ABSTRACT
Shanchol™, a WHO-prequalified oral cholera vaccine (OCV), has been used to control endemic cholera in Asia, as well as in emergencies and outbreaks elsewhere. The vaccine has not been used by public health systems in cholera-endemic settings of Africa although several outbreak response campaigns have been conducted. Here we present experiences from a mass vaccination campaign in a cholera-endemic setting of Ethiopia in which Shanchol™ was introduced through the public health system. The vaccination site was selected based on cholera cases reported in previous years. Social mobilization involved sensitization of community leaders, household visits, and mass distribution of banners, posters and leaflets. The vaccination was implemented after careful microplanning of logistics and cold chain, manpower, transportation, vaccine supply and supervision and monitoring of adverse events. Vaccine administration was recorded on individual vaccination cards. Vaccine delivery costs were collected and analyzed after vaccination. As there was no experience with Shanchol™ in Ethiopia, a bridging trial was conducted to demonstrate safety and immunogenicity of the vaccine in the local population prior to the mass vaccination. Oral cholera vaccination was conducted in two rounds of four days each in February 2015 and March 2015 in 10 selected villages of Shashemenae rural district of Ethiopia. A total of 62,161 people targeted. 47,137 people (76%) received the first dose, and 40,707 (65%) received two doses. The financial cost of the vaccination campaign was estimated at US $2·60 per dose or US $5·64 per fully immunized person. The cost of vaccine delivery excluding vaccine procurement was $0·68 per dose or $1·48 per fully immunized person. The study demonstrates that mass cholera vaccination administered through the public health system in Ethiopia is feasible, can be implemented through the existing health system at an affordable cost, and the vaccine is acceptable to the community. The lessons from this study are useful for deploying OCV in other African endemic settings through the public health system and may guide future immunization policy decisions.
KEYWORDS: cholera, mass campaign, vaccination
Introduction
Cholera, an acute life-threatening diarrheal disease caused by the bacterium Vibrio cholerae, is a food- and water-borne disease found worldwide, particularly in low- and middle-income countries.1 In 2015, the World Health Organization (WHO) reported 172,454 cases and 1,304 deaths from 42 countries.2 Cholera cases and deaths are thought to be significantly underreported by countries due to surveillance limitations and fear that notification might negatively impact the economy by influencing travel and trade.3 It is estimated that about 28,000 to 142,000 people die of cholera among 1·4 to 4·3 million annual cases in endemic countries.3 Although cholera is underreported, 41% of WHO reported cholera cases in 2015 were from Africa where the case fatality rate was higher than other regions, contributing to 72% of cholera deaths worldwide.2
Because cholera is a fecal-oral transmitted disease, improving water quality and sanitation has been the primary intervention for long-term sustained prevention and control in endemic settings. During outbreaks, providing safe potable water and personal hygiene are complementary to the provision of early and effective medical services to affected people. In addition, the WHO recommends that vaccination be considered as a further intervention for the prevention and control of cholera in both routine and outbreak situations.1 The oral cholera vaccine (OCV), Dukoral® (Crucell Sweden AB), has been on the market for a long time, but is primarily used as a travelers' vaccine due to its high cost and need for co-administration with a buffer, which complicates its use in field settings.4 Another oral cholera vaccine, Shanchol™ (Shantha Biotechnics Limited, India), was WHO-prequalified in 2011.The vaccine is available for use in endemic and epidemic settings, is low cost, and does not need a buffer.5 The vaccine has been used in the global oral cholera vaccine stockpile, which was created to help control cholera epidemics. The OCV was deployed in several endemic settings in Asia6 and in several outbreaks in Africa7,8 and Haiti,9 and was found to be effective.10-13 Euvichol®, new oral cholera vaccine, entered the market more recently and was WHO prequalified in 2015.14
The lower cost and easier administration of Shanchol™ (compared with Dukoral) fulfill the programmatic needs of cholera vaccination in routine public health settings.15 In India, the vaccine was administered through the public health system, demonstrating the feasibility, acceptability and effectiveness of Shanchol in routine use.6,11,16 Although the vaccine was used in outbreaks and emergency settings in Africa, the feasibility, acceptability and cost of cholera vaccination campaigns have not been studied in endemic African settings. Ethiopia is the second most populous country in sub-Saharan Africa and is cholera-endemic, with more than 100,000 cases occurring annually.17 making it well-placed to conduct a pilot OCV campaign in an endemic setting. We herein present our findings and lessons learned from oral cholera vaccine delivery through the public health setting in Ethiopia.
