Abstract
Rationale: Increasing awareness of the prevalence and significance of Preserved Ratio Impaired Spirometry (PRISm), alternatively known as restrictive or Global Initiative for Chronic Obstructive Lung Disease (GOLD)-unclassified spirometry, has expanded the body of knowledge on cross-sectional risk factors. However, longitudinal studies of PRISm remain limited.
Objectives: To examine longitudinal patterns of change in lung function, radiographic characteristics, and mortality of current and former smokers with PRISm.
Methods: Current and former smokers, aged 45 to 80 years, were enrolled in COPDGene (phase 1, 2008–2011) and returned for a 5-year follow-up (phase 2, 2012–2016). Subjects completed questionnaires, spirometry, chest computed tomography scans, and 6-minute-walk tests at both study visits. Baseline characteristics, longitudinal change in lung function, and mortality were assessed by post-bronchodilator lung function categories: PRISm (FEV1/FVC < 0.7 and FEV1 < 80%), GOLD0 (FEV1/FVC > 0.7 and FEV1 > 80%), and GOLD1–4 (FEV1/FVC < 0.7).
Measurements and Main Results: Although the prevalence of PRISm was consistent (12.4–12.5%) at phases 1 and 2, subjects with PRISm exhibited substantial rates of transition to and from other lung function categories. Among subjects with PRISm at phase 1, 22.2% transitioned to GOLD0 and 25.1% progressed to GOLD1–4 at phase 2. Subjects with PRISm at both phase 1 and phase 2 had reduced rates of FEV1 decline (−27.3 ± 42.1 vs. −33.0 ± 41.7 ml/yr) and comparable proportions of normal computed tomography scans (51% vs. 52.7%) relative to subjects with stable GOLD0 spirometry. In contrast, incident PRISm exhibited accelerated rates of lung function decline. Subjects with PRISm at phase 1 had higher mortality rates relative to GOLD0 and lower rates relative to the GOLD1–4 group.
Conclusions: PRISm is highly prevalent, is associated with increased mortality, and represents a transitional state for significant subgroups of subjects. Additional studies to characterize longitudinal progression in PRISm are warranted.
Keywords: spirometry classification, spirometry statistics and numerical data, spirometry mortality, lung disease epidemiology
At A Glance Commentary
Scientific Knowledge on the Subject
Preserved Ratio Impaired Spirometry (PRISm), more commonly known as “restrictive spirometry,” is a heterogeneous group associated with increased mortality. Longitudinal phenotypes within PRISm, which may be associated with differential risk, remain poorly characterized.
What This Study Adds to the Field
We examine longitudinal demographic, spirometric, chest computed tomography, and mortality data in the largest cohort of subjects with PRISm reported to date. We confirm the association between PRISm and increased mortality, present evidence to support the concept that PRISm represents a transitional state in a significant proportion of individuals, and describe potentially higher (“incident PRISm”)- and lower (“stable PRISm”)-risk subtypes, which should be examined in future studies.
Proportionate reductions in the FEV1 and FVC on spirometry, variably referred to in the medical literature as restrictive, Global Initiative for Chronic Obstructive Lung Disease (GOLD)-unclassified (1), nonspecific (2), or Preserved Ratio Impaired Spirometry (PRISm) (3) patterns, are estimated to have a cross-sectional prevalence between 5% and 19% (3–6) and are associated with increased respiratory symptoms (7–9) and mortality (5, 6, 10–14). Risk factors for this spirometric pattern include female sex, ever smoking cigarettes, diabetes mellitus, advanced age, extremes of body mass (both high and low), and increased central adiposity (7, 15–17). Globally, a history of tuberculosis (18–20) and exposure to biomass combustion products (21) have been identified as additional risk factors for PRISm.
Knowledge gaps in our understanding of PRISm persist, in part because of the historical exclusion of individuals with this spirometric abnormality from pulmonary research studies and to uncertainty regarding the significance of this lung function pattern. Emerging data from a modest number of longitudinal cohorts suggest that PRISm represents a transitional stage for a subset of individuals, with significant subgroups either progressing to classic airflow obstruction (defined by a reduced FEV1/FVC ratio) or normalizing their lung function over time (2, 10, 22). In this article, we examine 1) cross-sectional prevalence of PRISm at baseline and 5 years later, 2) mortality, and 3) longitudinal patterns of progression in lung function and radiographic features in a large, deeply phenotyped cohort of current and former smokers enrolled in the COPDGene study (23). We hypothesized that a substantial fraction of COPDGene patients would transition into and out of the PRISm group and that subjects transitioning into this group would have different characteristics relative to those who persisted in the PRISm group. A portion of the work contained in this manuscript has been previously published in abstract form (24).
Methods
Study Population and Data
Subjects were participants in COPDGene (ClinicalTrial.gov identifier: NCT000608764), an observational study of self-identified non-Hispanic white and African American current and former smokers from 21 clinical centers throughout the United States (23). Institutional review board approval was obtained at each participating site, and all subjects provided written informed consent. At baseline, subjects (phase 1, 2008–2011) were between the ages of 45 and 80 years, with 10 or more pack-years of cigarette smoking; exclusion criteria included a history of significant lung disease other than chronic obstructive pulmonary disease (COPD) or asthma and history of lung volume reduction surgery or lung transplantation (23). Subjects enrolled in phase 1 were invited to participate in a 5-year follow-up study visit (phase 2, 2012–2016). The study protocol and related documents are available at www.copdgene.org.
Subjects completed 1) questionnaires including the modified American Thoracic Society Respiratory Epidemiology Questionnaire, modified Medical Research Council dyspnea scale, St. George’s Respiratory Questionnaire (SGRQ); 2) spirometry before and after the administration of inhaled albuterol; 3) 6-minute-walk (6MW) test; and 4) inspiratory and expiratory chest computed tomography (CT) scanning at both study visits. Quantitative imaging analysis was performed using VIDA and Thirona software to determine percentage emphysema (percentage of voxels on inspiratory CT scan with an attenuation < −950 Hounsfield units), percentage gas trapping (percentage of lung with an attenuation< −856 Hounsfield units on expiratory CT scan), and TLC. Airway wall thickness was calculated as the square root of the wall area of a hypothetical airway with a 10-mm internal perimeter (Pi10). Visual scoring of phase 1 chest CT data was performed by at least two trained observers to denote the presence or absence of radiographic abnormalities (25). Vital status was assessed both centrally and at individual clinical centers using the Social Security Death Index (December 18, 2016 dataset; see online supplement). Acute respiratory events, defined as worsening respiratory symptoms requiring the use of systemic steroids and/or antibiotics, were assessed through the COPD Longitudinal Follow-up Program (26).
Variable Definitions
Percent predicted and lower limit of normal (LLN) values were calculated using National Health and Nutrition Examination Survey III reference equations for spirometry (27). PRISm was defined as a post-bronchodilator FEV1/FVC greater than or equal to 0.7 and an FEV1 less than 80% predicted (3). The GOLD0 group was defined as an FEV1/FVC greater than or equal to 0.7 and FEV1 greater than or equal to 80% predicted, and classically obstructed lung disease was defined as FEV1/FVC less than 0.7 (GOLD1–4). Subjects who had a lung transplant or lung volume reduction surgery between phase 1 and phase 2 visits were excluded from lung function analysis at phase 2.
Statistical Analyses
Univariate comparisons between subjects with PRISm and subjects with GOLD0 and GOLD1–4 spirometry were made using chi-square or Fisher exact test for categorical variables and Student’s t test or Wilcoxon rank sum test for continuous or ordinal variables. Univariate and Cox proportional hazards models adjusting for baseline age, race, sex, current smoking status, cumulative smoke exposure, and body mass index (BMI) were constructed to examine survival by lung function group. All analyses were conducted using the R statistical software (version 3.4.3) using the base, rmngb (28), survival (29), and survminer (30) packages.
