Abstract
Rationale: No medical intervention has been identified that decreases acute kidney injury and improves renal outcome at 1 year after cardiac surgery.
Objectives: To determine whether administration of nitric oxide reduces the incidence of postoperative acute kidney injury and improves long-term kidney outcomes after multiple cardiac valve replacement requiring prolonged cardiopulmonary bypass.
Methods: Two hundred and forty-four patients undergoing elective, multiple valve replacement surgery, mostly due to rheumatic fever, were randomized to receive either nitric oxide (treatment) or nitrogen (control). Nitric oxide and nitrogen were administered via the gas exchanger during cardiopulmonary bypass and by inhalation for 24 hours postoperatively.
Measurements and Main Results: The primary outcome was as follows: oxidation of ferrous plasma oxyhemoglobin to ferric methemoglobin was associated with reduced postoperative acute kidney injury from 64% (control group) to 50% (nitric oxide group) (relative risk [RR], 0.78; 95% confidence interval [CI], 0.62–0.97; P = 0.014). Secondary outcomes were as follows: at 90 days, transition to stage 3 chronic kidney disease was reduced from 33% in the control group to 21% in the treatment group (RR, 0.64; 95% CI, 0.41–0.99; P = 0.024) and at 1 year, from 31% to 18% (RR, 0.59; 95% CI, 0.36–0.96; P = 0.017). Nitric oxide treatment reduced the overall major adverse kidney events at 30 days (RR, 0.40; 95% CI, 0.18–0.92; P = 0.016), 90 days (RR, 0.40; 95% CI, 0.17–0.92; P = 0.015), and 1 year (RR, 0.47; 95% CI, 0.20–1.10; P = 0.041).
Conclusions: In patients undergoing multiple valve replacement and prolonged cardiopulmonary bypass, administration of nitric oxide decreased the incidence of acute kidney injury, transition to stage 3 chronic kidney disease, and major adverse kidney events at 30 days, 90 days, and 1 year.
Clinical trial registered with ClinicalTrials.gov (NCT01802619).
Keywords: nitric oxide, hemolysis, acute kidney injury, chronic renal insufficiency, rheumatic heart disease
At a Glance Commentary
Scientific Knowledge on the Subject
Kidney damage after cardiac surgery requiring prolonged cardiopulmonary bypass is a common and serious complication. Over the past decades all attempts to decrease kidney injury after heart surgery failed. Promising animal studies showed that administration of nitric oxide decreased renal dysfunction during hemolysis by oxidation of plasma oxyhemoglobin to methemoglobin.
What This Study Adds to the Field
In a randomized clinical trial in China of 244 adults undergoing elective, multiple valve replacement surgery, due mostly to rheumatic fever, administration of 80 parts per million of nitric oxide during and after prolonged cardiopulmonary bypass reduced the incidence of acute kidney injury and improved renal function at follow-up 1 year after surgery. Nitric oxide gas is the first pharmacological intervention to show a reduction in the incidence of acute kidney injury and an improvement of long-term kidney function in cardiac–surgical patients after prolonged cardiopulmonary bypass. These results should be assessed in non-Chinese patients without rheumatic fever.
Acute kidney injury (AKI) is a common and serious complication of cardiac surgical procedures that require prolonged (>90 min) cardiopulmonary bypass (CPB) (1, 2). Although the presence of AKI after CPB is associated with increased mortality, no medical interventions have yet been shown to be associated with improved long-term kidney function (1–7).
The mechanisms leading to AKI are multifactorial and not fully elucidated. However, hemolysis has been shown to be closely associated with postsurgery AKI (8–13). During hemolysis, Hb is released into the circulation in the form of oxyhemoglobin (Oxy-Hb). Plasma Oxy-Hb is filtered by the kidneys and facilitates development of AKI by intrarenal oxidative reactions (14). Furthermore, plasma oxyhemoglobin depletes vascular nitric oxide (NO) via the dioxygenation reaction to form methemoglobin (Met-Hb) (15, 16). Endogenous NO is a potent vasodilator that relaxes vascular smooth muscle, and NO depletion by plasma Oxy-Hb produces vasoconstriction, impairs tissue perfusion, and causes inflammation (17–22). The administration of therapeutic levels of 80 parts per million (ppm) exogenous NO gas oxidizes plasma Oxy-Hb to Met-Hb. The oxidized iron (Met-Hb) species is unable to deplete plasma NO (10, 14, 16, 23). In a human model of blood transfusion, we found that breathing NO at 80 ppm was safe and prevented depletion of plasma NO by circulating plasma Hb (17). In an experimental canine model of free water-induced hemolysis, Minneci and colleagues showed that plasma hemoglobin oxidized by NO inhalation reduced serum creatinine and renal dysfunction (19).
We hypothesized that administration of 80 ppm NO during and for 24 hours after prolonged CPB would convert plasma Oxy-Hb to Met-Hb and prevent intrarenal oxidative reactions and NO scavenging by plasma Oxy-Hb, thereby preserving kidney function. We performed a randomized trial in cardiac surgery patients undergoing multiple valve replacements requiring prolonged CPB to test whether NO could prevent AKI due to high levels of plasma Oxy-Hb caused by acute hemolysis. We monitored patients for up to 1 year after surgery to assess survival and evaluate whether patients who received NO benefited from improved renal function. Some of the results of these studies have been previously reported in the form of an abstract (24).
Methods
Study Design
This study was designed to determine whether NO administered during and after cardiac surgery requiring prolonged CPB reduces postoperative AKI. Patients were studied at 90 days and 1 year after surgery to assess the incidence of chronic kidney disease (CKD) and major adverse kidney events (MAKEs) (25).
We performed a prospective, randomized controlled trial comparing treatment with NO versus nitrogen (N2) in adult patients undergoing multiple valve cardiac surgery at the Departments of Anesthesiology and Cardiovascular Surgery of Xijing Hospital (Xi’an, China). The participants, caregivers, and investigators analyzing data and assessing the outcomes were blinded to group assignment. One perfusionist and one ICU physician were unblinded and prepared the appropriate test gas tanks and NO/N2 meters, which were then covered and blinded to others.
Treatment gases, NO at 80 ppm or N2, were given via the CPB machine and after CPB via a mechanical ventilator. Treatment gases were commenced at the onset of CPB and lasted for 24 hours or less if patients were ready to be extubated early. Treatment gases were weaned off over a period of 2 hours.