Results
Mass oral cholera vaccination was conducted in two rounds (20–25 February, 2015 and 13–18 March, 2015) in 10 selected villages of Shashemenae rural district, West Arsi Zone, Oromia Regional State. A total 48 teams consisting of a vaccinator, recorder and crowd controller managed the vaccination posts. The first round vaccination was conducted for four days in eight kebeles. As there were vaccines remaining from first round allocation, the vaccination was expanded to two additional kebeles for two more days. The vaccination teams were dispatched to the vaccination posts early in the morning so that actual vaccinations could take place from 8:00am to 4:00pm. The timing appears to have suited the vaccination recipients except on market day when most came only in the morning. In the first round, all those who received the vaccine were registered in the vaccination registration book and they were given a vaccination card. During the second round, vaccination was provided to those who received the first dose as per their vaccination cards. The cards helped identify those who had received the first dose and facilitated correct recording of vaccinations on the register.
A total of 62,161 people targeted after excluding children <1y and pregnant women and as per the administrative coverage 47,137 people (76%) received the first dose of OCV and 40,707 (65%) received two doses (Table 1). The dropout rate between the first round and second round was 14%. The vaccine wastage rate was less than 3% of procured vaccine mainly due to broken vials and spitting (forcing out contents from the mouth) of vaccine during administration. Total 9,185 doses remained unused at the end of second round. There were no reported cases of serious adverse events except for mild abdominal pain, diarrhea, vomiting, and loss of appetite among the few reported cases, which were managed symptomatically.
Table 1.
Targeted population and vaccinated people in each Kebele in two rounds, Feb.-Mar. 2015, Shashemanae, Ethiopia.
| Population |
Vaccination rounds |
||||||
|---|---|---|---|---|---|---|---|
| S. N | Kebeles | Total | Target | One | % | Two | % |
| 1 | Awash Dhanqu | 8678 | 8089 | 6431 | 80 | 5774 | 71 |
| 2 | Faji Gole | 8322 | 7767 | 5205 | 67 | 4740 | 61 |
| 3 | Faji Goba | 7409 | 6914 | 4814 | 69 | 4299 | 62 |
| 4 | Chefa Guta | 4021 | 3753 | 3138 | 84 | 2937 | 78 |
| 5 | Chebi | 6102 | 5695 | 4704 | 83 | 4148 | 73 |
| 6 | Bura | 6058 | 5653 | 5032 | 89 | 4311 | 76 |
| 7 | Kore Rogicha | 5333 | 5147 | 4167 | 81 | 3767 | 73 |
| 8 | Chulule | 4298 | 4010 | 3384 | 84 | 3107 | 77 |
| 9 | Alelu Ilu | 7216 | 6734 | 5079 | 75 | 3438 | 51 |
| 10 | Idola Burka | 9001 | 8399 | 5183 | 62 | 4186 | 50 |
| Total | 66438 | 62161 | 47137 | 76 | 40707 | 65 | |
The total financial cost of the vaccination program representing sum total costs incurred by the health system was US $229,600 for 88,173 doses utilized with an average cost of US $2·60 per dose and US $5·64 per fully immunized person (Table 2). The economic cost representing combined financial and already paid-up existing resources used for the vaccination as described in methods section was US $8,483 higher than the financial cost, comprising an additional US $0·10 per dose delivered or additional US $0·21 per fully immunized person. The financial cost of vaccine delivery, excluding the vaccine procurement cost, was US $0·68 per dose or US $1·48 per fully immunized person. Vaccine procurement was the costliest item followed by vaccine administration. It is worth to note that all financial costs are paid by the project funded by the donors and the marginal economic costs were borne by the government. About 35% and 44% of vaccine administration financial costs were staff allowance for round 1 and round 2 respectively. Rest was spent on materials and supplies. When looked at economic costs, 9% and 11% were personnel costs for round 1 and round 2 respectively. Whereas, the marginal economic costs on material an supplies was about 1% (0.8% for round 1 and 1.2% for round 2).
Table 2.
Financial and economic costs of vaccine delivery.
