To the Editor:
Pulmonary arterial hypertension (PAH) is a fatal disease of the pulmonary vasculature, characterized by abnormal remodeling of pulmonary vessels and elevated pulmonary arterial pressure, ultimately leading to right ventricular (RV) failure and death. MEF2 (myocyte enhancer factor 2) is a transcription factor that regulates multiple genes that are important during cardiovascular development and for vascular homeostasis (1), with MEF2C known to play a crucial role in regulating endothelial integrity, survival, and angiogenesis (2). A wealth of evidence supports regulation of MEF2 activity by the class IIA subset of HDACs (histone deacetylases), which directly bind to and inhibit its transcriptional activity (3). Our previous study demonstrated the involvement of the HDAC IIA–MEF2 axis in PAH, where increased HDAC IIA activity in pulmonary artery endothelial cells (PAECs) from subjects with PAH was strongly associated with impaired MEF2 function (4). Here, we used experimental rodent pulmonary hypertension (PH) models to determine whether genetic or pharmacologic targeting of the HDAC IIA–MEF2 axis can affect PH pathogenesis. Some of the results of these studies have been previously reported in the form of an abstract (5).
Methods
To investigate the role of endothelial MEF2 function in the pathogenesis of PH, we generated adult mice with inducible, endothelial-specific deletion of the Mef2c allele, using a tamoxifen-responsive Cdh5 promoter–driven Cre recombinase crossed to mice with Mef2cfl/fl alleles (henceforth called Mef2cECKO mice) (6, 7). Invasive hemodynamic and morphometric parameters were assessed at baseline and after exposure to 3 weeks of chronic hypoxia (10% FiO2). For the SU-5416/hypoxia rat models, male Sprague-Dawley rats (200–250 g) were injected subcutaneously with SU-5416 (Sigma; 20 mg/kg body weight). For drug administration, rats were given injections of either vehicle or TMP269 (50 mg/kg of body weight intraperitoneally; DC Chemical), or Tasquinimod (10 mg/kg body weight by oral gavage; DC Chemical) daily.
Results and Discussion
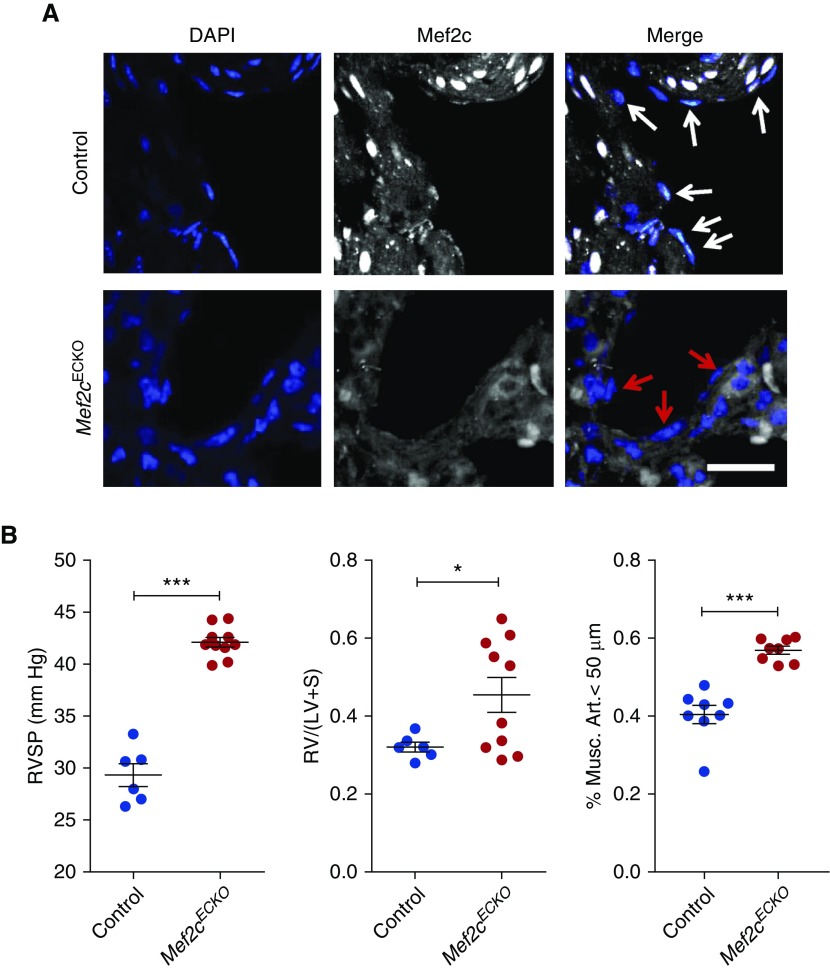
Immunofluorescent staining confirmed the endothelial-specific deletion of Mef2c in the lungs after injection of tamoxifen (Figure 1A). These mice did not display any appreciable pulmonary vascular phenotype up to 6 weeks after Mef2c deletion, but on chronic hypoxia exposure, the Mef2cECKO mice developed significantly higher right ventricular systolic pressure (Figure 1B). We also observed increased right ventricle to left ventricle plus septum weight ratios in the Mef2cECKO mice, although there was variability in the extent of right ventricular hypertrophy (Figure 1B). Lung morphometric analyses showed significantly increased percentage of smooth muscle actin–positive muscularized pulmonary arterioles in Mef2cECKO mice compared with controls. Overall, these findings provide the first genetic evidence that endothelial MEF2C is crucial for maintaining pulmonary vascular homeostasis in vivo, and that its genetic deletion results in exacerbation of PH.