Outcomes
The primary endpoint of this study was the incidence of AKI. AKI was defined as either an increase in serum creatinine by 50% within 7 days of surgery, or an increase in serum creatinine by 0.3 mg/dl within 2 days of surgery from preoperative baseline levels of serum creatinine (26). Secondary outcomes included the development of stage 3 CKD (estimated glomerular filtration rate [eGFR], <60 ml/min/1.73 m2) (26, 27), loss of 25% of eGFR compared with baseline, and MAKE (defined as a composite outcome of loss of 25% of eGFR from baseline, end-stage renal disease requiring a continuous renal replacement therapy, and mortality) (27) at 30 days, 90 days, and 1 year after ICU admission. Together with the renal outcomes, other single-organ dysfunction, self-care activities (28), and overall mortality were assessed. One-year follow-up visits after surgery and laboratory studies including plasma Hb, NO consumption, and urine biomarkers of kidney injury were completed in 2017.
Statistical Analysis
Continuous variables were expressed as mean ± SD or median (interquartile range). Differences between the two cohorts of patients were tested using a parametric unpaired Student’s t test or nonparametric Mann-Whitney U test, as appropriate. Categorical variables were described as frequency (%). The relative risk (RR) and the median differences (NO group vs. control group), including 95% confidence intervals (CIs), were used to describe the differences in perioperative characteristics, the occurrence of AKI, and differences in intrahospital, 30-day, 90-day and 1-year postoperative outcomes. We performed an intention-to-treat data analysis including patients who received hydroxyethyl starch in the cardiopulmonary bypass priming solution and a per-protocol data analysis excluding patients who received hydroxyethyl starch in the cardiopulmonary bypass priming solution.
Please refer to the online supplement for the detailed study protocol.
Results
Demographic Data
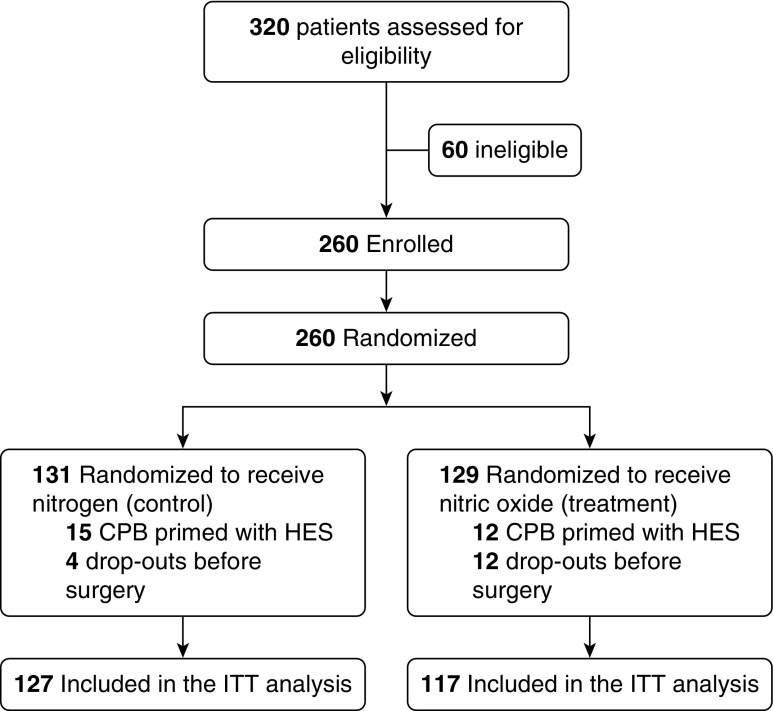
Three hundred and twenty patients were screened and consented to participate in the trial. Sixty patients were excluded before randomization because their surgery was either cancelled (n = 46) or the surgical plan was changed to single valve replacement (n = 14). Thus, 260 patients were randomized either to receive NO or N2. Sixteen patients dropped out from the study before surgery because, at the time of initiation of CPB, the gas treatment (NO or N2) was not available (see Table E1 in the online supplement). Thus, 244 patients were included in the analysis. One-hundred and twenty-seven patients received N2 gas (control group, n = 127) whereas 117 patients received NO (NO group, n = 117) (Figure 1). Equally balanced patient and surgical characteristics of the two groups are assessed in Tables 1 and E2. Surgical procedures and indications were similar between the two groups (Tables 1 and E3). Elapsed surgical time and CPB duration were slightly higher in the NO group.
Figure 1.
Consolidated Standards of Reporting Trials flow diagram: enrollment, allocation, and analysis. CPB = cardiopulmonary bypass; HES = hydroxyethyl starch; ITT = intention-to-treat.
Table 1.
Baseline Characteristics
| Control (n = 127) | NO (n = 117) | |
|---|---|---|
| Age, yr | 48.4 ± 8.6 | 48.7 ± 9.5 |
| Sex | ||
| Female | 75 (59) | 65 (56) |
| Male | 52 (41) | 52 (44) |
| BMI, kg/m2 | 22.1 ± 2.8 | 21.8 ± 2.8 |
| BSA, m2 | 1.63 (1.52–1.71) | 1.63 (1.51–1.74) |
| Admission | ||
| Rheumatic disease | 119 (94) | 107 (91) |
| Congenital valve disease | 2 (1) | 2 (2) |
| Infectious valve disease | 6 (5) | 8 (7) |
| Surgery | ||
| AVR | 1 (1) | 0 (0) |
| MVR+AVR | 45 (35) | 49 (42) |
| MVR+AVR+TVP/R | 79 (62) | 68 (58) |
| MVR+TVP/R | 2 (2) | 0 (0) |
| NYHA | ||
| II | 53 (42) | 45 (39) |
| III | 73 (57) | 69 (59) |
| IV | 0 (0) | 1 (1) |
| Undisclosed | 1 (1) | 2 (2) |
| PAH | 61 (48) | 63 (54) |
| EuroSCORE II, % | 1.1 (0.9–1.6) | 1.2 (0.9–1.6) |
| Serum creatinine, mg/dl | 1.06 (0.97–1.17) | 1.04 (0.96–1.14) |
| eGFR, ml/min/1.73 m2 | 70.3 ± 12.4 | 71.1 ± 15.0 |
| eGFR < 60 ml/min/1.73 m2 | 22 (17) | 24 (21) |
Definition of abbreviations: AVR = aortic valve replacement; BMI = body mass index; BSA = body surface area; eGFR = estimated glomerular filtration rate; EuroSCORE II = European system for cardiac operative risk evaluation; MVR = mitral valve replacement; NO = nitric oxide; NYHA = New York Heart Association; PAH = pulmonary artery hypertension, defined as systolic pulmonary arterial pressure > 38 mm Hg at rest on transthoracic echocardiography before surgery; TVP/R = tricuspid valve plasty or replacement.