| Financial cost (in US$) |
Economic costs(in US$) |
|||||
|---|---|---|---|---|---|---|
| Cost item | Total costs | Cost per dose | Percentage | Total costs | Cost per dose | Percentage |
| 1.Vaccine procurement | $169,495 | $1.92 | 73.82% | $169,592 | $1.92 | 71.23% |
| 1.1.Purchase price | $163,120 | $1.85 | 71.05% | $163,120 | $1.85 | 68.51% |
| 1.2.Add-on costs | $6,374 | $0.07 | 2.78% | $6,472 | $0.07 | 2.72% |
| 2.Vaccination program preparation | $9,145 | $0.10 | 3.98% | $11,083 | $0.13 | 4.66% |
| 2.1.Mirco-planning | $13 | $0.00 | 0.01% | $117 | $0.00 | 0.05% |
| 2.2.Training | $2,325 | $0.03 | 1.01% | $2,796 | $0.03 | 1.17% |
| 2.3.Sensitization & social mobilization | $6,807 | $0.08 | 2.96% | $8,170 | $0.09 | 3.43% |
| 3.Vaccine administration | $49,123 | $0.56 | 21.39% | $54,003 | $0.61 | 22.68% |
| 3.1.Vaccination round 1 | $26,736 | $0.30 | 11.64% | $28,602 | $0.32 | 12.01% |
| 3.2. Vaccination round 2 | $22,387 | $0.25 | 9.75% | $25,4011 | $0.29 | 10.67% |
| 4. AEFI | $1,836 | $0.02 | 0.80% | $3,404 | $0.04 | 1.43% |
| Total/ average | $229,600 | $2.60 | 100.00% | $238,083 | $2.70 | 100.00% |
AEFI = adverse events following immunizations.
Discussion
The OCV vaccination study is use of Shanchol™ in an endemic public health setting in Africa. Shanchol™ was introduced through the public health setting in Odisha State, India in 2011 and cholera vaccination was found to be acceptable, feasible6,16 and effective although vaccination recipients bore significant indirect costs.18 As with Odisha, we found oral cholera vaccination was feasible and acceptable in Ethiopia, as suggested by the sufficient vaccination coverage. The financial cost of US $1.48 per vaccine dose delivered through the public sector was higher than the US $1·13 estimated in Odisha, India in 20116 but it was lower than the OCV delivery cost in Bangladesh (US $1·63 in 2011),19 Guinea (US $1·90 in 2012)7 and South Sudan (US $3·77 in 2012).20 This difference could be due to the nature of the health care setting where vaccination was conducted, the lowest costs being in India and Ethiopia where vaccinations were conducted through the existing public health system. The more costly vaccinations in Guinea and South Sudan were conducted in outbreaks and refugee camp settings.
The Ethiopian experience and outcomes are valuable for the expansion of OCV use to other cholera-endemic African settings through existing public health systems. Currently, the use of OCV in epidemic and endemic settings has been financed by Gavi, the Vaccine Alliance through the global oral cholera vaccine stockpile mechanism managed by WHO. Although Gavi has a learning agenda to further explore cholera vaccine use in endemic settings,21 using vaccines from the stockpile for routine vaccination has not been possible due to the limited global supply of oral cholera vaccines.14 Thus, stockpile deployments have been typically only made for emergencies or outbreaks.5,9 Consequently, findings from this study may provide helpful information on OCV use in endemic settings and can inform Gavi's 2018 vaccine investment strategy, as well vaccination strategies for endemic settings in Africa.21,22
One of the key lessons learned is that WHO prequalification of a vaccine or availability of a vaccine stockpile does not necessarily create vaccine demand or lead to vaccine use in endemic disease situations. Despite WHO prequalification of the oral cholera vaccine, we had to conduct further clinical research to prove the vaccine was safe and efficacious in the local population to obtain the in-country vaccine import permit and licensure, which was time-consuming and resource-intensive. A strong in-country advocate to champion for the introduction of a new vaccine is necessary; in this case, EPHI, the research wing of the Ministry of Health, served that role. Thorough community-based participatory microplanning involving grass roots health workers and community leaders, supported by a dynamic site management team, was key to success. The vaccination campaign also needed strong technical and financial support, which was obtained through partnerships with external agencies and donors for vaccine procurement, licensure, and development of the implementation plan. These factors may contribute a lot for the feasibility of vaccination through public health systems in future.
This pilot oral cholera vaccine introduction in Africa provides rich ground for future vaccinations. Efforts should be made to secure resources to document and disseminate the lessons learnt and to understand vaccine impact and cost-effectiveness in cholera and diarrheal disease control, particularly in the context of overall cholera control strategies that involve improving water and sanitation.
Limitations
There are several limitations in the study. The regulatory challenge faced in Ethiopia for obtaining import permit was unique because Shanchol was not used in African population by then (2013). Now that OCV is widely used in African population, regulatory challenges should not be that substantial. As a part of the campaign, age and sex of the vaccine recipients was not documented in the vaccination register and thus age and sex categorization of administrative coverage of vaccine could not be performed. The second dose of OCV was given to those who presented their vaccination cards provided during the administration of first dose. The loss of vaccination card may have minimized the recipient's ability to obtain second dose. As there was no financial support, research activities such as acceptability survey, out of pocket expenditures to vaccine recipients, evaluation of impact estimates etc. were not conducted. Future vaccination campaign budgets should include minimal research components.
