frequency is calculated as the percentage of mutant-positive droplets divided by the total amount of DNA-containing droplets. Multiple wells were merged for analysis and Poisson confidence intervals defined using QuantaSoft software (Bio-Rad, Hercules, CA, USA). Samples were deemed “positive” when the 95% Poisson confidence intervals did not overlap the wild-type negative control. (Figure 2). Although some samples showed a few positive droplets (Supplementary Table 1 and Supplementary Figure 2), when the 95% confidence intervals overlapped wild-type these samples were deemed negative. Additional statistical analysis performed in R-Studio (Boston, MA, USA).
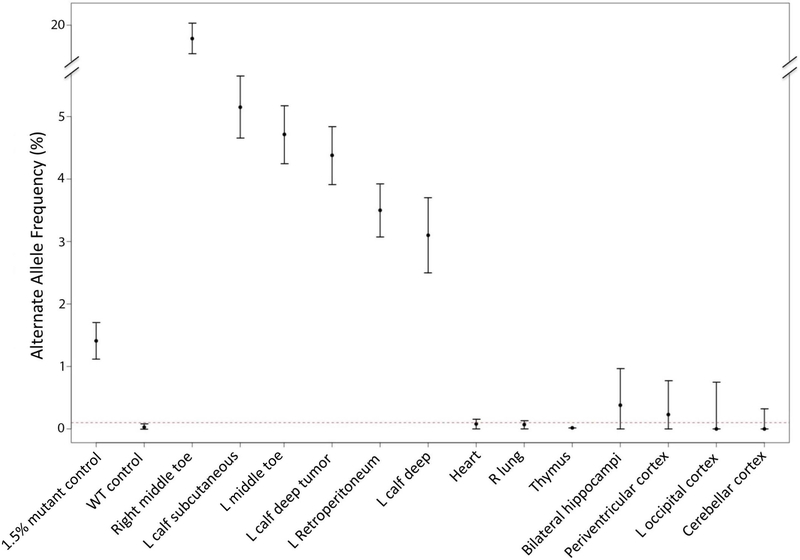
Figure 2. Alternative Allele Fraction of PIK3CA p.E545K across multiple tissues in a single patient:
Y axis represents AAF. 95% Poisson confidence intervals represented as vertical lines. 1.5% AAF mutant positive control as well as wild type (0% AAF) negative control indicated, along with 13 other patient specimens. Not all tissues tested in this study are represented in this figure. The dotted red line shows the top of the 95% confidence interval for the wild-type specimen; samples whose confidence interval crossed this boundary were deemed mutation negative. The Y- axis is broken to show the AAF from the right toe (~20%) in the same table.
Targeted NGS Megaplex™ Assay.
This assay sequences all exons of PIK3CA as well 32 other genes (Supplementary Table 2). Per sample coverage ranged from to 240 to greater than 1400 sequencing reads per bp, with an average coverage of 647. Genomic regions were captured using biotinylated RNA oligonucleotides (SureSelect) prepared in pared-end libraries with ~200-bp insert size, and sequernced on an Illumina HiSeq2500 with 100-bp read lengths, in a modification of previously published pipeline. (Mirzaa et al., 2016; Pritchard et al., 2012)
CASE REPORT
Clinical History:
This male infant was the first born child to unrelated parents of Caucasian ancestry. Fetal ultrasound at 20 weeks of gestation identified overgrowth involving the left leg and left side of the trunk. He was born at 38+6 weeks of gestation via vaginal delivery. He was small for gestational age, with a birth weight of 2.43 kg (3rd percentile for age and gender), and microcephalic with birth head circumference 31.5 cm (3 standard deviations below the mean for age and gender). Delivery was complicated by poor respiratory effort and poor tone, requiring continuous positive airway pressure (CPAP), with an arterial cord blood pH of 6.9. Initial physical examination revealed multiple abnormalities, most notably an extensive vascular-lymphatic malformation involving the left leg, scrotum, and abdomen causing markedly asymmetric overgrowth (Figure 1). The right leg was also segmentally overgrown with digital enlargement of toes 2–4, but without an overlying vascular malformation (Figure 1J,K). Other than a unilateral low-set left ear, no dysmorphic features were noted.