Results
Among 10,199 current and former smokers from phase 1 and 6,284 subjects from phase 2, 10,133 (phase 1) and 5,621 (phase 2) had valid spirometry data available. The prevalence of PRISm among individuals with spirometry data available remained relatively unchanged at 12.4% (phase 1) and 12.5% (phase 2). Characteristics of the subjects with PRISm relative to subjects with GOLD0 and GOLD1–4 spirometry at phases 1 and 2 are shown in Table 1. At both phases 1 and 2, subjects with PRISm have the highest average BMI, are enriched for African Americans and current smokers, and have the lowest TLC and quantitative emphysema measurements.
Table 1.
Cohort Characteristics at Phase 1 and Phase 2 by Spirometric Lung Function Class
| Phase 1 |
Phase 2 |
|||||
|---|---|---|---|---|---|---|
| PRISm | GOLD0 | GOLD1–4 | PRISm | GOLD0 | GOLD1–4 | |
| Subjects, n (%) | 1,260 (12.4) | 4,389 (43.3) | 4,484 (44.3) | 703 (12.5) | 2,424 (43.1) | 2,494 (44.4) |
| Female sex, n (%) | 678 (53.8) | 2,068 (47.1)* | 1,977 (44.1)* | 390 (55.5) | 1,283 (52.9) | 1,113 (44.6)* |
| African American, n (%) | 541 (42.9) | 1,807 (41.2) | 1,018 (22.7)* | 291 (41.4) | 836 (34.5)* | 589 (23.6)* |
| Age at study visit, yr | 57.2 (8.2) | 56.6 (8.4)* | 63.1 (8.6)* | 62.4 (8.1) | 63.3 (8.3)* | 68.0 (8.3)* |
| BMI, kg/m2 | 31.9 (7.3) | 28.9 (5.8)* | 27.9 (6.1)* | 32 (7.5) | 29.2 (6.0)* | 28 (6.2)* |
| Current smoker, n (%) | 802 (63.7) | 2,619 (59.7)* | 1,944 (43.4)* | 346 (49.3) | 967 (39.9)* | 881 (35.3)* |
| Pack-years | 42.6 (24.2) | 37.2 (20.2)* | 51.6 (27.2)* | 41.9 (23.1) | 37.5 (20.7)* | 50.8 (25.2)* |
| FEV1% predicted | 70.2 (8.4) | 97.4 (11.5)* | 57.4 (22.8)* | 70.1 (8) | 97.7 (11.8)* | 60.6 (22.7)* |
| FVC % predicted | 71.5 (9.2) | 96.6 (11.9)* | 81.8 (20.3)* | 70.8 (8.9) | 95.8 (11.7)* | 82.5 (20.0)* |
| TLC % predicted† | 80.3 (13.4) | 92.7 (14.8)* | 102.2 (16.8)* | 81.5 (13.4) | 94.2 (14.2)* | 101.5 (16)* |
| Percent emphysema† | 1.6 (2.6) | 2.1 (2.7)* | 12.0 (12.4)* | 1.4 (3.3) | 1.7 (2.5)* | 10.8 (11.9)* |
| BDR, n (%)‡ | 171 (13.8) | 431 (10.0)* | 1495 (33.6)* | 95 (13.6) | 247 (10.3)* | 767 (30.8)* |
| SGRQ | 29.8 (23.1) | 17 (18)* | 36.9 (22.9)* | 24.5 (21.3) | 14.6 (16.7)* | 32.0 (21.8)* |
| 6MWD, m | 385.9 (112.0) | 454.8 (106.8)* | 375.7 (124.3)* | 369.0 (123.7) | 432.5 (121.9)* | 360.2 (135.5) |
| Chronic bronchitis, n (%) | 225 (17.9) | 552 (12.6)* | 1,164 (26.0)* | 80 (11.4) | 210 (8.7)* | 561 (22.5)* |
Definition of abbreviations: 6MWD = 6-minute-walk distance; BDR = bronchodilator responsive; BMI = body mass index; GOLD = Global Initiative for Chronic Obstructive Lung Disease; PRISm = Preserved Ratio Impaired Spirometry; SGRQ = St. George’s Respiratory Questionnaire.
Data are expressed as mean (SD) unless otherwise noted.
P value < 0.05 relative to PRISm.
Numbers of subjects with quantitative computed tomography imaging available: PRISm = 1,139, GOLD0 = 4,104, GOLD1–4 = 4,178 (phase 1); PRISm = 465, GOLD0 = 1,819, GOLD1–4 = 1,831 (phase 2).
BDR defined as ≥200 ml and ≥12% increase in either FEV1 or FVC after administration of inhaled albuterol. Numbers of subjects with BDR data: PRISm = 1,243, GOLD0 = 4,309, GOLD1–4 = 4,451 (phase 1); PRISm = 700, GOLD0 = 2,390, GOLD1–4 = 2,491 (phase 2).
Mortality by Lung Function Category
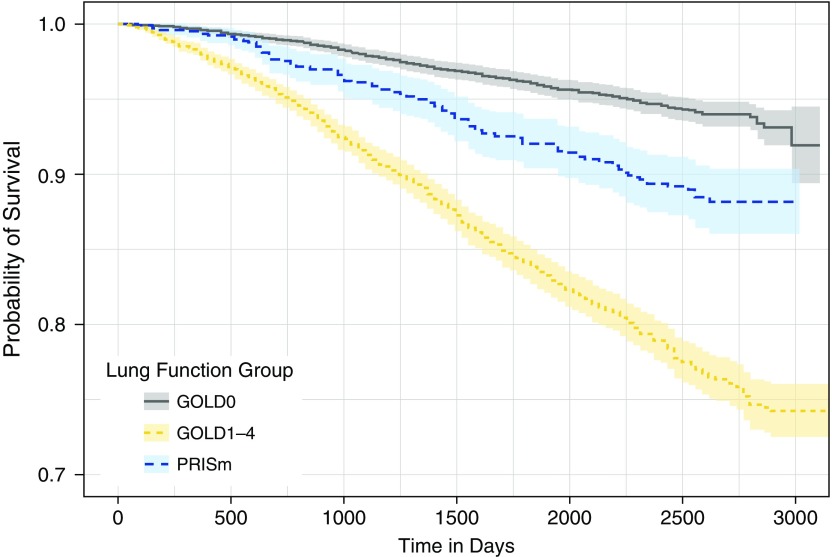
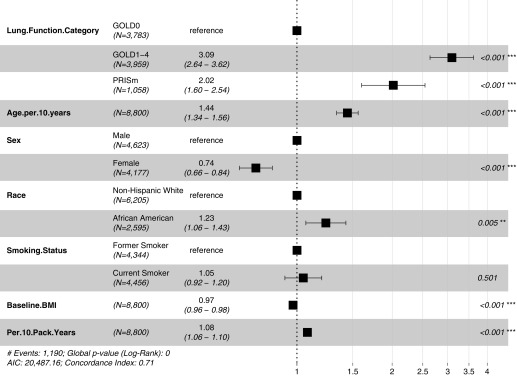
Subjects with lung function data at phase 1 and subsequent assessment of vital status available (ntotal = 8,800) were included in an analysis of mortality rates by lung function category. Subjects with PRISm at phase 1 (PRISm-P1) had unadjusted mortality rates intermediate between subjects with GOLD0 and GOLD1–4 spirometry (Figure 1). Hazard ratios by lung function category from Cox proportional hazards models adjusted for age, sex, race, current smoking status, BMI, and pack-years are shown in Figure 2. PRISm remained a significant predictor for increased mortality in models that adjusted for center of enrollment (data not shown) and when BMI was examined as a three-level categorical variable (see Figure E1 in the online supplement). When we examined mortality rates in PRISm relative to individual GOLD1–4 spirometry categories, unadjusted mortality rates for PRISm were intermediate between GOLD1 and GOLD2 strata (Figure E2). In Cox proportional hazards models adjusted for age, sex, race, current smoking, and BMI, PRISm mortality rates were not significantly different from the GOLD2 strata (Figure E3). In a subgroup analysis of individuals with PRISm-P1, African American race was no longer a risk factor for increased mortality, and increasing BMI was associated with a trend toward increased risk instead of decreased risk, as noted in the full cohort model (Figure E4).
