Abstract
Rationale: Cigarette smoking is prevalent in the United States and is the leading cause of preventable diseases. A prominent complication of smoking is an increase in lower respiratory tract infections (LRTIs). Although LRTIs are known to be increased in subjects that smoke, the mechanism(s) by which this occurs is poorly understood.
Objectives: Determine how cigarette smoke (CS) reduces reactive oxygen species (ROS) production by the phagocytic NOX2 (NADPH oxidase 2), which is essential for innate immunity in lung macrophages.
Methods: NOX2-derived ROS and Rac2 (Ras-related C3 botulinum toxin substrate 2) activity were determined in BAL cells from wild-type and Rac2−/− mice exposed to CS or cadmium and in BAL cells from subjects that smoke. Host defense to respiratory pathogens was analyzed in mice infected with Streptococcus pneumoniae.
Measurements and Main Results: NOX2-derived ROS in BAL cells was reduced in mice exposed to CS via inhibition of the small GTPase Rac2. These mice had greater bacterial burden and increased mortality compared with air-exposed mice. BAL fluid from CS-exposed mice had increased levels of cadmium, which mediated the effect on Rac2. Similar observations were seen in human subjects that smoke. To support the importance of Rac2 in the macrophage immune response, overexpression of constitutively active Rac2 by lentiviral administration increased NOX2-derived ROS, decreased bacterial burden in lung tissue, and increased survival compared with CS-exposed control mice.
Conclusions: These observations suggest that therapies to maintain Rac2 activity in lung macrophages restore host defense against respiratory pathogens and diminish the prevalence of LRTIs in subjects that smoke.
Keywords: NADPH oxidase 2, heavy metals, respiratory tract infections, innate immunity
At a Glance Commentary
Scientific Knowledge on the Subject
Cigarette smoking is the leading cause of preventable disease in the United States. One important complication of cigarette smoking is an increased risk of lower respiratory tract infections. The link between cigarette smoking and lung infections is well established; however, the mechanism(s) by which this occurs is poorly understood and remains to be thoroughly investigated.
What This Study Adds to the Field
Cigarette smoke diminished reactive oxygen species production in lung macrophages, which are critical for host defense. We determined that cadmium from cigarette smoke mediated these changes by inhibiting the small GTPase Rac2 (Ras-related C3 botulinum toxin substrate 2). These observations suggest that therapies to maintain Rac2 activity in lung macrophages restore host defense against respiratory pathogens and diminish the prevalence and severity of lower respiratory tract infections in subjects who smoke.
Cigarette smoking remains prevalent in the United States, with approximately 17% of adults currently smoking (1). The incidence of lower respiratory tract infections (LRTIs) is increased in individuals that smoke tobacco. LRTIs are the most common infection that results in hospitalization and are the leading infectious cause of death (2–5). Although the link between cigarette smoking and LRTIs is well described (4), the molecular pathogenesis of cigarette smoke–induced LRTI is not known.
Lung macrophages play a critical role in host defense to respiratory pathogens (6–9). Although neutrophils are often considered the essential cell in combating bacterial infections, studies have questioned the effectiveness of neutrophils in bacterial killing and bacterial clearance as compared with macrophages (6, 8). Studies show in models of bacterial pneumonia that lung macrophage depletion decreases containment and clearance of bacteria, as well as impairs survival (6, 10).
One mechanism lung macrophages use for host defense includes the generation of reactive oxygen species (ROS) by the NOX2 (NADPH oxidase 2) complex. NOX2 is a large complex composed of membrane components gp91phox and p22phox and the four cytosolic proteins p67phox, p47phox, p40phox, and the small GTP-binding protein Rac (Ras-related C3 botulinum toxin substrate). Importantly, the generation of NOX2-derived ROS in lung macrophages requires activation of Rho GTPases. Rac2 binding of cytosolic p67phox and its translocation to the membrane are required for the formation of NOX2 (9, 11). Dominant negative mutations of Rac2 in infant children are associated with an increased risk of infection (12, 13).
Cigarette smoke (CS) contains more than 4,500 compounds, some of which are carcinogens, toxins, oxidants, and metals (1). Of all the noxious compounds in CS, metals are known to play a critical role in the threat of diseases associated with smoking (14). Respiratory diseases, cardiovascular disease, and bone frailty have also been associated with metal exposure (15–18). Cadmium is one metal found in CS that has no physiological function in humans. CS is the main source of cadmium toxicity. Cadmium has been implicated in altering phagocytosis and bacterial clearance in lung macrophages, whereas other immune cells are unaffected (19).
Here, we assessed the role of CS in altering lung macrophage host defense. We determined that CS diminished NOX2-derived ROS generation in BAL cells and led to increased lung bacterial burden in CS-exposed mice compared with air-exposed mice. Of all the metals in CS, we found that cadmium was increased in the BAL fluid (BALF) of mice and humans that smoked. Cadmium altered Rac2 activity, which is required for the generation of membrane-derived ROS in lung macrophages. Recovery of Rac2 activation by the administration of a lentiviral vector overexpressing constitutively active Rac2 to mice promoted lung macrophage innate immunity, reduced lung bacterial burden, and led to increased survival. Some of the results of these studies have been previously reported in the form of an abstract (20).
Methods
Additional methods are presented in the online supplement.
Human Subjects
We obtained human lung macrophages and BALF, as previously described (21), from smokers and nonsmokers without lung disease under approved protocols E150318008 and F120404001 by the University of Alabama at Birmingham (UAB) Institutional Review Board. Human BAL specimens were used for research only. All subjects provided prior written consent to participate in the study.
Animal Studies
Protocols were approved by the UAB Institutional Animal Care and Use Committee. Eight- to 12-week-old male and female wild-type C57BL6 and Rac2−/− mice were used. Mice were exposed in whole-body chambers to air or 40 times diluted mainstream cigarette smoke for 10 consecutive days, using a SCIREQ “inExpose” smoke machine. Mice were exposed to whole-body mainstream CS generated from 3R4F research cigarettes (9.4 mg tar/0.726 mg nicotine; University of Kentucky) with removed filters, to increase the potency of the cigarettes (22), as previously described (23).
In vivo transduction studies were performed with constitutively active Rac2 (Rac2CA) lentivirus, which was a generous gift from Y. Cao (Nankai University, Tianjin, China) (24). Full-length Flag-Rac2CA cDNA was subcloned into pLVX-IRES-tdTomato lentiviral vector (Clontech). Concentrated lentiviral supernatant (5 × 107 TU in 100 μl) was delivered intratracheally as described (25). Thirty minutes before intratracheal instillation, all viral supernatants were mixed with Lipofectamine 2000 (final concentration, 5% [vol/vol]; Invitrogen). Acute inflammation was allowed to resolve for 6 weeks after transduction.
For in vivo bacterial infection studies, mice were administered sterile saline or 107 Streptococcus pneumoniae (strain A66.1, type 3) intratracheally and were killed after 2 days. Bacterial infections were performed after the last smoke exposure on Day 10 or after 5 days of cadmium exposure. Mice were monitored every 4–6 hours after bacterial exposure for the duration of the exposure.
Inductively Coupled Plasma Mass Spectrometry
Metal levels (Mn, Cd, Fe, Cu, As, Pb, and Tl) in BAL samples were measured by inductively coupled plasma-mass spectrometry (ICP-MS) (7500a ICP-MS; Agilent). An aliquot (500 μl) of each sample was diluted in 4.5 ml of 2% nitric acid (trace metal basis). Samples were analyzed in sextuplicate and the concentration of each metal was calculated using a standard calibration curve. All solutions used for the analysis were treated with Chelex 100 resin (BioRad) to remove cations.
Statistical Analysis
Statistical comparisons were performed using an unpaired two-tailed Student t test when only two groups of data are presented, one-way ANOVA with a Tukey post hoc test when multiple data groups are present, a Mann-Whitney U test, or Kaplan-Meier analysis in survival studies. All statistical analysis data are expressed as mean ± SEM unless otherwise indicated; P < 0.05 was considered to be significant. GraphPad Prism 5 statistical software (GraphPad Software) was used for analysis.
