Abstract
Store-operated Ca2+ entry (SOCE) is a Ca2+ entry mechanism activated by depletion of intracellular Ca2+ stores. In skeletal muscle, SOCE is mediated by an interaction between stromal-interacting molecule-1 (STIM1), the Ca2+ sensor of the sarcoplasmic reticulum, and ORAI1, the Ca2+-release-activated-Ca2+ (CRAC) channel located in the transverse tubule membrane. This review focuses on the molecular mechanisms and physiological role of SOCE in skeletal muscle, as well as how alterations in STIM1/ORAI1-mediated SOCE contribute to muscle disease. Recent evidence indicates that SOCE plays an important role in both muscle development/growth and fatigue. The importance of SOCE in muscle is further underscored by the discovery that loss- and gain-offunction mutations in STIM1 and ORAI1 result in an eclectic array of disorders with clinical myopathy as central defining component. Despite differences in clinical phenotype, all STIM1/ORAI1 gain-of-function mutations-linked myopathies are characterized by the abnormal accumulation of intracellular membranes, known as tubular aggregates. Finally, dysfunctional STIM1/ORAI1-mediated SOCE also contributes to the pathogenesis of muscular dystrophy, malignant hyperthermia, and sarcopenia. The picture to emerge is that tight regulation of STIM1/ORAI1-dependent Ca2+ signaling is critical for optimal skeletal muscle development/function such that either aberrant increases or decreases in SOCE activity result in muscle dysfunction.
Keywords: Ca2+ signaling, Ca2+-release-activated-Ca2+ (CRAC), muscle fatigue, tubular aggregate myopathy (TAM)
1. Physiological Role of STIM1/ORAI1 Store-operated Ca2+ Entry in Skeletal Muscle.
Calcium (Ca2+) is a ubiquitous second messenger that controls a plethora of cellular functions including proliferation, differentiation, apoptosis, exocytosis, neurotransmitter release, and muscle contraction. Ca2+ signaling occurs when cytoplasmic levels, kept at very low concentrations under resting conditions (~10−7 M), rise upon either the release of Ca2+ from intracellular stores and/or entry of Ca2+ into the cell from the extracellular space. Store-operated Ca2+ entry (SOCE), first discovered in salivary gland cells by James Putney in 1986 [1] and referred to as “capacitative Ca2+ entry,” is one of the most important pathways for extracellular Ca2+ influx in non-excitable cells. After its initial discovery, SOCE in mast cells was shown to be mediated by Ca2+ release-activated calcium (CRAC) channels [2]. However, identification of the molecular components that coordinate SOCE remained elusive for two decades until it was shown that stromal-interacting molecule 1 (STIM1) was the Ca2+ sensor in the endoplasmic reticulum (ER) membrane [3] and ORAI1 [4] was the Ca2+-permeable CRAC channel in the plasma membrane (Figure 1). The mechanism for SOCE activation was subsequently elucidated by a series of elegant studies conducted primarily in non-excitable cells [5]. Specifically, agonist-mediated Ca2+ release through activation of inositol-1,4,5trisphosphate receptor channels, sufficient to deplete ER Ca2+ stores, results in Ca2+ dissociation from luminal STIM1 N-terminal EF-hand domains. Subsequent global conformational changes in STIM1 promotes oligomerization of STIM1 proteins that then interact and activate highly, Ca2+-selective ORAI1 channels localized in discrete junctional regions (~8–10 nm), or puncta, between the ER and plasma membrane [6]. While Ca2+ entry through activated ORAI1 channels is used to replenish ER Ca2+ stores, it is now clear that SOCE plays an important role in a wide range of Ca2+dependent physiological functions including gene transcription, neurotransmitter release, and muscle contraction. Moreover, alterations in SOCE activity result in loss of fine control of Ca2+-mediated processes that lead to pathological conditions including immunodeficiency and myopathy. In this section, we discuss the molecular mechanism and physiological role of SOCE in skeletal muscle.
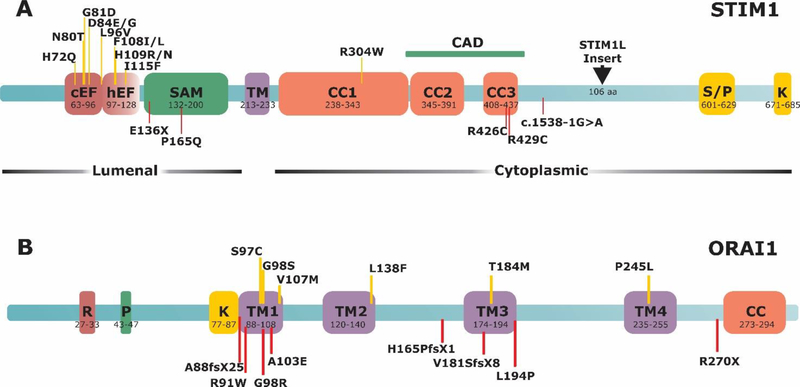
Figure 1. Schematic representation of STIM1 and ORAI1 proteins and location of associated disease mutations.
A) STIM1 protein structure. cEF, canonical EF-hand; hEF, hidden EF-hand; SAM, sterile α-motif; TM, transmembrane; CC, coiled-coil region; S/P serine-proline-rich domain; K, lysine-rich domain; CAD, channel activation domain. B) ORAI1 protein structure. R/P, arginine-proline-rich domain; TM, transmembrane domain; CC, coiled-coil domain. Upper, yellow lines indicate gain-of-function mutations. Lower, red lines indicate loss-of-function mutations.
1.1. Control of Ca2+ Signaling in Skeletal Muscle.
The primary function of skeletal muscle is to generate force for movement. Muscle contraction and relaxation are regulated by rapid (millisecond time frame) changes in myoplasmic free Ca2+ concentration. To produce a rapid increase in myoplasmic Ca2+ levels, muscle fibers utilize a highly-organized sarcotubular membrane apparatus termed the Ca2+ release unit (CRU) or “triad,” which is composed of a transverse tubule (TT), a specialized invagination of the sarcolemma that runs transversally to the long axis of the muscle fiber, flanked by two terminal cisternae of the sarcoplasmic reticulum (SR). The SR and TT membranes of the CRU are closely opposed (~12–15 nm), thus providing the structural framework for excitation-contraction (EC) coupling, the process whereby an action potential in the TT is used to trigger Ca2+ release from the SR used to drive muscle contraction. At the molecular level, EC coupling in skeletal muscle is mediated by mechanical coupling between the dihydropyridine receptor (DHPR), the voltage sensor in the TT membrane, and ryanodine receptor type 1 (RYR1) Ca2+ release channel in the SR terminal cisternae [7]. Several other junctional proteins, including calsequestrin type 1 (CASQ1), triadin, junctin, JP-45, Stac3, and FK-506 binding protein 12 interact with the DHPR-RYR1 macromolecular machinery to influence Ca2+ release during EC coupling [8–10]. As one example, CASQ1, a highly acidic protein in the lumen of SR terminal cisternae that binds Ca2+ with moderate affinity, but high capacity [11–13], functions both as a luminal Ca2+ buffer and a regulator of RYR1 Ca2+ release channel activity [14, 15]. The SR also contains a high-level of sarco/endoplasmic reticulum Ca2+ ATPase-1 pumps that efficiently transport Ca2+ released during EC coupling back into the SR to terminate contraction and refill SR Ca2+ stores. The combination of both high capacity SR Ca2+ buffer (CASQ1) and robust Ca2+ reuptake makes it difficult to fully deplete SR Ca2+ stores in adult skeletal muscle fibers. As a result, a single twitch contraction results in only ~10% reduction of the releasable SR Ca2+ pool [16]; enabling twitch contractions to continue for long periods of time following complete removal of extracellular Ca2+ [17]. For many years it was believed that Ca2+ entry had no role in skeletal muscle. However, 45Ca2+ tracer studies revealed that significant Ca2+ uptake from the extracellular space occurs during 40 Hz stimulation of intact muscles, in a manner that is not prevented by inhibitors of voltage-gated L-type Ca2+ channels [18]. In addition, muscle performance is reduced and muscle fiber types are altered in mouse models that reduce SOCE in skeletal muscle [19, 20]. Muscle specific force is also reduced during repetitive, high-frequency ex vivo stimulation under conditions that inhibit SOCE, particularly following acute exercise [21]. Finally, mutations in the STIM1 and ORAI1 genes result in human disorders that include a range of muscle phenotypes such as hypotonia, atrophy, and tubular aggregate myopathy (see Section 2.0). The following sections focus on recent advances in elucidating the molecular determinants, properties and physiological role of SOCE in muscle.
1.2. SOCE in Skeletal Muscle
Activation of a Ca2+ entry pathway in muscle by depletion of SR Ca2+ stores was first demonstrated in myotubes in 1996 by Steinhardt and colleagues [22]. Kurebayashi and Ogawa reported a similar process in adult muscle fibers five years later [23]. These findings motivated subsequent studies to identify the molecular determinants and physiological role of SOCE in muscle. Initial studies suggested that SOCE in muscle was determined by coupling of either RYRs or inositol-1,4,5-trisphosphate receptors in the SR membrane with transient receptor potential cation channels (TRPC) in the external membrane [24–29]. However, inositol-1,4,5-trisphosphate receptor expression in adult muscle is low and primarily restricted to the nuclear envelope [30] and SOCE persists in the absence of either RYR1 [27, 31] or TRPC3 [32]. Following the discovery that STIM1 and ORAI1 mediate SOCE in non-excitable cells [3, 4], subsequent work focused on assessing the roles of STIM1 and ORAI1 in skeletal muscle.
1.2.1. Molecular Components of SOCE
Several observations provide strong evidence for SOCE in muscle being coordinated by a functional interaction between STIM1 in the SR and ORAI1 in the TT. First, STIM1 and ORAI1 are abundantly expressed in both myotubes and adult skeletal muscle fibers [33, 34]. Second, using Ca2+ entry and Mn2+ quench assays, Lyfenko and Dirksen showed that SOCE is abolished by either knocking-down STIM1 or expressing a dominant-negative ORAI1 construct (E106Q) that eliminates ion permeation through the channel [31]. Third, myotubes from both global [33] and muscle-specific STIM1-knockout mice [35] lack SOCE, consistently with STIM1 being the SR Ca2+ sensor for SOCE activation in muscle. Fourth, SOCE is also abolished in adult muscle fibers from mice with either muscle-specific expression of dominant-negative ORAI1 [19] or muscle-specific ORAI1 knockout [20]. These studies provide compelling evidence that STIM1 and ORAI1 are required for SOCE in skeletal muscle. It should be noted that while ORAI1 functions in skeletal muscle as a SOCE channel, STIM1 has been proposed to be a multipurpose stress transducer activated by diverse stimuli (depletion, oxidation, temperature, hypoxia and acidification) that may regulate multiple downstream targets including different ion channels (e.g. ORAI1, ORAI2, ORAI3, TRPC channels), pumps/exchangers, adaptor proteins, ER chaperones, signaling enzymes and ER stress/remodeling proteins [36]. Finally, while transcripts for STIM2 [37], ORAI2 and ORAI3 [31] are also expressed in myotubes, the precise role(s) of these other isoforms in muscle Ca2+ homeostasis and function remain largely unresolved.
1.2.2. Properties of SOCE
In non-excitable cells, STIM1 and ORAI1 proteins are diffusely distributed throughout the ER and the PM, respectively, under resting conditions. Upon store depletion, STIM1 oligomerizes to form punctuate clusters in junctional regions between the ER and plasma membrane (of 10–25 nm gap). Within these puncta, STIM1 oligomers interact and activate highly Ca2+-selective ORAI1 (CRAC) channels in the plasma membrane [6]. In T-lymphocytes, this entire process, from store depletion to CRAC channel activation, occurs over ~1 minute [38]. In muscle, however, some authors have proposed that Ca2+ influx can be activated very quickly (<1 second) following Ca2+ store depletion [16, 39]. This rapid activation of SOCE might in part be possible in muscle because highly-organized, pre-formed SR-TT junctions are already present. Under resting conditions with a Ca2+ replete SR, ORAI1 is located within the TT system [19, 21] and STIM1 is positioned both within the SR terminal cisternae and throughout the free SR within the I-band region of the sarcomere [19, 21, 33]. The localization of a fraction of STIM1 with ORAI1 within pre-existing triad junctions is proposed to underlie rapid SOCE activation in muscle since neither STIM1 redistribution nor SR remodeling upon store depletion are required [40]. Consistent with functional STIM1/ORAI1 localization within the triad junction, muscle fibers from mice lacking mitsugumin-29, a synaptophysin protein that contributes to triad formation and maintenance, exhibit swollen and irregular TTs, altered triad structure, and a marked impairment of SOCE [27, 41].
1.2.3. Proposed Roles of STIM1S and STIM1L
Although the above studies provide evidence for pre-localization of ORAI1 and a fraction of STIM1 to the triad in resting muscle, the precise nature and role of STIM1 present within the free SR at the I-band require more investigation. Stiber and colleagues [33] suggested that muscle contains two functionally distinct pools of STIM1 proteins: i) one pool located at the triad that mediates rapid SOCE activation and ii) a reserve pool within the free SR at the I-band that is recruited following store depletion to produce graded SOCE. A recent study provides a potential molecular explanation for these two different pools of STIM1 [42]. This work discovered a STIM1 splice variant highly expressed in skeletal muscle, STIM1L (L for long, as it encodes an extra 106 amino acids), that is the consequence of alternative splicing of exon 11. The unique residues in STIM1L were found to interact with cytoskeletal actin in a manner that results in the formation of permanent clusters with ORAI1 even under conditions of fully replete Ca2+ stores [42]. On the other hand, the more common, shorter STIM1 variant (STIM1S, S for short) was more diffusely distributed throughout the SR at rest and required >1 min following store depletion to form clusters [42, 43]. As the rate-limiting-step for SOCE activation in non-excitable cells involves the time required for STIM1S oligomers to migrate into clusters and then interact/activate ORAI1 channels [43–45], pre-formed STIM1L-ORAI1 clusters within the triad junction could account for rapid SOCE activation upon sustained or repetitive Ca2+ release sufficient to transiently deplete Ca2+ within the terminal SR. Conversely, as STIM1S is localized throughout the free SR and is not pre-localized with ORAI1, STIM1S might mediate graded recruitment of additional SOCE activity when needed.