Figure 1.
Kaplan-Meier plot of mortality by lung function category in the COPDGene cohort (nGOLD0 = 3,783, nPRISm = 1,058, nGOLD1–4 = 3,959). PRISm: FEV1/FVC ≥ 0.7 and FEV1 < 80% predicted; GOLD0: FEV1/FVC ≥ 0.7 and FEV1 ≥ 80% predicted; GOLD1–4: FEV1/FVC < 0.7. GOLD = Global Initiative for Chronic Obstructive Lung Disease; PRISm = Preserved Ratio Impaired Spirometry.
Figure 2.
Forest plot of hazard ratios for mortality from Cox proportional hazards model. PRISm: FEV1/FVC ≥ 0.7 and FEV1 < 80% predicted; GOLD0: FEV1/FVC ≥ 0.7 and FEV1 ≥ 80% predicted; GOLD1–4: FEV1/FVC < 0.7. **P < 0.01; ***P < 0.001. AIC = Akaike information criterion; BMI = body mass index; GOLD = Global Initiative for Chronic Obstructive Lung Disease; PRISm = Preserved Ratio Impaired Spirometry.
Longitudinal Change in Lung Function
Of the 6,284 COPDGene subjects who returned for the 5-year follow-up visit, 5,593 subjects had valid lung function data available at both phases 1 and 2. The rate of FEV1 and FVC decline per year by lung function category is shown in Table 2; as a group, subjects with PRISm-P1 had lower average rates of lung function decline relative to subjects with either GOLD0 or GOLD1–4 spirometry.
Table 2.
Rate of Lung Function Decline by Lung Function Category at Enrollment
| Lung Function Category at Phase 1 |
|||
|---|---|---|---|
| PRISm (n = 684) | GOLD0 (n = 2,641) | GOLD1–4 (n = 2,268) | |
| ΔFEV1, ml/yr | −18.9 (52.6) | −41.8 (47.1)* | −38.9 (54.5)* |
| ΔFVC, ml/yr | −9.8 (68.1) | −41.9 (63.5)* | −63.4 (90.4)* |
Definition of abbreviations: GOLD = Global Initiative for Chronic Obstructive Lung Disease; PRISm = Preserved Ratio Impaired Spirometry.
Data are expressed as mean (SD).
P < 0.05 relative to PRISm.
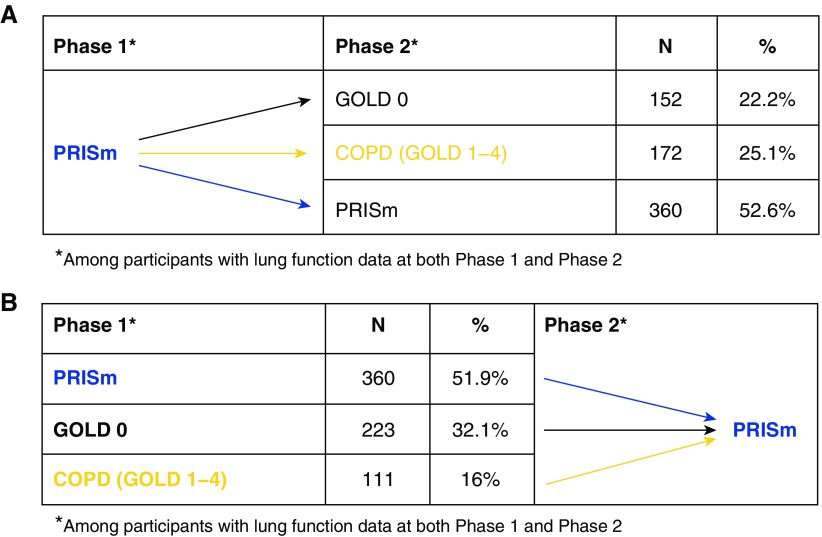
The modest decline in average lung function among individuals with PRISm-P1 is due, in part, to a substantial minority (22.2%) who improved their lung function at phase 2 (i.e., transitioned from PRISm to GOLD0 spirometry); Figures 3and E5 illustrate the transitions between lung function categories of subjects with PRISm-P1 and PRISm-P2 (PRISm-P2). A higher proportion of subjects with PRISm-P1 transitioned to a different lung function category at phase 2 (47.4%) relative to individuals with GOLD0 spirometry or GOLD1–4 spirometry (20.4% and 11.8%, respectively).
Figure 3.
Change in lung function categories among subjects with Preserved Ratio Impaired Spirometry (PRISm) at (A) phase 1 and (B) phase 2. COPD = chronic obstructive pulmonary disease; GOLD = Global Initiative for Chronic Obstructive Lung Disease.
Longitudinal Lung Function Change in Subjects with PRISm-P1
A significant number of subjects with PRISm-P1 transitioned to a different lung function category at phase 2. Baseline characteristics of individuals with PRISm-P1 by lung function category at phase 2 are shown in Table 3. There were no differences in sex, race, baseline symptoms (modified Medical Research Council dyspnea scale, SGRQ), or exercise capacity (6MW distance) associated with future lung function category at phase 2. Reduction in BMI was associated with transitioning from PRISm-P1 to GOLD0 spirometry at phase 2 (Table 3). In a multivariate model adjusting for age, sex, race, current smoking status, and pack-years, decreasing BMI remained a significant predictor of the PRISm-P1 to GOLD0 in phase 2 transition (Table E1). In a secondary analysis of subjects with PRISm-P1, which also adjusted for change in TLC (nΔTLCavailable = 474), decreasing BMI remained a significant predictor of transitioning from PRISm to GOLD0 spirometry (data not shown).
Table 3.