The cost data were collected retrospectively which may have introduced recall bias which was minimized by cross verifying reported costs/cost items to recorded ones. The capital costs such as buildings, cold chain rooms and equipment, vehicles etc. was not included in data collection. This may have contributed to underestimation of the costs. As described in the methods we did not include the costs incurred by household members to travel to vaccination sites and to receive free OCV which also may have contributed to the underestimation of the costs.
Conclusion
In conclusion, this study demonstrates that mass oral cholera vaccination through the Ethiopian public health system is feasible and acceptable. It also suggests that OCV campaigns in cholera-endemic settings through existing health care systems may be less expensive than OCV campaigns in emergency settings. The practical lessons from this study will be useful for deploying OCV elsewhere in Africa, for future OCV financing decisions by Gavi, and for policy decisions on cholera control by WHO, and regional and national authorities as a complementary measure to existing, longer-term efforts to improve water and sanitation.
Methods
The vaccination feasibility and costing study was a partnership among the Ethiopian Public Health Institute (EPHI) in Addis Ababa, Ethiopia; the Oromia Regional Health Bureau of Oromia Region, Ethiopia; and the International Vaccine Institute (IVI) in Seoul, Republic of Korea. The study was approved by IVI's Institutional Review Board (IRB) and the National Ethics Committee, Ethiopia. Study site selection, regulatory approvals, microplanning, vaccine shipment, transport and storage, and sensitization and social mobilization leading to the mass vaccination are described below.
Selection of study sites
To identify the cholera hotspots to target with the oral cholera vaccine, we conducted a review of cholera cases (acute watery diarrhea) reported from 2006 to 2010 in Ethiopia (Fig 1), and listed the regions by descending order based on the number of cases reported. Cholera outbreaks of varying sizes were reported by Ethiopia since 1996, and the most significant outbreak occurred in 2006, resulting in 51,074 cases and 565 deaths. Among the 10 regions that reported a total of 142,580 cases, including 1,650 deaths, over the five years, three were notably affected: Oromia, Amhara, and the Southern Nations Nationalities and People Region. Shashemene town, located on the highway linking several Ethiopian towns in the south, reported over half of all cases of cholera in Oromia in 2009. At the district level, Shashemene rural district appeared to be a cholera hotspot, reporting cases every year for the last several years, and was therefore chosen for the vaccination feasibility and costing study. As the district was large with a population of 251,225, we selected smaller administrative units known as kebeles. Taking into account the number of cases and risk factors for cholera and resource limitations, 10 out of 38 kebeles in Shashemene rural district were selected for the vaccination campaign (Fig 2).
Figure 1.
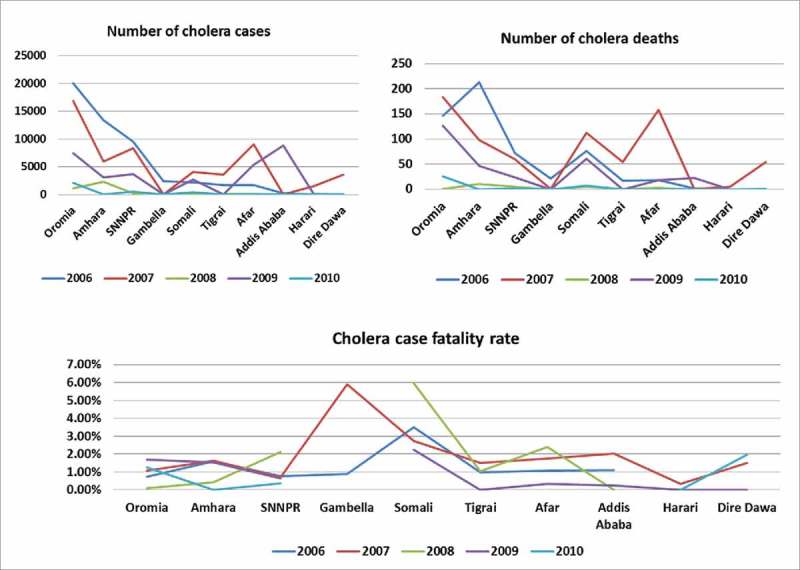
Reported Cases of Cholera 2006–2010 by Region.
Figure 2.