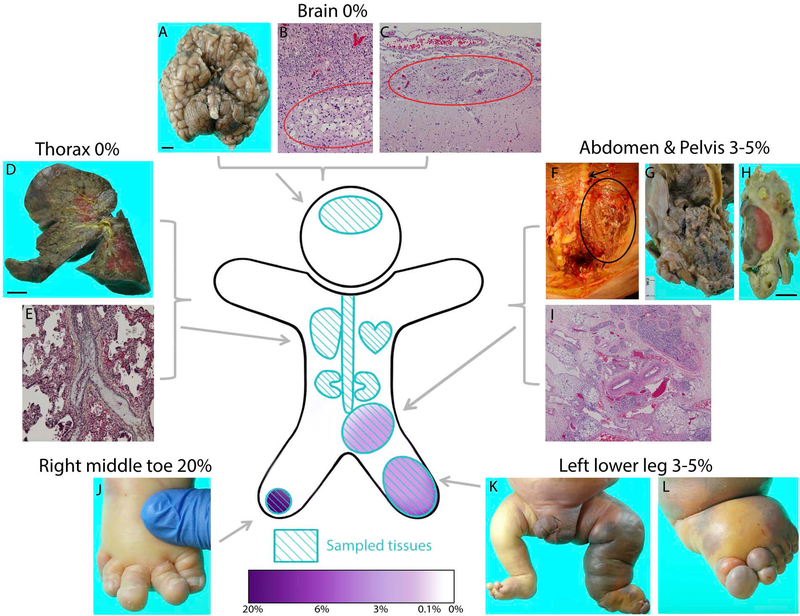
Figure 1. PIK3CA p.E545K mutation levels and autopsy findings:
Black scale bars correspond to 1 cm. Tissues sampled for ddPCR testing (light blue) and presence of PIK3CA muation (purple) represented in cartoon figure. Not all sampled tissues are pictured. A: external gross brain anatomy indicates normal development except for generalized cerebellar atrophy. Brain size smaller than average (390 gm). B: Periventricular leukomalacia (circle). C: Leptomeningeal glioneuronal heterotopia (circle) near R thalamus. D: Right lung demonstrating chronic neonatal lung disease and E: remote thromboembolus (central vessel with luminal fibrous occlusion). F: Pelvic and retroperitoneal extension of lymphatic malformation (circle) near spine (arrow) G: Posterior view of vascular malformation near bifurcation of abdominal aorta, encasing left common iliac. H: Vascular malformation surrounding left kidney. I: The rectum is surrounded by loose fibrous tissue containing variably-sized vascular spaces and small islands of adipose and lymphoid tissue with hemorrhage/congestion. J: Right foot exhibiting macrodactyly of toes two, three, and four. K: Significant overgrowth and dusky, violaceous discoloration seen in left leg. L: Left foot exhibiting overgrowth, edema, and discoloration.
Magnetic resonance imaging (MRI) demonstrated a large, thinly septated, fluid filled mass within the left retroperitoneum, extending from the diaphragm to the pelvic floor and involving the left lower extremity and scrotum. This mass displaced the left kidney, distal aorta, inferior vena cava, and bladder. Macro and micro-cystic features were noted, as was a persistent sciatic vein in the left leg.
His medical course was complicated by abdominal distension and fluid overload, hepatomegaly with ascites and cholestatic liver dysfunction, transfusion dependent thrombocytopenia/anemia/neutropenia, consumptive coagulopathy with a non-occlusive right internal jugular thrombus, recurrent E. coli sepsis/possible meningitis, sick euthyroid syndrome, acute kidney injury, total parenteral nutrition dependency, and gastrointestinal (GI) bleeding. Brain MRI at age one week demonstrated ischemia and acute infarcts in the central grey and white matter (more prominent on the left side), with periventricular and thalamic infarcts and evidence of deep cerebral vein thrombosis . Brain MRI at 1 month of age showed evolving cystic encephalomalacia with ex vacuo dilation of lateral and third ventricles (Supplementary Figure 2), with foci of restricted diffusion in the bilateral frontal white matter indicating new infarcts.
Treatment with low-dose (0.2 mg/m2/dose) sirolimus was initiated at 25 days of life after blood cultures were conclusively negative; however, no appreciable effect was observed during the 37 days of treatment.
At age 40 days, E. coli sepsis with respiratory failure and severe ascites recurred, prompting a reconsideration of goals of care. Palliative management was initiated, and at 62 days of age, life support was withdrawn and the child passed away due to multi-organ failure.