Characteristics of Subjects with Preserved Ratio Impaired Spirometry at Phase 1 by Lung Function Category at Phase 2
| Lung Function Category at Phase 2 |
|||
|---|---|---|---|
| PRISm (n = 360) | GOLD0 (n = 152) | GOLD1–4 (n = 172) | |
| Months between phase 1 and phase 2 visits | 67.6 (9.6) | 67.5 (9.4) | 67.3 (9.0) |
| Age, yr | 56.7 (8.1)*† | 58.6 (8.1) | 59.3 (8.8) |
| Female sex, n (%) | 200 (55.6) | 94 (61.8) | 89 (51.7) |
| African American race, n (%) | 141 (39.2) | 61 (40.1) | 69 (40.1) |
| BMI, kg/m2 | 32.7 (7.7)† | 32.4 (6.7) | 31.2 (7.2) |
| Current smoker, n (%) | 219 (60.8)* | 77 (50.7) | 97 (56.4) |
| Pack-years | 40.4 (22.3)† | 38.8 (20.6)‡ | 45.9 (25.0) |
| FEV1% predicted, baseline | 69.5 (8.1)* | 74.8 (5.5)‡ | 68.2 (8.8) |
| FVC% predicted, baseline | 70.2 (8.9)*† | 75.0 (6.8)‡ | 71.9 (9.7) |
| TLCCT % predicted§ | 79.3 (13.2)† | 80.5 (13.9)‡ | 84.2 (15.0) |
| Chronic bronchitis, n (%) | 63 (17.5)* | 15 (9.9) | 28 (16.3) |
| mMRC | 1.4 (1.4) | 1.4 (1.4) | 1.4 (1.4) |
| SGRQ | 28.1 (23.2) | 25.2 (19.5) | 29.1 (23.5) |
| 6MWD, m | 402.1 (111.4) | 401.7 (102.6) | 384.4 (106.6) |
| Percent emphysema§ | 1.5 (3.2)† | 1.6 (2.1)‡ | 2.2 (2.9) |
| Percent gas trapping|| | 8.2 (6.6)† | 9.7 (7.6)‡ | 12.6 (8.2) |
| Pi10, mm** | 3.72 (0.12) | 3.72 (0.12) | 3.71 (0.14) |
| Acute respiratory events/yr†† | 0.3 (0.6)† | 0.3 (0.8) | 0.4 (0.8) |
| ΔFEV1, ml/yr | −27.2 (42.2)*† | 28.0 (44.5)‡ | −42.9 (53.8) |
| ΔFVC, ml/yr | −32.6 (56.8)*† | 35.7 (59.4)‡ | −2.6 (75.2) |
| ΔBMI, kg/m2 | 0.0 (3.8)* | −1.3 (3.8)‡ | −0.4 (3.5) |
| ΔAdjusted lung density, g/L‡‡ | −0.2 (12.5) | −2.4 (11.2) | −2.7 (12.1) |
Definition of abbreviations: 6MWD = 6-minute-walk distance; BMI = body mass index; CT = computed tomography; GOLD = Global Initiative for Chronic Obstructive Lung Disease; mMRC = modified Medical Research Council; P1 = phase 1; P2 = phase 2; Pi10 = square root of the wall area (in mm) of a hypothetical airway with an internal perimeter of 10 mm; PRISm = Preserved Ratio Impaired Spirometry; SGRQ = St. George’s Respiratory Questionnaire; TLCCT = total lung capacity by computed tomography.
Data are expressed as mean (SD) unless otherwise noted. Data are shown for subjects with lung function data at P1 and P2.
P < 0.05 between PRISm-P2 and GOLD0 spirometry at P2.
P < 0.05 between PRISm-P2 and GOLD1–4 spirometry at P2.
P < 0.05 between GOLD0 and GOLD1–4 at P2.
Subjects with quantitative CT imaging available for analysis: PRISm = 331, GOLD0 = 138, GOLD1–4 = 154.
Subjects with quantitative CT imaging available for analysis: PRISm = 272, GOLD0 = 112, GOLD1–4 = 122.
Subjects with quantitative CT imaging available for analysis: PRISm = 329, GOLD0 = 138, GOLD1–4 = 152.
Subjects with longitudinal follow-up data on acute respiratory events as assessed by interview every 6 months: PRISm = 341, GOLD0 = 142, GOLD1–4 = 166.
Subjects with change in CT lung density data: PRISm = 250, GOLD0 = 108, GOLD1–4 = 116.
Subgroup Analysis: Stable PRISm
Approximately half of the subjects who had PRISm-P1 continued to demonstrate PRISm at P2 (i.e., stable PRISm). The average rate of decline in FEV1 among subjects with stable PRISm was significantly less than that observed in subjects with stable GOLD0 spirometry at phases 1 and 2 (−27.2 ± 42.2 ml/yr vs. −33 ± 41.6 ml/yr, respectively). This difference remained significant in multivariate regression models adjusting for age, sex, race, cumulative cigarette smoking, and current smoking status at enrollment (Table E2). Subjects with stable PRISm had comparable rates of decline in FVC relative to subjects with stable GOLD0 spirometry (data not shown). Among subjects with visual scoring of baseline CT scan data available (nstablePRISm = 297, nstableNormal = 1,857), subjects with stable PRISm had a comparable proportion of scans judged by expert reviewers to be normal relative to subjects with stable GOLD0 spirometry (51.2% vs. 52.8%, P = 0.6).
Subgroup Analysis: PRISm to GOLD1–4 Transition
Subjects who transitioned from PRISm-P1 to GOLD1–4 at phase 2 exhibited higher mean rates of decline in FEV1 and lower mean rates of decline in FVC relative to other subjects with PRISm at phase 1 (Table 3); higher variances in the rates of lung function decline were also observed. These subjects also had the highest average cumulative cigarette smoke exposure, TLC% predicted, percent emphysema, and percent gas trapping at baseline relative to subjects who remained PRISm or normalized their lung function. In analyses of subjects with available quantitative CT data using multivariate logistic regression models, baseline TLC% predicted and percentage gas trapping remained significant predictors of transitioning to GOLD1–4 at phase 2 (Table E3).
Incident PRISm Exhibits Accelerated Lung Function Decline
Approximately one-third of the subjects with PRISm-P2 had GOLD0 spirometry at phase 1 (i.e., incident PRISm). The subjects who developed incident PRISm had a higher average rate of decline in both FEV1 (−88 ± 42.5 ml/yr) and FVC (−105.4 ± 60.7 ml/yr) relative to all other COPDGene subjects with lung function data available at phase 1 and phase 2 (−35.8 ± 50.7 ml/yr of FEV1 and −44.3 ± 77.6 ml/yr of FVC). In multivariate models adjusting for age, sex, race, current smoking status at enrollment, and cumulative cigarette smoke exposure, incident PRISm status remained independently associated with an accelerated rate of decline in FEV1 (Table E4). The rate of decline in FEV1 in incident PRISm also exceeded the rate of decline in subjects who developed incident classical obstruction (GOLD0-P1 to GOLD1–4; data not shown).
Baseline characteristics of subjects who developed incident PRISm-P2 relative to subjects with GOLD0 spirometry at baseline who did not develop PRISm are shown in Table E5. Subjects who developed incident PRISm were younger and had higher average BMI as well as increased proportions of current smokers and African Americans. Subjects who developed incident PRISm had less radiographic emphysema and gas trapping but greater airway wall thickness (Pi10) relative to subjects who did not develop incident PRISm. Subjects who developed incident PRISm also had more dyspnea and worse functional status (as indicated by higher SGRQ scores and lower 6MW distance) at baseline and the largest increase in average BMI between phase 1 and phase 2. Such subjects were also more likely to have bronchodilator responsiveness at baseline. In both univariate and multivariate models, decreased baseline TLC% predicted and percentage emphysema and greater change in BMI were robust independent predictors of incident PRISm status at phase 2 among individuals with GOLD0 spirometry at phase 1 (Table E6).
LLN Analyses
Because the use of fixed threshold cutoffs can lead to overestimation of obstructive lung disease in older individuals, we repeated selected key analyses using LLN thresholds (29) to define PRISm-LLN (FEV1/FVC ≥ LLN and FEV1 < 80% predicted), COPD-LLN (FEV1/FVC < LLN), and normal-LLN (FEV1/FVC ≥ LLN, FEV1 ≥ 80% predicted). Among subjects with lung function measurements available, the prevalence of PRISm-LLN was 14.9% at phase 1 and 16.5% at phase 2. Subjects with PRISm-LLN at phase 1 continued to demonstrate increased rates of transitioning to a different lung function category at phase 2 (42.0%) relative to normal-LLN (17.0%) and COPD-LLN (15.6%). Among PRISm-LLN phase 1 subjects with lung function measurements available at phase 2, 20.2% transitioned to COPD-LLN (Figure E6). In Cox proportional hazards models adjusted for age, sex, race, current smoking status, pack-years, and BMI, PRISm-LLN was associated with increased mortality relative to the normal-LLN group (Figure E7).