1.2.4. Effect of Exercise on SOCE
What conditions might drive graded recruitment of STIM1Smediated SOCE in skeletal muscle? A recent study found that a single period of acute treadmill exercise triggers a reorganization of the sarcotubular membrane system in extensor digitorum longus muscle fibers of wild type male mice. The sarcotubular reorganization results in the formation of new junctional contacts between the SR and TT membrane within the I-band of the sarcomere [21]. The width of the junctional gap in these new SR/TT junctions is only ~7–8 nm, narrower than that of triads containing RYR1 feet (12–15 nm). In contrast to the translocation of STIM1 oligomers toward ORAI1 in the plasma membrane following store depletion in non-excitable cells, in skeletal fibers exercise drives the TT membrane containing ORAI1 to elongate into the I-band, toward the Z-line, to form junctions with elongated stacks of free SR membranes that contain STIM1 proteins (Figure 2). Immunofluorescence and immuno-gold for electron microscopy studies found that, following exercise, STIM1 localization remains largely within the Iband, while a fraction of ORAI1 moves toward the Z-line, promoting a significant increase in STIM1/ORAI1 co-localization in the I-band. Prior studies reported that overexpression of STIM1 in non-muscle cells triggers a similar remodeling of ER membranes to form stacks of flat-parallel cisternae [46, 47]. Following acute treadmill exercise, extensor digitorum longus muscles were more resistant to fatigue during repetitive, high-frequency stimulation in the presence of extracellular Ca2+, a difference abolished by experimental interventions that reduce SOCE (e.g. 0 Ca2+ or the addition of the SOCE inhibitors BTP-2 or 2-APB) [21]. Together, these results indicate that acute exercise triggers the formation of new SR-TT junctions within the I-band that enable recruitment of additional STIM1/ORAI1 interactions needed to enhance SOCE and muscle performance during rigorous activity.
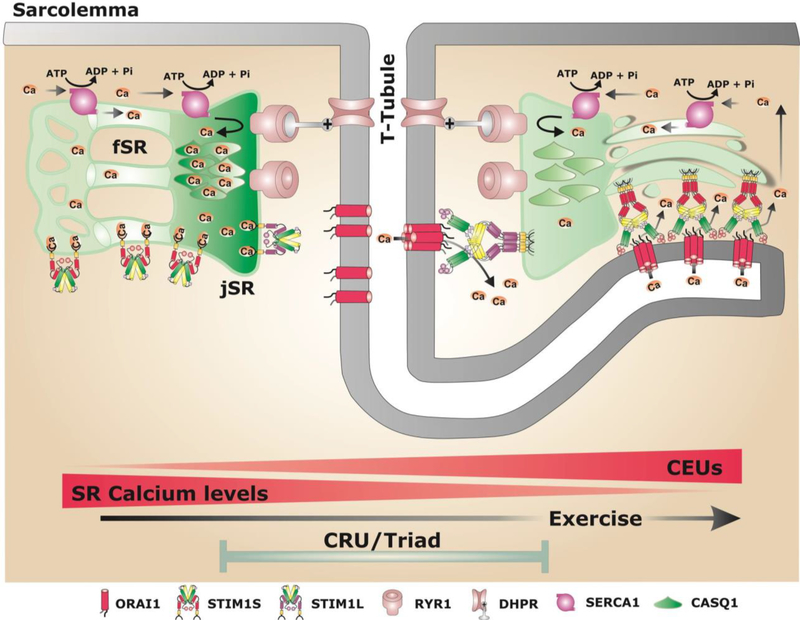
Figure 2. Schematic model showing potential sites of STIM1/ORAI1 coupling in skeletal muscle under resting conditions and after exercise.
The SR of adult fibers is divided in two compartments: i) the junctional SR (jSR, or SR terminal cisternae) that contains CASQ1 and RYR1 and is closely associated with the T-tubule (TT) that contains DHPR, to form the triad (or CRU), the site of excitation-contraction (EC) coupling; ii) the free SR (fSR) that does not interact with TT and is localized throughout I band. Both jSR and fSR contain high levels of the sarco/endoplasmic Ca2+ ATPase-1 (SERCA1), a Ca2+ ATPase that pumps Ca-2+ ions Ca2+ released during EC coupling from the myoplasm back into the lumen of the SR. Two different splice variants are expressed in skeletal muscle: STIM1S and STIM1L. Under resting conditions (left side), ORAI1 is located within the TT system at the triad, while STIM1S and STIM1L are located in the fSR and jSR, respectively. After exercise (right side): i) the fSR and TT undergo a striking remodeling to form new junctions composed by multiple layers of flat parallel stacks of fSR cisternae and an extension of the TT from the triad into the I-band toward the Z-line [21]. These new junctions promote increased STIM1/ORAI1 colocalization at the I-band as TT elongation allows ORAI1 to interact with STIM1S proteins in the fSR. These new junctions are proposed to function as Calcium Entry Units (CEUs) [21].
Given the potential role for these junctions in mediating STIM1/ORAI1 SOCE after acute exercise, they were referred to as Calcium Entry Units (CEUs) in order to differentiate them from classical CRUs (Figure 2). In contrast to rapid activation of SOCE in muscle following store depletion [16, 39], exercise-induced formation of CEUs occurs over a relatively long period time (minutes/hours). It is also important to note that CEUs similar to those formed after exercise are also present in muscle fibers from mice not subjected to acute exercise, although they are less frequent and smaller in size [21]. Several unanswered issues regarding CEUs remain to be addressed. For example, the relative roles of STIM1S, STIM1L, and ORAI1 in exercise-dependent sarcotubular remodeling, CEU formation and SOCE in muscle remain unclear. Second, the relative contribution of STIM1/ORAI1 coupling within the triad (i.e. CRUs) versus CEUs to SOCE in muscle at rest and after exercise also remains to be determined.
1.3. Physiological Role of SOCE in Skeletal Muscle Development and Function
Recent evidence indicates that SOCE plays an important role in both muscle development and maintaining contractile activity during periods of prolonged, high-frequency stimulation.
1.3.1. Role of SOCE in Muscle Development
STIM1/ORAI1 coupling regulates long-term muscle function (e.g. differentiation, development and growth) through activation of downstream Ca2+-dependent signals including nuclear factor of activated T-cells (NFAT), mitogen-activated protein (MAP) kinase, and extracellular signal-regulated kinase 1 and 2 (ERK 1/2) [48, 49]. The role of STIM1/ORAI1 SOCE in muscle development is supported by studies showing increased expression of both proteins and enhanced SOCE activity during differentiation of myoblasts to myotubes [33, 49–51]. In addition, global STIM1 KO mice exhibit delayed muscle development and a lethal congenital myopathy [33, 35], while muscle-specific STIM1 KO results in reduced activation of multiple Ca2+-dependent signal transduction pathways (including calcineurin, MAP kinase, ERK1/2, and AKT) involved in muscle maturation and growth [35]. Muscle-specific STIM1 KO mice and transgenic mice with muscle-specific expression of dominant negative E108Q ORAI1 (dnORAI1 mice) both exhibit reductions in whole-body and muscle mass, which are due in part to a marked decrease in muscle fiber cross-sectional area [19, 35]. Consistent with these findings, mice with constitutive, muscle-specific knockout of ORAI1 (cORAI1 KO mice), in which ORAI1 is absent throughout development, exhibit parallel reductions in both body/muscle mass and muscle fiber cross-sectional area [20]. Slow twitch soleus muscles from cORAI1 KO mice exhibit reduced content and cross-sectional area of oxidative, fatigue-resistant type I fibers [20]. Together, these studies indicate that STIM1/ORAI1-dependent signals promote muscle fiber maturation, growth and specification of oxidative, fatigue-resistant fibers. However, as muscle mass, fiber cross-sectional area, and fatigue-resistant fiber content are not altered following postnatal, muscle-specific ORAI1 ablation [20], the impact of ORAI1 on muscle fiber specification and growth occurs during an early developmental time point. Taken together, these studies provide strong evidence for STIM1/ORAI1-dependent activation of Ca2+-dependent downstream signaling cascades that promote muscle fiber growth and differentiation during early development.
1.3.2. Role of SOCE in Maintaining Contractile Function
In addition to playing a role in muscle development and growth, STIM1/ORAI1-dependent SOCE also promotes sustained force generation during periods of prolonged activity. Under these conditions, muscle is activated by repetitive, high-frequency train of action potentials (e.g. 150 Hz) to produce tetanic force. Such sustained stimulation triggers massive Ca2+ release that can result in a transient depletion of SR Ca2+ content, especially at sites of active Ca2+ release. As the Ca2+ current through ORAI1 channels exhibits strong inward rectification [52], significant Ca2+ influx through activated ORAI1 channels would be expected to occur primarily during intervals between succeeding high-frequency trains of action potentials after the membrane potential has returned to a negative value (e.g. −90 mV). The ability of muscle fibers to recover Ca2+ ions from the extracellular space via STIM1/ORAI1-mediated SOCE represents one mechanism to promote SR store refilling needed to maintain Ca2+ release during periods of repetitive, high-frequency stimulation. Consistent with this, removal of extracellular Ca2+ and addition of SOCE channel inhibitors (e.g., 2-APB, BTP-2) reduce the ability of skeletal muscle to maintain contractile force during prolonged stimulation [21, 27]. Muscles from mitsugumin-29 knockout mice, which display reduced SOCE activity, exhibit an increased susceptibility to fatigue [27]. Conversely, mice that lack sarcalumenin, a Ca2+-binding protein in the free SR, display increased mitsugumin-29 expression, increased SOCE activity, and enhanced resistance to muscle fatigue [53]. More recently, Stretye and colleagues reported that myostatin-deficiency is accompanied by parallel reductions in STIM1 and ORAI1 expression, SOCE activity, SR Ca2+ content and depolarization-evoked Ca2+ release [54], while Boncompagni and colleagues found that exercise-induced formation of CEUs correlates with an increased ability of muscle to maintain force during sustained contractions in the presence of extracellular Ca2+ [21]. Together, these studies support a close relationship between STIM1/ORAI1-dependent SOCE, SR Ca2+ content, and resistance to fatigue.
Studies using STIM1 and ORAI1 loss-of-function mouse models provided important additional insights into the physiological role of STIM1/ORAI1-dependent Ca2+ entry on muscle contractile function [19, 20, 33, 35]. STIM1-deficient myotubes lack SOCE and exhibit a marked reduction in the ability to maintain myoplasmic Ca2+ transients during repetitive KCl-induced depolarization as a consequence of reduced SR Ca2+ content [33]. Muscle-specific STIM1 ablation results in loss of SOCE and the inability of muscle fibers to maintain Ca2+ transient amplitude during repetitive stimulation in spite of EC coupling being unaffected [35]. However, as neither global nor muscle-specific STIM1 knockout mice survive into adulthood, these studies do not provide insight into the physiological role of STIM1/ORAI1 coupling in adult muscle. On the other hand, muscle-specific dnORAI1 transgenic mice lack SOCE in muscle and thrive into adulthood [19]. Thus, dnORAI1 transgenic mice provided the first opportunity to assess the physiological role of ORAI1-dependent SOCE in adult skeletal muscle. Adult dnORAI1 mice exhibit increased susceptibility to exhaustion during rotarod and treadmill exercise. Single muscle fibers from dnORAI1 mice show decreases in both SR Ca2+ store content and ability to maintain Ca2+ transient amplitude during repetitive stimulation. Consistent with this, extensor digitorum longus muscles exhibit a reduced ability to maintain contractile force when subjected to the same repetitive stimulation protocol [19]. Subsequent studies using cORAI1 KO mice confirmed the physiological role of STIM1/ORAI1mediated SOCE in promoting SR Ca2+ refilling and sustained muscle activity during high-frequency stimulation. [20]. Interestingly, while cORAI1 KO mice exhibit reduced performance in treadmill and rotarod endurance tests, this was not observed following muscle-specific ablation of ORAI1 in adult mice using tamoxifen-inducible ORAI1 KO mice [20]. This finding suggests that the reduced endurance observed in cORAI1 KO mice primarily results from the reduction in both cross-sectional area and fractional contribution of fatigue-resistant type I fibers observed with early developmental ablation of ORAI1 (section 1.3.1).
2. Mutations in STIM1 and ORAI1 Genes Cause Muscle Disease
Since their discovery over a decade ago, mutations in STIM1 and ORAI1 have been linked to multiple human diseases including various forms of immunodeficiency and myopathy (Table 1 and Figure 1). The fact that mutations in STIM1 and ORAI1 result in disorders characterized by clinical myopathy is consistent with the discussion above that STIM1/ORAI1-mediated SOCE plays an important role in muscle function.
Table 1. Genetic, mechanistic, histological, and clinical muscle phenotypes of patients with loss-of-function and gain-of-function STIM1 and ORAI1 mutations.
CID, combined immunodeficiency; CAD, channel activation domain; CC, coiled-coil domain; CK, creatine kinase; cEF, canonical EF-hand domain; hEF, hidden EF-hand domain; ER, endoplasmic reticulum; GF, gain-of-function mutation; LF, loss-of-function mutation; ND, not determined; CID, severe combined immunodeficiency; SOCE, store-operated Ca2+ entry; STIM1, stromal interaction molecule-1; TA, tubular aggregates; TAM, tubular aggregate myopathy; YPS, York platelet syndrome.