Results
Cigarette Smoke Increases Lung Bacterial Burden
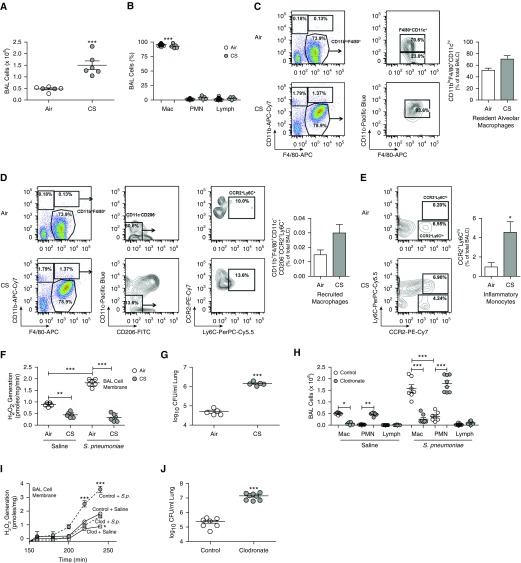
Studies show that CS alters the innate immune response; however, CS increased the total number of BAL cells threefold (Figure 1A). Although macrophages were the predominant cell type (Figure 1B), we questioned the source of the BAL cells. No differences were detected in resident alveolar macrophages after CS exposure (Figure 1C) or recruited macrophages (Figure 1D), whereas CCR2+/Ly6Chi inflammatory monocytes were significantly increased in CS-exposed mice (Figure 1E).
Figure 1.
Cigarette smoke (CS) increases lung bacterial burden. (A) Total number of BAL cells (n = 6), (B) cell differential (n = 6), and (C–E) percentage of resident macrophages (CD11blo/F4/80+/CD11chi) (n = 3) (C), recruited macrophages (CD11b+/F4/80+/CD11c−/CD206−/CCR2+/Ly6C+) (n = 3) (D), and early inflammatory monocytes (CCR2+/Ly6Chi) (n = 3) (E) from air- and CS-exposed mice (16 cigarettes/d for 10 d). (F) Lung macrophage NOX2 (NADPH oxidase 2)-derived reactive oxygen species (ROS) generation (n = 6) and (G) lung colony-forming units (cfu) from air- and CS-exposed mice infected with vehicle or Streptococcus pneumoniae (107 cfu for 48 h, strain A66.1, type 3) (n = 5). (H) Cell differential, total number (n = 7–8), (I) lung macrophage NOX2-derived ROS generation (n = 5), and (J) lung colony-forming units from mice treated with control or clodronate liposomes and infected with vehicle or S. pneumoniae (n = 7–8). *P < 0.05; **P < 0.001; ***P < 0.0001. Values shown represent means ± SEM. Two-tailed t test statistical analysis was used for A, G, and J. Mann-Whitney U statistical analysis was used for C–E. One-way ANOVA followed by Tukey’s multiple comparison test was performed on B, F, H, and I. Results from A, B, F, and G were repeated at least five times; C–E were conducted once with representative plots of three shown, and H–J were repeated three times. BALC = BAL cells; Clod = clodronate; FITC = fluorescein isothiocyanate; Lymph = lymphocyte; Mac = macrophage/monocyte; PMN = polymorphonuclear neutrophil; S.p. = Streptococcus pneumoniae.
We investigated the relationship of CS exposure and bacterial lung infection in vivo. The total number of BAL cells was increased with CS exposure and was increased further after exposure to Streptococcus pneumoniae (strain A66.1, type 3), as a model of LRTI (see Figure E1A in the online supplement). Macrophages were the predominant cell type in BALF from CS-exposed mice; however, S. pneumoniae exposure increased neutrophil recruitment as well (Figure E1B). Proinflammatory cytokines tumor necrosis factor (TNF)-α, macrophage inflammatory protein (MIP)-2, and IL-6 were increased in response to CS alone, but there was a significant further increase to a similar level in response to S. pneumoniae (Figures E1C–E1E).
ROS production by NOX2 in the cell membrane is necessary for bacterial killing and has an important role in macrophage innate immunity (6, 8, 10). CS exposure significantly decreased membrane-derived ROS generation in BAL cells (n = 6) (Figure 1F). Although BAL cells from S. pneumoniae–exposed mice had significantly increased NOX2-derived ROS generation, CS exposure abrogated this increase. The CS-mediated reduction in membrane-derived ROS was associated with a significant increase in colony-forming units from the lungs of mice (Figure 1G).
To investigate the significance of lung macrophages in bacterial lung infection, macrophages were depleted in mice, using clodronate liposomes. Macrophage depletion did not alter the total BAL cell count after S. pneumoniae exposure (Figure E1F), but there was an increase in number (Figure 1H) and percentage of neutrophils in the BAL (Figure E1G). S. pneumoniae infection in mice depleted of macrophages showed a significant reduction in membrane-derived ROS compared with BAL cells from mice treated with control liposomes (Figure 1I). In addition, macrophage depletion significantly increased colony-forming units in lungs (Figure 1J). Confirming the significance of lung macrophages, no difference in lung colony-forming units was detected in mice depleted of neutrophils compared with controls (Figures E1H and E1I). These data indicate that lung macrophages have a critical role in host defense to respiratory pathogens.
Macrophage Rac2 Regulates NOX2-derived ROS
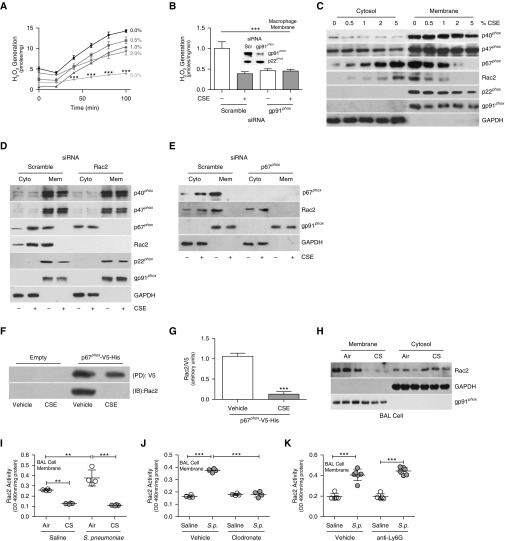
To determine the role of the impaired lung macrophage immune response in CS-mediated development of LRTI, macrophages were exposed to cigarette smoke extract (CSE). Membrane-derived ROS generation was markedly reduced in macrophages exposed to CSE in a dose-dependent manner (Figure 2A). Cell viability was not minimized by CSE (data not shown). Silencing gp91phox had a similar effect as exposing cells to CSE (Figure 2B). In addition, phorbol myristate acetate (PMA)–exposed macrophages generated significantly more membrane-derived ROS than vehicle-exposed macrophages, and PMA generated no ROS after CSE exposure (Figures E2A and E2B).
Figure 2.
Macrophage Rac2 (Ras-related C3 botulinum toxin substrate 2) regulates NOX2 (NADPH oxidase 2)-derived reactive oxygen species (ROS). (A and B) Membrane-derived ROS generation in THP-1 cells treated with the indicated concentration of cigarette smoke extract (CSE) for 3 hours (n = 3) (A) and THP-1 cells expressing scrambled or gp91phox siRNA treated with vehicle or CSE (5%) for 3 hours (n = 3) (B). (B, inset) Immunoblot analysis confirming gp91phox silencing. (C and D) Immunoblot analysis of NOX2 complex proteins in isolated membrane or cytosol fractions from MH-S cells treated with the indicated concentrations of CSE (C) and THP-1 cells expressing scrambled or Rac2 siRNA treated with vehicle or CSE (D). (E) Immunoblot analysis of THP-1 cells expressing scrambled or p67phox siRNA treated with vehicle or CSE. (F) Histidine (His) pull-down in MH-S cells expressing empty or p67phox-V5-His and treated with vehicle or CSE and (G) quantification. (H) Immunoblot analysis of BAL cells from air- or CS-exposed mice (n = 3). (I and J) Rac2 activity in BAL cells from air- and CS-exposed mice infected with vehicle or Streptococcus pneumoniae (n = 4) (I) and mice treated with control or clodronate liposomes and infected with vehicle or S. pneumoniae (n = 4) (J). (K) Rac2 membrane activity in BAL cells from control and anti-Ly6G–treated mice infected with vehicle or S. pneumoniae (n = 5–6). **P < 0.001; ***P < 0.0001. Values shown represent means ± SEM. One-way ANOVA followed by Tukey’s multiple comparison test was used for A, B, and I–K. Results from A, B, and J were repeated three times; immunoblot analyses were performed three times, with representative immunoblots shown in C–F and H; I was conducted at least five times. Cyto = cytosol; IB = immunoblot; Mem = membrane; PD = pull-down; Scr = scrambled; S.p. = Streptococcus pneumoniae.