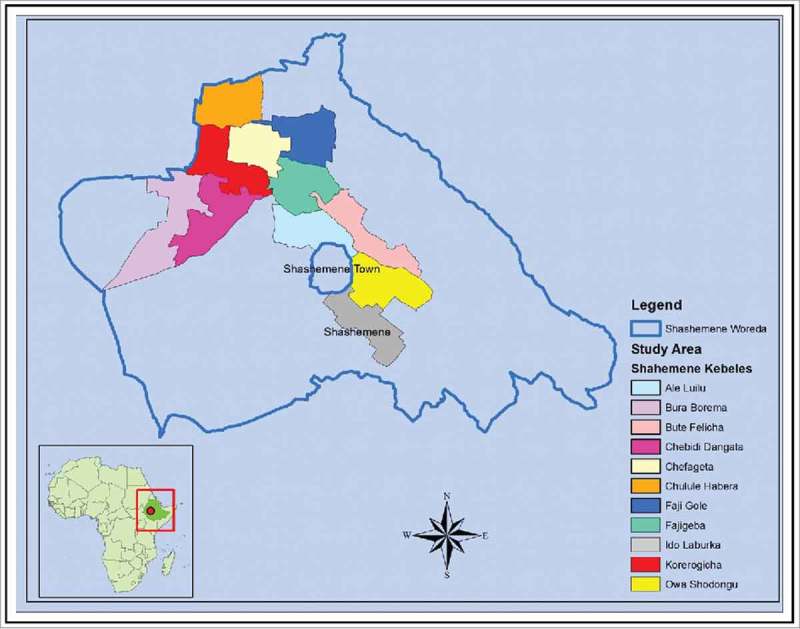
Sites selected for feasibility and costing of oral cholera vaccination in Ethiopia. Created by ArcGIS 10.2 for Desktop.
Regulatory and ethical approvals
Shanchol™ is manufactured and licensed in India and obtained WHO-prequalification based on clinical safety and efficacy data generated in India. At the time of protocol submission in April 2013, although the vaccine was used in Asia, it was not previously used or tested in endemic Ethiopian settings. Therefore, prior to the mass vaccination, the Ethiopian regulatory authority (Food, Medicine and Health Administration and Control Authority-FMHACA) required safety and efficacy data in the Ethiopian population in order for us to obtain the necessary approvals for license to import and use of the OCV in the country. We conducted a clinical trial to demonstrate safety and immunogenicity of Shanchol™ in the Ethiopian population,23 resulting in import permit of the vaccine in Ethiopia in November 2014.24 Obtaining ethical approval for the clinical trial involving children was challenging because in-country law does not permit involvement of children in biomedical research. Approval to include children in the trial was finally received from the national regulatory authority after the Ministry of Health confirmed the plan to use the vaccine as recommended in all age groups, including persons above one year old. However this process delayed the vaccination campaign for more than 1·5 years.24
Microplanning
A vaccination implementation team was established that included committees for technical support, training, logistics management, and social mobilization. The committee members developed a “bottom-up” approach to participatory microplanning, involving public health field staff and community leaders. Efforts were made to ensure the identification of appropriate sites for vaccine administration, standard and consistent assessment of cold chain materials, vaccine needs, and campaign personnel. A detailed logistics plan for vaccine storage, transportation and administration was developed. Monitoring and supervision plans were developed for training, social mobilization, communication, and adverse events following immunization (AEFI).
Training
A multi-layered training was conducted starting from training of trainers. Training of all staff involved in vaccination was conducted at the district level. Forty data collectors and 40 vaccinators were trained for a full day together with 8 supervisors for their role in in the campaign. Social mobilisers and crowd controllers had different roles and were trained separately.
Vaccine shipment, transport and storage
A total of 100,030 doses of Shanchol™ (1·5 ml liquid formulation in 2·5 ml vials) were shipped from Hyderabad, India, as per international vaccine air transport guidelines, packed in 65 cartons, each 7 square meters in size. On arrival, the temperature indicators in the vaccine packages were examined and found to be in the recommended range of 2–8°C. The vaccines were kept in the airport cold room until customs clearance and were then transported in refrigerated trucks to the central cold room at EPHI in Addis Ababa where they were maintained at + 2–8°C. From the central store, the vaccines were transported to the Shashemene district office 300 kilometers away in refrigerated vehicles two days prior to each round of vaccination to avoid overloading the district cold chain. On arrival, the vaccines were stored in cold boxes at the district health office and transported to the vaccination sites on a daily basis using vaccine carriers. Cold boxes containing vaccines were also pre-positioned at some vaccination posts to replenish vaccines without the need for the district's main storage facility. Regular cold chain assessments were conducted by local and district health offices with support from WHO Ethiopia. Refrigerators, cold boxes, and vaccine carriers mobilized from adjacent kebeles to vaccination sites at health facilities and health offices were sufficient for each round of vaccination. Cold chain equipment functionality and readiness to receive the vaccines were ensured before the start of the campaign.
Sensitization and social mobilization
Sensitization involved convening a meeting with the district's executive committee that was responsible for overseeing and supporting the overall vaccination campaign, and convening two meetings with local leaders who were responsible for supporting community mobilization with the participation of kebele community mobilization volunteers. These meetings involved key opinion leaders in the community whose support and common understanding was necessary for effective execution of the campaign.