Figure 1.
Endothelial-specific deletion of Mef2c leads to worsening pulmonary hypertension. (A) Immunofluorescent staining for Mef2c (white) in the lungs of Mef2cECKO mice. White and red arrows designate endothelial cell nuclei. DAPI (blue) is also shown. Scale bar: 50 μm. (B) (Left) Right ventricular systolic pressure (RVSP) measurements of control and Mef2cECKO mice after exposure to chronic hypoxia. ***P < 0.001. n = 6–10 per group. (Middle) Right ventricle to left ventricle + septum [RV/(LV + S)] weight ratios for control and Mef2cECKO mice after exposure to chronic hypoxia. *P < 0.05. (Right) Quantification of muscularized pulmonary vessels in Mef2cECKO mice and controls. ***P < 0.001. Mef2c = myocyte enhancer factor 2c; Musc. Art. = muscularized pulmonary arterioles.
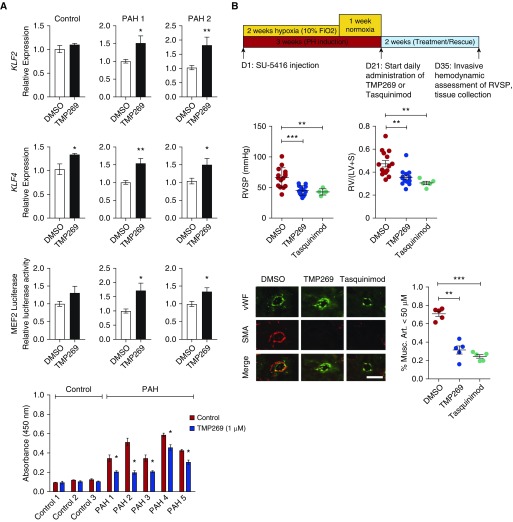
We sought to further investigate the efficacy of HDAC IIA inhibition in inducing MEF2 activation. Stimulation with TMP269 (8), a highly selective HDAC IIA inhibitor, led to significant increase in KLF2 and KLF4 expression and increased MEF2 luciferase reporter activity in two independent PAH PAEC lines (Figure 2A). Next, we found that treatment of PAECs from patients with PAH, which maintain a higher proliferation rate compared with control PAECs, displayed significantly decreased proliferation rate when treated with TMP269. TMP269 had a negligible effect on proliferation of control PAECs. To determine in vivo whether augmenting MEF2 activity via selective HDAC IIA inhibition can reverse PH pathogenesis, we tested TMP269, as well as a second HDAC IIA inhibitor that has been used in cancer clinical trials (Tasquinimod), in the SU-5416/hypoxia rat PH model (9, 10). Although use of male rats is a limitation, they develop more severe PH and were used for our studies. Rats were treated for 2 weeks with daily administration of either TMP269 or Tasquinimod after they were induced to develop PH (Figure 2B). We found a significant reduction in right ventricular systolic pressure and RV hypertrophy in rats treated with TMP269 or Tasquinimod compared with vehicle controls. Morphometric analyses of the lungs demonstrated a significantly decreased number of muscularized arterioles in both the TMP269 and Tasquinimod groups. Endothelial transcriptional machinery is an emerging therapeutic target in PAH (11, 12). Overall, our findings show the importance of a transcriptional pathway mediated by HDAC IIA–MEF2 signaling in PH, via demonstration that endothelial-specific deletion of Mef2c exacerbates hypoxia-induced PH and two independent HDAC IIA–selective inhibitors, including one (Tasquinimod) that has been administered in a large phase 3 clinical trial, can rescue the severe