Autopsy findings:
Autopsy confirmed the presence of a large, predominantly microcystic vascular malformation of the left lower extremity infiltrating the left retroperitoneum, left inguinal canal, and pelvis, partially encasing and displacing the spleen, distal colon, rectum, bladder, left kidney, left testes and left internal/external iliac arteries (Figure 1). This malformation consisted of superficial capillary and both superficial and deep lymphatic and venous structures, with several large cystic fibrinopurulent abcesses and foci of extramedullary hematopoeisis. There was evidence of platelet and clotting factor sequestration with fibrinous attachments between the cystic mass and adjacent abdominal organs. The heart, liver, and spleen were enlarged (Supplemental Text). Histologic appearance of the lungs showed mild bronchopulmonary dysplasia due to chronic mechanical ventilation and evidence of remote pulmonary thromboemboli (Figure 1E). Examination of the liver showed diffuse hepatic injury secondary to hyperalimentation and endotoxin-related sepsis, as well as some ischemic injury.
Histologic examination of the brain confirmed the presence of bilateral severe diffuse periventricular leukomalacia, as well as neuronal depletion and gliosis, attributed to remote hypoxia/ischemia (Figure 1B). Although antemortem imaging suggested venous thrombosis, no thrombotic foci were identified. The meninges appeared hypercellular and fibrotic, possibly related to remote hemorrhage but without evidence of acute or chronic meningitis. There were a few areas in the brain and spinal cord suspicious for focal abnormalties not necessarily secondary to brain injury, but these were subtle. These included a focal area of possible perithalamic leptomeningeal glioneural heterotopia (Figure 1C), unilateral hippocampal gyral dysplasia, and some evidence of dysplastic neurons and prominent arterioles within sections of hypertrophic lumbosacral spinal cord. No vascular malformation was identified in the brain or spinal cord.
Molecular Results:
27 tissues from this patient were tested for the following hotspot PIK3CA mutations: p. E542K, p. E545K, p.H1047L and p.H1047R. A PIK3CA mutation (p.E545K) was identified in five tissues (Figure 2, Supplementary Table 1). Mutation levels in positive samples ranged from 3% to 20% AAF. (Figure 2). The subcutaneous tissues of the left calf and toe, and deeper tissues of the left calf and left retroperitoneum represented the most severely affected tissues of the body (Figure 1), with no significant difference between mutation levels in these tissues (Figure 2). The tissue with the highest detected AAF (20%) was the macrodactylous third toe of the right foot (Figure 1J). Although small numbers of mutation positive droplets were identified in other tissues tested (Supplementary Figure 1), none of these tissues were significantly different from wild-type control DNA (Figure 2).
OVERGROWTH-VASCULAR MALFORMATION COHORT
We retrospectively reviewed all patients referred to the University of Washington for Megaplex™ panel testing over a 2 year period. The Megaplex™ is a targetted NGS assay that sequences 37 genes implicated in overgrowth and vascular malformations (Supplemental Text). Only patients with the clinical indication of somatic overgrowth or vascular malformation were included; patients with brain overgrowth were specifically excluded. A total of 22 individuals met these criteria, with age at time of testing ranging from 21 days to 53 years (Table 1). We identified nine mutation-positive individuals (41%) with pathogenic PIK3CA mutations, three of which (p.K111_N114del, Q546R, and N1044S) have not previously been reported in PROS (Table 1). None of these variants are present at any frequency within EXAC or gnomAD (exac.broadinstitute.org/, gnomad.broadinstitute.org/). The glutamine at position 546 and the asparagine at position 1044 are highly evolutionarily conserved, and mutations at both of these sites are present in tumors within the catalogue of somatic mutations in cancer (COSMIC) database. The p.N1044S substitution was present at higher AAF in affected tissue (40%) than in unaffected tissue (7%), and has been reported as pathogenic by another clinical lab in ClinVar. The p.K111_N114del in-frame deletion overlaps a missense variant (p.I112N) reported as pathogenic in a patient with megalencephaly and is supported by functional evidence. (Di Donato et al., 2016) Overall AAF values for mutations ranged from 1.9% to 50%, with an average value of 18%. In all nine mutation positive individuals “affected” tissue was submitted for sequencing, five of whom had undetectable mutation levels in paired blood-derived DNA. For five of the thirteeen patients in which a PIK3CA mutation was not detected, only blood was available for testing. In all samples, the per base pair coverage of PIK3CA was >200X, with most samples (78%) having >400X coverage. Four patients had hotspot mutations. In one patient (#6) the PIK3CA AAF was 50%, suggesting the possibility of a non-mosaic mutation, but non-lesional tissue (e.g. blood) was not available to prove this. No mutations were identified in the other 32 genes within the Megaplex™ panel in these 22 patients.