| Clinical and Histological Muscle Features Resulting from STIM1 and ORAI1 Gene Mutations | ||||||
|---|---|---|---|---|---|---|
| Disease | Gene/Position | Mutation | Lost or gain of function/others | Histological features | Muscle phenotype | Reference |
| CID | STIM1/SAM | p.E128RfsX9(or E136X) | LF Abolishes STIM1 expression | No abnormalities | No progressive muscular hypotonia, partial iris hypoplasia, mydriasis Onset: early | [58] |
| STIM1/SAM | p.P165Q | LF Reduces STIM1 protein expression | ND | No progressive muscular hypotonia Onset: early | [156] | |
| CID | STIM1/CAD | p.R426C | LF Impairs STIM1/ORAI1 co-localization | ND | No muscular hypotonia Onset: early | [157, 158] |
| STIM1/CAD | p.R429C | LF Normal STIM1 expression, impairs STIM1-ORAI1 interaction | ND | Mild muscular hypotonia, mydriasis Onset: early | [59, 60, 159] | |
| STIM1/Exon8 | c. 1538–1G>ASplice-site mutation | LF Low STIM1 mRNA levels, abolishes STIM1 protein expression | ND | ND Onset: early | [160] | |
| CID | ORAI1/TM1 | p.R91W | LF Abolishes ORAI1 channel conductance | Predominance of type I fibers, atrophic type II fibers, fiber size variations | Muscular hypotonia Onset: early | [4, 56] |
| ORAI1/TM1 | p.G98R | LF Normal ORAI1 mRNA Abolish ORAI1 protein expression | No abnormalities by H&E Mitochondrial damage by EM | Muscular hypotonia, mydriasis, partial iris hipoplasia Onset: early | [57] | |
| ORAI1/TM1 | p.A88SfsX25 | LF Abolishes ORAI1 mRNA/protein expression | ND | Muscular hypotonia Onset: early | [56] | |
| ORAI1/TM1 | p.A103E | LF Abolishes ORAI1 protein expression | ND | Muscular hypotonia Onset: early | [56] | |
| ORAI1/TM3 | p.V181SfsX8 | LF Normal ORAI1 mRNA Abolish ORAI1 protein expression | Predominance of type I fibers and atrophic type II fiber | Muscular hypotonia, esotropia Onset: early | [57] | |
| ORAI1/TM3 | p.L194P | LF Normal ORAI1 mRNA Abolishes ORAI1 protein expression | No abnormalities by H&E Mitochondrial damage by EM | Muscular hypotonia Onset: early | [56, 57] | |
| ORAI1/2nd loop | p.H165PfsX1 | LF Abolishes ORAI1 protein expression | ND | Muscular hypotonia Onset: early | [161] | |
| ORAI1/C-terminus | p.R270X | LF Impairs ORAI1 association with STIM1 | ND | Hypotonia Onset: early | [162] | |
| YPS/Stormorken syndrome | STIM1/CC1 | p.R304W | GF Constitutive SOCE Impairs STIM1 inhibition Increase in basal Ca2+ levels Decreases ER Ca2+ content | TAs, predominance of atrophic type II fibers | Muscle weakness Cramps Miosis Elevated CK levels Onset: early, mild and late | [64–66, 83] |
| Stormorken-like syndrome/TAM | STIM1/hEF | p.I115F | GF | TAs, type I fiber prevalence, type II fiber hypotrophy Mild increase in internal nuclei Fiber size variability Fatty infiltration and muscle atrophy | Slowly progressive, girdle muscle weakness and contractures of ischiocrural muscles. Elevate CK levels Onset: early | [66, 163] |
| ORAI1/TM4 | p.P245L | GF Not constitutively activated Impairs slow Ca2+ dependent inactivation Requires STIM1 association | TAs in type I & II fibers Fiber size variation Fibro fatty infiltration Internal nuclei | Progressive proximal of distal upper limbs, proximal lower limb weakness Cramps Miosis Elevate CK levels Onset: late | [64, 164] | |
|
TAM TAM |
STIM1/cEF | p.D84E | Constitutive STIM1 activation (clusters) | TAs in type I fibers, type I fiber prevalence, type II fiber atrophy, fiber size variation Internal nuclei | Proximal muscle weakness with lower limb predominance Diffuse cramps Elevate CK levels Hypocalcemia Onset: late | [165] |
| ORAI1/TM1 | p.G98S | GF Constitutive SOCE Increase in basal Ca2+ levels Normal ORAI & STIM1 protein expression (1/6 patient analyzed) | TAs in Type I & II fibers Single internal nuclei Type I fiber predominance Fiber size variation | Slowly progressive muscle weakness, lower and upper proximal limbs, myalgia, cramps, stiffness Marked replacement of soleus and gastrocnemius muscles with fat Severe atrophy and fat infiltration of trapezius, latissimus dorsi, gluteus medius, hamstrings and adductor thigh muscles Bilateral ankle joint contractures, rigid spine and hypocalcemia Elevate CK levels Onset: early | [63, 166] | |
|
TAM TAM |
ORAI1/TM2 | p.L138F | GF Constitutive SOCE Increase in basal Ca2+ levels | TAs in type I & II fibers Single/multiple internal nuclei Type I fiber predominance Fiber size variation | Slowly progressive, diffuse muscle weakness. Modest increase in CK levels Onset: mild | [63] |
| ORAI1/TM1 | p.S97C | GF Constitutive SOCE Ca2+ oscillations | TAs in type I & II fibers with predominance in type II fibers Type I fiber prevalence | Weakness in proximal and distal lower limbs and proximal upper limbs Cramps Miosis Elevate CK levels Onset: mild & late | [85] | |
| ORAI1/TM1 | p.V107M | GF Constitutive SOCE Increase in basal Ca2+ levels | TAs in type I & II fibers Fiber size variability Internal nuclei | Proximal lower limb and upper limb weakness Stiffness, cramps, and myalgia Calf hypertrophy Elevate CK levels Onset: early | [166] | |
| ORAI1/TM3 | p.T184M | GF No constitutive SOCE Requires STIM1 association Increase in basal Ca2+ levels | TAs in type I & II fibers Fiber size variability Internal nuclei | Mild general weakness Cramps and myalgia | [166] | |
| STIM1/cEF | p.D84G | GF Constitutive STIM1 activation Impairs STIM1 Ca2+ sensing Increase in basal Ca2+ levels Normal STIM1 expression | TAs in type I & II fibers Fiber size variation Type I fiber prevalence | Mild-slowly progressive Proximal lower limb weakness, mild and diffuse upper limb weakness Elevate CK levels Onset: early & mild | [62] | |
| STIM1/cEF | p.H72Q | GF Constitutive STIM1 activation Impaired STIM1 Ca2+ sensing | TAs in type I & II fibers Fiber size variation Type I fiber prevalence Type II fiber atrophy | No muscle weakness Elevate CK levels Onset: asymptomatic | [62] | |
| STIM1/cEF | p.G81D | GF Constitutive SOCE Increase in basal Ca2+ levels Impairs STIM1 Ca2+ sensing | TAs with prevalence in type I fibers Type II fiber atrophy | Lower and upper limb muscle weakness, Mild-severe contractures, rigid spine, stiff neck, scapular winging, muscle atrophy Elevate CK levels Onset: early | [167] | |
| STIM1/hEF | p.H109Rp.H109N | GF Constitutive STIM1 activation Impairs STIM1 Ca2+ sensing | TAs in type I & II fibers Type I fiber prevalence Type II fiber atrophy | Proximal lower limb weakness, mild proximal upper limb weakness Elevate CK levels Onset: early, mild & late | [62, 163, 168] | |
| STIM1/cEF | p.N80T | GF Constitutive STIM1 activation (clusters) Impairs STIM1 Ca2+ sensing | TAs with prevalence in type II fibers Fiber size variability Internal nuclei and fibrosis | Proximal and lower limb muscle weakness Myalgia Heel contractures Eye movement defects Elevate CK levels Onset: early & late | [168] | |
| STIM1/cEF | p.L96V | GF Constitutive STIM1 activation (clusters) Impaired STIM1 Ca2+ sensing | TAs with prevalence in type II fibers Fiber size variability Internal nuclei | Muscle weakness in lower limbs Myalgia Elevated CK levels Onset: early | [168] | |
| STIM1/hEF | p.F108I | GF Constitutive STIM1 activation (clusters) Impairs STIM1 Ca2+ sensing | TAs Fiber size variability Type I fiber prevalence Internal nuclei, fibrosis | Proximal muscle weakness Myalgia Elevated CK levels Onset: early | [168] | |
| STIM1/hEF | p.F108L | GF Moderate constitutive STIM1 activation (clusters) Impairs STIM1 Ca2+ sensing | TAs Atrophy of Type II fibers | No muscle weakness Myalgia Normal CK levels Onset: late | [168] | |
2.1. Loss-of-Function Mutations in STIM1 and ORAI1 Genes
The involvement of SOCE dysfunction in skeletal muscle disease became clear with the initial identification of ORAI1 as the pore-forming subunit for CRAC activity [4]. Using a modified linkage analysis with single-nucleotide polymorphism arrays and Drosophila RNA interference screen approaches, a loss-of-function missense mutation in ORAI1 (p.R91W) was identified in patients with combined immunodeficiency (CID) with severe skeletal muscle myopathy [4, 55]. A subsequent clinical report from a patient with this mutation also revealed a predominance of type I fibers and atrophic type II fibers, consistent with ORAI1 exhibiting a critical role in fiber type differentiation/maintenance [56]. Similarly, a gastrocnemius muscle biopsy taken from a patient with an ORAI1 p.V181SfsX9 loss-of-function mutation exhibited an almost complete absence of type II muscle fibers [57].
To date, 13 different loss-of-function mutations in the STIM1 and ORAI1 genes, which result in a marked reduction of SOCE function, have been described (Figure 1). Loss-of-function mutations in the STIM1 gene have been reported to result in severe, early-onset immunodeficiency with muscle hypotonia, mydriasis, iris hypoplasia, ectodermal dysplasia, splenomegaly, lymphadenopathy, and multiple auto-immune manifestations including thrombocytopenia and hemolytic anemia (Table 1). As one example, patients homozygous for the E136X (p.E128RfsX9) mutation in STIM1 exhibit CID coincident with progressive muscular hypotonia as a consequence of a lack of SOCE [58]. Similarly, significant muscular hypotonia was reported in patients with a loss-of-function p.R429C point mutation in STIM1 [59], a conserved residue in the third coiled-coil domain required for oligomerization of STIM1 dimers and subsequent binding/activation of ORAI1 channels [60]. In many cases, individuals with loss-of-function mutations in STIM1 and ORAI1 exhibit a common clinical phenotype. For example, patients carrying STIM1 (p.E128RfsX9, p.P165Q, p.R426C, or p.R429C) and ORAI1 (p.A88SfsX25, p.R91W, p.G98R, p.H165PfsX1, p.V181SfsX8, p.L194P) mutations experience CID, atrophy/hypotonia of skeletal muscle, myopathy and a severe chronic pulmonary problem due to respiratory muscle weakness [49, 57, 61] (Table 1).
2.2. Gain-of-Function Mutations in STIM1 and ORAI1 Genes
Recently, multiple gain-of-function mutations in both the STIM1 and ORAI1 genes (Figure 1), which result in constitutively activated SOCE, were found to be linked to three overlapping diseases with presentations ranging from non-syndromic tubular aggregate myopathy (TAM) [62, 63] to more complex pathologies such as Stormorken and York Platelet syndromes [64–66] (Table 1).
2.2.1. Tubular Aggregate Myopathy (TAM)
TAM is a relatively rare skeletal muscle disorder characterized by progressive muscle weakness, cramps, and myalgia [67] that exhibits both autosomal dominant and recessive modes of inheritance [68]. Despite differences in clinical phenotype and symptomatology among TAM patients, a consistent histopathological feature is the presence of tubular aggregates (TAs). TAs are abnormal structures in muscle composed of an unusual accumulation of highly-ordered and tightly packed SR tubule membranes (Figure 3) that contain CASQ1, SERCA, and sarcalumenin [67, 69]. The first identification and characterization of TAs were made in 1970 by Engel and colleagues using electron microscopy to observe TAs in muscle biopsies from dyskalemic patients [70]. Under electron microscopy and freeze fracture, TAs appear as single- or double-walled parallel aligned tubules in longitudinal sections (Figure 3A-C) and as honeycomb-like structures in cross-sections (Figure 3D-F). The tubules can either appear empty or containing electron-dense material. Under light microscopy, TAs are detected using two distinct staining procedures: 1) as organized inclusions of bright red materials located either in the center or periphery of the fiber upon Gomori trichrome staining or 2) as dark stained regions with NADH-tetrazolium reductase staining. Interestingly, TAs stain negatively for succinate dehydrogenase, consistent with their SR, rather than mitochondrial, origin.
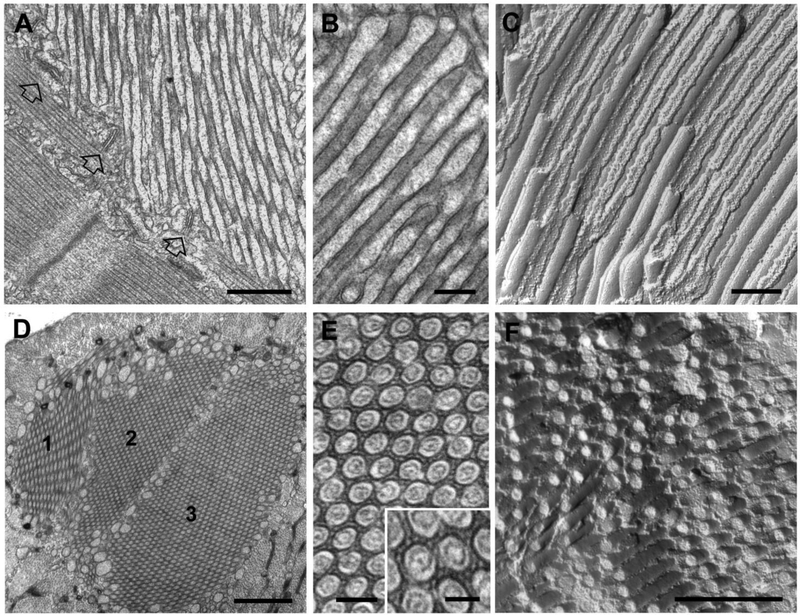
Figure 3. Tubular aggregates (TAs) in extensor digitorum longus fibers from 2 year old male wild type mice.
A-C) EM images of longitudinal views of TAs in thin sections (A and B) and freeze fracture (C). Empty arrows in panel A point to jSR-TT junctions (or triads) located at the periphery of a TA. Tubes of TAs are typically filled with electron-dense material, most likely CASQ1 (B). The regular and straight shape of the tubules are emphasized in freeze fracture replicas, where tubules appear as long cylinders showing alternated views of the luminal and cytoplasmic leaflets (see [75] for additional detail). D-F) EM images of cross-sectional views of TAs in thin sections (D and E) and freeze fracture (F). Large TAs often result from the association of multiple TAs of smaller size (panel D: 1–3). In the core of each domain, tubes forming the TA display uniform diameters and appear ordered in a hexagonal pattern (E). Note the presence of small linkages that bridge the gap between membranes of adjacent tubules (E, inset). Bars: A, D and F: 0.5 μm; B and C: 0.2 μm; E: 0.1 μm (inset: 0.05 μm)
Subsequent to their description in dyskalemic patients, TAs were found in a variety of other pathological conditions affecting muscle including hypoxia [71], periodic paralysis, congenital myasthenic syndromes, and myotonic disorders [72–74]. Thus, TAs are a relatively non-specific structural alteration present in patients across a wide variety of neuromuscular disorders. Although having not yet been confirmed in humans with age, TAs are abundant in fast twitch muscle of aged (>24 months old) male mice and stain positively for CASQ1 and SERCA (Figure 3) [67, 75].
A relationship between altered Ca2+ homeostasis and the formation of TAs has long been suggested [69, 73]. The hypothesis that TAs are formed as a consequence of altered Ca2+ handling in skeletal muscle fibers, is strengthened by the fact that gain-of-function mutations in both STIM1 and ORAI1 result in myopathies characterized by the presence of TAs (see sections 2.2.2 and 2.2.3 below). Thus, increased SOCE via constitutively-active STIM1/ORAI1 proteins likely serves as an upstream trigger for TA formation. While the precise downstream cellular and molecular mechanisms that underlie TA assembly remain to be elucidated, several hypotheses have been advanced. One possibility is that enhanced SOCE promotes an aggregation and fusion of free SR vesicles/membranes into TAs. Alternatively, TAs may represent an adaptive mechanism triggered by constitutive SOCE designed to limit Ca2+-mediated muscle fiber hypercontraction and necrosis.