Data represent n (%), mean ± SD, or median (IQR). P > 0.05 for all comparisons.
Primary Outcome
Significantly fewer patients in the NO group developed AKI within 7 days of surgery compared with the control group (intention-to-treat analysis: 50% vs. 64%; RR, 0.78; 95% CI, 0.62–0.97; P = 0.014 [Table 2]; per-protocol analysis, excluding patients receiving hydroxyethyl starch: 50% vs. 63%; RR, 0.78; 95% CI, 0.62–0.99; P = 0.022 [Table E4]).
Table 2.
Intrahospital Study Outcomes, Plasma Chemistry, and Clinical Outcomes at 30 Days, 90 Days, and 1 Year after Cardiac Surgery: Intention-to-Treat Analysis
| Control (n = 127) | NO (n = 117) | RR or Median Difference (95% CI) | P Value | |
|---|---|---|---|---|
| Primary outcome | ||||
| AKI within 1 wk | 81 (64) | 58 (50) | 0.78 (0.62 to 0.97) | 0.014 |
| AKI stage | ||||
| I | 66 (52) | 49 (42) | 0.81 (0.61 to 1.06) | 0.058 |
| II | 8 (6) | 4 (3) | 0.54 (0.17 to 1.75) | 0.153 |
| III | 7 (6) | 5 (4) | 0.78 (0.25 to 2.38) | 0.328 |
| Secondary outcomes | ||||
| RRT | 6 (5) | 4 (3) | 0.72 (0.21 to 2.50) | 0.305 |
| Intubation time, h | 24.6 (21.8 to 27.5) | 24.4 (21.8 to 27.2) | 0.3 (−1.42 to 0.8) | 0.292 |
| ICU LOS, d | 2.8 (1.9 to 3.6) | 2.8 (1.9 to 3.7) | 0.02 (−0.09 to 0.16) | 0.346 |
| Intrahospital LOS, d | 10 (8 to 12) | 10 (9 to 12) | 0 (0 to 1) | 0.351 |
| Intrahospital mortality | 7 (6) | 2 (2) | 0.31 (0.07 to 1.46) | 0.068 |
| 30 d | ||||
| Stage 3 CKD | 29 (24)/120 | 19 (17)/114 | 0.69 (0.41 to 1.16) | 0.080 |
| >25% loss of eGFR | 11 (9)/120 | 3 (3)/114 | 0.29 (0.08 to 1.00) | 0.025 |
| MAKE30 | 19 (15)/127 | 7 (6)/116 | 0.40 (0.18 to 0.92) | 0.016 |
| 90 d | ||||
| Stage 3 CKD | 39 (33)/120 | 24 (21)/115 | 0.64 (0.41 to 0.99) | 0.024 |
| >25% loss of eGFR | 11 (9)/120 | 2 (2)/115 | 0.19 (0.04 to 0.84) | 0.014 |
| MAKE90 | 19 (15)/127 | 7 (6)/117 | 0.40 (0.17 to 0.92) | 0.015 |
| 1 yr | ||||
| Stage 3 CKD | 34 (31)/108 | 19 (18)/103 | 0.59 (0.36 to 0.96) | 0.017 |
| >25% loss of eGFR | 7 (6)/108 | 1 (1)/103 | 0.15 (0.02 to 1.20) | 0.037 |
| MAKE1 | 16 (14)/116 | 7 (6)/108 | 0.47 (0.20 to 1.10) | 0.041 |
| Mortality | 8 (6)/127 | 3 (3)/117 | 0.41 (0.11 to 1.50) | 0.088 |
Definition of abbreviations: AKI = acute kidney injury; CI = confidence interval; CKD = chronic kidney disease; eGFR = estimated glomerular filtration rate; IQR = interquartile range; LOS = length of stay; MAKE30, MAKE90, MAKE1 = major adverse kidney events index at 30 days, 90 days, and 1 year, respectively; NO = nitric oxide; RR = relative risk; RRT = renal replacement therapy.
Data represent n (%), median (IQR), or n (%)/N unless otherwise noted. The P values given are one-sided and based on the sample size calculation.
Plasma Biomarkers of Hemolysis
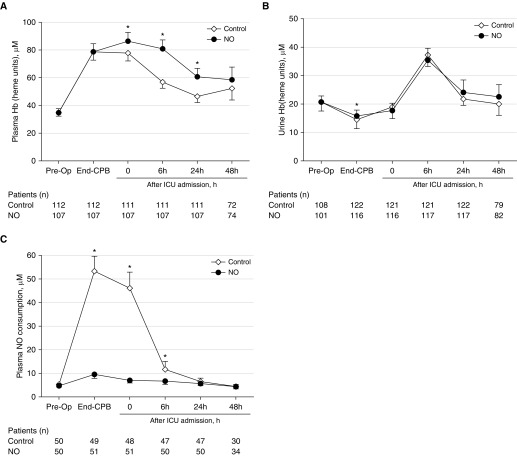
Although plasma Hb levels increased at the end of CPB, there were no differences between the NO and control groups. However, at 0 hours (P = 0.016), 6 hours (P < 0.001), and 24 hours (P = <0.001) after ICU admission, plasma Hb was higher in the NO group (Figure 2A). With the exception of a single time point (end of CPB; P = 0.034), urine Hb levels did not differ between the treatment groups during the study period (Figure 2B).
Figure 2.
Analysis of plasma hemolysis biomarkers. (A) Analysis of plasma Hb before and up to 48 hours after surgery. (B) Analysis of urine Hb before and up to 48 hours after surgery. (C) Analysis of plasma nitric oxide (NO) consumption before and up to 48 hours after surgery. Data are expressed as means with 95% confidence intervals. *P < 0.05 versus control group. CPB = cardiopulmonary bypass.
To determine whether administration of NO successfully oxidized circulating Oxy-Hb to Met-Hb, we measured NO consumption in plasma samples from 51 patients in the NO group and 50 patients in the control group. In the NO treatment group, plasma NO consumption was significantly lower as compared with patients treated with N2 (end of CPB, P < 0.001; ICU admission, P < 0.001; 6 h, P = 0.012) (Figure 2C).