TABLE 1:
PIK3CA mutations identified in Vascular Malformation/Somatic Overgrowth Cohort
| Patient # | Diagnosis | Gender | Age | Genomic position (hg19) | cDNA (NM_006218.2) | Amino acid change | Previously reported? | Tissue | AAF | Average Coverage |
|---|---|---|---|---|---|---|---|---|---|---|
| VAN_1 | CapM, asymmetric OVG (lower limbs, abdomen) | M | 44 days | chr3:178928079 | c.1357G>A | E453K | PMID 24374682, 25557259 | FB | 0.21 | 1228 |
| Blood | 0 | 1433 | ||||||||
| VAN_2 | CLOVES | F | 3 yrs | chr3:178936095 | c.1637A>G | Q546R | novel | FB | 0.03 | 354 |
| Blood | 0 | 422 | ||||||||
| VAN_3 | facial OVG (soft tissue, bone, skin, parotid) and sebaceous nevus | F | 45 yrs | 3:178936082 | c.1624G>A | E542K | hotspot | Affected tissue | 0.27 | 836 |
| Blood | 0 | 891 | ||||||||
| VAN_4 | L hemihypertophy, facial OVG with neck mass, asymmetric macroglossia, exostoses | M | 23 months | 3:178952085 | c.3140A>G | H1047R | hotspot | FFPE (tonsil) | 0.02 | 253 |
| blood | 0 | 795 | ||||||||
| VAN_5 | VLM of anterior mediastinum and BL upper arms | M | 1 yr | 3:178936082 | c.1624G>A | E542K | hotspot | FFPE (VLM lesion) | 0.019 | 1070 |
| VAN_6 | OVG (scapular) | M | 4 yrs | chr3:178916945-178916956 | c.332_343del | K111_N114del | novel | FFPE (scapular mass) | 0.5 | 608 |
| FFPE (scapular mass) | 0.5 | 937 | ||||||||
| VAN_7 | L Hemihypertrophy, unspecified vascular malformation | F | 10 yrs | chr3:178952076 | c.3131A>G | N1044S | novel | Affected tissue | 0.4 | 406 |
| Unaffected tissue | 0.07 | 522 | ||||||||
| VAN_8 | Macrodactyly, cystic lung lesion, lipoma gastric fundus and segmental OVG | M | 53 yrs | 3:178936082 | c.1624G>A | E542K | hotspot | Affected tissue | 0.17 | 461 |
| E542K | Blood | 0 | 241 | |||||||
| VAN_9 | CILF | F | 8 yrs | chr3:178928079 | c.1357G>A | E453K | PMID 24374682, 25557259 | Affected tissue | 0.09 | 641 |
| VAN_10 | venous malformation R hand, infantile hemangioma, epilepsy with cortical dysplasia | M | 4 yrs | – | – | – | – | ND | NA | 306 |
| VAN_11 | Congenital intestinal lymphangiectasia | F | 2 yrs | – | – | – | – | Retroperitoneal soft tissue | NA | 697 |
| VAN_12 | Lymphatic malformation R pelvis and thigh. Possible Kaposiform lymphangiomatosis |
F | 11 yrs | – | – | – | – | Blood | NA | 432 |
| VAN_13 | Vascular malformation, predominantly capillary, L thigh | M | 16 yrs | – | – | – | – | FB | NA | 272 |
| VAN_14 | MLT | M | 17 yrs | – | – | – | – | Blood | NA | 1118 |
| VAN_15 | VLM chest and abdomen | M | 5 yrs | – | – | – | – | Blood | NA | 1268 |
| VAN_16 | VLM head and neck | F | ND | – | – | – | – | FB | NA | 530 |
| VAN_17 | CLOVES | F | 12 | – | – | – | – | FB | NA | 677 |
| VAN_18 | Macrocystic LM | F | 8 yrs | – | – | – | – | FFPE (LM lesion) | NA | 271 |
| VAN_19 | CapM, very mild leg hemihypertrophy (R>L), ASD and VSD | M | 21 days | – | – | – | – | Blood | NA | 432 |
| – | – | – | – | FFPE (capM) | NA | 254 | ||||
| VAN_20 | CLOVES | F | 23 months | – | – | – | – | Skin (unaffected side of body) | NA | 738 |
| – | – | – | – | FB (unaffected side of body) | NA | 853 | ||||
| – | – | – | – | Blood | NA | 418 | ||||
| VAN_21 | VLM L arm | F | 15 yrs | – | – | – | – | Blood | NA | 1291 |
| VAN_22 | unspecified OVG | F | 14 months | – | – | – | – | Blood | NA | 944 |
Abbreviations: AAF (alternate allele frequency), ASD (atrioseptal defect), CapM (Capillary Malformation), CILF (congenital inflitrating lipomatosis of the face), CLOVES (congenital lipomatous overgrowth with vascular abnormaltieis, epidermal nevi and skeletal/spinal abnormalities), FB (fibroblast), MLT (multifocal lymphangioendotheliomatosis with thrombocytopenia), OVG (overgrowth), VLM (mixed venolymphatic malformation), VSD (ventriculosepatal defect).