A second hypothesis is that a dysfunction in Ca2+ homeostasis could result in protein misfolding and aggregation due to altered proteostasis, which in turn, drives morphological changes in the SR structure that lead to TA formation [76]. In this regard, protein N-glycosylation plays a critical role in the correct maintenance of ER proteostasis, such that a reduction in protein N-glycosylation can induce ER/SR stress and increase the accumulation of unfolded proteins [77, 78]. Recessive mutations in the glutamine-fructose-6-phosphate transaminase 1 gene, an enzyme that produces UDP-N-acetylglucosamine needed for N-linked glycosylation in the ER/SR, results in congenital myasthenic syndromes with a limb-girdle dystrophy phenotype that are associated with TAs [79]. Interestingly, mutation of an N-glycosylation site in STIM1 correlates with strong activation of ORAI1 channels that could drive TAs formation in skeletal muscle [80]. It is also possible that TAs are driven by conditions (e.g. hypoxia, aging, constitutive SOCE) that promote abnormal clustering of STIM1 oligomers. Consistent with this idea, EM images of TAs reveal the presence of discrete, evenly spaced electron-dense bridges within the gap between adjacent tubules (Figure 3 E). Similar bridging structures were described in TAs of patients with TAM [67] and between stacks of flat parallel cisternae in CEUs following exercise [21]. The molecular nature of these electron-dense strands remains unknown, though aggregates of STIM1 oligomers represent a reasonable candidate.
This possibility is supported by immuno-gold detection of STIM1 in the SR stacks following exercise in adult skeletal muscle [21] and in the ER stacks after STIM1 overexpression in HeLa cells [46]. In addition, Endo and colleagues showed that TAs in skeletal muscle from TAM patients are positive for STIM1 and ORAI1 immunostaining, consistent with the two proteins being sequestered in TAs [63]. However, a separate study found that STIM1 is not included in TAs, but rather is co-localized with RYR1 around the TA periphery, in muscle biopsies of TAM patients with STIM1 gain-of-function mutations [62]. Thus, while a relationship between altered SOCE function and Ca2+ homeostasis in TA formation in muscle is clear, additional studies are needed to more precisely determine the precise molecular components of TAs and the cellular/molecular mechanisms involved in their formation.
2.2.2. STIM1 Gain-of-Function Mutations
The majority of autosomal dominant mutations in STIM1 linked to TAM are located in the N-terminal luminal cEF-hand or hEF-hand (EF1/2) domains (Figure 1). These domains function as a sensor for the Ca2+ concentration in the SR lumen and are critical for initiating a complex series of conformational changes that lead to STIM1 oligomerization and ORAI1 channel activation following SR Ca2+ depletion. Bohm and colleagues were the first to describe four heterozygous dominant missense mutations in four families with dominant TAM, each affecting a highly-conserved EF1/2 hand amino acid residue [62]. Members from these families displayed a clinical spectrum of myopathic features including elevated serum creatine kinase levels, wrist contractures, ophthalmoplegia, miosis, as well as mild and slowly progressive lower-limb muscle weakness [62] (Table 1). Ten different STIM1 EF-hand mutations, five each in both the cEF1- and hEF2-hands, have been reported to date. By disrupting the ability of EF1/2 hands to bind Ca2+ and/or destabilizing the interaction with the sterile α-motif (SAM) domain, these mutations promote STIM1 oligomerization and ORAI1 channel activation even in the absence of SR Ca2+ depletion. Consistent with this, in contrast to wild type STIM1, the STIM1 EF-hand mutants promote clustering of STIM1 monomers independently from thapsigargin-induced store depletion following expression in C2C12 myoblasts. Moreover, myoblasts obtained from a patient with one of these mutations (p.D84G) were shown to exhibit elevated levels of resting Ca2+ and increased thapsigargin-induced and thapsigargin-independent Ca2+ influx compared to that of control myoblasts [62].
Similar to TAM, patients affected by Stormorken disease, a rare autosomal-dominantly inherited disorder characterized by a complex clinical picture including thrombocytopenia, platelet defects, congenital miosis, and proximal muscle weakness (Table 1), also exhibit increased serum levels of creatine kinase and skeletal muscle fibers with histological evidence of myopathy with TAs [81, 82]. A mutation in the first cytosolic coiled-coil domain of STIM1 (p.R304W), a domain that plays a critical role in maintaining STIM1 in a closed, inactive state until SR Ca2+ levels are reduced enough to dissociate Ca2+ from the EF-hand domains, was shown to be associated with Stormorken disease [64, 65, 83]. The p.R304W mutation is proposed to disrupt the conformation of an inhibitory helix in the first coiled-coil domain that unlocks the inhibitory state of STIM1, thus resulting in a gain-of-function effect on STIM1 function [83]. Indeed, the p.R304W mutation promotes formation of STIM1 puncta and CRAC channel activation even in the absence of thapsigargin-induced store depletion [64, 65, 83]. In addition, a recent study reported a muscle degenerative phenotype in p.R304W knock-in mice [84].
2.2.3. ORAI1 Gain-of-Function Mutations
To date, four autosomal dominant inherited gain-of-function mutations in ORAI1 have been reported in families with TAM (p.G98S and p.L138F) and Stormoken-like disease (p.S97C and p.245L) (Figure 1). The two TAM mutations, p.G98S and p.L138F, located in transmembrane domains 1 and 2, respectively, result in constitutively activatedORAI1 channels that permit Ca2+ influx independently of Ca2+ store depletion and STIM1 oligomerization [63]. The p.P245L mutation, located in transmembrane domain 4, results in a Stormorken-like phenotype characterized by myopathy with TAs and congenital miosis, but in the absence of thrombocytopenia and platelet defects [64]. Heterologous co-expression of STIM1 and either wild type or p.P245L ORAI1 in HEK 293 cells revealed that this mutation markedly reduces Ca2+ dependent inactivation of ORAI1, and thus, enhances SOCE, but still requires STIM1 to be activated [64]. The p.S97C mutation in ORAI1, located in the transmembrane domain 1, is associated with mild, late-onset TAM with congenital miosis, consistent with a Stormorken-like syndrome in the absence of thrombocytopathy (Table 1). Similar to the adjacent p.G98S TAM mutation, the p.S97C mutation results in a constitutively active channel that increases Ca2+ influx both in the absence and presence of store depletion [85]. Functional assays conducted in both transfected HEK 293 cells and patient-derived myotubes revealed that the p.S97C mutation in ORAI1 increases the rate of SOCE, consistent with a gain-of-function effect on the channel. The reason(s) for the markedly earlier onset and more severe clinical presentation observed in TAM patients possessing the p.G98S mutation compared to those with milder Stormorken-like syndrome of patients with the p.S97C mutation is not entirely clear. As one possibility, the difference in phenotypic expression could be due to a larger effect of the p.G98S mutations on heterotypic ORAI1 channel function and/or other genetic or environmental factors that influence the clinical phenotype [85].
3. SOCE Activity as a Modifier of Skeletal Muscle Disorders.
In addition to loss-of-function and gain-of-function mutations in STIM1 and ORAI1 directly causing muscle disease, altered STIM1/ORAI1 function also indirectly contributes to or amplifies the pathogenesis of other muscle disorders including muscular dystrophy, malignant hyperthermia, and sarcopenia.
3.1. SOCE and Muscular Dystrophy
Muscular dystrophy (MD) comprises a heterogeneous group of muscle diseases characterized by weakness and progressive muscle degeneration. The most common MD is Duchenne muscular dystrophy (DMD), an incurable X-linked disorder resulting from loss-of-function mutations to the gene that encodes dystrophin [86]. Dystrophin, a 427 KDa structural protein on the cytoplasmic face of the sarcolemma [87], anchors actin filaments and microtubules to a group of proteins collectively known as the dystrophin-associated protein complex [88]. The dystrophin-associated protein complex stabilizes sarcolemmal integrity during mechanical stress [89] by linking the cytoskeleton to laminin in the extracellular matrix [90]. While disruption of sarcolemmal integrity is a common feature among different MDs, the exact mechanism(s) that underlies progressive muscle fiber degeneration remains controversial. A longstanding hypothesis for muscle fiber degeneration in DMD is that alterations in the sarcolemma stability promote excessive Ca2+ entry that triggers calpain- and mitochondrial-mediated cell death [91]. Consistent with this hypothesis, defective Ca2+ homeostasis has been shown to play a role in the pathogenesis of the dystrophic phenotype. For example, both Ca2+ influx and total Ca2+ content are increased in muscle biopsies from DMD patients and in skeletal muscle fibers from mdx mice (a murine dystrophin-deficient model of DMD) [92, 93]. Myoplasmic Ca2+ overload could trigger an array of intracellular pathways/mechanisms responsible for the dystrophic phenotype including: mitochondrial Ca2+ overload, enhanced oxidative stress, and activation of the Ca2+-dependent proteases [94–96]. However, the mechanisms for enhanced Ca2+ influx in dystrophic fibers, including through “microtears” in the sarcolemma or specific ion channels, remain uncertain and continue to be debated. Over the past two decades, increased activity of several different classes of Ca2+ channels (e.g. Ca2+ leak channels, stretch-activated channels, receptor-operated channels and SOCE channels) have all been proposed to promote the dystrophic phenotype in DMD [97–104].
A growing number of reports provide evidence for a modulatory role of SOCE in the pathogenesis of DMD. Early studies found that SOCE activity is upregulated in muscle from mdx mice [93], though the molecular components at that time remained unknown. Prior to the identification of STIM1 and ORAI1 in coordinating SOCE in muscle (see section 1.2.1 above), Vandebrouck and colleagues found that upregulation of TRPC1 and TRPC3 voltage-independent Ca2+-permeable channels sensitive to thapsigargin- and caffeine-mediated store depletion increases Ca2+ influx in muscle fibers from mdx mice [100]. Consistent with these results, overexpression of TRPC3 in muscle enhanced, while dominant negative TRPC4 expression reduced, the dystrophic phenotype of mdx and δ-sarcoglycan-deficient (Sgcd−/−) mice [101]. A similar reduction in the dystrophic phenotype was also observed following overexpression of dominant negative stretch-activated TRPV2 channels [105]. Moreover, different levels of stretch-activated TRPC1 channel activity correlates with DMD in mice [106] and muscle damage following eccentric contractions is reduced in TRPC1 knockout mice [107].
Several studies provide evidence for a modulatory contribution of STIM1/ORAI1-dependent Ca2+ influx to the dystrophic phenotype of mdx mice. First of all, muscle fibers from mdx mice exhibit increased STIM1/ORAI1 expression and SOCE function, including a shift in SOCE activation and deactivation thresholds to higher SR luminal Ca2+ concentrations [39, 108]. In line with these findings, Zhao and colleagues found an increase in both ORAI1 mRNA and protein levels coincident with enhanced SOCE activity and SR Ca2+ storage in muscle from mdx mice, although STIM1 levels were unaltered in this study [102]. This enhanced SOCE and SR Ca2+ storage were reduced by either shRNA-mediated ORAI1 knockdown or treatment of animals with BTP-2, a potent CRAC channel inhibitor. Together, these studies suggest that increased STIM1/ORAI1dependent SOCE contributes to Ca2+-mediated muscle fiber degeneration in mdx mice. Consistent with this idea, muscle-specific STIM1 overexpression enhances SOCE and promotes a dystrophic phenotype (e.g. increased inflammation, fibrosis, necrosis, mitochondrial swelling, and serum CK levels) similar to that observed in mdx and Sgcd−/− mice [103]. Importantly, both SOCE and MD of mdx and Sgcd−/− mice are reduced by crossing with transgenic mice with muscle-specific expression of dominant-negative ORAI1 (dnORAI1) [103]. These findings support a modulatory role for STIM1/ORAI1-mediated Ca2+ entry in the pathogenesis of MD.
3.2. SOCE and Malignant Hyperthermia
Malignant hyperthermia (MH) susceptibility is an inherited pharmacogenetic disorder characterized by a life-threatening response to halogenated anesthetics (i.e. halothane, isoflurane) and certain muscle relaxants (e.g. succinylcholine) commonly used during surgical procedures [109]. The exposure of susceptible individuals to these agents triggers a hypermetabolic reaction characterized by skeletal muscle rigidity, rise in core body temperature (e.g. hyperthermia), rupture of skeletal muscle fibers (e.g. rhabdomyolysis) and dangerous increases in serum levels of creatine kinase and potassium. MH crises can be lethal if not immediately corrected by suspension of triggering agents, cooling and administration of dantrolene, the only FDA-approved drug to treat MH reactions. At the molecular level, exposure of MH susceptible individuals to triggering agents induces an uncontrolled sustained rise in myoplasmic Ca2+ concentration as a result of activation of RYR1 Ca2+ release channels in the SR [109]. In ~70% of cases, MH susceptibility is caused by mutations in the RYR1 gene [110, 111]. Mutations in the α1S-subunit of the DHPR account for another ~1% of cases [112, 113]. The genetic cause of the remaining ~30% of cases remains unknown. While mice lacking CASQ1 (CASQ1-null) exhibit a phenotype resembling MH in humans [114, 115], mutations in CASQ1 were not identified in a large North American cohort of MH patients [116]. An in vitro caffeine-halothane contracture test (IVCT or CHCT), which measures the sensitivity of a muscle biopsies to caffeine- and halothane-induced contractures, was developed as a diagnostic assay for MH susceptibility in the late 1980s and continues to be the gold standard for MH diagnosis [117, 118].
For many years it was assumed that the sustained rise in myoplasmic Ca2+ during an MH event was solely the consequence of an uncontrolled activation of RYR1 Ca2+ release from the SR. However, early optimization studies for the diagnostic IVCT/CHCT found that the assay only worked when extracellular Ca2+ is included in the solution bathing the muscle biopsy [119–121], implicating a critical role for extracellular Ca2+ in the enhanced sensitivity of MH susceptible muscle. More recently, MH triggering agents were proposed to promote sustained RYR1-dependent Ca2+ release sufficient to deplete SR Ca2+ stores [114, 122–124], which in turn, activates SOCE channels to trigger Ca2+ influx from an essentially infinite extracellular compartment. The combined effects of sustained SR Ca2+ release, store depletion, and massive SOCE-dependent Ca2+ influx result in the myoplasmic Ca2+ overload, hypercontractures, heat generation and rhabdomyolysis that occur during an MH event. The first direct evidence for a role of SOCE in MH pathogenesis was provided by Steele and colleagues who used mechanically-skinned fibers from normal and MH susceptible individuals to show that therapeutic levels of halothane induce SR Ca2+ release and store depletion sufficient to activate STIM1-dependent Ca2+ influx in muscle fibers from MH susceptible, but not normal, individuals [125]. Consistent with this, Cully et al (2018) used a skinned fiber approach to show that muscle fibers from MH-susceptible individuals exhibit enhanced RYR1 Ca2+ leak, persistent SOCE activity, and a compensatory increase in capacity to extrude Ca2+ across the TT system compared with that observed for muscle fibers from control individuals [126].