CSE did not alter p40phox or p47phox membrane localization or disrupt p22phox or gp91phox presence in the membrane; however, CSE inhibited p67phox and Rac2 membrane recruitment in a dose-dependent manner (Figure 2C). Because Rac2 binds p67phox to translocate it to the membrane (7, 9, 11), we confirmed that silencing Rac2 dramatically abolished p67phox membrane localization (Figures 2D, E2C, and E2D). The other NOX2 components were not altered by Rac2 silencing. Similarly, silencing p67phox inhibited Rac2 membrane localization (Figures 2E, E2E, and E2F). Validating the intracellular interaction between p67phox and Rac2, macrophages expressing p67phox-V5-His had increased binding to Rac2, and CSE inhibited this interaction (Figures 2F and 2G). CSE also inhibited Rac2 membrane activity in a dose-dependent manner (Figure E2G).
Because Rac2 is critical for the formation of NOX2, we determined that CS attenuated Rac2 membrane localization of BAL cells from mice (Figure 2H). CS exposure also inhibited Rac2 membrane activity in BAL cells from S. pneumoniae–exposed mice (Figure 2I).
Although there is an increase in neutrophils with S. pneumoniae infection, CSE elicited no change in Rac2 activity (Figure E2H) or membrane-derived ROS (Figure E2I) in isolated membranes from neutrophils. Furthermore, Rac2 activity was significantly increased in isolated membranes from BAL cells after S. pneumoniae exposure, and macrophage depletion abrogated this response (Figure 2J), whereas mice depleted of neutrophils showed no difference in membrane Rac2 activity in BAL cells compared with controls (Figure 2K). The alteration in macrophage Rac2 activity directly regulated NOX2-derived ROS generation (Figure E2J).
Rac2−/− Mice Have Impaired Host Defense
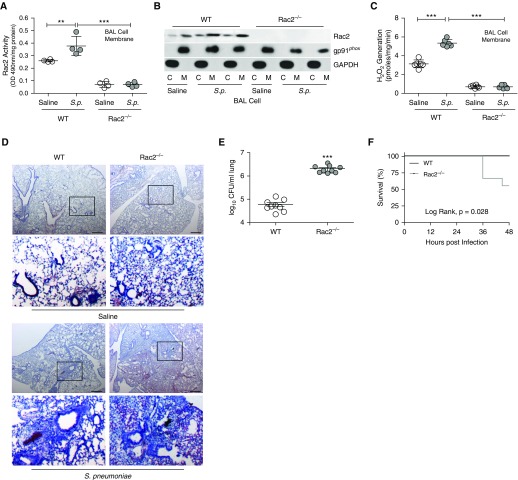
Although the Rac2-dependent immune response of BAL cells from mice exposed to CS has a key role in the development of LRTI, wild-type and Rac2−/− mice had a similar increase in BAL cells (Figure E3A) and cell differential (Figure E3B) after S. pneumoniae exposure. Wild-type mice infected with S. pneumoniae had increased Rac2 activity in the BAL cell membrane (Figure 3A). Rac2 activity in Rac2−/− mice was below that of saline controls and was not altered with S. pneumoniae infection. Rac2 membrane localization was enhanced in BAL cells from infected wild-type mice (Figure 3B). The absence of Rac2 markedly reduced ROS generation in the BAL cell membrane (Figure 3C).
Figure 3.
Rac2−/− mice have impaired host defense. Wild-type and Rac2−/− mice were infected with Streptococcus pneumoniae. (A) Rac2 activity in BAL cell membrane (n = 4–5), (B) immunoblot analysis in cytosol and membrane fractions of BAL cells, and (C) NOX2 (NADPH oxidase 2)-derived reactive oxygen species generation in BAL cell membrane (n = 5). (D) Hematoxylin and eosin staining of lung tissues was performed (n = 4–5); and (E) lung colony-forming units (n = 9) and (F) Kaplan-Meier survival curves (n = 8–9) were determined. Scale bars, 600 μm. The second and fourth rows in D show magnifications of the boxed regions in the first and third rows, respectively. **P < 0.001; ***P < 0.0001. Values shown represent means ± SEM. One-way ANOVA followed by Tukey’s multiple comparison test was used for A and C; two-tailed Student’s t-test statistical analysis was used for E, and a log-rank (Mantel-Cox) test was used for F. Results from A–F were repeated three times with representative immunoblot and histology micrographs shown. C = cytosol; M = membrane; Rac2 = Ras-related C3 botulinum toxin substrate 2; S.p. = Streptococcus pneumoniae; WT = wild type.
The proinflammatory cytokines TNF-α and MIP-2 (Figures E3C and E3D) showed similar responses to infection in wild-type and Rac2−/− BALF, whereas IL-6 was increased in Rac2−/− BALF compared with wild type (Figure E3E). The deletion of Rac2−/− did not alter normal lung architecture (Figure 3D); however, Rac2−/− mice infected with S. pneumoniae had lobar consolidation associated with hemorrhage, whereas the wild-type mice had peribronchial infiltrates. The histological findings were confirmed as Rac2−/− mice had significantly greater lung colony-forming units than did wild-type mice (Figure 3E). Moreover, the Rac2 deletion increased mortality after infection (Figure 3F).
We then determined the role of CS exposure in Rac2−/− mice. Both strains had a similar increase in BAL cells after CS and S. pneumoniae exposure (Figure E3F), and no differences were detected in the cell differential (Figure E3G). CS exposure reduced NOX2-derived ROS generation in wild-type BAL cell membrane to a level similar to that seen in Rac2−/− mice (Figure E3H). CS significantly increased colony-forming units in the lungs from wild-type mice to a similar level as seen in Rac2−/− mice (Figure E3I). Furthermore, CS-exposed wild-type mice had similar mortality to the Rac2−/− mice (Figure E3J). Taken together, these data suggest that CS exposure increases the severity of LRTI by modulating Rac2.
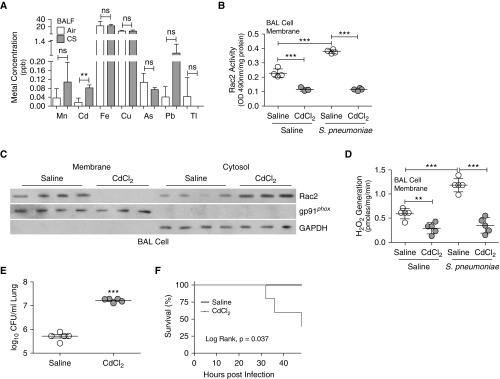
Cadmium in CS Impairs Macrophage Innate Immunity by Inhibiting Rac2
Studies suggest that significantly higher levels of metals are present in lung tissue of smokers (26, 27). We found that the concentration of cadmium was 11-fold greater in BALF from CS-exposed than air-exposed mice (n = 4); however, no difference was detected for other metals (Figure 4A). We determined that cadmium had a similar effect as CS. CdCl2 significantly diminished Rac2 membrane activity in empty- and Flag-Rac2WT-transfected macrophages and had no effect on macrophages expressing constitutively active Rac2 (YFP-Rac2CA) (Figure E4A). CdCl2 significantly reduced NOX2-derived ROS to the level present in macrophages with Rac2 silenced (Figure E4B). The other major GTPase in macrophages, Rac1, was not altered by CdCl2 exposure (Figure E4C).
Figure 4.