Urinary Biomarkers and Plasma Chemistry
To normalize for diverse urinary output rates, we examined the ratio of urinary kidney injury molecule-1 to urinary creatinine (KIM-1u/creau). The value of this ratio was significantly higher 0 hours (P = 0.003) and 24 hours after ICU admission (P = 0.009) in the NO group. Similarly, the ratio of urinary neutrophil gelatinase-associated lipocalin to urinary creatinine (NGALu/creau) increased in both groups from the end of CPB to 24 hours after ICU admission. NGALu/creau increased significantly more starting at the end of CPB until 24 hours after ICU admission in the NO treatment group as compared with the N2 group. The ratio of urinary N-acetyl-β-d-glucosaminidase to urinary creatinine (NAGu/creau) increased in both groups from baseline to ICU admission. The NAGu/creau ratio increased significantly more at ICU admission (P = 0.008) in the NO treatment as compared with the N2 group.
Urinary creatinine levels did not differ between the groups until 48 hours after ICU admission. Urinary creatinine levels and unadjusted levels of urinary biomarkers are reported in Table E5. Also, whole blood hemoglobin, white blood cell concentration, platelet concentration, and plasma bilirubin levels did not differ between the two treatment groups (Table E6).
Secondary Outcomes
Nitric oxide administration resulted in fewer patients transitioning to stage 3 CKD. Patients treated with NO also had a lower MAKE index at 30 days, 90 days, and 1 year compared with the control group (intention-to-treat analysis [Table 2]; per-protocol analysis [Table E4]).
Twenty-four patients (21%) in the NO group and 22 (17%) in the control group had an eGFR lower than 60 ml/min/1.73 m2 at baseline, suggesting stage 3 CKD before surgery (Table 1).
By 90 days, an eGFR below 60 ml/min/1.73 m2 was found in 24 patients (21%) in the NO group, whereas the number of patients with stage 3 CKD increased to 39 (33%) in the control group (RR, 0.64; 95% CI, 0.41–0.99; P = 0.024); and by 1 year, 19 patients (18%) in the NO group had an eGFR below 60 ml/min/1.73 m2 compared with 34 (31%) in the control group (RR, 0.59; 95% CI, 0.36–0.96; P = 0.017) (Table 2). At 30 days, only three patients in the NO treatment group (<3%) evidenced more than a 25% eGFR loss from baseline value as compared with 11 patients in the control group (9%) (RR, 0.29; 95% CI, 0.08–1.00; P = 0.025); at 90 days, there were two patients (2%) in the NO treatment group versus 11 (9%) in the control group (RR, 0.19; 95% CI, 0.04–0.84; P = 0.014); and, at 1 year, one patient in the NO treatment group (1%) versus seven patients in the control group (6%) evidencing more than a 25% eGFR reduction (RR, 0.15; 95% CI, 0.02–1.20; P = 0.037). There was a trend of decreased mortality in the NO group intrahospital and at 1 year after cardiac surgery. Taken together, these results show that the major adverse kidney events (MAKEs) were markedly decreased in the NO group at 30 days (RR, 0.40; 95% CI, 0.18–0.92; P = 0.016), at 90 days (RR, 0.40; 95% CI, 0.17–0.92; P = 0.015), and at 1 year (RR, 0.47; 95% CI, 0.20–1.10; P = 0.041) (Table 2). Per-protocol analysis is shown in the online supplement (Table E4) and confirms the renal-protective effects of NO on the above-mentioned secondary outcomes.
No difference was found between the groups in other intrahospital outcomes or in other long-term outcome variables (e.g., intrahospital and 1-yr occurrence of other organ injury, independence in activities of daily living, hospital readmission rate, and mortality up to 1 yr) (Tables 2 and E4, E7, E8, and E9).
Safety of 80 ppm NO Delivery
Nitric oxide delivery levels were never reduced for safety concerns. Continuous measurement of NO2 showed values always below 1 ppm in all patients during the entire 24 hours of NO treatment. Plasma Met-Hb significantly increased from baseline to the end of CPB in the NO group and was significantly higher at the end of CPB (P < 0.001) and at 0 hours (P < 0.001), 6 hours (P < 0.001), and 24 hours after ICU admission (P < 0.001) compared with the control group. The highest value of Met-Hb measured in the NO group was 9.3%, and no patient exceeded 10% Met-Hb at any time (Table E6).
In the NO treatment group, no patient experienced postoperative hemorrhage requiring multiple blood transfusions or reoperation (Table E7). Seventy-six percent of the patients in the control group and 68% in the NO treatment group required blood transfusion in the perioperative period (Table E2). There were no adverse events, complications, or other organ dysfunction associated with the use of NO (Table E7). All patients at 24 hours of treatment after CPB commenced were weaned off NO or nitrogen over a period of 2 hours. If patients were extubated before 24 hours after CPB, the time of treatment gas weaning was considered less than the intubation time. The total period of gas administration did not differ between the groups (P = 0.457; Table E2). No patient required reinstitution of gas treatment.
Discussion
We investigated the effects on renal function of NO administration during and after multiple valve replacement heart surgery requiring more than 90 minutes of CPB in a largely ethnic Han Chinese population with rheumatic heart disease. This phase 2b prospective, randomized controlled trial showed that NO reduced the incidence of AKI, transition to stage 3 CKD, and MAKE index at 1 year after cardiac surgery whether hydroxyethyl starch was added to the priming solution of the CPB or not.
The beneficial clinical impact of using NO was associated with a 22% relative risk reduction in the rate of perioperative AKI (from 64% in the N2 group to 50% in the NO group). Although not significantly different, intrahospital renal replacement therapy (RRT) was initiated in 3% of the patients in the NO group versus 5% in the control N2 group, and the mortality rates at 1 year were 3% and 6%, respectively. Favorable short-term effects of perioperative administration of NO translated into a 42% relative reduction of stage 3 CKD at 1 year (from 31% in the N2 group to 18% in the NO group). Overall, the rate of MAKE at 30 days, 90 days, and 1 year was reduced in patients treated with NO.
At a biochemical level, plasma Hb concentration increased similarly at the end of CPB in the two treatment groups, indicating extensive hemolysis. However, exposure to 80 ppm NO during and after CPB maintained lower levels of perioperative plasma NO consumption. Plasma NO consumption in the control N2 group increased 10-fold when it was compared with levels before surgery. Taken together, these biochemical results suggest that administration of exogenous NO during hemolysis expedites the transition of the highly unstable plasma Oxy-Hb to its reduced and inert form Met-Hb, which is unable to deplete NO from the vasculature.