SU-5416/hypoxia model of PH in rats. Although the focus of our current study has been the effect of HDAC IIA–MEF2 signaling on pulmonary vascular remodeling, future implications of this signaling axis include the effect on the RV itself, especially given recent studies demonstrating that Mef2c expression is increased in compensated RV but lost in decompensated RV, further implicating the importance of MEF2 in preservation of RV function in PAH (13). Studies to selectively investigate the RV implications of the HDAC IIA–MEF2 axis, through use of models such as pulmonary artery banding, will be critical as we seek to better understand the RV–pulmonary artery coupling in PAH.
Figure 2.
Histone deacetylase IIA inhibition activates MEF2 (myocyte enhancer factor 2) and ameliorates experimental pulmonary hypertension (PH). (A) (Top two rows) Expression of KLF2 (Krüppel-like factor 2) and KLF4 in response to TMP269 (1 μM) in pulmonary artery endothelial cells (PAECs) from two patients with pulmonary arterial hypertension (PAH). *P < 0.05; **P < 0.01. (Third row) Luciferase reporter assay using PAH PAECs infected with a lentiviral MEF2-responsive promoter–driven luciferase reporter in response to stimulation with TMP269 (1 μM). *P < 0.05. (Bottom) Human PAECs from subjects with PAH or from control subjects were treated with TMP269 for 48 hours and proliferation was assessed with WST-1 assay. *P < 0.05. (B) (Top) Overall schematic of the experimental design of PH rescue in Sprague-Dawley rats. (Middle) Right ventricular systolic pressure (RVSP) (left) and right ventricle to left ventricle + septum [RV/(LV + S)] weight ratios (right) in SU-5416/hypoxia Sprague-Dawley rats treated with TMP269 or Tasquinimod. **P < 0.01; ***P < 0.001. n = 5–15 per group. (Bottom) Representative images and quantification of muscularized pulmonary vessels in the different experimental groups. Scale bar: 50 μm. **P < 0.01; ***P < 0.001. Musc. Art. = muscularized pulmonary arterioles; SMA = smooth muscle actin; vWF = von Willebrand factor.
Acknowledgments
Acknowledgment
The authors thank R. Adams for the Cdh5-CreERT2 mice; S. Park for assistance with mouse genotyping; X. Hu, N. Barucci, and W. Zavadowski for assistance with the experimental rodent studies; and R. Webber and N. Copeland for animal care.
Footnotes
This work was supported by funding from the NIH/NHLBI (HL119345 to H.J.C., HL007778 to A.S., and HL060917 and HL081064 to S.C.E.; Vascular Intervention/Innovation and Therapeutic Advances Program Contract Number: HHSN268201700009C), American Heart Association (Established Investigator Award to H.J.C.), and National Research Foundation of Korea Grant funded by the Korean Government (NRF 2014S1A2A2028520 to J.K. and H.J.C.).
Originally Published in Press as DOI: 10.1164/rccm.201805-0817LE on August 14, 2018
Author disclosures are available with the text of this letter at www.atsjournals.org.