For mass communication, banners, posters and leaflets were designed, pretested, and distributed in the targeted community. Attention was given to carefully choosing locally appropriate language and developing messaging for the printed materials as cholera is a very sensitive issue for the government. Exceptional care was also taken to prevent conveying a false impression of an impending or ongoing epidemic in the area. Besides print materials, interpersonal communication was widely used for social mobilization. Information about the OCV and the planned mass vaccination were communicated to local leaders and health care workers and at the household level in order to mobilize the respective residents. Each kebele has a local development unit with about 30 households that is divided into a health development army network consisting of one to five households. This network was used to facilitate community awareness and to mobilize the population for mass vaccination.
Mass vaccination
All community members aged one year and above and non-pregnant women of Shashemene were invited to the vaccination. A total of 48 teams, each consisting of a vaccinator, recorder and crowd controller managed the vaccination sites. Informed consent was taken before vaccination. Additional support was obtained from local volunteers for social mobilization, as well as for crowd control at sites with high participation. Supervisors provided hands-on support to vaccination teams, and each supervisor managed five teams to conduct monitoring and supervision to ensure quality of vaccine delivery and follow-up of AEFIs. They also mobilized support from local leaders and managed operational issues such as vaccine replenishment from the district office and ensuring availability of additional stock at nearby health centers.
AEFI monitoring was carried out by vaccination teams who observed vaccination recipients at the site for thirty minutes, and later by AEFI monitors located at health centers and hospitals.
Vaccination administrative coverage estimation
At the vaccination sites, the address of each individual was recorded in a register. Vaccination administrative coverage by round was calculated as a proportion of people vaccinated upon the census-projected population size of the kebele (projected census data for the year 2012).
Costs of vaccine delivery
A Microsoft Excel tool (CHOLTOOL) was developed by IVI and used to estimate the vaccination campaign costs in Ethiopia.25 The tool was built based on IVI's previous experience of estimating OCV delivery costs in Odisha, India,6 and it distinguishes between financial costs and economic costs. The vaccine delivery cost estimation was done from health system perspective. Financial costs include the value of items purchased or consumed for the vaccination campaign directly such as microplanning, training, social mobilization, transportation, and allowances. They represent the additional funds required to conduct the OCV campaign through the existing health care delivery system (i.e., Ministry of Health) in Shashemene. Economic costs include the value of items already provided for by the Ministry of Health or other sources and constitutes items such as existing health workers' time and salaries, time of volunteers, existing cold chain and logistics, and donations. They represent the complete picture of resources consumed for the vaccination campaign irrespective of the payer. As the vaccination campaign was linked to a research project, all costs related to research were excluded. The costs were converted to United States Dollars (US $) using the mean exchange rate between US $ and Ethiopian Birr (1 USD = 20·5 Birr) for 2015 published by the National Bank of Ethiopia.26
The delivery costs were collected on CHOLTOOL. Study staff visited EPHI in Addis Ababa and the Primary Health Center in Shashemane two months after the OCV campaign. The program managers and coordinators involved in planning and implementing the vaccination campaign were interviewed on-site to identify and chronologically list the cost items required for the vaccination campaign. The unit cost for each item was then determined using a micro-costing approach to estimate the component costs based on interview notes and review of the financial records maintained at the respective offices. The reported costs and expenditure records were cross-checked for validity. Finally, the number of cost items consumed and unit cost per item were collected to estimate total costs. The main cost items collected on CHOLTOOL includes: vaccine and procurement costs such as vaccine price, insurance, custom clearance and handling; program preparation costs such as microplanning, sensitization, communication materials, social mobilization, training; vaccine administration costs such as vaccine storage and transport, manpower and incentives, supplies and logistics, and waste management for two rounds of campaign; and management of AEFIs. The cost of both consumed and wasted vaccines was included in vaccine and procurement costs. Capital costs such as buildings, cold rooms, cold chain equipment and vehicles were excluded from the analysis. Similarly, time cost of staff from international organizations who supported the overall activity was not included. Finally, the cost items were categorized and the results were presented based on recommendations from a recent review on OCV delivery costs.27 People received vaccine free of costs and the costs borne by vaccine recipients or their households were not included in the analysis.
Funding Statement
We thank LG Electronics for funding the project.