Last, no adverse events occurred due to the administration of 80 ppm NO for 24 hours, and total blood Met-Hb levels remained below 10% in all patients throughout.
Postoperative AKI is a common and major complication after cardiac surgery, with associated increases in short-term and long-term morbidity and mortality. In a study from Duke University, 54% of 4,217 adult patients undergoing coronary artery bypass grafting surgery developed AKI (4). In the United States, and in Europe, patients undergoing valve replacement have a rate of AKI as high as 60–70% (4, 8–16), requiring RRT in up to 16% of patients (8). In a chart review of 146 cases of multiple valve replacement from December 2012 to June 2013, we found a similar incidence of AKI (68%) with a 6% incidence of RRT requirements after multiple valve surgery in a Han Chinese population affected by rheumatic heart disease at Xijing Hospital (Xi’an, China) (data not published). In prior epidemiological studies, even a minimal rise in serum creatinine showed a strong association with increased long-term complications and increased mortality in cardiac surgery patients (29–31). In attempts to alleviate the burden of postoperative AKI, several trials have tested medical interventions without any success (3, 5–7). By monitoring patients for up to 1 year after surgery, our trial shows that a reduction in the rate of postoperative AKI by NO therapy resulted in improved long-term kidney outcomes.
Our trial focuses on the prevention of renal injury caused by hemolysis when complex cardiac surgery requires prolonged CPB. Plasma Hb and heme, products of hemolysis, are scavenged by haptoglobin and hemopexin, respectively. However, during extensive hemolysis plasma Hb accumulates in the circulation (20, 22), causing vasoconstriction (32, 33), and impairs tissue perfusion by scavenging nitric oxide (8, 32, 33), resulting in renal injury (9, 19, 34, 35). The levels of circulating plasma Hb have been shown to be associated with the rate and severity of postsurgery AKI (9). In animal and human physiological exploratory studies, administration of therapeutic exogenous NO gas has been shown to oxidize plasma Oxy-Hb to Met-Hb, preventing pulmonary (17, 36) and systemic vasoconstriction (33, 36) and organ injury (19, 32, 37). We found that prolonged CPB causes hemolysis with an increased concentration of plasma Hb and increased plasma NO consumption compared with levels measured before surgery. In contrast, exposing plasma to NO gas in the CPB oxygenator and after surgery for an additional 24 hours by inhaling 80 ppm NO prevented the depletion of plasma NO, which was associated with a decrease in AKI rate and transition to stage 3 CKD at 90 days and 1 year after surgery.
Other than preventing vasoconstriction due to Oxy-Hb, NO might have improved pulmonary perfusion in this study by its well-described selective pulmonary vasodilator properties, which might have increased cardiac output especially in patients with PAH (37, 38). To account for an elevated baseline PAH, patients with preoperative PAH were allocated equally to both groups during the randomization process. However, during surgery cardiac function was not measured by transesophageal echocardiography or pulmonary artery pressure monitoring with an indwelling pulmonary artery catheter, as these are not standardly monitored at Xijing Hospital.
In addition to its pulmonary vasodilator effects, others have suggested that gaseous NO administration has protective properties against systemic inflammation and reperfusion injury (39, 40). In a pediatric cardiac surgery study by Checchia and colleagues (37), NO gas delivered via the CPB circuit resulted in lower troponin and natriuretic peptide levels, improved diuresis, and led to a better postoperative intensive care unit course. The authors suggested that in children, NO delivered by CPB decreased ischemia–reperfusion injury, thereby improving cardiac and renal function. In a follow-up study, James and colleagues randomized 198 children to either receive intraoperative 20 ppm NO via the CPB bypass oxygenator, or have standard conduct of bypass. The authors showed that NO gas reduced the incidence of a low cardiac output syndrome postoperatively (38).
Despite the remarkable improvement of kidney function in both short- and long-term renal outcomes, we observed a transiently higher peak in urinary biomarkers of kidney injury in patients who received NO at the end of CPB and surgery compared with the control N2 group (Table E4). There are three considerations that should be noted when interpreting the observed discrepancy in this study between plasma creatinine levels and urinary biomarkers. First, KIM-1 was higher in the NO group at only two time points—at ICU admission and at 24 hours—and NAG levels were higher only at ICU admission. KIM-1 and NAG are sensitive markers of tubule stress and might not reflect the overall function of the nephrons (GFR), especially if NO has a vasodilatory effect maintaining GFR despite some mild tubular injury.
Second, secretions of urinary biomarkers are triggered by a variety of renal and extrarenal stimuli. For example, NGAL is an iron-transporting protein and its release is regulated by plasma iron levels. In the present study, plasma levels of Hb were higher in the NO group at ICU admission compared with the control N2 group (Figure 2A). The increased plasma Hb, and possibly iron levels, in the NO group may have increased NGAL secretion. Third, renal biomarkers have their own nephron-protective properties. Therefore, it cannot be excluded that NO gas might directly increase secretion of these urinary biomarkers. This uncoupling phenomenon between the filtration of serum creatinine and secretion of urinary biomarkers is intriguing and might be a focus for future research elucidating the mechanisms regulating the secretion of urinary biomarkers and kidney repair during NO treatment. More recently, Friedrich and colleagues cast doubts on the clinical use of urine NGAL as a predictor of AKI and severity of renal injury (41). Likewise, we were unable to determine the benefits of using renal biomarkers to predict long-term kidney outcomes in this randomized trial.
In cardiac surgery, perioperative AKI is defined by rising serum creatinine levels in association with increased short-term and long-term renal complications and increased mortality (1, 2, 4). We herein demonstrate the safety and efficacy of one of the first medical interventions, namely NO gas, to prevent AKI and decrease long-term adverse kidney events, a common and serious complication of cardiac surgery requiring prolonged CPB.
Limitations
First limitation
This trial selected “younger and healthier” patients undergoing valve replacement because of rheumatic heart disease as compared with the typical cardiac surgery patient profiles reported in the Western literature.
The present study selected a predominantly Han Chinese population of patients affected by rheumatic heart disease requiring multiple valve replacement. However, most cardiac surgery studies reported today are from Europe and North America, where degenerative valvular disease is the most common valve disease. It is important to recognize, however, that rheumatic heart disease remains a major cause of morbidity and premature death in the developing world. This high prevalence of rheumatic heart disease and the growing number of cardiac surgical procedures done in Asian countries make this study unique (42, 43). Rheumatic heart disease is estimated to be responsible for more than 500,000 deaths each year in Asia, with millions of patients waiting for definitive heart surgery (42). The extraordinarily rapid economic development of many Asian countries, together with advances in surgical technologies and surgical skills, now gives thousands of these patients access to definite surgical treatment, rendering rheumatic heart surgery the primary reason for heart valve procedures in Asia. Although the results of this study cannot be generalized to all races and pathologies requiring prolonged CPB for cardiac surgery, this study addresses one of the most common worldwide causes of heart disease and what will likely be the most common reason globally for prolonged CPB in cardiac surgery for the next decades.