Evidence from validated mouse models of MH susceptibility (Ryr1Y522S/+ and Ryr1R163C/+ RYR1 knock-in mice) provides additional support for a role of SOCE in MH pathogenesis. As is observed for other MH-causative mutations in RYR1, the Y522S and R163C promote RYR1 Ca2+ leak and increased sensitivity of the release channel to be opened by a wide variety of activators (including halothane, caffeine, 4-chloro-m-cresol, DHPR voltage sensor) [109]. Following homologous expression in RYR1-null myotubes, the Y522S MH mutation produces the highest degree of RYR1 Ca2+ leak, sensitization and SR Ca2+ store depletion of the MH-causative mutations studied in this system [127, 128]. Consistent with these findings, RYR1 channel permeability is increased (>2fold) and resting SR Ca2+ store content reduced (~50%) in muscle fibers from Ryr1Y522S/+ mice [129], which would be expected to increase SOCE and explain the elevation in resting Ca2+ levels [130, 131]. A similar increased RYR1 Ca2+ leak linked to massive reduction in SR Ca2+ content is observed in muscle fibers from CASQ1-null mice [114, 132, 133]. Consistent with an increased loss of SR Ca2+ via RYR, the rate of SOCE activation is accelerated in myotubes from both Ryr1Y522S/+ and CASQ1-null mice [134]. Dantrolene directly inhibits RYR1 channel activity in a calmodulin- [135] and Mg2+-dependent manner [136], but does not directly inhibit SOCE channels [134].
However, as SOCE activation lies downstream of store depletion, dantrolene indirectly inhibits SOCE by protecting stores from becoming depleted as a result of reducing RYR1 Ca2+ leak.
Myotubes derived Ryr1R163C/+ mice exhibit increased basal sarcolemmal divalent cation influx that results in a chronically elevated resting Ca2+ concentration [137]. Both elevated basal divalent cation influx and resting Ca2+ levels were reduced by inhibitors of ORAI1-dependent SOCE (BTP2, Gd3+, expression of dominant-negative ORAI1) and TRPC channels (Gd3+, GsMTx-4). Importantly, elegant in vivo measurements of resting Ca2+ levels in vastus lateralis muscles of control and Ryr1R163C/+ mice during exposure to 1.5% halothane vapor, revealed that local application of BTP-2, Gd3+ or GsMTx-4 all markedly reduced halothane-induced elevations in resting Ca2+ [137]. Taken together, studies conducted in muscle from MH susceptible individuals and validated murine models of MH support a paradigm by which SOCE serves as a modifier or amplifier of MH pathogenesis such that STIM1/ORAI1 coupling could represent a potential therapeutic target for the treatment of MH in humans.
3.3. SOCE and Age-related Muscle Dysfunction
Aging is a complex, irreversible process characterized by morphological alterations and progressive decline of multiple biological/physiological functions. Age-related loss of skeletal muscle function (sarcopenia) is characterized by a marked reduction in muscle mass, lowered muscle strength, increased susceptibility to fatigue, and reduced velocity of contraction [138, 139]. The underlying causes of sarcopenia includes loss of muscle mass (atrophy) due to reduced fiber number and size [140, 141], muscle stem cell depletion that leads to neuromuscular junction degeneration [142], and loss (1015%) and remodeling of motor units [143]. However, these effects alone are not sufficient to account for the degree of muscle weakness observed with aging. Impairments in intrinsic muscle fiber capacity for Ca2+ release and mitochondrial ATP production contribute to a reduction in specific force production. A decrease in DHPR expression leads to an uncoupling between DHPR and RYR1 proteins that results in a reduction in Ca2+ available to activate the contractile filaments [144, 145]. A decrease in number/area and altered morphology of CRUs also contributes to the reduction in electrically-evoked Ca2+ release and skeletal muscle force production [146, 147]. Finally, alterations in mitochondrial structure, function, and number also play a role in age-related decreases in muscle performance [148–150]. Thus, impairments in Ca2+ release and mitochondrial energy production contribute to the observed age-dependent decline in muscle specific force.
A reduction in STIM1/ORAI1-mediated SOCE is also proposed to contribute to the decline in muscle contractile force and increase in susceptibility to fatigue in aging. In support of this idea, muscle fatigue during repetitive, high-frequency stimulation is accelerated [27] and SOCE is reduced in skeletal muscle from aged (2 year old) mice [151]. Interestingly, the reduction of SOCE in muscle from aged mice was reported to occur in the absence of a change in transcript levels for either STIM1 or ORAI1 [151]. However, a significant reduction in the expression levels of mitsugumin-29 was reported in aged muscles [151], consistent with reduced mitsugumin-29 expression causing a disruption of triad architecture and decrease in SOCE [27]. Thornton and colleagues showed that SOCE is required to maintain contractile force during repetitive contractions in soleus muscles from young mice, but not from aged mice [152]. Together, these studies suggest that an inability to recover Ca2+ ions from the extracellular space via SOCE during periods of intense activity contributes to a reduction in force generation and increase in susceptibility to fatigue in aging. This idea is further supported by the fact that pharmacological inhibition of SOCE in muscles from young mice similarly results in a reduction in force generation and increase in susceptibility to fatigue [21, 27, 152]. However, the potential role of altered SOCE in sarcopenia is controversial. For example, Payne and colleagues reported that while sustained contraction during high-frequency stimulation depends on extracellular Ca2+ in a subpopulation of muscle fibers from aged mice, this was due to an effect of extracellular Ca2+ on excitability rather than insufficient SOCE [153]. In addition, Edwards and colleagues concluded that in spite of a 40% reduction in STIM1 expression (in the absence of a change in ORAI1 expression), SOCE is unaltered in muscle fibers from aged mice [154]. Thus, the relative role of STIM1/ORAI1-dependent SOCE in the age-dependent decline of skeletal muscle performance remains enigmatic. Clearly, additional studies are needed to resolve this issue.
4. Conclusions and Future Directions
Over the past two decades, the importance of Ca2+ entry in skeletal muscle has garnered significant attention. As a result, STIM1/ORAI1-mediated SOCE in muscle has unequivocally been demonstrated, shown to promote muscle growth/differentiation and limit force decay during sustained muscle activity. In addition, defects in STIM1/ORAI1-mediated SOCE both directly and indirectly contribute to a wide range of different clinical myopathies. Nevertheless, several important unresolved issues and open questions remain to be addressed.
Boncompagni and colleagues reported that acute treadmill exercise triggers a remodeling of sarcotubular membranes that results in an elongation of the TT from the triad into the I-band, toward the Z-line, to form junctional contact with flat-parallel stacks of SR cisternae [21]. As STIM1 and ORAI1 proteins are present within these newly formed regions of SR-TT contact, the junctions were termed CEUs. However the precise molecular mechanisms that control exercise-dependent remodeling of SR and TT membranes are unknown. For example, future studies will need to determine the specific exercised-induced signals, as well as the relative roles of STIM1S, STIM1L and ORAI1, in both remodeling of the free SR into well-ordered flat-parallel stacks of cisternae and extensions of the TT into these stacks of membranes. The potential role of proteins known to be involved in TT biogenesis and maintenance (e.g. junctophilins, myotubularin-1, dysferylin, BIN-1; [155]), in exercise-induced elongation of the TT and CEU formation, as well as the time course for the formation and disassembly of CEUs, need to be determined. Finally, although extensor digitorum longus muscles from mice subjected to exercise exhibit an increased frequency of CEUs containing STIM1 and ORAI1 proteins and enhanced resistance to fatigue [21], the relative impact of CEUs on the magnitude and properties of SOCE after exercise, as well as opposing Ca2+ efflux mechanisms, compared to that observed under resting conditions needs to be determined. Such an analysis would also provide insight into the relative contribution of STIM1/ORAI1 coupling from CRUs versus CEUs in SOCE in muscle at rest and after exercise.
Another major unresolved issue involves the precise relationship between SOCE function and the formation of TAs in skeletal muscle. It is now clear that gain-of-function mutations in both STIM1 and ORAI1 result in clinical myopathies with TAs being a central defining histopathological characteristic. However, the mechanism(s) by which constitutively active SOCE leads to the formation of TAs is unknown. Another unresolved issue is whether the formation and properties of TAs resulting from gain-of-function STIM1 and ORAI1 mutations are similar or fundamentally distinct from those observed in aging in which SOCE may be reduced. In addition, it is unclear if TAs contribute to the hypotonia and muscle weakness experienced by individuals with TAM or rather if their formation is a protective compensatory response designed to limit Ca2+ induced damage (e.g. increased proteolysis, oxidative stress, activation of the mitochondrial permeability transition pore). Alternatively, analogous to how physiological hypertrophy may initially be protective to the heart while later being detrimental, TAs could initially be protective by providing a sink for constitutive SOCE, but over time, become detrimental as larger and more extensive TAs begin to interfere with the EC coupling and myofilament apparatus. In any event, answers to these questions will require animal models that faithfully recapitulate the clinical and histopathological phenotype of human TAM patients. Finally, as loss- and gain-of-function mutations in both STIM1 and ORAI1 directly cause several muscle pathologies (see section 2.0) and SOCE dysfunction contributes to the pathogenesis of other muscle disorders (see section 3.0), STIM1/ORAI1-mediated SOCE in muscle could emerge as an intriguing potential new therapeutic target to treat a wide range of debilitating human myopathies in the future, though potential side effects of inhibiting ORAI1 channels in muscle (e.g. iris sphincter dysfunction) would need to be appropriately managed.
Highlights.
STIM1 and ORAI1 coordinate SOCE in skeletal muscle.
STIM1/ORAI1 SOCE promotes muscle development/growth and maintains Ca2+ store content during periods of prolonged, high-frequency stimulation.
Loss-of-function and gain-of-function mutations in STIM1 and ORAI1 result in an eclectic array of disorders with clinical myopathy as a central defining component.
Dysfunctional STIM/ORAI1 SOCE also contributes to the pathogenesis of other muscle disorders including muscular dystrophy, malignant hyperthermia, and sarcopenia.
Tight regulation of STIM1/ORAI1 SOCE is critical for optimal skeletal muscle function.
List of frequently used abbreviations:
- Ca2+
calcium
- CID
combined immunodeficiency
- CASQ1
calsequestrin-1
- CRAC
Ca2+-released-activated-Ca2+
- CEU
Ca2+ entry unit
- CRU
Ca2+ release unit
- DHPR
dihydropyridine receptor
- EC coupling
excitation-contraction coupling
- DMD
Duchenne muscular dystrophy
- ER
endoplasmic reticulum
- MH
malignant hyperthermia
- MD
muscular dystrophy
- RYR1
ryanodine receptor type-1
- SOCE
store-operated Ca2+ entry
- SR
sarcoplasmic reticulum
- STIM1
stromal interaction molecule-1
- TA
tubular aggregates
- TAM
tubular aggregate myopathy
- TRP
transient receptor potential cation
- TT
transverse tubule.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- [1].Putney JW Jr., A model for receptor-regulated calcium entry, Cell Calcium, 7 (1986) 1–12. [DOI] [PubMed] [Google Scholar]
- [2].Hoth M, Penner R, Depletion of intracellular calcium stores activates a calcium current in mast cells, Nature, 355 (1992) 353–356. [DOI] [PubMed] [Google Scholar]
- [3].Roos J, DiGregorio PJ, Yeromin AV, Ohlsen K, Lioudyno M, Zhang S, Safrina O, Kozak JA, Wagner SL, Cahalan MD, Velicelebi G, Stauderman KA, STIM1, an essential and conserved component of store-operated Ca2+ channel function, J Cell Biol, 169 (2005) 435–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Feske S, Gwack Y, Prakriya M, Srikanth S, Puppel SH, Tanasa B, Hogan PG, Lewis RS, Daly M, Rao A, A mutation in Orai1 causes immune deficiency by abrogating CRAC channel function, Nature, 441 (2006) 179–185. [DOI] [PubMed] [Google Scholar]
- [5].Prakriya M, Lewis RS, Store-Operated Calcium Channels, Physiol Rev, 95 (2015) 1383–1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Lewis RS, The molecular choreography of a store-operated calcium channel, Nature, 446 (2007) 284287. [DOI] [PubMed] [Google Scholar]
- [7].Melzer W, Herrmann-Frank A, Luttgau HC, The role of Ca2+ ions in excitation-contraction coupling of skeletal muscle fibres, Biochim Biophys Acta, 1241 (1995) 59–116. [DOI] [PubMed] [Google Scholar]
- [8].Zhang L, Kelley J, Schmeisser G, Kobayashi YM, Jones LR, Complex formation between junctin, triadin, calsequestrin, and the ryanodine receptor. Proteins of the cardiac junctional sarcoplasmic reticulum membrane, J Biol Chem, 272 (1997) 23389–23397. [DOI] [PubMed] [Google Scholar]
- [9].Dulhunty AF, Wei-LaPierre L, Casarotto MG, Beard NA, Core skeletal muscle ryanodine receptor calcium release complex, Clin Exp Pharmacol Physiol, 44 (2017) 3–12. [DOI] [PubMed] [Google Scholar]