DISCUSSION
PIK3CA-related overgrowth spectrum autopsy patient.
Here we report a severely affected patient with widespread overgrowth due to a hotspot (p.E545K) PIK3CA mutation. This patient succumbed to multi-organ failure after a complicated medical course that included coagulopathy, ischemic brain injury, and sepsis. There was some suggestion of hippocampal dysplasia and glioneuronal heterotopia at autopsy, although these findings were subtle and not supported by molecular analysis, which showed no evidence of the p.E545K PIK3CA mutation within 10 samples from the brain and spinal cord (Figure 2 and Supplemental Table 1). The brain findings are intriguing, however, as heterotopias and hippocampal dysplasia have been identified in individuals with PI3K-AKT-MTOR related disorders. (Alcantara et al., 2017; Mirzaa et al., 2016) Thus, this patient represents an atypical example of brain and body involvement due to a PIK3CA hotspot mutation. Notably, among hundreds of reported patients with PROS, (Keppler-Noreuil et al., 2014; Kuentz et al., 2017; Mirzaa et al., 2016), only two (LR12–033 in Mirzaa, 2016 and patient #33 in Keppler, 2014) have had features of both CLOVES and megalencephaly. It is also possible that the brain findings are secondary to this patient’s medical course (e.g. ischemic injury) or are incidental developmental anomalies.
This case also emphasizes the risk of coagulopathy in PROS. Pulmonary embolism, spinal thrombosis, and neonatal cerebral infarcts have been reported in patients with PROS. (Alomari, 2009; Keppler-Noreuil et al., 2015) Our patient had evidence of platelet sequestration and fibrous organization within the abdomen and pulmonary embolism (Supplemental Text). This coagulopathy may have been secondary to the presence of sepsis in this patient, but the underlying genetic condition, and the presence of the large microcystic vascular malformation, may also have been contributing factors. Although the magnitude of the risk of coagulopathy is uncertain at present, we recommend that patients with more severe PROS be evaluated by hematology, particularly in the neonatal or perioperative periods when there is already increased predisposition to coagulopathy.
The most severely affected tissues in LR16–397 had mutation levels of only 3–5% AAF, while the more mildly affected right leg had the highest level of detected PIK3CA mutation (20% AAF). A lack of correlation of disease severity and alternative allele frequency across a large group of patients with PROS has been previously reported, (Keppler-Noreuil et al., 2015) but this is the first report of a lack of correlation among multiple tissues within a single patient. There are several possible explanations for this. The severe disease processes occuring in the left leg may have recruited non-mutant cells, driving the mutation levels down. Another possibility is non-cell autonomous signaling between mutant and non-mutant cells within the left leg. (Baek et al., 2015; Enomoto, Vaughen, & Igaki, 2015) However, the most likely explanation is simply limitations of tissue sampling. More widespread sampling of tissues, in particular the unaffected right leg of this patient, could be helpful in exploring these possibilities. Unfortunately no samples were available from this part of the body.
Vascular Anomaly and Somatic Overgrowth Cohort.