Abbreviations
- AEFI
adverse events following immunization
- EPHI
Ethiopian Public Health Institute
- FMHACA
Food, Medicine and Health Administration and Control Authority
- IRB
Institutional Review Board
- IVI
International Vaccine Institute
- OCV
Oral cholera vaccine
- WHO
World Health Organization
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
We are thankful to the project staff, medical officers and volunteers and residents of Shashemene Rural District in West Arsi Zone, Ethiopia for supporting the vaccination. We sincerely thank Ann Levin and Win Morgan who developed the cholera vaccine delivery cost estimation tool, CHOLTOOL, under the guidance of Vittal Mogasale from the International Vaccine Institute and Raymond Hutubessy from the World Health Organization, Geneva, and pilot tested it in Ethiopia. We thank staff at Ethiopian public Health Institute and the Ethiopian Pharmaceutical Fund and Supply Agency for vaccine transport and logistical support. We are also thankful to Deborah G Hong and Jean-Louis Excler of the International Vaccine Institute for reviewing and editing the manuscript.
References
- 1.WHO Cholera vaccines: WHO position paper-Recommendations. Vaccine. 2010;28(30):4687–8. doi: 10.1016/j.vaccine.2010.05.008. PMID:20483196. [DOI] [PubMed] [Google Scholar]
- 2.Cholera, 2015 Releve epidemiologique hebdomadaire / Section d'hygiene du Secretariat de la Societe des Nations = Weekly epidemiological record / Health Section of the Secretariat of the League of Nations. 2016;91(38):433–40. [PubMed] [Google Scholar]
- 3.WHO Cholera. Fact sheet N°107. Updated October 2016. 2015 [cited 20163rdJune]. Available from: http://www.who.int/mediacentre/factsheets/fs107/en/.
- 4.Clemens J, Shin S, Sur D, Nair GB, Holmgren J. New-generation vaccines against cholera. Nat Rev Gastroenterol Hepatol. 2011;8(12):701–10. doi: 10.1038/nrgastro.2011.174. PMID:22064524. [DOI] [PubMed] [Google Scholar]
- 5.Martin S, Lopez AL, Bellos A, Deen J, Ali M, Alberti K, Anh DD, Costa A, Grais RF, Legros D, et al.. Post-licensure deployment of oral cholera vaccines: a systematic review. Bull World Health Organ. 2014;92(12):881–93. doi: 10.2471/BLT.14.139949. PMID:25552772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kar SK, Sah B, Patnaik B, Kim YH, Kerketta AS, Shin S, Rath SB, Ali M, Mogasale V, Khuntia HK, et al.. Mass vaccination with a new, less expensive oral cholera vaccine using public health infrastructure in India: The Odisha model. PLoS Med. 2014;8(2):e2629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ciglenecki I, Sakoba K, Luquero FJ, Heile M, Itama C, Mengel M, Grais RF, Verhoustraeten F, Legros D. Feasibility of mass vaccination campaign with oral cholera vaccines in response to an outbreak in Guinea. PLoS medicine. 2013;10(9):e1001512. doi: 10.1371/journal.pmed.1001512. PMID:24058301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Porta MI, Lenglet A, de Weerdt S, Crestani R, Sinke R, Frawley MJ, Van Herp M, Zachariah R. Feasibility of a preventive mass vaccination campaign with two doses of oral cholera vaccine during a humanitarian emergency in South Sudan. Trans R Soc Trop Med Hyg. 2014;108(12):810–5. doi: 10.1093/trstmh/tru153. PMID:25311798. [DOI] [PubMed] [Google Scholar]
- 9.Hall RH, Sack DA. Introducing cholera vaccination in Asia, Africa and Haiti: A meeting report. Vaccine. 2015;33(4):487–92. doi: 10.1016/j.vaccine.2014.11.031. PMID:25437100. [DOI] [PubMed] [Google Scholar]
- 10.Lucas ME, Deen JL, von Seidlein L, Wang XY, Ampuero J, Puri M, Ali M, Ansaruzzaman M, Amos J, Macuamule A, et al.. Effectiveness of mass oral cholera vaccination in Beira, Mozambique. N Engl J Med. 2005;352(8):757–67. doi: 10.1056/NEJMoa043323. PMID:15728808. [DOI] [PubMed] [Google Scholar]
- 11.Wierzba TF, Kar SK, Mogasale VV, Kerketta AS, You YA, Baral P, Khuntia HK, Ali M, Kim YH, Rath SB, et al.. Effectiveness of an oral cholera vaccine campaign to prevent clinically-significant cholera in Odisha State, India. Vaccine. 2015;33(21):2463–9. doi: 10.1016/j.vaccine.2015.03.073. PMID:25850019. [DOI] [PubMed] [Google Scholar]