Cadmium in cigarette smoke (CS) impairs BAL cell innate immunity via Rac2 inhibition. (A) BAL fluid samples from air- and CS-exposed wild-type mice were subjected to inductively coupled plasma mass spectrometry to determine concentrations of the indicated metals (n = 4). Wild-type mice were treated with vehicle or CdCl2 (100 ng/kg intratracheally for 5 d) and then infected with Streptococcus pneumoniae. (B) Rac2 (Ras-related C3 botulinum toxin substrate 2) activity in BAL cell membrane (n = 3–4), (C) immunoblot analysis in cytosol and membrane fractions of BAL cells, and (D) NOX2 (NADPH oxidase 2)-derived reactive oxygen species generation in BAL cell membrane were determined (n = 5). (E) Lung colony-forming units (n = 5) and (F) Kaplan-Meier survival curves were determined (n = 6–7). **P < 0.001; ***P < 0.0001. Values shown represent means ± SEM. Two-tailed Student’s t-test statistical analysis was used for A and E, one-way ANOVA with Tukey statistical analysis was used for B and D, and a log-rank (Mantel-Cox) test for F. Results were repeated two times for A. Results were repeated three times for B–F, with a representative immunoblot shown. BALF = BAL fluid; ns = not significant.
To determine whether cadmium had a similar effect as CS regarding host defense, we exposed mice to cadmium at the mean concentration found in BALF from CS-exposed mice. CdCl2- and S. pneumoniae–exposed wild-type mice showed an increase in the total number of BAL cells (Figure E4D), with the majority being lung macrophages (Figure E4E). Rac2 activity was increased in isolated BAL membranes from S. pneumoniae–exposed mice, and CdCl2 exposure significantly reduced its activity below that of saline controls (Figure 4B). Rac2 membrane localization in BAL cells was also absent in CdCl2-exposed mice (Figure 4C). Infection with S. pneumoniae dramatically induced membrane-derived H2O2 generation in BAL cells, whereas BAL cells from CdCl2-exposed mice exhibited a significant reduction in ROS (Figure 4D). Although manganese is known to be increased in lung epithelial cells from patients with chronic obstructive pulmonary disease (28), Rac2 activity was not altered in BAL cells from MnCl2-exposed mice, using the concentration found in BALF from CS-exposed mice (Figure E4F), suggesting that this is a specific effect of cadmium.
As seen with CS exposure, the proinflammatory cytokines TNF-α and MIP-2 (Figures E4G and E4H) were similar in BALF from infected mice; however, IL-6 expression was significantly increased in CdCl2-exposed mice after S. pneumoniae infection (Figure E4I). CdCl2 exposure resulted in significantly greater lung colony-forming units (Figure 4E), which was associated with increased mortality (Figure 4F) compared with saline-exposed mice. These data indicate CS and cadmium have similar effects in mediating Rac2 inhibition in lung macrophages, resulting in diminished host defense against bacterial pathogens.
We determined the role of cadmium exposure in Rac2−/− mice. Both strains had an increase in BAL cells (Figure E4J) and had a similar cell differential (Figure E4K). CdCl2 exposure reduced ROS generation in wild-type BAL cells to levels seen in Rac2−/− mice (Figure E4L). This resulted in similar colony-forming units (Figure E4M) and mortality as seen in Rac2−/− mice (Figure E4N).
CSE and Cadmium Alter Rac2 Isoprenylation
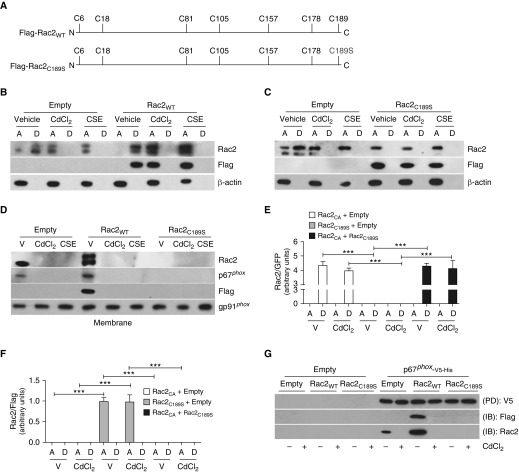
Rac2 has seven cysteine residues, and the C-terminal cysteine residue of Rho GTPases is modified by isoprenylation. This post-translational modification is required for activation and its interaction with other proteins (29, 30) (Figure 5A). Because CdCl2 and CSE altered Rac2 activity, we separated lysates into aqueous (nonprenylated) and detergent (prenylated) fractions. CdCl2 and CSE impaired isoprenylation as Rac2 expression remained in the aqueous fraction (Figure 5B). In vehicle-treated cells, Rac2 was present in the detergent fraction, indicating it was active, and these effects were enhanced with overexpression of Flag-Rac2WT. We confirmed that Cys189 was required for isoprenylation by mutating the C-terminal cysteine residue to a serine (Flag-Rac2C189S), as Rac2 remained in the aqueous fraction regardless of the exposure (Figure 5C).
Figure 5.
Cigarette smoke extract (CSE) and cadmium alter Rac2 (Ras-related C3 botulinum toxin substrate 2) isoprenylation. (A) Schematic diagram of full-length Rac2WT construct showing location of cysteine residues and Rac2 construct with Cys (C)→Ser (S) mutation at amino acid 189. (B) MH-S cells expressing empty or Flag-Rac2WT were treated with vehicle, CdCl2 (80 μM, 3 h), or CSE and were separated into aqueous or detergent fractions. Immunoblot analysis was performed. (C) MH-S cells expressing empty or Flag-Rac2C189S were treated with vehicle, CdCl2, or CSE and were separated into aqueous or detergent fractions. Immunoblot analysis was performed. (D) Macrophages expressing empty, Flag-Rac2WT, or Flag-Rac2C189S were treated with vehicle, CdCl2, or CSE. Immunoblot analysis was performed in MH-S cells in isolated membrane fraction. MH-S cells coexpressing empty and YFP-Rac2CA, or Flag-Rac2C189S, or YFP-Rac2CA and Flag-Rac2C189S were treated with vehicle or CdCl2. Quantification of immunoblot analysis was performed in isolated aqueous or detergent fractions for (E) GFP-Rac2 (n = 3) and (F) Flag-Rac2 (n = 3). (G) Histidine (His) pull-down in MH-S cells coexpressing empty or p67phox-V5-His together with Flag-Rac2WT or Flag-Rac2C189S treated with vehicle or CdCl2. ***P < 0.0001. Values shown represent means ± SEM. One-way ANOVA with Tukey statistical analysis was used for E and F. Results were repeated three times for all data in B–D and G, with representative immunoblots shown. A = aqueous; D = detergent; IB = immunoblot; PD = pull-down; V = vehicle; WT = wild type.
Rac2 isoprenylation is mediated through the mevalonate pathway, which is also responsible for protein farnesylation, cholesterol production, and ubiquinone generation. CdCl2 and CSE did not modulate these branches of the pathway (Figures E5A–E5C).
Unlike vehicle-treated macrophages expressing Flag-Rac2WT, Rac2 membrane localization was inhibited by CdCl2 and CSE (Figure 5D). In contrast, the Flag-Rac2C189S construct remained in the cytoplasm under all conditions (Figure E5D). Expression of p67phox followed the expression pattern of Rac2 in both fractions. Flag-Rac2WT and YFP-Rac2CA increased macrophage Rac2 activity in the membrane fraction of vehicle-treated cells (Figure E5E). CSE inhibited Rac2 activity only in macrophages expressing Flag-Rac2WT. Because macrophages expressing YFP-Rac2CA were able to abrogate the effects of CSE, we found that Rac2 remained isoprenylated in cells expressing YFP-Rac2CA (Figures 5E, 5F, and E5F). YFP-Rac2CA altered Rac2 localization when coexpressed with Flag-Rac2C189S. Because Rac2 and p67phox must directly interact for membrane recruitment, we determined that Rac2-p67phox interaction required Rac2 isoprenylation (Figures 5G and E5G). In aggregate, these data suggest that macrophage function is impaired by inhibiting Rac2 isoprenylation at Cys189.