Compared with available published literature on adults requiring cardiac surgery, the relatively young age of our patient population (mean age, 48 yr), the absence of preoperative severe chronic kidney disease (eGFR, <30 ml/min/1.73 m2), and other cardiovascular comorbidities (i.e., diabetes, obesity, and atherosclerosis) should be considered to avoid generalization. Future studies will evaluate whether NO confers similar renoprotective properties in older patients and those with more comorbidities.
Second limitation
The trial focused on prolonged (>90 min) CPB procedures: Hemolysis is closely associated with the duration of CPB (20, 22). Although off-pump procedures for coronary artery bypass graft and transcatheter valve replacement to avoid open-heart surgery have been adopted with a certain degree of success, most open-heart surgeries at major heart centers in the United States require more than 90 minutes of CPB time, as documented in the literature (1, 44, 45).
Third limitation
This trial did not show a decreased mortality rate or benefits in a cost–benefit analysis: Our study was not powered to test whether NO exposure could reduce mortality, nevertheless, at 1 year, the rate of mortality in the NO treatment group was 3% compared with 6% in the control group (P = 0.088; Table 2). In addition, NO gas is expensive and, to the best of our knowledge, was used for the first time in China in this trial. However, promising and economically viable alternative NO delivery systems are emerging (46–48).
Because of the current high cost of NO gas, the renal-protective effects of NO gas need to be reproduced in phase 3 clinical trials before implementation in clinical practice.
Conclusions
Among Chinese patients requiring prolonged CPB for multiple heart valve replacement, 80 ppm NO gas exposure during and after prolonged CPB is safe, decreased the incidence of AKI, and reduced transition to stage 3 CKD at 90 days and 1 year.
Acknowledgments
Acknowledgment
The authors thank the members of the Data Safety Monitoring Board of this trial: John Prowle M.D., Carl Rosow, M.D., Ph.D., and Li Yang, M.D.
Footnotes
Supported in part by the National Natural Science Foundation of China (grant 81370011), the Xijing Hospital Foundation (grant XJZT11Z01), the National Key Technology Research and Development Program of the Ministry of Science and Technology of China (grant 2012BA111B02), the Changjiang Scholars and Innovative Research Team in University of China (grant IRT-14R08), NIH/NHLBI grant 1 K23 HL128882-01A1, NIH grants R37 DK39773 and R01 DK072381, and the Department of Anesthesia, Critical Care and Pain Medicine at Massachusetts General Hospital.
Author Contributions: C.L., L.B., L.X., and W.M.Z. conceptualized the study. C.L., L.B., H.D., S.Y., Z.J., and L.X. revised and finalized the protocol. C.L., H.D., L.H., and M.C. collected patients’ descriptive data and collected plasma and urine samples. L.H. and M.C. conducted standardized anesthesia. S.Y., W.C., H.W., and Q.Z. screened patients and conducted surgery. J.S. delivered nitric oxide (NO) to patients. Z.J. and T.C. conducted cardiopulmonary bypass. R.Z. conducted postoperative intensive care to patients. E.R. and B.Y. measured plasma NO consumption. E.C. and V.S.S. measured urine biomarkers of kidney injury. E.R. and F.N. led the data analysis. C.L., L.B., E.R., J.V.B., L.X., and W.M.Z. led data interpretation. C.L., L.B., and E.R. wrote the first report. J.V.B., L.X., and W.M.Z. critically reviewed and revised the initial draft. All authors gave final approval of the version to be published and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy and integrity of any part of the work are appropriately investigated and resolved.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1164/rccm.201710-2150OC on June 22, 2018
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Wrobel K, Stevens SR, Jones RH, Selzman CH, Lamy A, Beaver TM, et al. Influence of baseline characteristics, operative conduct, and postoperative course on 30-day outcomes of coronary artery bypass grafting among patients with left ventricular dysfunction: results from the surgical treatment for ischemic heart failure (STICH) trial. Circulation. 2015;132:720–730. doi: 10.1161/CIRCULATIONAHA.114.014932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Karkouti K, Wijeysundera DN, Yau TM, Callum JL, Cheng DC, Crowther M, et al. Acute kidney injury after cardiac surgery: focus on modifiable risk factors. Circulation. 2009;119:495–502. doi: 10.1161/CIRCULATIONAHA.108.786913. [DOI] [PubMed] [Google Scholar]
- 3.Meybohm P, Bein B, Brosteanu O, Cremer J, Gruenewald M, Stoppe C, et al. RIPHeart Study Collaborators. A multicenter trial of remote ischemic preconditioning for heart surgery. N Engl J Med. 2015;373:1397–1407. doi: 10.1056/NEJMoa1413579. [DOI] [PubMed] [Google Scholar]
- 4.Kertai MD, Zhou S, Karhausen JA, Cooter M, Jooste E, Li YJ, et al. Platelet counts, acute kidney injury, and mortality after coronary artery bypass grafting surgery. Anesthesiology. 2016;124:339–352. doi: 10.1097/ALN.0000000000000959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Billings FT, IV, Hendricks PA, Schildcrout JS, Shi Y, Petracek MR, Byrne JG, et al. High-dose perioperative atorvastatin and acute kidney injury following cardiac surgery: a randomized clinical trial. JAMA. 2016;315:877–888. doi: 10.1001/jama.2016.0548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bove T, Zangrillo A, Guarracino F, Alvaro G, Persi B, Maglioni E, et al. Effect of fenoldopam on use of renal replacement therapy among patients with acute kidney injury after cardiac surgery: a randomized clinical trial. JAMA. 2014;312:2244–2253. doi: 10.1001/jama.2014.13573. [DOI] [PubMed] [Google Scholar]