References
- 1.Potthoff MJ, Olson EN. MEF2: a central regulator of diverse developmental programs. Development. 2007;134:4131–4140. doi: 10.1242/dev.008367. [DOI] [PubMed] [Google Scholar]
- 2.Lu YW, Lowery AM, Sun LY, Singer HA, Dai G, Adam AP, et al. Endothelial myocyte enhancer factor 2c inhibits migration of smooth muscle cells through fenestrations in the internal elastic lamina. Arterioscler Thromb Vasc Biol. 2017;37:1380–1390. doi: 10.1161/ATVBAHA.117.309180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kang Y, Kim J, Anderson JP, Wu J, Gleim SR, Kundu RK, et al. Apelin-APJ signaling is a critical regulator of endothelial MEF2 activation in cardiovascular development. Circ Res. 2013;113:22–31. doi: 10.1161/CIRCRESAHA.113.301324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim J, Hwangbo C, Hu X, Kang Y, Papangeli I, Mehrotra D, et al. Restoration of impaired endothelial myocyte enhancer factor 2 function rescues pulmonary arterial hypertension. Circulation. 2015;131:190–199. doi: 10.1161/CIRCULATIONAHA.114.013339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sofer A, Lee S, Papangeli I, Hwangbo C, Adachi T, Park S, et al. Selective HDAC IIA inhibition augments MEF2 activity and improves survival in experimental pulmonary hypertension [abstract]; Presented at the 12th PVRI Annual World Congress. January 21–25, 2018, Singapore. [Google Scholar]
- 6.Wang Y, Nakayama M, Pitulescu ME, Schmidt TS, Bochenek ML, Sakakibara A, et al. Ephrin-B2 controls VEGF-induced angiogenesis and lymphangiogenesis. Nature. 2010;465:483–486. doi: 10.1038/nature09002. [DOI] [PubMed] [Google Scholar]
- 7.Vong LH, Ragusa MJ, Schwarz JJ. Generation of conditional Mef2cloxP/loxP mice for temporal- and tissue-specific analyses. Genesis. 2005;43:43–48. doi: 10.1002/gene.20152. [DOI] [PubMed] [Google Scholar]
- 8.Lobera M, Madauss KP, Pohlhaus DT, Wright QG, Trocha M, Schmidt DR, et al. Selective class IIa histone deacetylase inhibition via a nonchelating zinc-binding group. Nat Chem Biol. 2013;9:319–325. doi: 10.1038/nchembio.1223. [DOI] [PubMed] [Google Scholar]
- 9.Taraseviciene-Stewart L, Kasahara Y, Alger L, Hirth P, Mc Mahon G, Waltenberger J, et al. Inhibition of the VEGF receptor 2 combined with chronic hypoxia causes cell death-dependent pulmonary endothelial cell proliferation and severe pulmonary hypertension. FASEB J. 2001;15:427–438. doi: 10.1096/fj.00-0343com. [DOI] [PubMed] [Google Scholar]
- 10.Isaacs JT, Antony L, Dalrymple SL, Brennen WN, Gerber S, Hammers H, et al. Tasquinimod Is an Allosteric Modulator of HDAC4 survival signaling within the compromised cancer microenvironment. Cancer Res. 2013;73:1386–1399. doi: 10.1158/0008-5472.CAN-12-2730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shatat MA, Tian H, Zhang R, Tandon G, Hale A, Fritz JS, et al. Endothelial Krüppel-like factor 4 modulates pulmonary arterial hypertension. Am J Respir Cell Mol Biol. 2014;50:647–653. doi: 10.1165/rcmb.2013-0135OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chun HJ, Bonnet S, Chan SY. Translational advances in the field of pulmonary hypertension. translating microRNA biology in pulmonary hypertension: it will take more than “miR” words. Am J Respir Crit Care Med. 2017;195:167–178. doi: 10.1164/rccm.201604-0886PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Paulin R, Sutendra G, Gurtu V, Dromparis P, Haromy A, Provencher S, et al. A miR-208-Mef2 axis drives the decompensation of right ventricular function in pulmonary hypertension. Circ Res. 2015;116:56–69. doi: 10.1161/CIRCRESAHA.115.303910. [DOI] [PubMed] [Google Scholar]