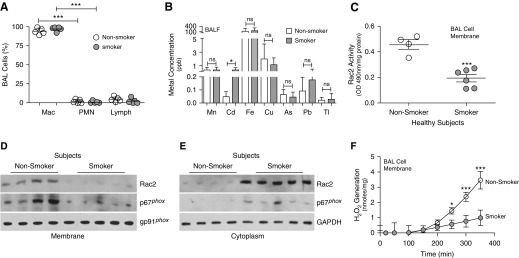
Rac2 Activity Is Reduced in BAL Cells from Smokers
BAL samples were obtained from nonsmokers and current smokers. Other than smoking history, demographics were similar between nonsmokers and smokers (Table 1). Lung macrophages were the predominant cells in BALF from both groups (Figure 6A). Similar to the cadmium concentration in CS-exposed mice, we found that BALF from smokers had a ninefold greater cadmium concentration compared with nonsmokers (Figure 6B). No other metal showed a significant difference. Isolated membrane from the BAL cells of current smokers had a significant reduction in Rac2 activity (Figure 6C). Rac2 and p67phox membrane localization was essentially absent in BAL cells from current smokers (Figures 6D and E6A), as the proteins remained in the cytosol (Figures 6E and E6B). The absence of Rac2 recruitment to the membrane corresponded to a significant diminution in membrane-derived ROS generation in current smokers (Figure 6F), suggesting that smoking mediates a defect in lung macrophage host defense by inhibiting Rac2.
Table 1.
Clinical Characteristics of Subjects
| Total (n = 10) | Nonsmoker (n = 4) | Current Smoker (n = 6) | P Value | |
|---|---|---|---|---|
| Age, yr | 52 ± 7 | 56 ± 7 | 50 ± 5 | 0.165 |
| Male sex | 5 (50%) | 2 (50%) | 3 (50%) | 1.000 |
| White race | 6 (60%) | 2 (50%) | 4 (67%) | 0.598 |
| Pack-years | 21 ± 19 | 2 ± 2 | 35 ± 10 | <0.0001 |
| FEV1 post-BD, L | 2.98 ± 0.59 | 2.85 ± 0.77 | 3.11 ± 0.54 | 0.597 |
| FEV1 post-BD, % | 96 ± 11 | 97 ± 11 | 95 ± 13 | 0.777 |
| FVC post-BD, L | 3.75 ± 0.85 | 3.48 ± 1.0 | 4.12 ± 0.55 | 0.364 |
| FVC post-BD, % | 94 ± 11 | 94 ± 14 | 95 ± 9 | 0.892 |
| FEV1/FVC | 0.81 ± 0.03 | 0.82 ± 0.02 | 0.80 ± 0.03 | 0.337 |
Definition of abbreviation: BD = bronchodilator.
Data expressed as means ± SD or n (%). Independent Student’s t test was used to compare means for continuous variables, and χ2 test was used for categorical variables.
Figure 6.
Rac2 (Ras-related C3 botulinum toxin substrate 2) activity is reduced in BAL cells from smokers. BAL was obtained from nonsmoking and smoking subjects (n = 4–6). (A) Cell differential was determined from BAL cells (n = 4–6). (B) Inductively coupled plasma mass spectrometry was performed for the indicated metals from BAL fluid (n = 4–6). (C) Rac2 activity in BAL cell membrane (n = 4–6). Immunoblot analysis of BAL cells was performed in (D) isolated membrane and (E) cytosol fractions. (F) NOX2 (NADPH oxidase 2)-derived reactive oxygen species generation in BAL cell membrane (n = 4). *P < 0.05; ***P < 0.0001. Values shown represent means ± SEM. One-way ANOVA with Tukey statistical analysis was used for A, and two-tailed Student’s t-test statistical analysis was used for B, C, and F. Results are from one experiment with n = 4–6 subjects. BALF = BAL fluid; Lymph = lymphocyte; Mac = macrophage/monocyte; ns = not significant; PMN = polymorphonuclear neutrophil.
Rac2 Ameliorates CS-mediated Lung Infection
To determine whether Rac2 deficiency (Rac2+/−) was similar to Rac2−/−, we found that there was no difference in lung bacterial burden compared with CdCl2-exposed wild-type mice (Figure E7A). CdCl2 increased mortality in wild-type mice to a similar extent as Rac2+/− mice (Figure E7B).
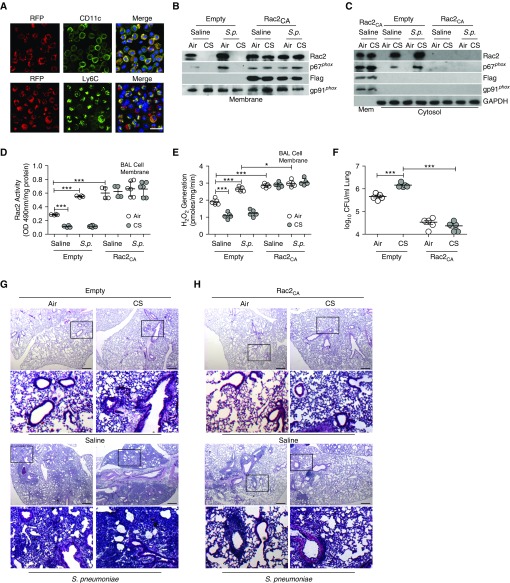
Because lung macrophages are effectively transduced by lentiviral vectors (25), we confirmed that resident alveolar macrophages (CD11c+) were transduced by lentiviruses (RFP+) (Figure 7A). We also found that lentiviral administration transduced early recruited monocytes (Ly6C+) 2 days after infection, as they are increased with CS exposure.
Figure 7.
Rac2 (Ras-related C3 botulinum toxin substrate 2) ameliorates cigarette smoke (CS)–mediated lung infection. Wild-type mice were administered pLVX-IRES-tdTomato (empty) or pLVX-IRES-tdTomato-Rac2CA (Rac2CA) lentivirus. (A) Representative confocal images of CD11c-, Ly6c-, and RFP-positive BAL cells counterstained with DAPI. Scale bar, 25 μm (n = 3). Wild-type mice were transduced with empty or Rac2CA lentivirus. After 6 weeks, mice were exposed to air or CS and then infected with saline or Streptococcus pneumoniae. (B) Immunoblot analysis of isolated BAL cell membrane. (C) Immunoblot analysis of isolated BAL cell membrane and cytosol with corresponding (D) Rac2 activity in BAL cell membrane (n = 4–8). (E) NOX2 (NADPH oxidase 2)-derived reactive oxygen species generation in BAL cell membrane (n = 5). (F) Lung colony-forming units (cfu; n = 5). Hematoxylin and eosin staining was performed in (G) empty-transduced (n = 5) and (H) Rac2CA-transduced wild-type mice (n = 5). Scale bars, 600 μm. The second and fourth rows in G and H show magnifications of the boxed regions in the first and third rows, respectively. *P < 0.05; ***P < 0.0001. One-way ANOVA with Tukey statistical analysis were used for D–F. Representative immunoblots and histology micrographs are shown. Mem = membrane; OD = optical density; RFP = red fluorescent protein; S.p. = Streptococcus pneumoniae.
Six weeks after lentivirus administration, lung macrophages were the predominant cell in BALF from mice transduced with tdTomato (empty) or Flag-Rac2CA-tdTomato (Rac2CA) lentiviral vector after CS exposure and S. pneumoniae infection (Figure E7C). Neither lentiviral administration nor S. pneumoniae infection altered CdCl2 concentration (data not shown). Rac2 and p67phox localization in the membrane fraction was significantly increased with Rac2CA transduction (Figures 7B, E7D, and E7E), whereas Rac2 and p67phox were in the cytosol in empty-transduced mice after CS (Figure 7C). The content of Rac2 in the membrane correlated with Rac2 activity, which was significantly increased in macrophages from Rac2CA-transduced mice regardless of exposure (Figure 7D). This difference in Rac2 activity was not due to increased Rac2 content as Rac2 expression in whole-cell isolates was similar in BAL cells isolated from empty- or Rac2CA-transduced mice (Figure E7F). Membrane-derived ROS generation mirrored the Rac2 activity in Rac2CA-transduced mice (n = 5) (Figure 7E).
The reduction in membrane-derived ROS production was associated with a significant difference in lung colony-forming units from Rac2CA-transduced mice (Figure 7F). S. pneumoniae infection led to complete lobar consolidation in empty-transduced CS-exposed mice, whereas the Rac2CA-transduced mice had peribronchial infiltrates (Figures 7G and 7H).