Discussion
Increasing awareness of the prevalence and significance of proportional impairments in FEV1 and FVC on spirometry has led to an increase in the average number of publications on the topic from 15 per year (1996–2000) to 47 per year (2010–2015) (31) (Table E7). The vast majority of studies are cross-sectional, with a small number examining change in cross-sectional prevalence over time (32). Longitudinal studies of PRISm remain limited but are beginning to demonstrate trends that may help to inform patterns of progression and prognosis.
Although the cross-sectional prevalence of PRISm within COPDGene remained unchanged, individual membership in PRISm was fluid, with nearly half of individuals either transitioning to or from another lung function category. The increased rate of transitions was observed regardless of whether fixed threshold or LLN criteria were used. Frequent transitions between lung function groups among patients with PRISm have been reported in other cohorts; similar proportions of transitions over 5 years were observed in heavy smokers enrolled in the Lovelace Smokers Cohort (22). During more than 24 years of follow-up in the TESAOD (Tuscon Epidemiological Study of Airway Obstructive Disease) cohort (10), more than half of the cohort with PRISm demonstrated either an “inconsistent” or “mixed” pattern of lung function impairment. Artifactual transitions in lung function resulting from “noise” in spirometry testing among individuals who straddle cutoff thresholds between categories are possible; however, the consistently increased proportion of individuals with PRISm who transition between lung function categories in independent cohorts supports the concept that these changes in lung function may reflect time-varying biological or pathological processes or potentially greater spirometric variability in the PRISm group.
We have confirmed the association between PRISm and increased mortality relative to individuals with GOLD0 spirometry (5, 10) and have expanded our analyses to consider the effects of race, sex, current smoking, and cumulative cigarette smoke exposure. Among the entire COPDGene cohort, female sex and increased BMI were both protective from mortality, whereas increasing age, cumulative cigarette smoke exposure, and African American race were associated with increased risk of death. These results were consistent regardless of whether PRISm was defined using fixed threshold or LLN criteria. Within the subgroup of individuals with PRISm at phase 1, African American race was not significantly associated with differential mortality, whereas increasing BMI was associated with increased, rather than decreased, mortality risk. This apparent “reversal” in the effect of BMI is likely due to the higher mean BMI among subjects with PRISm and the well-established J-shaped relationship between BMI and mortality; many subjects in the PRISm group reside firmly on the “ascending” arm of BMI mortality (33). The lack of significance of African American race as a predictor for increased mortality among PRISm may be due to a loss of statistical power in the PRISm subgroup analysis. Alternatively, less-accurate lung function prediction equations for African Americans may have led to an artifactual enrichment in PRISm rather than being indicative of lung pathology.
A second important finding was that the aggregate rate of lung function decline in PRISm was lower than the rate for other subjects enrolled in COPDGene, despite increased rates of current smoking at enrollment. This finding is both counterintuitive and distinct from a study of Peruvian adults, in whom PRISm was associated with accelerated decline in FEV1 (34). Differences in the populations examined in each of the studies (ethnicity, exposures, etc.) as well as considerable heterogeneity within PRISm populations likely contributed to these discrepancies. As noted above, although the aggregate rate of lung function decline in PRISm was modest, certain subgroups (e.g., incident PRISm) demonstrated accelerated rates of lung function decline, and other groups (resolving PRISm) demonstrated improvements in lung function.
Characterizing longitudinal patterns among patients with PRISm will be critical in informing the development of clinical management strategies. We hypothesize that, given the heterogeneity evident within PRISm, higher- and lower-risk subgroups likely exist. Between 15% and 40% of individuals with PRISm progress to develop classic airflow obstruction (i.e., COPD) (2, 10, 22). Because many of these prior studies examined older populations with the use of fixed ratio-defined obstruction, it is possible that a proportion of these PRISm-to-obstruction transitions resulted from age-related decline in the FEV1/FVC ratio. Within COPDGene, the rate of transition from PRISm to classic obstruction decreased modestly when using LLN relative to fixed-ratio thresholds but continued to account for a significant proportion of subjects. Given the increased mortality observed in COPD (5), as well as longitudinal data suggesting that individuals who transition between PRISm and classically obstructed states have increased mortality (10), we assert that this represents a higher-risk subgroup within PRISm. Although radiographic features such as higher TLC% predicted and increased gas trapping were independent predictors of developing classically obstructed spirometry in the future within the PRISm population in COPDGene, significant heterogeneity within this group exists, and additional features and methods to identify the subset at risk are needed.
Individuals who transition from GOLD0 spirometry to PRISm (incident PRISm) may also warrant consideration as a higher-risk subgroup. Accelerated lung function decline and increased respiratory morbidity in the incident PRISm subgroup were observed in our cohort and have also been reported in an independent study of young adults over nearly 30 years of follow-up (35). In the TESAOD cohort, the subset of subjects with incident PRISm demonstrated a trend toward increased all-cause mortality, which did not reach statistical significance (10). Within the Lovelace Smokers Cohort, recent-onset PRISm was associated with increased rates of “beneficial transitions,” especially in individuals with excess weight (22); a similar trend is implied from the association of change in BMI with both incident PRISm and resolving PRISm.
Of the longitudinal phenotypes observed in our study, a subset of subjects with stable PRISm may qualify for consideration as a lower-risk subtype. Subjects with stable PRISm appear to have normal, or even subnormal, rates of lung function decline and a comparable proportion of chest CT scans judged by expert reviewers to be entirely normal relative to individuals with stable normal spirometry. We hypothesize that some members of the stable PRISm subgroup may represent individuals who did not attain maximal lung function in early adulthood and, without additional pathological processes, have typical age-related decline in lung function (36, 37). Given reduced reserve, these individuals would be expected to become symptomatic earlier and may have increased mortality relative to their counterparts with normal pulmonary reserve, an observation that appears to be supported indirectly by data from the TESAOD cohort (10, 38). Although low maximal lung function has been shown to be a risk factor for the development of classic obstructive lung disease, the same has not been explicitly investigated for PRISm. Additional longitudinal studies of this stable PRISm subgroup are warranted to determine their rate of lung function decline and their risk of mortality.
In summary, we have demonstrated that PRISm 1) is associated with increased mortality relative to individuals with GOLD0 spirometry, 2) has a high rate of transitioning to other lung function categories, and 3) is likely composed of distinct subgroups. The major strengths of our study include the large number of deeply phenotyped individuals with longitudinal lung function, radiographic, and clinical acute respiratory event data; the use of post-bronchodilator spirometry (4); and the inclusion of African Americans. Limitations include the lack of races/ethnicities other than non-Hispanic whites and African Americans (39), longitudinal assessments at only two time points, and the exclusion of nonsmokers and lighter smokers (<10 pack-years cumulative exposure) as well as individuals younger than 45 years of age. Given the increasing prevalence of light smoking (which includes nondaily and social smoking) among young adults (40, 41) and increased prevalence of respiratory symptoms in this population (42), studies that include these individuals are needed. Last, differential rates of subject participation in phase 2 could lead to either under- or overestimation of effects. Within our cohort, individuals with worse lung function at baseline had lower rates of enrollment in phase 2 (Table E8), suggesting that our cohort may have a “healthy subject” bias (analogous to the “healthy worker” bias), whereby individuals who are more ill are less likely to participate. We acknowledge these relative weaknesses limit the generalizability of our findings, but we contend that the size and detail within our cohort allowed for hypothesis generation regarding the existence of clinically relevant subgroups within PRISm. Future studies, both within COPDGene and in additional independent cohorts with longitudinal data, are warranted to refine and quantify the consequences and trajectories of progression of distinct PRISm subtypes.