In addition to detailed analysis of this single patient, we also report clinical diagnostic results of PIK3CA sequencing in 22 patients with vascular malformations and somatic overgrowth. Pathogenic PIK3CA mutations in were identified in 9 patients across a wide range of AAFs (1.9% to 50%). The difference in the average AAF among non-hotspot mutations (25%) not significantly different (Welch Two Sample t-test) than that of hotspot mutations (12%), though the sample size is small (n=9).
We identified three novel PIK3CA variants (p.K111_N114del, Q546R, and N1044S) in our cohort. The p.K111_N114del in frame deletion is located in the linker region of PIK3CA that mediates binding with PIK3R2, a negative regulator of the PI3K lipid kinase. Interestingly, both this in-frame deletion, and an overlapping pathogenicv missense variant (p.I112N) appear to be present at non-mosaic (i.e. constitutional) levels. We hypothesize that mutations that disrupt the stochiometry of PIK3R2-PIK3CA binding may produce milder PI3K activation that does not produce embryonic lethality when present in the non-mosaic state. Analysis of additional, non-lesional tissue from this patient (VAN_6) is needed to prove that this variant is indeed constitutional.
In addition to these novel variants, we identified one previously reported PIK3CA variant (p.E543K) in two unrelated patients, as well as hotspot mutations in four patients. Although ddPCR is a highly sensitive and efficient method for the identification of PIK3CA variants, it is an allele-specific method and would have missed over half (5/9) of the detected PIK3CA mutations. In our cohort, mutations were never identified from blood derived DNA, even at excellent sequencing depths (241–1433 per bp), demonstrating that the negative predictive value of blood based PIK3CA sequencing is nil. Removing the five patients in the cohort (VAN_12,14,15,21, and 22) for which only blood was available for sequencing gives an overall diagnostic yield in this cohort of 53% (9/17), which roughly agrees with that of reports of similar cohorts. (Kuentz et al., 2017) We conclude that full gene sequencing of affected tissues and not blood is the most sensitive method for molecular diagnosis of patients with PROS.
In summary, we report a patient with somatic overgrowth and vascular malformation of the left leg, pelvis and peritoneum due to a hotspot (p.E545K) mutation in PIK3CA. This patient had a particularly severe presentation of PROS, complicated by coagulopathy, ischemic brain injury, and sepsis, which resulted in multi-organ failure and death at age 2 months. In addition to an extensive primarily microcystic lymphatic malformation, pulmonary thrombosis and evidence of platelet sequestration, autopsy findings included some brain abnormalities (heterotopia, hippocampal dysplasia) that may not be entirely secondary to ischemia and infection. Twenty seven tissues collected at autopsy were analyzed by ddPCR and provided a picture of the mutation distribution across multiple tissues (Figure 1). We found no evidence of a correlation between mutation levels and tissue specific disease severity, nor did we identify the presence of PIK3CA mutation within any brain tissue tested. We also tested a series of 22 individuals with somatic overgrowth and/or vascular-lymphatic malformations using a targeted Next Generation Sequencing panel and identified PIK3CA mutations in 53% (9/17) individuals for whom non-blood tissue was available, and identified three novel PIK3CA variants. This data expands the clinical and molecular spectrum of PROS, emphasizes that different molecular methods can be complementary in the diagnosis of these disorders, and highlights the risk of coagulopathy in a subset of patients with PIK3CA-related overgrowth.
Supplementary Material
ACKNOWLEDGEMENTS
We sincerely thank the family of patient LR16–397 for their support of our research. This study was funded by the Seattle Children’s Hospital Guild Funding Focus Award, NIH RO1 NS092772, and NIGMS-ID 905693 (JP); the National Institutes of Neurological Disorders and Stroke (NINDS) grant K08NS092898 and Jordan’s Guardian Angels (GM); and the Burroughs Wellcome Fund Career Award 1014700 for Medical Scientists (JTB). The content is solely the responsibility of the authors, and does not necessarily represent the official views of the National Institutes of Health. The funding sources had no role in the design and conduct of the study, collection, management, analysis and interpretation of the data, preparation, review or approval of the manuscript, or decision to submit the manuscript for publication. We thank Dr. Brian Gordon from the Seattle Children’s Research Institute for providing technical expertise regarding ddPCR, and Dr. Murat Maga for assistance with generating Figure 2.