Macrophages were the primary cell type in the BAL from empty- and Rac2CA-transduced mice exposed to saline or CdCl2 (Figure E7G). The macrophages in BALF showed a reduction in resident lung macrophages and a dramatic increase in recruitment of early inflammatory monocytes after infection (Figures E7H and E7I); however, no neutrophils were positive for RFP expression (data not shown). Similar to mice exposed to CS, Rac2CA-transduced mice exposed to CdCl2 showed significantly less lung colony-forming units than empty-transduced mice (Figure E7J).
Lung Macrophage Rac2 Promotes Macrophage Host Defense
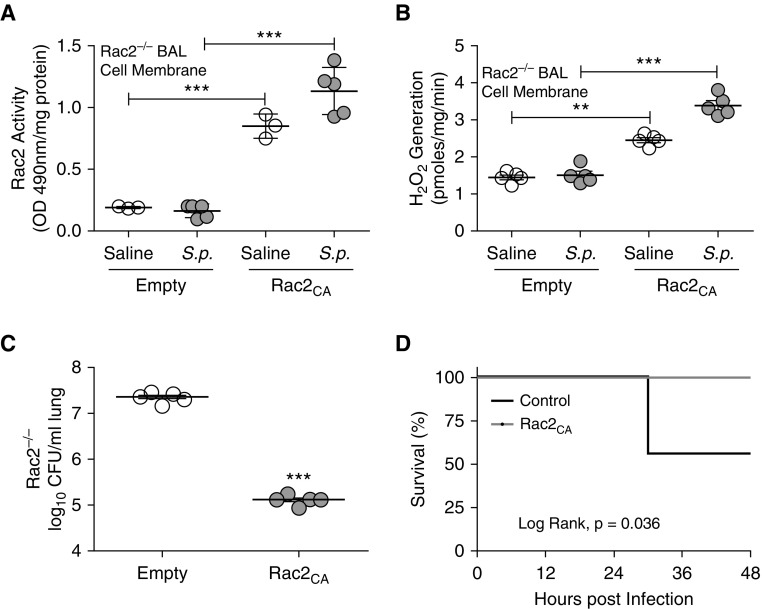
Because selective expression of Rac2CA in lung macrophages was protective in CS-exposed mice, we determined whether lentiviral transduction with constitutive active Rac2 in Rac2−/− mice enhanced host defense. Administration of Rac2CA lentivirus rescued Rac2 activity in isolated BAL cell membrane from Rac2−/− mice (Figure 8A). Rac2 and p67phox expression were also localized in membrane isolated from the BAL cells of Rac2−/− mice infected with Rac2CA lentivirus (Figures E8A and E8B). Type II alveolar epithelial cells isolated from Rac2CA-transduced wild-type mice showed no Flag expression, suggesting these cells were not transduced by the lentivirus (Figure E8C), because vesicular stomatitis virus protein G binds to receptors located on the basolateral side of the epithelium (25, 31).
Figure 8.
Lung macrophage Rac2 (Ras-related C3 botulinum toxin substrate 2) promotes macrophage host defense. Rac2−/− mice were administered pLVX-IRES-tdTomato (empty) or pLVX-IRES-tdTomato-Rac2CA (Rac2CA) lentivirus (5 × 107 TU, intratracheally). Six weeks later mice were exposed to saline or Streptococcus pneumoniae. (A) Rac2 activity in BAL cell membrane from Rac2−/− mice (n = 3–5). (B) NOX2 (NADPH oxidase 2)-derived reactive oxygen species generation in BAL cell membrane was determined (n = 5). (C) Lung colony-forming units (cfu; n = 5) and (D) Kaplan-Meier survival curves were performed (n = 7–9). **P < 0.001; ***P < 0.0001. Values shown represent means ± SEM. One-way ANOVA followed by Tukey’s multiple comparison test was used for A and B, two-tailed t-test statistical analysis was used for C, and a log-rank (Mantel-Cox) test was used for D. OD = optical density; S.p. = Streptococcus pneumoniae.
Rac2−/− mice transduced with Rac2CA lentivirus showed increased membrane-derived ROS generation (Figure 8B). Recovery of Rac2 activity in the Rac2−/− mice led to a reduction in lung bacterial burden (Figure 8C) and increased survival (Figure 8D). In aggregate, these observations uncover a molecular mechanism by which CS increases the incidence of LRTI and suggest that potentiating Rac2 activation in mononuclear phagocytes is a novel therapeutic modality to lessen the severity of infection in those that smoke.
Discussion
Accumulating data indicate that CS compromises the immune system by suppressing the clearance of bacteria from lungs (2–4, 32). Cadmium, which can generate oxidative stress, is one of the metal compounds present in CS, as a single cigarette contains nearly 3 μg of cadmium and has a high rate of transfer from tobacco to CS (33, 34). Lung tissue from smokers has increased cadmium content (26, 35). Our data demonstrate that cadmium levels are significantly increased in BALF from healthy smokers and uncover a mechanism by which cadmium from CS attenuates host defense.
Cadmium is not redox-active, but the generation of ROS is a critical mediator for cadmium-triggered tissue injury (36). Studies indicate that cadmium-induced oxidative stress is mediated through cellular redox disruption by depletion of antioxidant enzymes (37). Our data are the first to indicate that cadmium within CS alters the isoprenylation of Rac2, thereby inhibiting Rac2 activation.
NOX2 is the primary source of ROS generation in macrophages and participates in host defense and cellular signaling (38). Increased membrane-derived ROS is critical for removing damaged tissue and eradicating invading microbes (39, 40), whereas decreased NOX2-derived ROS is associated with the clearance of apoptotic cells (41). Some studies show that lung macrophages from smokers have increased antioxidant activity (42, 43), whereas others suggest that macrophages from smokers generate significantly greater levels of ROS than those from nonsmokers (44). The validity of this finding is uncertain as ROS was measured after stimulation with PMA, a known inducer of NOX2. The present study indicates that lung macrophages from healthy smokers have diminished NOX2-derived ROS generation. It is known that macrophage depletion decreases bacterial containment and survival (6, 10). Here we provide a molecular mechanism by which this occurs.
Lung macrophages facilitate the clearance of bacteria as well as the resolution of inflammation by removing apoptotic neutrophils by phagocytosis (8). We determined that macrophage-depleted mice showed a significant increase in neutrophil number compared with mice with intact macrophages. This imbalance may be due to the impaired neutrophil clearance by lung macrophages, thus potentiating the bacterial burden. Although we were unable to completely deplete lung macrophages, we cannot rule out the possible role of dendritic cells as clodronate also depletes these cells. CCR2-mediated monocyte recruitment is known to exacerbate infection after Listeria monocytogenes inoculation (45). In contrast, only a high inoculum of Mycobacterium tuberculosis mediates a serious infection in CCR2−/− mice (46). CCR2 has been shown to improve the innate immune response as well as play no role in protective immunity, which may be strain dependent (47, 48). Our data show that early infiltrating monocytes play a critical role in innate immunity, whereas neutrophils have less of an effect against bacterial respiratory pathogens.
Although we cannot rule out that other toxic agents in CS may alter lung macrophage host defense, we have identified that cadmium, which is increased in the BALF, abrogates NOX2-derived ROS production, leading to increased bacterial burden. One study showed that NOX2-derived ROS is not important in mediating acrolein-induced lung dysfunction (49). In addition, N-acetylcysteine antioxidant therapies have failed to improve lung function or quality of life (50), suggesting that approaches reliant on scavenging oxidants are not effective therapeutic strategies. Our observations suggest that targeting Rac2 is required for innate immunity in lung macrophages and that potentiating its activity is a therapeutic modality to reduce the severity of CS-induced bacterial pneumonia.
Acknowledgments
Acknowledgment
The authors thank the UAB Neuroscience NINDS Vector and Virus Core for generating the lentiviral constructs (NINDS P30NS047466), Dr. Igor Chesnokov for use of the Beckman TL-100 ultracentrifuge, and Michael Glogauer (University of Toronto) for his generous gift of Rac2−/− mice.
Footnotes
Supported by NIH grants 2R01ES015981-10 and 1R56ES027464-01 to A.B.C. and by a Parker B. Francis fellowship, American Lung Association biomedical research grant RG-507440, and a UAB Faculty development grant to J.L.L.-C. Additional support was provided by NIH grants R35HL135710, R01HL077783, R01HL114439, R01HL110950, and R01HL126596 to J.E.B. and R01AI118805 to D.E.B.