- 7.Dieleman JM, Nierich AP, Rosseel PM, van der Maaten JM, Hofland J, Diephuis JC, et al. Dexamethasone for Cardiac Surgery (DECS) Study Group. Intraoperative high-dose dexamethasone for cardiac surgery: a randomized controlled trial. JAMA. 2012;308:1761–1767. doi: 10.1001/jama.2012.14144. [DOI] [PubMed] [Google Scholar]
- 8.Vermeulen Windsant IC, de Wit NC, Sertorio JT, van Bijnen AA, Ganushchak YM, Heijmans JH, et al. Hemolysis during cardiac surgery is associated with increased intravascular nitric oxide consumption and perioperative kidney and intestinal tissue damage. Front Physiol. 2014;5:340. doi: 10.3389/fphys.2014.00340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vermeulen Windsant IC, Snoeijs MG, Hanssen SJ, Altintas S, Heijmans JH, Koeppel TA, et al. Hemolysis is associated with acute kidney injury during major aortic surgery. Kidney Int. 2010;77:913–920. doi: 10.1038/ki.2010.24. [DOI] [PubMed] [Google Scholar]
- 10.Baek JH, D’Agnillo F, Vallelian F, Pereira CP, Williams MC, Jia Y, et al. Hemoglobin-driven pathophysiology is an in vivo consequence of the red blood cell storage lesion that can be attenuated in guinea pigs by haptoglobin therapy. J Clin Invest. 2012;122:1444–1458. doi: 10.1172/JCI59770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boretti FS, Buehler PW, D’Agnillo F, Kluge K, Glaus T, Butt OI, et al. Sequestration of extracellular hemoglobin within a haptoglobin complex decreases its hypertensive and oxidative effects in dogs and guinea pigs. J Clin Invest. 2009;119:2271–2280. doi: 10.1172/JCI39115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Deininger S, Hoenicka M, Müller-Eising K, Rupp P, Liebold A, Koenig W, et al. Renal function and urinary biomarkers in cardiac bypass surgery: a prospective randomized trial comparing three surgical techniques. Thorac Cardiovasc Surg. 2016;64:561–568. doi: 10.1055/s-0035-1567871. [DOI] [PubMed] [Google Scholar]
- 13.Mamikonian LS, Mamo LB, Smith PB, Koo J, Lodge AJ, Turi JL. Cardiopulmonary bypass is associated with hemolysis and acute kidney injury in neonates, infants, and children. Pediatr Crit Care Med. 2014;15:e111–e119. doi: 10.1097/PCC.0000000000000047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Deuel JW, Schaer CA, Boretti FS, Opitz L, Garcia-Rubio I, Baek JH, et al. Hemoglobinuria-related acute kidney injury is driven by intrarenal oxidative reactions triggering a heme toxicity response. Cell Death Dis. 2016;7:e2064. doi: 10.1038/cddis.2015.392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Arnold EV, Bohle DS, Jordan PA. Reversible and irreversible hemichrome generation by the oxygenation of nitrosylmyoglobin. Biochemistry. 1999;38:4750–4756. doi: 10.1021/bi982729e. [DOI] [PubMed] [Google Scholar]
- 16.Olson JS, Foley EW, Rogge C, Tsai AL, Doyle MP, Lemon DD. No scavenging and the hypertensive effect of hemoglobin-based blood substitutes. Free Radic Biol Med. 2004;36:685–697. doi: 10.1016/j.freeradbiomed.2003.11.030. [DOI] [PubMed] [Google Scholar]
- 17.Berra L, Pinciroli R, Stowell CP, Wang L, Yu B, Fernandez BO, et al. Autologous transfusion of stored red blood cells increases pulmonary artery pressure. Am J Respir Crit Care Med. 2014;190:800–807. doi: 10.1164/rccm.201405-0850OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Meyer C, Heiss C, Drexhage C, Kehmeier ES, Balzer J, Mühlfeld A, et al. Hemodialysis-induced release of hemoglobin limits nitric oxide bioavailability and impairs vascular function. J Am Coll Cardiol. 2010;55:454–459. doi: 10.1016/j.jacc.2009.07.068. [DOI] [PubMed] [Google Scholar]
- 19.Minneci PC, Deans KJ, Zhi H, Yuen PS, Star RA, Banks SM, et al. Hemolysis-associated endothelial dysfunction mediated by accelerated NO inactivation by decompartmentalized oxyhemoglobin. J Clin Invest. 2005;115:3409–3417. doi: 10.1172/JCI25040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rezoagli E, Ichinose F, Strelow S, Roy N, Shelton K, Matsumine R, et al. Pulmonary and systemic vascular resistances after cardiopulmonary bypass: role of hemolysis. J Cardiothorac Vasc Anesth. 2017;31:505–515. doi: 10.1053/j.jvca.2016.06.009. [DOI] [PubMed] [Google Scholar]
- 21.Risbano MG, Kanias T, Triulzi D, Donadee C, Barge S, Badlam J, et al. Effects of aged stored autologous red blood cells on human endothelial function. Am J Respir Crit Care Med. 2015;192:1223–1233. doi: 10.1164/rccm.201501-0145OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim-Campbell N, Gretchen C, Callaway C, Felmet K, Kochanek PM, Maul T, et al. Cell-free plasma hemoglobin and male gender are risk factors for acute kidney injury in low risk children undergoing cardiopulmonary bypass. Crit Care Med. 2017;45:e1123–e1130. doi: 10.1097/CCM.0000000000002703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reiter CD, Wang X, Tanus-Santos JE, Hogg N, Cannon RO, III, Schechter AN, et al. Cell-free hemoglobin limits nitric oxide bioavailability in sickle-cell disease. Nat Med. 2002;8:1383–1389. doi: 10.1038/nm1202-799. [DOI] [PubMed] [Google Scholar]
- 24.Lei C, Berra L, Rezoagli E, Yu B, Strelow S, Nordio F, et al. Prevention of acute kidney injury by nitric oxide during and after prolonged cardiopulmonary bypass: a double blind randomized controlled trial [abstract 20991] Circulation. 2015;132:2274–2275. [Google Scholar]
- 25.Billings FT, IV, Shaw AD. Clinical trial endpoints in acute kidney injury. Nephron Clin Pract. 2014;127:89–93. doi: 10.1159/000363725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Khwaja A. KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin Pract. 2012;120:c179–c184. doi: 10.1159/000339789. [DOI] [PubMed] [Google Scholar]
- 27.National Kidney Foundation. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002;39(2) Suppl 1:S1–S266. [PubMed] [Google Scholar]