REFERENCES
- Alcantara D, Timms AE, Gripp K, Baker L, Park K, Collins S, . . . Mirzaa GM (2017). Mutations of AKT3 are associated with a wide spectrum of developmental disorders including extreme megalencephaly. Brain, 140(10), 2610–2622. doi:10.1093/brain/awx203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alomari AI (2009). Characterization of a distinct syndrome that associates complex truncal overgrowth, vascular, and acral anomalies: a descriptive study of 18 cases of CLOVES syndrome. Clin Dysmorphol, 18(1), 1–7. doi:10.1097/MCD.0b013e328317a716 [DOI] [PubMed] [Google Scholar]
- Baek ST, Copeland B, Yun EJ, Kwon SK, Guemez-Gamboa A, Schaffer AE, . . . Gleeson JG (2015). An AKT3-FOXG1-reelin network underlies defective migration in human focal malformations of cortical development. Nat Med, 21(12), 1445–1454. doi:10.1038/nm.3982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Donato N, Rump A, Mirzaa GM, Alcantara D, Oliver A, Schrock E, . . . O’Driscoll M (2016). Identification and Characterization of a Novel Constitutional PIK3CA Mutation in a Child Lacking the Typical Segmental Overgrowth of “PIK3CA-Related Overgrowth Spectrum”. Hum Mutat, 37(3), 242–245. doi:10.1002/humu.22933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enomoto M, Vaughen J, & Igaki T (2015). Non-autonomous overgrowth by oncogenic niche cells: Cellular cooperation and competition in tumorigenesis. Cancer Sci, 106(12), 1651–1658. doi:10.1111/cas.12816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gymnopoulos M, Elsliger MA, & Vogt PK (2007). Rare cancer-specific mutations in PIK3CA show gain of function. Proc Natl Acad Sci U S A, 104(13), 5569–5574. doi:10.1073/pnas.0701005104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hindson BJ, Ness KD, Masquelier DA, Belgrader P, Heredia NJ, Makarewicz AJ, . . . Colston BW (2011). High-throughput droplet digital PCR system for absolute quantitation of DNA copy number. Anal Chem, 83(22), 8604–8610. doi:10.1021/ac202028g [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keppler-Noreuil KM, Rios JJ, Parker VE, Semple RK, Lindhurst MJ, Sapp JC, . . . Biesecker LG (2015). PIK3CA-related overgrowth spectrum (PROS): diagnostic and testing eligibility criteria, differential diagnosis, and evaluation. Am J Med Genet A, 167A(2), 287–295. doi:10.1002/ajmg.a.36836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keppler-Noreuil KM, Sapp JC, Lindhurst MJ, Parker VE, Blumhorst C, Darling T, . . . Biesecker LG (2014). Clinical delineation and natural history of the PIK3CA-related overgrowth spectrum. Am J Med Genet A, 164A(7), 1713–1733. doi:10.1002/ajmg.a.36552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuentz P, St-Onge J, Duffourd Y, Courcet JB, Carmignac V, Jouan T, . . . Riviere JB (2017). Molecular diagnosis of PIK3CA-related overgrowth spectrum (PROS) in 162 patients and recommendations for genetic testing. Genet Med. doi:10.1038/gim.2016.220 [DOI] [PubMed] [Google Scholar]
- Luks VL, Kamitaki N, Vivero MP, Uller W, Rab R, Bovee JV, . . . Murillo R (2015). Lymphatic and other vascular malformative/overgrowth disorders are caused by somatic mutations in PIK3CA. J Pediatr, 166(4), 1048–1054 e1041–1045. doi:10.1016/j.jpeds.2014.12.069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirzaa G, Timms AE, Conti V, Boyle EA, Girisha KM, Martin B, . . . Dobyns WB (2016). PIK3CA-associated developmental disorders exhibit distinct classes of mutations with variable expression and tissue distribution. JCI Insight, 1(9). doi:10.1172/jci.insight.87623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pritchard CC, Smith C, Salipante SJ, Lee MK, Thornton AM, Nord AS, . . . Walsh T (2012). ColoSeq provides comprehensive lynch and polyposis syndrome mutational analysis using massively parallel sequencing. J Mol Diagn, 14(4), 357–366. doi:10.1016/j.jmoldx.2012.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riviere JB, Mirzaa GM, O’Roak BJ, Beddaoui M, Alcantara D, Conway RL, . . . Dobyns WB (2012). De novo germline and postzygotic mutations in AKT3, PIK3R2 and PIK3CA cause a spectrum of related megalencephaly syndromes. Nat Genet, 44(8), 934–940. doi:10.1038/ng.2331 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.