- [10].Rebbeck RT, Karunasekara Y, Board PG, Beard NA, Casarotto MG, Dulhunty AF, Skeletal muscle excitation-contraction coupling: who are the dancing partners?, Int J Biochem Cell Biol, 48 (2014) 28–38. [DOI] [PubMed] [Google Scholar]
- [11].Meissner G, Isolation and characterization of two types of sarcoplasmic reticulum vesicles, Biochim Biophys Acta, 389 (1975) 51–68. [DOI] [PubMed] [Google Scholar]
- [12].Yano K, Zarain-Herzberg A, Sarcoplasmic reticulum calsequestrins: structural and functional properties, Mol Cell Biochem, 135 (1994) 61–70. [DOI] [PubMed] [Google Scholar]
- [13].Campbell KP, MacLennan DH, Jorgensen AO, Mintzer MC, Purification and characterization of calsequestrin from canine cardiac sarcoplasmic reticulum and identification of the 53,000 dalton glycoprotein, J Biol Chem, 258 (1983) 1197–1204. [PubMed] [Google Scholar]
- [14].Beard NA, Sakowska MM, Dulhunty AF, Laver DR, Calsequestrin is an inhibitor of skeletal muscle ryanodine receptor calcium release channels, Biophys J, 82 (2002) 310–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Ikemoto N, Ronjat M, Meszaros LG, Koshita M, Postulated role of calsequestrin in the regulation of calcium release from sarcoplasmic reticulum, Biochemistry, 28 (1989) 6764–6771. [DOI] [PubMed] [Google Scholar]
- [16].Launikonis BS, Rios E, Store-operated Ca2+ entry during intracellular Ca2+ release in mammalian skeletal muscle, J Physiol, 583 (2007) 81–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Armstrong CM, Bezanilla FM, Horowicz P, Twitches in the presence of ethylene glycol bis( -aminoethyl ether)-N,N’-tetracetic acid, Biochim Biophys Acta, 267 (1972) 605–608. [DOI] [PubMed] [Google Scholar]
- [18].Gissel H, Clausen T, Excitation-induced Ca2+ uptake in rat skeletal muscle, Am J Physiol, 276 (1999) R331–339. [DOI] [PubMed] [Google Scholar]
- [19].Wei-Lapierre L, Carrell EM, Boncompagni S, Protasi F, Dirksen RT, Orai1-dependent calcium entry promotes skeletal muscle growth and limits fatigue, Nat Commun, 4 (2013) 2805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Carrell EM, Coppola AR, McBride HJ, Dirksen RT, Orai1 enhances muscle endurance by promoting fatigue-resistant type I fiber content but not through acute store-operated Ca2+ entry, FASEB J, 30 (2016) 4109–4119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Boncompagni S, Michelucci A, Pietrangelo L, Dirksen RT, Protasi F, Exercise-dependent formation of new junctions that promote STIM1-Orai1 assembly in skeletal muscle, Sci Rep, 7 (2017) 14286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Hopf FW, Reddy P, Hong J, Steinhardt RA, A capacitative calcium current in cultured skeletal muscle cells is mediated by the calcium-specific leak channel and inhibited by dihydropyridine compounds, J Biol Chem, 271 (1996) 22358–22367. [DOI] [PubMed] [Google Scholar]
- [23].Kurebayashi N, Ogawa Y, Depletion of Ca2+ in the sarcoplasmic reticulum stimulates Ca2+ entry into mouse skeletal muscle fibres, J Physiol, 533 (2001) 185–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Kiselyov K, Xu X, Mozhayeva G, Kuo T, Pessah I, Mignery G, Zhu X, Birnbaumer L, Muallem S, Functional interaction between InsP3 receptors and store-operated Htrp3 channels, Nature, 396 (1998) 478482. [DOI] [PubMed] [Google Scholar]
- [25].Bennett DL, Bootman MD, Berridge MJ, Cheek TR, Ca2+ entry into PC12 cells initiated by ryanodine receptors or inositol 1,4,5-trisphosphate receptors, Biochem J, 329 ( Pt 2) (1998) 349–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Kiselyov KI, Shin DM, Wang Y, Pessah IN, Allen PD, Muallem S, Gating of store-operated channels by conformational coupling to ryanodine receptors, Mol Cell, 6 (2000) 421–431. [DOI] [PubMed] [Google Scholar]
- [27].Pan Z, Yang D, Nagaraj RY, Nosek TA, Nishi M, Takeshima H, Cheng H, Ma J, Dysfunction of store-operated calcium channel in muscle cells lacking mg29, Nat Cell Biol, 4 (2002) 379–383. [DOI] [PubMed] [Google Scholar]
- [28].Launikonis BS, Barnes M, Stephenson DG, Identification of the coupling between skeletal muscle store-operated Ca2+ entry and the inositol trisphosphate receptor, Proc Natl Acad Sci U S A, 100 (2003) 2941–2944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Rosenberg P, Hawkins A, Stiber J, Shelton JM, Hutcheson K, Bassel-Duby R, Shin DM, Yan Z, Williams RS, TRPC3 channels confer cellular memory of recent neuromuscular activity, Proc Natl Acad Sci U S A, 101 (2004) 9387–9392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Blaauw B, Del Piccolo P, Rodriguez L, Hernandez Gonzalez VH, Agatea L, Solagna F, Mammano F, Pozzan T, Schiaffino S, No evidence for inositol 1,4,5-trisphosphate-dependent Ca2+ release in isolated fibers of adult mouse skeletal muscle, J Gen Physiol, 140 (2012) 235–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Lyfenko AD, Dirksen RT, Differential dependence of store-operated and excitation-coupled Ca2+ entry in skeletal muscle on STIM1 and Orai1, J Physiol, 586 (2008) 4815–4824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Lee EH, Cherednichenko G, Pessah IN, Allen PD, Functional coupling between TRPC3 and RyR1 regulates the expressions of key triadic proteins, J Biol Chem, 281 (2006) 10042–10048. [DOI] [PubMed] [Google Scholar]
- [33].Stiber J, Hawkins A, Zhang ZS, Wang S, Burch J, Graham V, Ward CC, Seth M, Finch E, Malouf N, Williams RS, Eu JP, Rosenberg P, STIM1 signalling controls store-operated calcium entry required for development and contractile function in skeletal muscle, Nat Cell Biol, 10 (2008) 688–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Vig M, DeHaven WI, Bird GS, Billingsley JM, Wang H, Rao PE, Hutchings AB, Jouvin MH, Putney JW, Kinet JP, Defective mast cell effector functions in mice lacking the CRACM1 pore subunit of store-operated calcium release-activated calcium channels, Nat Immunol, 9 (2008) 89–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Li T, Finch EA, Graham V, Zhang ZS, Ding JD, Burch J, Oh-hora M, Rosenberg P, STIM1Ca(2+) signaling is required for the hypertrophic growth of skeletal muscle in mice, Mol Cell Biol, 32 (2012) 3009–3017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Soboloff J, Rothberg BS, Madesh M, Gill DL, STIM proteins: dynamic calcium signal transducers, Nat Rev Mol Cell Biol, 13 (2012) 549–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Oh MR, Lee KJ, Huang M, Kim JO, Kim DH, Cho CH, Lee EH, STIM2 regulates both intracellular Ca(2+) distribution and Ca(2+) movement in skeletal myotubes, Sci Rep, 7 (2017) 17936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Wu MM, Buchanan J, Luik RM, Lewis RS, Ca2+ store depletion causes STIM1 to accumulate in ER regions closely associated with the plasma membrane, J Cell Biol, 174 (2006) 803–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Edwards JN, Friedrich O, Cully TR, von Wegner F, Murphy RM, Launikonis BS, Upregulation of store-operated Ca2+ entry in dystrophic mdx mouse muscle, Am J Physiol-Cell Ph, 299 (2010) C42–C50. [DOI] [PubMed] [Google Scholar]
- [40].Dirksen RT, Checking your SOCCs and feet: the molecular mechanisms of Ca2+ entry in skeletal muscle, J Physiol-London, 587 (2009) 3139–3147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Nishi M, Komazaki S, Kurebayashi N, Ogawa Y, Noda T, Iino M, Takeshima H, Abnormal features in skeletal muscle from mice lacking mitsugumin29, J Cell Biol, 147 (1999) 1473–1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Darbellay B, Arnaudeau S, Bader CR, Konig S, Bernheim L, STIM1L is a new actin-binding splice variant involved in fast repetitive Ca2+ release, J Cell Biol, 194 (2011) 335–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Liou J, Fivaz M, Inoue T, Meyer T, Live-cell imaging reveals sequential oligomerization and local plasma membrane targeting of stromal interaction molecule 1 after Ca2+ store depletion, Proc Natl Acad Sci U S A, 104 (2007) 9301–9306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Navarro-Borelly L, Somasundaram A, Yamashita M, Ren D, Miller RJ, Prakriya M, STIM1-Orai1 interactions and Orai1 conformational changes revealed by live-cell FRET microscopy, J Physiol, 586 (2008) 5383–5401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Calloway N, Vig M, Kinet JP, Holowka D, Baird B, Molecular clustering of STIM1 with Orai1/CRACM1 at the plasma membrane depends dynamically on depletion of Ca2+ stores and on electrostatic interactions, Mol Biol Cell, 20 (2009) 389–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Orci L, Ravazzola M, Le Coadic M, Shen WW, Demaurex N, Cosson P, From the Cover: STIM1induced precortical and cortical subdomains of the endoplasmic reticulum, Proc Natl Acad Sci U S A, 106 (2009) 19358–19362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Perni S, Dynes JL, Yeromin AV, Cahalan MD, Franzini-Armstrong C, Nanoscale patterning of STIM1 and Orai1 during store-operated Ca2+ entry, Proc Natl Acad Sci U S A, 112 (2015) E5533–5542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Stiber JA, Rosenberg PB, The role of store-operated calcium influx in skeletal muscle signaling, Cell Calcium, 49 (2011) 341–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Kiviluoto S, Decuypere JP, De Smedt H, Missiaen L, Parys JB, Bultynck G, STIM1 as a key regulator for Ca2+ homeostasis in skeletal-muscle development and function, Skelet Muscle, 1 (2011) 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Darbellay B, Arnaudeau S, Konig S, Jousset H, Bader C, Demaurex N, Bernheim L, STIM1- and Orai1-dependent store-operated calcium entry regulates human myoblast differentiation, J Biol Chem, 284 (2009) 5370–5380. [DOI] [PubMed] [Google Scholar]
- [51].Lee KJ, Woo JS, Hwang JH, Hyun C, Cho CH, Kim DH, Lee EH, STIM1 negatively regulates Ca(2)(+) release from the sarcoplasmic reticulum in skeletal myotubes, Biochem J, 453 (2013) 187–200. [DOI] [PubMed] [Google Scholar]
- [52].Yarotskyy V, Dirksen RT, Temperature and RyR1 regulate the activation rate of store-operated Ca(2)+ entry current in myotubes, Biophys J, 103 (2012) 202–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Zhao X, Yoshida M, Brotto L, Takeshima H, Weisleder N, Hirata Y, Nosek TM, Ma J, Brotto M, Enhanced resistance to fatigue and altered calcium handling properties of sarcalumenin knockout mice, Physiol Genomics, 23 (2005) 72–78. [DOI] [PubMed] [Google Scholar]
- [54].Sztretye M, Geyer N, Vincze J, Al-Gaadi D, Olah T, Szentesi P, Kis G, Antal M, Balatoni I, Csernoch L, Dienes B, SOCE Is Important for Maintaining Sarcoplasmic Calcium Content and Release in Skeletal Muscle Fibers, Biophys J, 113 (2017) 2496–2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Feske S, ORAI1 and STIM1 deficiency in human and mice: roles of store-operated Ca2+ entry in the immune system and beyond, Immunol Rev, 231 (2009) 189–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].McCarl CA, Picard C, Khalil S, Kawasaki T, Rother J, Papolos A, Kutok J, Hivroz C, Ledeist F, Plogmann K, Ehl S, Notheis G, Albert MH, Belohradsky BH, Kirschner J, Rao A, Fischer A, Feske S, ORAI1 deficiency and lack of store-operated Ca2+ entry cause immunodeficiency, myopathy, and ectodermal dysplasia, J Allergy Clin Immunol, 124 (2009) 1311–1318 e1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Lian J, Cuk M, Kahlfuss S, Kozhaya L, Vaeth M, Rieux-Laucat F, Picard C, Benson MJ, Jakovcevic A, Bilic K, Martinac I, Stathopulos P, Kacskovics I, Vraetz T, Speckmann C, Ehl S, Issekutz T, Unutmaz D, Feske S, ORAI1 mutations abolishing store-operated Ca(2+) entry cause anhidrotic ectodermal dysplasia with immunodeficiency, J Allergy Clin Immunol, (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Picard C, McCarl CA, Papolos A, Khalil S, Luthy K, Hivroz C, LeDeist F, Rieux-Laucat F, Rechavi G, Rao A, Fischer A, Feske S, STIM1 mutation associated with a syndrome of immunodeficiency and autoimmunity, N Engl J Med, 360 (2009) 1971–1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Fuchs S, Rensing-Ehl A, Speckmann C, Bengsch B, Schmitt-Graeff A, Bondzio I, Maul-Pavicic A, Bass T, Vraetz T, Strahm B, Ankermann T, Benson M, Caliebe A, Folster-Holst R, Kaiser P, Thimme R, Schamel WW, Schwarz K, Feske S, Ehl S, Antiviral and regulatory T cell immunity in a patient with stromal interaction molecule 1 deficiency, J Immunol, 188 (2012) 1523–1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Maus M, Jairaman A, Stathopulos PB, Muik M, Fahrner M, Weidinger C, Benson M, Fuchs S, Ehl S, Romanin C, Ikura M, Prakriya M, Feske S, Missense mutation in immunodeficient patients shows the multifunctional roles of coiled-coil domain 3 (CC3) in STIM1 activation, Proc Natl Acad Sci U S A, 112 (2015) 6206–6211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Lacruz RS, Feske S, Diseases caused by mutations in ORAI1 and STIM1, Ann N Y Acad Sci, 1356 (2015) 45–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Bohm J, Chevessier F, Maues De Paula A, Koch C, Attarian S, Feger C, Hantai D, Laforet P, Ghorab K, Vallat JM, Fardeau M, Figarella-Branger D, Pouget J, Romero NB, Koch M, Ebel C, Levy N, Krahn M, Eymard B, Bartoli M, Laporte J, Constitutive activation of the calcium sensor STIM1 causes tubular-aggregate myopathy, Am J Hum Genet, 92 (2013) 271–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Endo Y, Noguchi S, Hara Y, Hayashi YK, Motomura K, Miyatake S, Murakami N, Tanaka S, Yamashita S, Kizu R, Bamba M, Goto Y, Matsumoto N, Nonaka I, Nishino I, Dominant mutations in ORAI1 cause tubular aggregate myopathy with hypocalcemia via constitutive activation of store-operated Ca(2)(+) channels, Hum Mol Genet, 24 (2015) 637–648. [DOI] [PubMed] [Google Scholar]