Acknowledgments
COPDGene Investigators—Core Units
Administrative Center: James D. Crapo (Principal Investigator), Edwin K. Silverman (Principal Investigator), Barry J. Make, and Elizabeth A. Regan.
Genetic Analysis Center: Terri Beaty, Ferdouse Begum, Peter J. Castaldi, Michael Cho, Dawn L. DeMeo, Adel R. Boueiz, Marilyn G. Foreman, Eitan Halper-Stromberg, Lystra P. Hayden, Craig P. Hersh, Jacqueline Hetmanski, Brian D. Hobbs, John E. Hokanson, Nan Laird, Christoph Lange, Sharon M. Lutz, Merry-Lynn McDonald, Margaret M. Parker, Dandi Qiao, Elizabeth A. Regan, Edwin K. Silverman, Emily S. Wan, Sungho Won, Phuwanat Sakornsakolpat, and Dmitry Prokopenko.
Imaging Center: Mustafa Al Qaisi, Harvey O. Coxson, Teresa Gray, MeiLan K. Han, Eric A. Hoffman, Stephen Humphries, Francine L. Jacobson, Philip F. Judy, Ella A. Kazerooni, Alex Kluiber, David A. Lynch, John D. Newell, Jr., Elizabeth A. Regan, James C. Ross, Raul San Jose Estepar, Joyce Schroeder, Jered Sieren, Douglas Stinson, Berend C. Stoel, Juerg Tschirren, Edwin Van Beek, Bram van Ginneken, Eva van Rikxoort, George Washko, and Carla G. Wilson.
PFT QA Center, Salt Lake City, UT: Robert Jensen.
Data Coordinating Center and Biostatistics, National Jewish Health, Denver, CO: Douglas Everett, Jim Crooks, Camille Moore, Matt Strand, and Carla G. Wilson.
Epidemiology Core, University of Colorado Anschutz Medical Campus, Aurora, CO: John E. Hokanson, John Hughes, Gregory Kinney, Sharon M. Lutz, Katherine Pratte, and Kendra A. Young.
Mortality Adjudication Core: Surya Bhatt, Jessica Bon, MeiLan K. Han, Barry Make, Carlos Martinez, Susan Murray, Elizabeth Regan, Xavier Soler, and Carla G. Wilson.
Biomarker Core: Russell P. Bowler, Katerina Kechris, and Farnoush Banaei-Kashani.
COPDGene Investigators—Clinical Centers
Ann Arbor VA, Ann Arbor, MI: Jeffrey L. Curtis, Carlos H. Martinez, and Perry G. Pernicano.
Baylor College of Medicine, Houston, TX: Nicola Hanania, Philip Alapat, Mustafa Atik, Venkata Bandi, Aladin Boriek, Kalpatha Guntupalli, Elizabeth Guy, Arun Nachiappan, and Amit Parulekar.
Brigham and Women’s Hospital, Boston, MA: Dawn L. DeMeo, Craig Hersh, Francine L. Jacobson, and George Washko.
Columbia University, New York, NY: R. Graham Barr, John Austin, Belinda D’Souza, Gregory D. N. Pearson, Anna Rozenshtein, and Byron Thomashow.
Duke University Medical Center, Durham, NC: Neil MacIntyre, Jr., H. Page McAdams, and Lacey Washington.
HealthPartners Research Institute, Minneapolis, MN: Charlene McEvoy and Joseph Tashjian.
Johns Hopkins University, Baltimore: Robert Wise, Robert Brown, Nadia N. Hansel, Karen Horton, Allison Lambert, and Nirupama Putcha.
Los Angeles Biomedical Research Institute at Harbor UCLA Medical Center, Torrance, CA: Richard Casaburi, Alessandra Adami, Matthew Budoff, Hans Fischer, Janos Porszasz, Harry Rossiter, and William Stringer.
Michael E. DeBakey VAMC, Houston, TX: Amir Sharafkhaneh and Charlie Lan.
Minneapolis VA, Minneapolis, MN: Christine Wendt and Brian Bell.
Morehouse School of Medicine, Atlanta, GA: Marilyn G. Foreman, Eugene Berkowitz and Gloria Westney.
National Jewish Health, Denver, CO: Russell Bowler and David A. Lynch.
Reliant Medical Group, Worcester, MA: Richard Rosiello and David Pace.
Temple University, Philadelphia, PA: Gerard Criner, David Ciccolella, Francis Cordova, Chandra Dass, Gilbert D’Alonzo, Parag Desai, Michael Jacobs, Steven Kelsen, Victor Kim, A. James Mamary, Nathaniel Marchetti, Aditi Satti, Kartik Shenoy, Robert M. Steiner, Alex Swift, Irene Swift, and Maria Elena Vega-Sanchez.
University of Alabama, Birmingham, AL: Mark Dransfield, William Bailey, Surya Bhatt, Anand Iyer, Hrudaya Nath, and J. Michael Wells.
University of California, San Diego, CA: Joe Ramsdell, Paul Friedman, Xavier Soler, and Andrew Yen.
University of Iowa, Iowa City, IA: Alejandro P. Comellas, Karin F. Hoth, John Newell, Jr., and Brad Thompson.
University of Michigan, Ann Arbor, MI: MeiLan K. Han, Ella Kazerooni, and Carlos H. Martinez.
University of Minnesota, Minneapolis, MN: Joanne Billings, Abbie Begnaud, and Tadashi Allen.
University of Pittsburgh, Pittsburgh, PA: Frank Sciurba, Jessica Bon, Divay Chandra, Carl Fuhrman, and Joel Weissfeld.
University of Texas Health Science Center at San Antonio, San Antonio, TX: Antonio Anzueto, Sandra Adams, Diego Maselli-Caceres, and Mario E. Ruiz.
Footnotes
Supported by Department of Veterans Affairs, Rehabilitation Research and Development grant IK2RX002165 and NIH grants NHLBI U01 HL089897, U01 HL089856, and K24 HL 138188. The COPDGene study (NCT00608764) is also supported by the COPD Foundation through contributions made to an Industry Advisory Committee composed of AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline, Novartis, Pfizer, Siemens, and Sunovion. The funding agencies had no role in the study design; collection, analysis, or interpretation of data; drafting of the manuscript; or decision to submit for publication.
A complete list of COPDGene Investigators may be found before the References.
Author Contributions: E.S.W., S.F., J.D.C., and E.K.S. were involved in the conception and design of the study. E.A.R., J.H., M.K.H., R.C., B.J.M., J.D.C., D.L.D., and E.K.S. were involved in study data collection. E.S.W., D.L.D., and E.K.S. were involved in data analysis and interpretation. All authors participated in drafting and revising the current manuscript and approve of the current submission.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1164/rccm.201804-0663OC on June 6, 2018
Author disclosures are available with the text of this article at www.atsjournals.org.