- 28.Katz S, Downs TD, Cash HR, Grotz RC. Progress in development of the index of ADL. Gerontologist. 1970;10:20–30. doi: 10.1093/geront/10.1_part_1.20. [DOI] [PubMed] [Google Scholar]
- 29.Hobson CE, Yavas S, Segal MS, Schold JD, Tribble CG, Layon AJ, et al. Acute kidney injury is associated with increased long-term mortality after cardiothoracic surgery. Circulation. 2009;119:2444–2453. doi: 10.1161/CIRCULATIONAHA.108.800011. [DOI] [PubMed] [Google Scholar]
- 30.Ryckwaert F, Boccara G, Frappier JM, Colson PH. Incidence, risk factors, and prognosis of a moderate increase in plasma creatinine early after cardiac surgery. Crit Care Med. 2002;30:1495–1498. doi: 10.1097/00003246-200207000-00016. [DOI] [PubMed] [Google Scholar]
- 31.Rydén L, Sartipy U, Evans M, Holzmann MJ. Acute kidney injury after coronary artery bypass grafting and long-term risk of end-stage renal disease. Circulation. 2014;130:2005–2011. doi: 10.1161/CIRCULATIONAHA.114.010622. [DOI] [PubMed] [Google Scholar]
- 32.Lei C, Yu B, Shahid M, Beloiartsev A, Bloch KD, Zapol WM. Inhaled nitric oxide attenuates the adverse effects of transfusing stored syngeneic erythrocytes in mice with endothelial dysfunction after hemorrhagic shock. Anesthesiology. 2012;117:1190–1202. doi: 10.1097/ALN.0b013e318272d866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yu B, Raher MJ, Volpato GP, Bloch KD, Ichinose F, Zapol WM. Inhaled nitric oxide enables artificial blood transfusion without hypertension. Circulation. 2008;117:1982–1990. doi: 10.1161/CIRCULATIONAHA.107.729137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Donadee C, Raat NJ, Kanias T, Tejero J, Lee JS, Kelley EE, et al. Nitric oxide scavenging by red blood cell microparticles and cell-free hemoglobin as a mechanism for the red cell storage lesion. Circulation. 2011;124:465–476. doi: 10.1161/CIRCULATIONAHA.110.008698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Graw JA, Mayeur C, Rosales I, Liu Y, Sabbisetti VS, Riley FE, et al. Haptoglobin or hemopexin therapy prevents acute adverse effects of resuscitation after prolonged storage of red cells. Circulation. 2016;134:945–960. doi: 10.1161/CIRCULATIONAHA.115.019955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Baron DM, Yu B, Lei C, Bagchi A, Beloiartsev A, Stowell CP, et al. Pulmonary hypertension in lambs transfused with stored blood is prevented by breathing nitric oxide. Anesthesiology. 2012;116:637–647. doi: 10.1097/ALN.0b013e318246ef77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Checchia PA, Bronicki RA, Muenzer JT, Dixon D, Raithel S, Gandhi SK, et al. Nitric oxide delivery during cardiopulmonary bypass reduces postoperative morbidity in children: a randomized trial. J Thorac Cardiovasc Surg. 2013;146:530–536. doi: 10.1016/j.jtcvs.2012.09.100. [DOI] [PubMed] [Google Scholar]
- 38.James C, Millar J, Horton S, Brizard C, Molesworth C, Butt W. Nitric oxide administration during paediatric cardiopulmonary bypass: a randomised controlled trial. Intensive Care Med. 2016;42:1744–1752. doi: 10.1007/s00134-016-4420-6. [DOI] [PubMed] [Google Scholar]
- 39.Hataishi R, Zapol WM, Bloch KD, Ichinose F. Inhaled nitric oxide does not reduce systemic vascular resistance in mice. Am J Physiol Heart Circ Physiol. 2006;290:H1826–H1829. doi: 10.1152/ajpheart.00938.2005. [DOI] [PubMed] [Google Scholar]
- 40.Varadarajan R, Golden-Mason L, Young L, McLoughlin P, Nolan N, McEntee G, et al. Nitric oxide in early ischaemia reperfusion injury during human orthotopic liver transplantation. Transplantation. 2004;78:250–256. doi: 10.1097/01.tp.0000128188.45553.8c. [DOI] [PubMed] [Google Scholar]
- 41.Friedrich MG, Bougioukas I, Kolle J, Bireta C, Jebran FA, Placzek M, et al. NGAL expression during cardiopulmonary bypass does not predict severity of postoperative acute kidney injury. BMC Nephrol. 2017;18:73. doi: 10.1186/s12882-017-0479-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Carapetis JR. Rheumatic heart disease in Asia. Circulation. 2008;118:2748–2753. doi: 10.1161/CIRCULATIONAHA.108.774307. [DOI] [PubMed] [Google Scholar]
- 43.Zhao J, Hei F. 2016 cardiac surgery and cardiopulmonary bypass report of People’s Republic of China [in Chinese] Chin J Extracorpor Circ. 2016;14:130–132. [Google Scholar]
- 44.Une D, Mesana L, Chan V, Maklin M, Chan R, Masters RG, et al. Clinical impact of changes in left ventricular function after aortic valve replacement: analysis from 3112 patients. Circulation. 2015;132:741–747. doi: 10.1161/CIRCULATIONAHA.115.015371. [DOI] [PubMed] [Google Scholar]
- 45.Steiner ME, Ness PM, Assmann SF, Triulzi DJ, Sloan SR, Delaney M, et al. Effects of red-cell storage duration on patients undergoing cardiac surgery. N Engl J Med. 2015;372:1419–1429. doi: 10.1056/NEJMoa1414219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Berra L, Rodriguez-Lopez J, Rezoagli E, Yu B, Fisher DF, Semigran MJ, et al. Electric plasma-generated nitric oxide: hemodynamic effects in patients with pulmonary hypertension. Am J Respir Crit Care Med. 2016;194:1168–1170. doi: 10.1164/rccm.201604-0834LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hajian B, De Backer J, Vos W, Van Holsbeke C, Ferreira F, Quinn DA, et al. Pulmonary vascular effects of pulsed inhaled nitric oxide in COPD patients with pulmonary hypertension. Int J Chron Obstruct Pulmon Dis. 2016;11:1533–1541. doi: 10.2147/COPD.S106480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pezone MJ, Wakim MG, Denton RJ, Gamero LG, Roscigno RF, Gilbert RJ, et al. Nitrogen dioxide reducing ascorbic acid technologies in the ventilator circuit leads to uniform NO concentration during inspiration. Nitric Oxide. 2016;58:42–50. doi: 10.1016/j.niox.2016.06.001. [DOI] [PubMed] [Google Scholar]