- [64].Nesin V, Wiley G, Kousi M, Ong EC, Lehmann T, Nicholl DJ, Suri M, Shahrizaila N, Katsanis N, Gaffney PM, Wierenga KJ, Tsiokas L, Activating mutations in STIM1 and ORAI1 cause overlapping syndromes of tubular myopathy and congenital miosis, Proc Natl Acad Sci U S A, 111 (2014) 4197–4202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Misceo D, Holmgren A, Louch WE, Holme PA, Mizobuchi M, Morales RJ, De Paula AM, Stray-Pedersen A, Lyle R, Dalhus B, Christensen G, Stormorken H, Tjonnfjord GE, Frengen E, A dominant STIM1 mutation causes Stormorken syndrome, Hum Mutat, 35 (2014) 556–564. [DOI] [PubMed] [Google Scholar]
- [66].Markello T, Chen D, Kwan JY, Horkayne-Szakaly I, Morrison A, Simakova O, Maric I, Lozier J, Cullinane AR, Kilo T, Meister L, Pakzad K, Bone W, Chainani S, Lee E, Links A, Boerkoel C, Fischer R, Toro C, White JG, Gahl WA, Gunay-Aygun M, York platelet syndrome is a CRAC channelopathy due to gain-of-function mutations in STIM1, Mol Genet Metab, 114 (2015) 474–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Chevessier F, Bauche-Godard S, Leroy JP, Koenig J, Paturneau-Jouas M, Eymard B, Hantai D, Verdiere-Sahuque M, The origin of tubular aggregates in human myopathies, J Pathol, 207 (2005) 313–323. [DOI] [PubMed] [Google Scholar]
- [68].Jain D, Sharma MC, Sarkar C, Suri V, Sharma SK, Singh S, Das TK, Tubular aggregate myopathy: a rare form of myopathy, J Clin Neurosci, 15 (2008) 1222–1226. [DOI] [PubMed] [Google Scholar]
- [69].Salviati G, Pierobon-Bormioli S, Betto R, Damiani E, Angelini C, Ringel SP, Salvatori S, Margreth A, Tubular aggregates: sarcoplasmic reticulum origin, calcium storage ability, and functional implications, Muscle Nerve, 8 (1985) 299–306. [DOI] [PubMed] [Google Scholar]
- [70].Engel WK, Bishop DW, Cunningham GG, Tubular aggregates in type II muscle fibers: ultrastructural and histochemical correlation, J Ultrastruct Res, 31 (1970) 507–525. [DOI] [PubMed] [Google Scholar]
- [71].Schiaffino S, Severin E, Cantini M, Sartore S, Tubular aggregates induced by anoxia in isolated rat skeletal muscle, Lab Invest, 37 (1977) 223–228. [PubMed] [Google Scholar]
- [72].Rosenberg NL, Neville HE, Ringel SP, Tubular aggregates. Their association with neuromuscular diseases, including the syndrome of myalgias/cramps, Arch Neurol, 42 (1985) 973–976. [DOI] [PubMed] [Google Scholar]
- [73].Pierobon-Bormioli S, Armani M, Ringel SP, Angelini C, Vergani L, Betto R, Salviati G, Familial neuromuscular disease with tubular aggregates, Muscle Nerve, 8 (1985) 291–298. [DOI] [PubMed] [Google Scholar]
- [74].Morgan-Hughes JA, Tubular aggregates in skeletal muscle: their functional significance and mechanisms of pathogenesis, Curr Opin Neurol, 11 (1998) 439–442. [DOI] [PubMed] [Google Scholar]
- [75].Boncompagni S, Protasi F, Franzini-Armstrong C, Sequential stages in the age-dependent gradual formation and accumulation of tubular aggregates in fast twitch muscle fibers: SERCA and calsequestrin involvement, Age (Dordr), 34 (2012) 27–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Schiaffino S, Tubular aggregates in skeletal muscle: just a special type of protein aggregates?, Neuromuscul Disord, 22 (2012) 199–207. [DOI] [PubMed] [Google Scholar]
- [77].Helenius A, Aebi M, Intracellular functions of N-linked glycans, Science, 291 (2001) 2364–2369. [DOI] [PubMed] [Google Scholar]
- [78].Groenendyk J, Sreenivasaiah PK, Kim DH, Agellon LB, Michalak M, Biology of endoplasmic reticulum stress in the heart, Circ Res, 107 (2010) 1185–1197. [DOI] [PubMed] [Google Scholar]
- [79].Senderek J, Muller JS, Dusl M, Strom TM, Guergueltcheva V, Diepolder I, Laval SH, Maxwell S, Cossins J, Krause S, Muelas N, Vilchez JJ, Colomer J, Mallebrera CJ, Nascimento A, Nafissi S, Kariminejad A, Nilipour Y, Bozorgmehr B, Najmabadi H, Rodolico C, Sieb JP, Steinlein OK, Schlotter B, Schoser B, Kirschner J, Herrmann R, Voit T, Oldfors A, Lindbergh C, Urtizberea A, von der Hagen M, Hubner A, Palace J, Bushby K, Straub V, Beeson D, Abicht A, Lochmuller H, Hexosamine biosynthetic pathway mutations cause neuromuscular transmission defect, Am J Hum Genet, 88 (2011) 162–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Kilch T, Alansary D, Peglow M, Dorr K, Rychkov G, Rieger H, Peinelt C, Niemeyer BA, Mutations of the Ca2+-sensing stromal interaction molecule STIM1 regulate Ca2+ influx by altered oligomerization of STIM1 and by destabilization of the Ca2+ channel Orai1, J Biol Chem, 288 (2013) 1653–1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Stormorken H, [Stormorken’s syndrome], Tidsskr Nor Laegeforen, 122 (2002) 2853–2856. [PubMed] [Google Scholar]
- [82].Mizobuchi M, Tanaka C, Sako K, Murakami N, Nihira A, Abe T, Tateno Y, Takahashi T, Nonaka I, [Muscle involvement of Stormorken’s syndrome], Rinsho Shinkeigaku, 40 (2000) 915–920. [PubMed] [Google Scholar]
- [83].Morin G, Bruechle NO, Singh AR, Knopp C, Jedraszak G, Elbracht M, Bremond-Gignac D, Hartmann K, Sevestre H, Deutz P, Herent D, Nurnberg P, Romeo B, Konrad K, Mathieu-Dramard M, Oldenburg J, Bourges-Petit E, Shen Y, Zerres K, Ouadid-Ahidouch H, Rochette J, Gain-of-Function Mutation in STIM1 (P.R304W) Is Associated with Stormorken Syndrome, Hum Mutat, 35 (2014) 1221–1232. [DOI] [PubMed] [Google Scholar]
- [84].Gamage TH, Gunnes G, Lee RH, Louch WE, Holmgren A, Bruton JD, Lengle E, Selnes Kolstad TR, Revold T, Svanstrøm Amundsen S, Dalen KT, Holme PA, Tjønnfjord GE, Christensen G, Westerblad H, Klungland A, Bergmeier W, Misceo D, Frengen E, STIM1 R304W causes muscle degeneration and impaired platelet activation in mice, Cell Calcium, (2018) https://doi.org/10.1016/j.ceca.2018.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Garibaldi M, Fattori F, Riva B, Labasse C, Brochier G, Ottaviani P, Sacconi S, Vizzaccaro E, Laschena F, Romero NB, Genazzani A, Bertini E, Antonini G, A novel gain-of-function mutation in ORAI1 causes late-onset tubular aggregate myopathy and congenital miosis, Clin Genet, 91 (2017) 780–786. [DOI] [PubMed] [Google Scholar]
- [86].Monaco AP, Neve RL, Colletti-Feener C, Bertelson CJ, Kurnit DM, Kunkel LM, Isolation of candidate cDNAs for portions of the Duchenne muscular dystrophy gene, Nature, 323 (1986) 646–650. [DOI] [PubMed] [Google Scholar]
- [87].Hoffman EP, Brown RH Jr., Kunkel LM, Dystrophin: the protein product of the Duchenne muscular dystrophy locus, Cell, 51 (1987) 919–928. [DOI] [PubMed] [Google Scholar]
- [88].Allen DG, Whitehead NP, Froehner SC, Absence of Dystrophin Disrupts Skeletal Muscle Signaling: Roles of Ca2+, Reactive Oxygen Species, and Nitric Oxide in the Development of Muscular Dystrophy, Physiol Rev, 96 (2016) 253–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Lynch GS, Rafael JA, Chamberlain JS, Faulkner JA, Contraction-induced injury to single permeabilized muscle fibers from mdx, transgenic mdx, and control mice, Am J Physiol Cell Physiol, 279 (2000) C1290–1294. [DOI] [PubMed] [Google Scholar]
- [90].Ibraghimov-Beskrovnaya O, Ervasti JM, Leveille CJ, Slaughter CA, Sernett SW, Campbell KP, Primary structure of dystrophin-associated glycoproteins linking dystrophin to the extracellular matrix, Nature, 355 (1992) 696–702. [DOI] [PubMed] [Google Scholar]
- [91].Durbeej M, Campbell KP, Muscular dystrophies involving the dystrophin-glycoprotein complex: an overview of current mouse models, Curr Opin Genet Dev, 12 (2002) 349–361. [DOI] [PubMed] [Google Scholar]
- [92].Bertorini TE, Bhattacharya SK, Palmieri GM, Chesney CM, Pifer D, Baker B, Muscle calcium and magnesium content in Duchenne muscular dystrophy, Neurology, 32 (1982) 1088–1092. [DOI] [PubMed] [Google Scholar]
- [93].Tutdibi O, Brinkmeier H, Rudel R, Fohr KJ, Increased calcium entry into dystrophin-deficient muscle fibres of MDX and ADR-MDX mice is reduced by ion channel blockers, J Physiol, 515 ( Pt 3) (1999) 859–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Whitehead NP, Pham C, Gervasio OL, Allen DG, N-Acetylcysteine ameliorates skeletal muscle pathophysiology in mdx mice, J Physiol, 586 (2008) 2003–2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Spencer MJ, Tidball JG, Calpain translocation during muscle fiber necrosis and regeneration in dystrophin-deficient mice, Exp Cell Res, 226 (1996) 264–272. [DOI] [PubMed] [Google Scholar]
- [96].Goonasekera SA, Lam CK, Millay DP, Sargent MA, Hajjar RJ, Kranias EG, Molkentin JD, Mitigation of muscular dystrophy in mice by SERCA overexpression in skeletal muscle, J Clin Invest, 121 (2011) 1044–1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Boittin FX, Petermann O, Hirn C, Mittaud P, Dorchies OM, Roulet E, Ruegg UT, Ca2+independent phospholipase A2 enhances store-operated Ca2+ entry in dystrophic skeletal muscle fibers, J Cell Sci, 119 (2006) 3733–3742. [DOI] [PubMed] [Google Scholar]
- [98].Fong PY, Turner PR, Denetclaw WF, Steinhardt RA, Increased activity of calcium leak channels in myotubes of Duchenne human and mdx mouse origin, Science, 250 (1990) 673–676. [DOI] [PubMed] [Google Scholar]
- [99].Franco A Jr., Lansman JB, Calcium entry through stretch-inactivated ion channels in mdx myotubes, Nature, 344 (1990) 670–673. [DOI] [PubMed] [Google Scholar]
- [100].Vandebrouck C, Martin D, Colson-Van Schoor M, Debaix H, Gailly P, Involvement of TRPC in the abnormal calcium influx observed in dystrophic (mdx) mouse skeletal muscle fibers, J Cell Biol, 158 (2002) 1089–1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Millay DP, Goonasekera SA, Sargent MA, Maillet M, Aronow BJ, Molkentin JD, Calcium influx is sufficient to induce muscular dystrophy through a TRPC-dependent mechanism, Proc Natl Acad Sci U S A, 106 (2009) 19023–19028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Zhao X, Moloughney JG, Zhang S, Komazaki S, Weisleder N, Orai1 mediates exacerbated Ca(2+) entry in dystrophic skeletal muscle, PLoS One, 7 (2012) e49862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Goonasekera SA, Davis J, Kwong JQ, Accornero F, Wei-LaPierre L, Sargent MA, Dirksen RT, Molkentin JD, Enhanced Ca(2)(+) influx from STIM1-Orai1 induces muscle pathology in mouse models of muscular dystrophy, Hum Mol Genet, 23 (2014) 3706–3715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Onopiuk M, Brutkowski W, Young C, Krasowska E, Rog J, Ritso M, Wojciechowska S, Arkle S, Zablocki K, Gorecki DC, Store-operated calcium entry contributes to abnormal Ca(2)(+) signalling in dystrophic mdx mouse myoblasts, Arch Biochem Biophys, 569 (2015) 1–9. [DOI] [PubMed] [Google Scholar]
- [105].Iwata Y, Katanosaka Y, Arai Y, Shigekawa M, Wakabayashi S, Dominant-negative inhibition of Ca2+ influx via TRPV2 ameliorates muscular dystrophy in animal models, Hum Mol Genet, 18 (2009) 824–834. [DOI] [PubMed] [Google Scholar]
- [106].Matsumura CY, Taniguti AP, Pertille A, Santo Neto H, Marques MJ, Stretch-activated calcium channel protein TRPC1 is correlated with the different degrees of the dystrophic phenotype in mdx mice, Am J Physiol Cell Physiol, 301 (2011) C1344–1350. [DOI] [PubMed] [Google Scholar]
- [107].Zhang BT, Whitehead NP, Gervasio OL, Reardon TF, Vale M, Fatkin D, Dietrich A, Yeung EW, Allen DG, Pathways of Ca(2)(+) entry and cytoskeletal damage following eccentric contractions in mouse skeletal muscle, J Appl Physiol (1985), 112 (2012) 2077–2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Cully TR, Edwards JN, Friedrich O, Stephenson DG, Murphy RM, Launikonis BS, Changes in plasma membrane Ca-ATPase and stromal interacting molecule 1 expression levels for Ca(2+) signaling in dystrophic mdx mouse muscle, Am J Physiol Cell Physiol, 303 (2012) C567–576. [DOI] [PubMed] [Google Scholar]
- [109].Rosenberg H, Sambuughin N, Riazi S, Dirksen R, Malignant Hyperthermia Susceptibility, in: Adam MP, Ardinger HH, Pagon RA, Wallace SE, Bean LJH, Stephens K, Amemiya A (Eds.) GeneReviews((R)), Seattle (WA), 1993. [Google Scholar]
- [110].Galli L, Orrico A, Lorenzini S, Censini S, Falciani M, Covacci A, Tegazzin V, Sorrentino V, Frequency and localization of mutations in the 106 exons of the RYR1 gene in 50 individuals with malignant hyperthermia, Hum Mutat, 27 (2006) 830. [DOI] [PubMed] [Google Scholar]
- [111].Robinson R, Carpenter D, Shaw MA, Halsall J, Hopkins P, Mutations in RYR1 in malignant hyperthermia and central core disease, Hum Mutat, 27 (2006) 977–989. [DOI] [PubMed] [Google Scholar]
- [112].Monnier N, Procaccio V, Stieglitz P, Lunardi J, Malignant-hyperthermia susceptibility is associated with a mutation of the alpha 1-subunit of the human dihydropyridine-sensitive L-type voltage-dependent calcium-channel receptor in skeletal muscle, Am J Hum Genet, 60 (1997) 1316–1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Carpenter D, Ringrose C, Leo V, Morris A, Robinson RL, Halsall PJ, Hopkins PM, Shaw MA, The role of CACNA1S in predisposition to malignant hyperthermia, BMC Med Genet, 10 (2009) 104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Dainese M, Quarta M, Lyfenko AD, Paolini C, Canato M, Reggiani C, Dirksen RT, Protasi F, Anesthetic- and heat-induced sudden death in calsequestrin-1-knockout mice, FASEB J, 23 (2009) 1710–1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Protasi F, Paolini C, Dainese M, Calsequestrin-1: a new candidate gene for malignant hyperthermia and exertional/environmental heat stroke, J Physiol, 587 (2009) 3095–3100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Kraeva N, Zvaritch E, Frodis W, Sizova O, Kraev A, MacLennan DH, Riazi S, CASQ1 gene is an unlikely candidate for malignant hyperthermia susceptibility in the North American population, Anesthesiology, 118 (2013) 344–349. [DOI] [PubMed] [Google Scholar]