Author Contributions: J.L.L.-C. and A.B.C. developed the concept and design of the study. J.L.L.-C., L.G., P.L.J., D.E.B., J.Y.H., J.E.B., J.M.W., A.V.F.M., S.E.L., J.S.D., Y.W., D.D., V.B.A., and A.B.C. assisted with conducting experiments. J.L.L.-C., L.G., J.E.B., J.M.W., A.V.F.M., S.E.L., J.S.D., Y.W., D.D., and A.B.C. acquired data. J.L.L.-C., P.L.J., D.E.B., J.Y.H., J.E.B., J.M.W., J.S.D., Y.W., V.B.A., and A.B.C. provided reagents. J.L.L.-C., D.E.B., A.V.F.M., S.E.L., J.S.D., Y.W., and A.B.C. provided analysis and interpretation of experiments and results. J.L.L.-C. and A.B.C. wrote the manuscript.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1164/rccm.201712-2388OC on June 13, 2018
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health. The health consequences of smoking—50 years of progress: a report of the surgeon general. Atlanta, GA: Centers for Disease Control and Prevention; 2014. [Google Scholar]
- 2.Garmendia J, Morey P, Bengoechea JA. Impact of cigarette smoke exposure on host–bacterial pathogen interactions. Eur Respir J. 2012;39:467–477. doi: 10.1183/09031936.00061911. [DOI] [PubMed] [Google Scholar]
- 3.Nuorti JP, Butler JC, Farley MM, Harrison LH, McGeer A, Kolczak MS, et al. Active Bacterial Core Surveillance Team. Cigarette smoking and invasive pneumococcal disease. N Engl J Med. 2000;342:681–689. doi: 10.1056/NEJM200003093421002. [DOI] [PubMed] [Google Scholar]
- 4.Stämpfli MR, Anderson GP. How cigarette smoke skews immune responses to promote infection, lung disease and cancer. Nat Rev Immunol. 2009;9:377–384. doi: 10.1038/nri2530. [DOI] [PubMed] [Google Scholar]
- 5.GBD 2016 Causes of Death Collaborators. Global, regional, and national age-sex specific mortality for 264 causes of death, 1980–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2017;390:1151–1210. doi: 10.1016/S0140-6736(17)32152-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Broug-Holub E, Toews GB, van Iwaarden JF, Strieter RM, Kunkel SL, Paine R, III, et al. Alveolar macrophages are required for protective pulmonary defenses in murine Klebsiella pneumonia: elimination of alveolar macrophages increases neutrophil recruitment but decreases bacterial clearance and survival. Infect Immun. 1997;65:1139–1146. doi: 10.1128/iai.65.4.1139-1146.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dorseuil O, Reibel L, Bokoch GM, Camonis J, Gacon G. The Rac target NADPH oxidase p67phox interacts preferentially with Rac2 rather than Rac1. J Biol Chem. 1996;271:83–88. doi: 10.1074/jbc.271.1.83. [DOI] [PubMed] [Google Scholar]
- 8.Knapp S, Leemans JC, Florquin S, Branger J, Maris NA, Pater J, et al. Alveolar macrophages have a protective antiinflammatory role during murine pneumococcal pneumonia. Am J Respir Crit Care Med. 2003;167:171–179. doi: 10.1164/rccm.200207-698OC. [DOI] [PubMed] [Google Scholar]
- 9.van Bruggen R, Anthony E, Fernandez-Borja M, Roos D. Continuous translocation of Rac2 and the NADPH oxidase component p67phox during phagocytosis. J Biol Chem. 2004;279:9097–9102. doi: 10.1074/jbc.M309284200. [DOI] [PubMed] [Google Scholar]
- 10.Hashimoto S, Pittet JF, Hong K, Folkesson H, Bagby G, Kobzik L, et al. Depletion of alveolar macrophages decreases neutrophil chemotaxis to Pseudomonas airspace infections. Am J Physiol. 1996;270:L819–L828. doi: 10.1152/ajplung.1996.270.5.L819. [DOI] [PubMed] [Google Scholar]
- 11.Diebold BA, Bokoch GM. Molecular basis for Rac2 regulation of phagocyte NADPH oxidase. Nat Immunol. 2001;2:211–215. doi: 10.1038/85259. [DOI] [PubMed] [Google Scholar]
- 12.Ambruso DR, Knall C, Abell AN, Panepinto J, Kurkchubasche A, Thurman G, et al. Human neutrophil immunodeficiency syndrome is associated with an inhibitory Rac2 mutation. Proc Natl Acad Sci USA. 2000;97:4654–4659. doi: 10.1073/pnas.080074897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Williams DA, Tao W, Yang F, Kim C, Gu Y, Mansfield P, et al. Dominant negative mutation of the hematopoietic-specific Rho GTPase, Rac2, is associated with a human phagocyte immunodeficiency. Blood. 2000;96:1646–1654. [PubMed] [Google Scholar]
- 14.Sopori M. Effects of cigarette smoke on the immune system. Nat Rev Immunol. 2002;2:372–377. doi: 10.1038/nri803. [DOI] [PubMed] [Google Scholar]
- 15.Staessen JA, Roels HA, Emelianov D, Kuznetsova T, Thijs L, Vangronsveld J, et al. Public Health and Environmental Exposure to Cadmium (PheeCad) Study Group. Environmental exposure to cadmium, forearm bone density, and risk of fractures: prospective population study. Lancet. 1999;353:1140–1144. doi: 10.1016/s0140-6736(98)09356-8. [DOI] [PubMed] [Google Scholar]
- 16.Navas-Acien A, Guallar E, Silbergeld EK, Rothenberg SJ. Lead exposure and cardiovascular disease: a systematic review. Environ Health Perspect. 2007;115:472–482. doi: 10.1289/ehp.9785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang CH, Jeng JS, Yip PK, Chen CL, Hsu LI, Hsueh YM, et al. Biological gradient between long-term arsenic exposure and carotid atherosclerosis. Circulation. 2002;105:1804–1809. doi: 10.1161/01.cir.0000015862.64816.b2. [DOI] [PubMed] [Google Scholar]
- 18.Moriyama H, Kobayashi M, Takada T, Shimizu T, Terada M, Narita J, et al. Two-dimensional analysis of elements and mononuclear cells in hard metal lung disease. Am J Respir Crit Care Med. 2007;176:70–77. doi: 10.1164/rccm.200601-134OC. [DOI] [PubMed] [Google Scholar]
- 19.Loose LD, Silkworth JB, Simpson DW. Influence of cadmium on the phagocytic and microbicidal activity of murine peritoneal macrophages, pulmonary alveolar macrophages, and polymorphonuclear neutrophils. Infect Immun. 1978;22:378–381. doi: 10.1128/iai.22.2.378-381.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Casey JL, Jackson PL, Antony VB, Carter AB. Cigarette smoke impairs alveolar macrophage host defense via Rac2 inhibition [abstract] Am J Respir Crit Care Med. 2017;195:A7370. [Google Scholar]
- 21.He C, Murthy S, McCormick ML, Spitz DR, Ryan AJ, Carter AB. Mitochondrial Cu,Zn-superoxide dismutase mediates pulmonary fibrosis by augmenting H2O2 generation. J Biol Chem. 2011;286:15597–15607. doi: 10.1074/jbc.M110.187377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu C, Feng S, van Heemst J, McAdam KG. New insights into the formation of volatile compounds in mainstream cigarette smoke. Anal Bioanal Chem. 2010;396:1817–1830. doi: 10.1007/s00216-010-3457-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wells JM, O’Reilly PJ, Szul T, Sullivan DI, Handley G, Garrett C, et al. An aberrant leukotriene A4 hydrolase-proline-glycine-proline pathway in the pathogenesis of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2014;190:51–61. doi: 10.1164/rccm.201401-0145OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wan YJ, Yang Y, Leng QL, Lan B, Jia HY, Liu YH, et al. Vav1 increases Bcl-2 expression by selective activation of Rac2-Akt in leukemia T cells. Cell Signal. 2014;26:2202–2209. doi: 10.1016/j.cellsig.2014.05.015. [DOI] [PubMed] [Google Scholar]