Contributor Information
Collaborators: for the COPDGene Investigators, James D. Crapo, Edwin K. Silverman, Barry J. Make, Elizabeth A. Regan, Terri Beaty, Ferdouse Begum, Peter J. Castaldi, Michael Cho, Dawn L. DeMeo, Adel R. Boueiz, Marilyn G. Foreman, Eitan Halper-Stromberg, Lystra P. Hayden, Craig P. Hersh, Jacqueline Hetmanski, Brian D. Hobbs, John E. Hokanson, Nan Laird, Christoph Lange, Sharon M. Lutz, Merry-Lynn McDonald, Margaret M. Parker, Dandi Qiao, Elizabeth A. Regan, Edwin K. Silverman, Emily S. Wan, Sungho Won, Phuwanat Sakornsakolpat, Dmitry Prokopenko, Mustafa Al Qaisi, Harvey O. Coxson, Teresa Gray, MeiLan K. Han, Eric A. Hoffman, Stephen Humphries, Francine L. Jacobson, Philip F. Judy, Ella A. Kazerooni, Alex Kluiber, David A. Lynch, John D. Newell, Jr., Elizabeth A. Regan, James C. Ross, Raul San Jose Estepar, Joyce Schroeder, Jered Sieren, Douglas Stinson, Berend C. Stoel, Juerg Tschirren, Edwin Van Beek, Bram van Ginneken, Eva van Rikxoort, George Washko, Carla G. Wilson, Robert Jensen, Douglas Everett, Jim Crooks, Camille Moore, Matt Strand, Carla G. Wilson, John E. Hokanson, John Hughes, Gregory Kinney, Sharon M. Lutz, Katherine Pratte, Kendra A. Young, Surya Bhatt, Jessica Bon, MeiLan K. Han, Barry Make, Carlos Martinez, Susan Murray, Elizabeth Regan, Xavier Soler, Carla G. Wilson, Russell P. Bowler, Katerina Kechris, Farnoush Banaei-Kashani, Jeffrey L. Curtis, Carlos H. Martinez, Perry G. Pernicano, Nicola Hanania, Philip Alapat, Mustafa Atik, Venkata Bandi, Aladin Boriek, Kalpatha Guntupalli, Elizabeth Guy, Arun Nachiappan, Amit Parulekar, Dawn L. DeMeo, Craig Hersh, Francine L. Jacobson, George Washko, R. Graham Barr, John Austin, Belinda D’Souza, Gregory D. N. Pearson, Anna Rozenshtein, Byron Thomashow, Neil MacIntyre, Jr., H. Page McAdams, Lacey Washington, Charlene McEvoy, Joseph Tashjian, Robert Wise, Robert Brown, Nadia N. Hansel, Karen Horton, Allison Lambert, Nirupama Putcha, Richard Casaburi, Alessandra Adami, Matthew Budoff, Hans Fischer, Janos Porszasz, Harry Rossiter, William Stringer, Amir Sharafkhaneh, Charlie Lan, Christine Wendt, Brian Bell, Marilyn G. Foreman, Eugene Berkowitz, Gloria Westney, Russell Bowler, David A. Lynch, Richard Rosiello, David Pace, Gerard Criner, David Ciccolella, Francis Cordova, Chandra Dass, Gilbert D’Alonzo, Parag Desai, Michael Jacobs, Steven Kelsen, Victor Kim, A. James Mamary, Nathaniel Marchetti, Aditi Satti, Kartik Shenoy, Robert M. Steiner, Alex Swift, Irene Swift, Maria Elena Vega-Sanchez, Mark Dransfield, William Bailey, Surya Bhatt, Anand Iyer, Hrudaya Nath, J. Michael Wells, Joe Ramsdell, Paul Friedman, Xavier Soler, Andrew Yen, Alejandro P. Comellas, Karin F. Hoth, John Newell, Jr., Brad Thompson, MeiLan K. Han, Ella Kazerooni, Carlos H. Martinez, Joanne Billings, Abbie Begnaud, Tadashi Allen, Frank Sciurba, Jessica Bon, Divay Chandra, Carl Fuhrman, Joel Weissfeld, Antonio Anzueto, Sandra Adams, Diego Maselli-Caceres, and Mario E. Ruiz
References
- 1.Wan ES, Hokanson JE, Murphy JR, Regan EA, Make BJ, Lynch DA, et al. COPDGene Investigators. Clinical and radiographic predictors of GOLD-unclassified smokers in the COPDGene study. Am J Respir Crit Care Med. 2011;184:57–63. doi: 10.1164/rccm.201101-0021OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Iyer VN, Schroeder DR, Parker KO, Hyatt RE, Scanlon PD. The nonspecific pulmonary function test: longitudinal follow-up and outcomes. Chest. 2011;139:878–886. doi: 10.1378/chest.10-0804. [DOI] [PubMed] [Google Scholar]
- 3.Wan ES, Castaldi PJ, Cho MH, Hokanson JE, Regan EA, Make BJ, et al. COPDGene Investigators. Epidemiology, genetics, and subtyping of preserved ratio impaired spirometry (PRISm) in COPDGene. Respir Res. 2014;15:89. doi: 10.1186/s12931-014-0089-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Backman H, Eriksson B, Hedman L, Stridsman C, Jansson SA, Sovijärvi A, et al. Restrictive spirometric pattern in the general adult population: methods of defining the condition and consequences on prevalence. Respir Med. 2016;120:116–123. doi: 10.1016/j.rmed.2016.10.005. [DOI] [PubMed] [Google Scholar]
- 5.Mannino DM, Buist AS, Petty TL, Enright PL, Redd SC. Lung function and mortality in the United States: data from the First National Health and Nutrition Examination Survey follow up study. Thorax. 2003;58:388–393. doi: 10.1136/thorax.58.5.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mannino DM, McBurnie MA, Tan W, Kocabas A, Anto J, Vollmer WM, et al. BOLD Collaborative Research Group. Restricted spirometry in the Burden of Lung Disease Study. Int J Tuberc Lung Dis. 2012;16:1405–1411. doi: 10.5588/ijtld.12.0054. [DOI] [PubMed] [Google Scholar]
- 7.Guerra S, Carsin AE, Keidel D, Sunyer J, Leynaert B, Janson C, et al. Health-related quality of life and risk factors associated with spirometric restriction. Eur Respir J. 2017;49:1602096. doi: 10.1183/13993003.02096-2016. [DOI] [PubMed] [Google Scholar]
- 8.Vaz Fragoso CA, McAvay G, Van Ness PH, Casaburi R, Jensen RL, MacIntyre N, et al. Phenotype of spirometric impairment in an aging population. Am J Respir Crit Care Med. 2016;193:727–735. doi: 10.1164/rccm.201508-1603OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marcus BS, McAvay G, Gill TM, Vaz Fragoso CA. Respiratory symptoms, spirometric respiratory impairment, and respiratory disease in middle-aged and older persons. J Am Geriatr Soc. 2015;63:251–257. doi: 10.1111/jgs.13242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guerra S, Sherrill DL, Venker C, Ceccato CM, Halonen M, Martinez FD. Morbidity and mortality associated with the restrictive spirometric pattern: a longitudinal study. Thorax. 2010;65:499–504. doi: 10.1136/thx.2009.126052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Scarlata S, Pedone C, Fimognari FL, Bellia V, Forastiere F, Incalzi RA. Restrictive pulmonary dysfunction at spirometry and mortality in the elderly. Respir Med. 2008;102:1349–1354. doi: 10.1016/j.rmed.2008.02.021. [DOI] [PubMed] [Google Scholar]
- 12.Honda Y, Watanabe T, Shibata Y, Otaki Y, Kadowaki S, Narumi T, et al. Impact of restrictive lung disorder on cardiovascular mortality in a general population: the Yamagata (Takahata) study. Int J Cardiol. 2017;241:395–400. doi: 10.1016/j.ijcard.2017.04.049. [DOI] [PubMed] [Google Scholar]
- 13.Vaz Fragoso CA, Gill TM, McAvay G, Yaggi HK, Van Ness PH, Concato J. Respiratory impairment and mortality in older persons: a novel spirometric approach. J Investig Med. 2011;59:1089–1095. doi: 10.231/JIM.0b013e31822bb213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nonato NL, Nascimento OA, Padilla RP, de Oca MM, Tálamo C, Valdivia G, et al. Occurrence of respiratory symptoms in persons with restrictive ventilatory impairment compared with persons with chronic obstructive pulmonary disease: the PLATINO study. Chron Respir Dis. 2015;12:264–273. doi: 10.1177/1479972315588004. [DOI] [PubMed] [Google Scholar]