- 12.Ivers LC, Hilaire IJ, Teng JE, Almazor CP, Jerome JG, Ternier R, Boncy J, Buteau J, Murray MB, Harris JB, et al.. Effectiveness of reactive oral cholera vaccination in rural Haiti: a case-control study and bias-indicator analysis. Lancet Glob Health. 2015;3(3):e162–8. doi: 10.1016/S2214-109X(14)70368-7. PMID:25701994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Luquero FJ, Sack DA. Effectiveness of oral cholera vaccine in Haiti. Lancet Glob Health. 2015;3(3):e120–1. doi: 10.1016/S2214-109X(15)70015-X. PMID:25701986. [DOI] [PubMed] [Google Scholar]
- 14.Desai SN, Pezzoli L, Martin S, Costa A, Rodriguez C, Legros D, Perea W. A second affordable oral cholera vaccine: implications for the global vaccine stockpile. Lancet Glob Health. 2016;4(4):e223–4. doi: 10.1016/S2214-109X(16)00037-1. PMID:27013303. [DOI] [PubMed] [Google Scholar]
- 15.Desai SN, Clemens JD. An overview of cholera vaccines and their public health implications. Curr Opin Pediatr. 2012;24(1):85–91. doi: 10.1097/MOP.0b013e32834eb625. PMID:22157363. [DOI] [PubMed] [Google Scholar]
- 16.Kar SK, Pach A, Sah B, Kerketta AS, Patnaik B, Mogasale V, Kim YH, Rath SB, Shin S, Khuntia HK, et al.. Uptake during an oral cholera vaccine pilot demonstration program, Odisha, India. Hum Vaccin Immunother. 2014;10(10):2834–42. doi: 10.4161/21645515.2014.971655. PMID:25483631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ali M, Nelson AR, Lopez AL, Sack DA. Updated global burden of cholera in endemic countries. PLoS Negl Trop Dis. 2015;9(6):e0003832. doi: 10.1371/journal.pntd.0003832. PMID:26043000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mogasale V, Kar SK, Kim J-H, Mogasale VV, Kerketta AS, Patnaik B, Rath SB, Puri MK, You YA, Khuntia HK, et al.. An estimation of private household costs to receive free oral cholera vaccine in Odisha, India. PLoS Negl Trop Dis. 2015;9(9):e0004072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Khan IA, Saha A, Chowdhury F, Khan AI, Uddin MJ, Begum YA, Riaz BK, Islam S, Ali M, Luby SP, et al.. Coverage and cost of a large oral cholera vaccination program in a high-risk cholera endemic urban population in Dhaka, Bangladesh. Vaccine. 2013;31(51):6058–64. doi: 10.1016/j.vaccine.2013.10.021. PMID:24161413. [DOI] [PubMed] [Google Scholar]
- 20.MSF Mass Oral Cholera Vaccination Campaign (OCV) in Maban County in the refugee camps and host population in the direct surroundings of the camps. Medecins sans Frontieres Holland. 2012. [Google Scholar]
- 21.Kallenberg J. Gavi's Vaccine Investment Strategy. WHO Product Development for Vaccines Advisory Committee Meeting, 2015,7–9, September, Geneva Report No. [Google Scholar]
- 22.Gavi Oral cholera vaccine support. June 8, 2016. Available from: http://www.gavi.org/support/nvs/cholera-vaccine/.
- 23.Desai SN, Akalu Z, Teshome S, Teferi M, Yamuah L, Kim DR, Yang JS, Hussein J, Park JY, Jang MS, et al.. A Randomized, Placebo-Controlled Trial Evaluating Safety and Immunogenicity of the Killed, Bivalent, Whole-Cell Oral Cholera Vaccine in Ethiopia. Am J Trop Med Hyg. 2015;93(3):527–33. doi: 10.4269/ajtmh.14-0683. PMID:26078323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hsiao A, Desai SN, Mogasale V, Exclerc J-L, Digilio L. Lessons learnt from 12 oral cholera vaccine campaigns in resource-poor settings. WHO Bull. 2017;95:303–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Morgan W, Levin A, Hutubessy RC, Mogasale V. Costing Oral Cholera Vaccine Delivery Using a Generic Oral Cholera Vaccine Delivery Planning and Costing Tool (CHOLTOOL). International Vaccine Insitute. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.National Bank Of Ethiopia Inter-bank Daily Foreign Exchange Rate in (USD)- Archive (January 2015 -Novemer 2015) [Internet]. 2015. [cited December8, 2015]. Available from: http://www.nbe.gov.et/market/dailyexchange.html
- 27.Mogasale V, Ramani E, Wee H, Kim J. Oral Cholera Vaccination Delivery Cost in Low- and Middle-Income Countries: An Analysis Based on Systematic Review. PLoS Negl Trop Dis. 2016;10(12):e0005124. doi: 10.1371/journal.pntd.0005124. PMID:27930668. [DOI] [PMC free article] [PubMed] [Google Scholar]