- 25.Wilson AA, Murphy GJ, Hamakawa H, Kwok LW, Srinivasan S, Hovav AH, et al. Amelioration of emphysema in mice through lentiviral transduction of long-lived pulmonary alveolar macrophages. J Clin Invest. 2010;120:379–389. doi: 10.1172/JCI36666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pinto E, Cruz M, Ramos P, Santos A, Almeida A. Metals transfer from tobacco to cigarette smoke: evidences in smokers’ lung tissue. J Hazard Mater. 2017;325:31–35. doi: 10.1016/j.jhazmat.2016.11.069. [DOI] [PubMed] [Google Scholar]
- 27.Philippot Q, Deslée G, Adair-Kirk TL, Woods JC, Byers D, Conradi S, et al. Increased iron sequestration in alveolar macrophages in chronic obstructive pulmonary disease. PLoS One. 2014;9:e96285. doi: 10.1371/journal.pone.0096285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hassan F, Xu X, Nuovo G, Killilea DW, Tyrrell J, Da Tan C, et al. Accumulation of metals in GOLD4 COPD lungs is associated with decreased CFTR levels. Respir Res. 2014;15:69. doi: 10.1186/1465-9921-15-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Osborn-Heaford HL, Ryan AJ, Murthy S, Racila AM, He C, Sieren JC, et al. Mitochondrial Rac1 GTPase import and electron transfer from cytochrome c are required for pulmonary fibrosis. J Biol Chem. 2012;287:3301–3312. doi: 10.1074/jbc.M111.308387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zeng PY, Rane N, Du W, Chintapalli J, Prendergast GC. Role for RhoB and PRK in the suppression of epithelial cell transformation by farnesyltransferase inhibitors. Oncogene. 2003;22:1124–1134. doi: 10.1038/sj.onc.1206181. [DOI] [PubMed] [Google Scholar]
- 31.Kobinger GP, Weiner DJ, Yu QC, Wilson JM. Filovirus-pseudotyped lentiviral vector can efficiently and stably transduce airway epithelia in vivo. Nat Biotechnol. 2001;19:225–230. doi: 10.1038/85664. [DOI] [PubMed] [Google Scholar]
- 32.Green GM, Carolin D. The depressant effect of cigarette smoke on the in vitro antibacterial activity of alveolar macrophages. N Engl J Med. 1967;276:421–427. doi: 10.1056/NEJM196702232760801. [DOI] [PubMed] [Google Scholar]
- 33.Hendrick DJ. Smoking, cadmium, and emphysema. Thorax. 2004;59:184–185. doi: 10.1136/thx.2003.018432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nandi M, Slone D, Jick H, Shapiro S, Lewis GP. Cadmium content of cigarettes. Lancet. 1969;2:1329–1330. doi: 10.1016/s0140-6736(69)90865-4. [DOI] [PubMed] [Google Scholar]
- 35.Grasseschi RM, Ramaswamy RB, Levine DJ, Klaassen CD, Wesselius LJ. Cadmium accumulation and detoxification by alveolar macrophages of cigarette smokers. Chest. 2003;124:1924–1928. doi: 10.1378/chest.124.5.1924. [DOI] [PubMed] [Google Scholar]
- 36.Wang Y, Fang J, Leonard SS, Rao KM. Cadmium inhibits the electron transfer chain and induces reactive oxygen species. Free Radic Biol Med. 2004;36:1434–1443. doi: 10.1016/j.freeradbiomed.2004.03.010. [DOI] [PubMed] [Google Scholar]
- 37.Gong Q, Hart BA. Effect of thiols on cadmium-induced expression of metallothionein and other oxidant stress genes in rat lung epithelial cells. Toxicology. 1997;119:179–191. doi: 10.1016/s0300-483x(96)03608-6. [DOI] [PubMed] [Google Scholar]
- 38.Bae YS, Lee JH, Choi SH, Kim S, Almazan F, Witztum JL, et al. Macrophages generate reactive oxygen species in response to minimally oxidized low-density lipoprotein: Toll-like receptor 4- and spleen tyrosine kinase-dependent activation of NADPH oxidase 2. Circ Res. 2009;104:210–218, 21p following 218. doi: 10.1161/CIRCRESAHA.108.181040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hodge S, Hodge G, Ahern J, Jersmann H, Holmes M, Reynolds PN. Smoking alters alveolar macrophage recognition and phagocytic ability: implications in chronic obstructive pulmonary disease. Am J Respir Cell Mol Biol. 2007;37:748–755. doi: 10.1165/rcmb.2007-0025OC. [DOI] [PubMed] [Google Scholar]
- 40.Hodge S, Matthews G, Mukaro V, Ahern J, Shivam A, Hodge G, et al. Cigarette smoke–induced changes to alveolar macrophage phenotype and function are improved by treatment with procysteine. Am J Respir Cell Mol Biol. 2011;44:673–681. doi: 10.1165/rcmb.2009-0459OC. [DOI] [PubMed] [Google Scholar]
- 41.Johann AM, von Knethen A, Lindemann D, Brüne B. Recognition of apoptotic cells by macrophages activates the peroxisome proliferator-activated receptor-γ and attenuates the oxidative burst. Cell Death Differ. 2006;13:1533–1540. doi: 10.1038/sj.cdd.4401832. [DOI] [PubMed] [Google Scholar]
- 42.Rahman I, MacNee W. Oxidant/antioxidant imbalance in smokers and chronic obstructive pulmonary disease. Thorax. 1996;51:348–350. doi: 10.1136/thx.51.4.348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schaberg T, Klein U, Rau M, Eller J, Lode H. Subpopulations of alveolar macrophages in smokers and nonsmokers: relation to the expression of CD11/CD18 molecules and superoxide anion production. Am J Respir Crit Care Med. 1995;151:1551–1558. doi: 10.1164/ajrccm.151.5.7735614. [DOI] [PubMed] [Google Scholar]
- 44.Kondo T, Tagami S, Yoshioka A, Nishimura M, Kawakami Y. Current smoking of elderly men reduces antioxidants in alveolar macrophages. Am J Respir Crit Care Med. 1994;149:178–182. doi: 10.1164/ajrccm.149.1.8111579. [DOI] [PubMed] [Google Scholar]
- 45.Kurihara T, Warr G, Loy J, Bravo R. Defects in macrophage recruitment and host defense in mice lacking the CCR2 chemokine receptor. J Exp Med. 1997;186:1757–1762. doi: 10.1084/jem.186.10.1757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Peters W, Scott HM, Chambers HF, Flynn JL, Charo IF, Ernst JD. Chemokine receptor 2 serves an early and essential role in resistance to Mycobacterium tuberculosis. Proc Natl Acad Sci USA. 2001;98:7958–7963. doi: 10.1073/pnas.131207398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Winter C, Taut K, Srivastava M, Länger F, Mack M, Briles DE, et al. Lung-specific overexpression of CC chemokine ligand (CCL) 2 enhances the host defense to Streptococcus pneumoniae infection in mice: role of the CCL2–CCR2 axis. J Immunol. 2007;178:5828–5838. doi: 10.4049/jimmunol.178.9.5828. [DOI] [PubMed] [Google Scholar]
- 48.Dessing MC, de Vos AF, Florquin S, van der Poll T. Monocyte chemoattractant protein 1 does not contribute to protective immunity against pneumococcal pneumonia. Infect Immun. 2006;74:7021–7023. doi: 10.1128/IAI.00977-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lu Q, Mundy M, Chambers E, Lange T, Newton J, Borgas D, et al. Alda-1 protects against acrolein-induced acute lung injury and endothelial barrier dysfunction. Am J Respir Cell Mol Biol. 2017;57:662–673. doi: 10.1165/rcmb.2016-0342OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Decramer M, Rutten-van Mölken M, Dekhuijzen PN, Troosters T, van Herwaarden C, Pellegrino R, et al. Effects of N-acetylcysteine on outcomes in chronic obstructive pulmonary disease (Bronchitis Randomized on NAC Cost-Utility Study, BRONCUS): a randomised placebo-controlled trial. Lancet. 2005;365:1552–1560. doi: 10.1016/S0140-6736(05)66456-2. [DOI] [PubMed] [Google Scholar]