- [117].Ellis FR, A Protocol for the Investigation of Malignant Hyperpyrexia (Mh) Susceptibility, Brit J Anaesth, 56 (1984) 1267–1269. [DOI] [PubMed] [Google Scholar]
- [118].Larach MG, Standardization of the caffeine halothane muscle contracture test. North American Malignant Hyperthermia Group, Anesth Analg, 69 (1989) 511–515. [PubMed] [Google Scholar]
- [119].Adnet PJ, Krivosic-Horber RM, Adamantidis MM, Reyford H, Cordonnier C, Haudecoeur G, Effects of calcium-free solution, calcium antagonists, and the calcium agonist BAY K 8644 on mechanical responses of skeletal muscle from patients susceptible to malignant hyperthermia, Anesthesiology, 75 (1991) 413–419. [DOI] [PubMed] [Google Scholar]
- [120].Fletcher JE, Huggins FJ, Rosenberg H, The importance of calcium ions for in vitro malignant hyperthermia testing, Can J Anaesth, 37 (1990) 695–698. [DOI] [PubMed] [Google Scholar]
- [121].Nelson TE, Bedell DM, Jones EW, Porcine malignant hyperthermia: effects of temperature and extracellular calcium concentration on halothane-induced contracture of susceptible skeletal muscle, Anesthesiology, 42 (1975) 301–306. [PubMed] [Google Scholar]
- [122].Yang T, Riehl J, Esteve E, Matthaei KI, Goth S, Allen PD, Pessah IN, Lopez JR, Pharmacologic and functional characterization of malignant hyperthermia in the R163C RyR1 knock-in mouse, Anesthesiology, 105 (2006) 1164–1175. [DOI] [PubMed] [Google Scholar]
- [123].Chelu MG, Goonasekera SA, Durham WJ, Tang W, Lueck JD, Riehl J, Pessah IN, Zhang P, Bhattacharjee MB, Dirksen RT, Hamilton SL, Heat- and anesthesia-induced malignant hyperthermia in an RyR1 knock-in mouse, FASEB J, 20 (2006) 329–330. [DOI] [PubMed] [Google Scholar]
- [124].Michelucci A, Paolini C, Boncompagni S, Canato M, Reggiani C, Protasi F, Strenuous exercise triggers a life-threatening response in mice susceptible to malignant hyperthermia, FASEB J, 31 (2017) 3649–3662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Duke AM, Hopkins PM, Calaghan SC, Halsall JP, Steele DS, Store-operated Ca2+ entry in malignant hyperthermia-susceptible human skeletal muscle, J Biol Chem, 285 (2010) 25645–25653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Cully TR, Choi RH, Bjorksten AR, Stephenson DG, Murphy RM, Launikonis BS, Junctional membrane Ca(2+) dynamics in human muscle fibers are altered by malignant hyperthermia causative RyR mutation, Proc Natl Acad Sci U S A, 115 (2018) 8215–8220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Avila G, Dirksen RT, Functional effects of central core disease mutations in the cytoplasmic region of the skeletal muscle ryanodine receptor, J Gen Physiol, 118 (2001) 277–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Dirksen RT, Avila G, Distinct effects on Ca2+ handling caused by malignant hyperthermia and central core disease mutations in RyR1, Biophys J, 87 (2004) 3193–3204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Manno C, Figueroa L, Royer L, Pouvreau S, Lee CS, Volpe P, Nori A, Zhou J, Meissner G, Hamilton SL, Rios E, Altered Ca2+ concentration, permeability and buffering in the myofibre Ca2+ store of a mouse model of malignant hyperthermia, J Physiol, 591 (2013) 4439–4457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Durham WJ, Aracena-Parks P, Long C, Rossi AE, Goonasekera SA, Boncompagni S, Galvan DL, Gilman CP, Baker MR, Shirokova N, Protasi F, Dirksen R, Hamilton SL, RyR1 S-nitrosylation underlies environmental heat stroke and sudden death in Y522S RyR1 knockin mice, Cell, 133 (2008) 53–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Andronache Z, Hamilton SL, Dirksen RT, Melzer W, A retrograde signal from RyR1 alters DHP receptor inactivation and limits window Ca2+ release in muscle fibers of Y522S RyR1 knock-in mice, Proc Natl Acad Sci U S A, 106 (2009) 4531–4536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Canato M, Scorzeto M, Giacomello M, Protasi F, Reggiani C, Stienen GJ, Massive alterations of sarcoplasmic reticulum free calcium in skeletal muscle fibers lacking calsequestrin revealed by a genetically encoded probe, Proc Natl Acad Sci U S A, 107 (2010) 22326–22331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Michelucci A, Paolini C, Canato M, Wei-Lapierre L, Pietrangelo L, De Marco A, Reggiani C, Dirksen RT, Protasi F, Antioxidants protect calsequestrin-1 knockout mice from halothane- and heatinduced sudden death, Anesthesiology, 123 (2015) 603–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Yarotskyy V, Protasi F, Dirksen RT, Accelerated activation of SOCE current in myotubes from two mouse models of anesthetic- and heat-induced sudden death, PLoS One, 8 (2013) e77633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Oo YW, Gomez-Hurtado N, Walweel K, van Helden DF, Imtiaz MS, Knollmann BC, Laver DR, Essential Role of Calmodulin in RyR Inhibition by Dantrolene, Mol Pharmacol, 88 (2015) 57–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Choi RH, Koenig X, Launikonis BS, Dantrolene requires Mg(2+) to arrest malignant hyperthermia, Proc Natl Acad Sci U S A, 114 (2017) 4811–4815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Eltit JM, Ding X, Pessah IN, Allen PD, Lopez JR, Nonspecific sarcolemmal cation channels are critical for the pathogenesis of malignant hyperthermia, FASEB J, 27 (2013) 991–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Evans WJ, What is sarcopenia?, J Gerontol A Biol Sci Med Sci, 50 Spec No (1995) 5–8. [DOI] [PubMed] [Google Scholar]
- [139].Roubenoff R, Hughes VA, Sarcopenia: current concepts, J Gerontol A Biol Sci Med Sci, 55 (2000) M716–724. [DOI] [PubMed] [Google Scholar]
- [140].Lexell J, Human aging, muscle mass, and fiber type composition, J Gerontol A Biol Sci Med Sci, 50 Spec No (1995) 11–16. [DOI] [PubMed] [Google Scholar]
- [141].Frontera WR, Suh D, Krivickas LS, Hughes VA, Goldstein R, Roubenoff R, Skeletal muscle fiber quality in older men and women, Am J Physiol Cell Physiol, 279 (2000) C611–618. [DOI] [PubMed] [Google Scholar]
- [142].Liu W, Klose A, Forman S, Paris ND, Wei-LaPierre L, Cortes-Lopez M, Tan A, Flaherty M, Miura P, Dirksen RT, Chakkalakal JV, Loss of adult skeletal muscle stem cells drives age-related neuromuscular junction degeneration, Elife, 6 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Faulkner JA, Larkin LM, Claflin DR, Brooks SV, Age-related changes in the structure and function of skeletal muscles, Clin Exp Pharmacol Physiol, 34 (2007) 1091–1096. [DOI] [PubMed] [Google Scholar]
- [144].Delbono O, O’Rourke KS, Ettinger WH, Excitation-calcium release uncoupling in aged single human skeletal muscle fibers, J Membr Biol, 148 (1995) 211–222. [DOI] [PubMed] [Google Scholar]
- [145].Renganathan M, Sonntag WE, Delbono O, L-type Ca2+ channel-insulin-like growth factor-1 receptor signaling impairment in aging rat skeletal muscle, Biochem Biophys Res Commun, 235 (1997) 784789. [DOI] [PubMed] [Google Scholar]
- [146].Boncompagni S, d’Amelio L, Fulle S, Fano G, Protasi F, Progressive disorganization of the excitation-contraction coupling apparatus in aging human skeletal muscle as revealed by electron microscopy: a possible role in the decline of muscle performance, J Gerontol A Biol Sci Med Sci, 61 (2006) 995–1008. [DOI] [PubMed] [Google Scholar]
- [147].Zampieri S, Pietrangelo L, Loefler S, Fruhmann H, Vogelauer M, Burggraf S, Pond A, GrimStieger M, Cvecka J, Sedliak M, Tirpakova V, Mayr W, Sarabon N, Rossini K, Barberi L, De Rossi M, Romanello V, Boncompagni S, Musaro A, Sandri M, Protasi F, Carraro U, Kern H, Lifelong physical exercise delays age-associated skeletal muscle decline, J Gerontol A Biol Sci Med Sci, 70 (2015) 163–173. [DOI] [PubMed] [Google Scholar]
- [148].Trounce I, Byrne E, Marzuki S, Decline in skeletal muscle mitochondrial respiratory chain function: possible factor in ageing, Lancet, 1 (1989) 637–639. [DOI] [PubMed] [Google Scholar]
- [149].Peterson CM, Johannsen DL, Ravussin E, Skeletal muscle mitochondria and aging: a review, J Aging Res, 2012 (2012) 194821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150].Pietrangelo L, D’Incecco A, Ainbinder A, Michelucci A, Kern H, Dirksen RT, Boncompagni S, Protasi F, Age-dependent uncoupling of mitochondria from Ca2(+) release units in skeletal muscle, Oncotarget, 6 (2015) 35358–35371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [151].Zhao X, Weisleder N, Thornton A, Oppong Y, Campbell R, Ma J, Brotto M, Compromised store-operated Ca2+ entry in aged skeletal muscle, Aging Cell, 7 (2008) 561–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [152].Thornton AM, Zhao X, Weisleder N, Brotto LS, Bougoin S, Nosek TM, Reid M, Hardin B, Pan Z, Ma J, Parness J, Brotto M, Store-operated Ca(2+) entry (SOCE) contributes to normal skeletal muscle contractility in young but not in aged skeletal muscle, Aging (Albany NY), 3 (2011) 621–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [153].Payne AM, Jimenez-Moreno R, Wang ZM, Messi ML, Delbono O, Role of Ca2+, membrane excitability, and Ca2+ stores in failing muscle contraction with aging, Exp Gerontol, 44 (2009) 261–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [154].Edwards JN, Blackmore DG, Gilbert DF, Murphy RM, Launikonis BS, Store-operated calcium entry remains fully functional in aged mouse skeletal muscle despite a decline in STIM1 protein expression, Aging Cell, 10 (2011) 675–685. [DOI] [PubMed] [Google Scholar]
- [155].Al-Qusairi L, Laporte J, T-tubule biogenesis and triad formation in skeletal muscle and implication in human diseases, Skelet Muscle, 1 (2011) 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [156].Schaballie H, Rodriguez R, Martin E, Moens L, Frans G, Lenoir C, Dutre J, Canioni D, Bossuyt X, Fischer A, Latour S, Meyts I, Picard C, A novel hypomorphic mutation in STIM1 results in a late-onset immunodeficiency, J Allergy Clin Immunol, 136 (2015) 816–819 e814. [DOI] [PubMed] [Google Scholar]
- [157].Wang S, Choi M, Richardson AS, Reid BM, Seymen F, Yildirim M, Tuna E, Gencay K, Simmer JP, Hu JC, STIM1 and SLC24A4 Are Critical for Enamel Maturation, J Dent Res, 93 (2014) 94S100S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [158].Muik M, Fahrner M, Schindl R, Stathopulos P, Frischauf I, Derler I, Plenk P, Lackner B, Groschner K, Ikura M, Romanin C, STIM1 couples to ORAI1 via an intramolecular transition into an extended conformation, EMBO J, 30 (2011) 1678–1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [159].Nakamura L, Sandrock-Lang K, Speckmann C, Vraetz T, Buhrlen M, Ehl S, Heemskerk JW, Zieger B, Platelet secretion defect in a patient with stromal interaction molecule 1 deficiency, Blood, 122 (2013) 3696–3698. [DOI] [PubMed] [Google Scholar]
- [160].Byun M, Abhyankar A, Lelarge V, Plancoulaine S, Palanduz A, Telhan L, Boisson B, Picard C, Dewell S, Zhao C, Jouanguy E, Feske S, Abel L, Casanova JL, Whole-exome sequencing-based discovery of STIM1 deficiency in a child with fatal classic Kaposi sarcoma, J Exp Med, 207 (2010) 23072312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [161].Chou J, Badran YR, Yee CSK, Bainter W, Ohsumi TK, Al-Hammadi S, Pai SY, Feske S, Geha RS, A novel mutation in ORAI1 presenting with combined immunodeficiency and residual T-cell function, J Allergy Clin Immunol, 136 (2015) 479–482 e471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [162].Badran YR, Massaad MJ, Bainter W, Cangemi B, Naseem SU, Javad H, Al-Tamemi S, Geha RS, Chou J, Combined immunodeficiency due to a homozygous mutation in ORAI1 that deletes the Cterminus that interacts with STIM 1, Clin Immunol, 166–167 (2016) 100–102. [DOI] [PubMed] [Google Scholar]
- [163].Hedberg C, Niceta M, Fattori F, Lindvall B, Ciolfi A, D’Amico A, Tasca G, Petrini S, Tulinius M, Tartaglia M, Oldfors A, Bertini E, Childhood onset tubular aggregate myopathy associated with de novo STIM1 mutations, J Neurol, 261 (2014) 870–876. [DOI] [PubMed] [Google Scholar]
- [164].Shahrizaila N, Lowe J, Wills A, Familial myopathy with tubular aggregates associated with abnormal pupils, Neurology, 63 (2004) 1111–1113. [DOI] [PubMed] [Google Scholar]
- [165].Noury JB, Bohm J, Peche GA, Guyant-Marechal L, Bedat-Millet AL, Chiche L, Carlier RY, Malfatti E, Romero NB, Stojkovic T, Tubular aggregate myopathy with features of Stormorken disease due to a new STIM1 mutation, Neuromuscul Disord, 27 (2017) 78–82. [DOI] [PubMed] [Google Scholar]
- [166].Bohm J, Bulla M, Urquhart JE, Malfatti E, Williams SG, O’Sullivan J, Szlauer A, Koch C, Baranello G, Mora M, Ripolone M, Violano R, Moggio M, Kingston H, Dawson T, DeGoede CG, Nixon J, Boland A, Deleuze JF, Romero N, Newman WG, Demaurex N, Laporte J, ORAI1 Mutations with Distinct Channel Gating Defects in Tubular Aggregate Myopathy, Hum Mutat, 38 (2017) 426–438. [DOI] [PubMed] [Google Scholar]
- [167].Walter MC, Rossius M, Zitzelsberger M, Vorgerd M, Muller-Felber W, Ertl-Wagner B, Zhang Y, Brinkmeier H, Senderek J, Schoser B, 50 years to diagnosis: Autosomal dominant tubular aggregate myopathy caused by a novel STIM1 mutation, Neuromuscul Disord, 25 (2015) 577–584. [DOI] [PubMed] [Google Scholar]
- [168].Bohm J, Chevessier F, Koch C, Peche GA, Mora M, Morandi L, Pasanisi B, Moroni I, Tasca G, Fattori F, Ricci E, Penisson-Besnier I, Nadaj-Pakleza A, Fardeau M, Joshi PR, Deschauer M, Romero NB, Eymard B, Laporte J, Clinical, histological and genetic characterisation of patients with tubular aggregate myopathy caused by mutations in STIM1, J Med Genet, 51 (2014) 824–833. [DOI] [PubMed] [Google Scholar]