- 15.Kurth L, Hnizdo E. Change in prevalence of restrictive lung impairment in the U.S. population and associated risk factors: the National Health and Nutrition Examination Survey (NHANES) 1988-1994 and 2007-2010. Multidiscip Respir Med. 2015;10:7. doi: 10.1186/s40248-015-0003-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pan J, Xu L, Lam TH, Jiang CQ, Zhang WS, Jin YL, et al. Association of adiposity with pulmonary function in older Chinese: Guangzhou Biobank Cohort Study. Respir Med. 2017;132:102–108. doi: 10.1016/j.rmed.2017.10.003. [DOI] [PubMed] [Google Scholar]
- 17.Vatrella A, Calabrese C, Mattiello A, Panico C, Costigliola A, Chiodini P, et al. Abdominal adiposity is an early marker of pulmonary function impairment: findings from a Mediterranean Italian female cohort. Nutr Metab Cardiovasc Dis. 2016;26:643–648. doi: 10.1016/j.numecd.2015.12.013. [DOI] [PubMed] [Google Scholar]
- 18.Amaral AF, Coton S, Kato B, Tan WC, Studnicka M, Janson C, et al. BOLD Collaborative Research Group. Tuberculosis associates with both airflow obstruction and low lung function: BOLD results. Eur Respir J. 2015;46:1104–1112. doi: 10.1183/13993003.02325-2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee JY, Hwang YI, Park YB, Park JY, Kim KU, Oh YM, et al. Prevalence of spirometrically-defined restrictive ventilatory defect in Korea: the fourth-2, 3, and fifth Korean National Health and Nutrition Examination Survey, 2008-2012. J Korean Med Sci. 2015;30:725–732. doi: 10.3346/jkms.2015.30.6.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pefura-Yone EW, Balkissou AD, Kengne AP. Determinants of restrictive spirometric pattern in a sub-Saharan urban setting: a cross-sectional population-based study. Open Respir Med J. 2016;10:86–95. doi: 10.2174/1874306401610010086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mukherjee S, Roychoudhury S, Siddique S, Banerjee M, Bhattacharya P, Lahiri T, et al. Respiratory symptoms, lung function decrement and chronic obstructive pulmonary disease in pre-menopausal Indian women exposed to biomass smoke. Inhal Toxicol. 2014;26:866–872. doi: 10.3109/08958378.2014.965560. [DOI] [PubMed] [Google Scholar]
- 22.Sood A, Petersen H, Qualls C, Meek PM, Vazquez-Guillamet R, Celli BR, et al. Spirometric variability in smokers: transitions in COPD diagnosis in a five-year longitudinal study. Respir Res. 2016;17:147. doi: 10.1186/s12931-016-0468-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Regan EA, Hokanson JE, Murphy JR, Make B, Lynch DA, Beaty TH, et al. Genetic epidemiology of COPD (COPDGene) study design. COPD. 2010;7:32–43. doi: 10.3109/15412550903499522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wan ES, Fortis S, Hokanson JE, DeMeo DL, Crapo JD, Silverman EK. Longitudinal lung function and mortality in preserved ratio impaired spirometry (PRISm) in the COPDGene study [abstract] Am J Respir Crit Care Med. 2017;195:A4988. doi: 10.1164/rccm.201804-0663OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barr RG, Berkowitz EA, Bigazzi F, Bode F, Bon J, Bowler RP, et al. COPDGene CT Workshop Group. A combined pulmonary-radiology workshop for visual evaluation of COPD: study design, chest CT findings and concordance with quantitative evaluation. COPD. 2012;9:151–159. doi: 10.3109/15412555.2012.654923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stewart JI, Moyle S, Criner GJ, Wilson C, Tanner R, Bowler RP, et al. COPDGene Investigators. Automated telecommunication to obtain longitudinal follow-up in a multicenter cross-sectional COPD study. COPD. 2012;9:466–472. doi: 10.3109/15412555.2012.690010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general U.S. population. Am J Respir Crit Care Med. 1999;159:179–187. doi: 10.1164/ajrccm.159.1.9712108. [DOI] [PubMed] [Google Scholar]
- 28.Pierucci AF.rmngb: miscellaneous collection of functions for medical data analysisR package version 0.6-1. 2014[accessed 2018 May 21]. Available from: https://www.rdocumentation.org/packages/rmngb/versions/0.6-1
- 29.Therneau T. A package for survival analysis in S_: version 2.38. 2015. [accessed 2018 May 21]. Available from: https://cran.r-project.org/web/packages/survival/index.html.
- 30.Kassambara A, Kosinski M.survminer: drawing survival curves using ‘ggplot2’R package version 0.4.2. 2018[accessed 2018 May 21]. Available from: https://cran.r-project.org/web/packages/survminer/index.html
- 31.Number of publications published by year returned using PubMed keyword search “restrictive spirometry” [accessed 2018 Feb 27] Available from: https://www.ncbi.nlm.nih.gov/pubmed.
- 32.Ford ES, Mannino DM, Wheaton AG, Giles WH, Presley-Cantrell L, Croft JB. Trends in the prevalence of obstructive and restrictive lung function among adults in the United States: findings from the National Health and Nutrition Examination surveys from 1988-1994 to 2007-2010. Chest. 2013;143:1395–1406. doi: 10.1378/chest.12-1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Aune D, Sen A, Prasad M, Norat T, Janszky I, Tonstad S, et al. BMI and all cause mortality: systematic review and non-linear dose-response meta-analysis of 230 cohort studies with 3.74 million deaths among 30.3 million participants. BMJ. 2016;353:i2156. doi: 10.1136/bmj.i2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Siddharthan T, Grigsby M, Miele CH, Bernabe-Ortiz A, Miranda JJ, Gilman RH, et al. Prevalence and risk factors of restrictive spirometry in a cohort of Peruvian adults. Int J Tuberc Lung Dis. 2017;21:1062–1068. doi: 10.5588/ijtld.17.0101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kalhan R, Dransfield MT, Colangelo LA, Cuttica MJ, Jacobs DR, Jr, Thyagarajan B, et al. Respiratory symptoms in young adults and future lung disease: the CARDIA Lung Study. Am J Respir Crit Care Med. 2018;197:1616–1624. doi: 10.1164/rccm.201710-2108OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lange P, Celli B, Agustí A, Boje Jensen G, Divo M, Faner R, et al. Lung-function trajectories leading to chronic obstructive pulmonary disease. N Engl J Med. 2015;373:111–122. doi: 10.1056/NEJMoa1411532. [DOI] [PubMed] [Google Scholar]
- 37.Stocks J, Sonnappa S. Early life influences on the development of chronic obstructive pulmonary disease. Ther Adv Respir Dis. 2013;7:161–173. doi: 10.1177/1753465813479428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vasquez MM, Zhou M, Hu C, Martinez FD, Guerra S. Low lung function in young adult life is associated with early mortality. Am J Respir Crit Care Med. 2017;195:1399–1401. doi: 10.1164/rccm.201608-1561LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vaz Fragoso CA, McAvay G, Gill TM, Concato J, Quanjer PH, Van Ness PH. Ethnic differences in respiratory impairment. Thorax. 2014;69:55–62. doi: 10.1136/thoraxjnl-2013-203631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li X, Loukas A, Perry CL. Very light smoking and alternative tobacco use among college students. Addict Behav. 2018;81:22–25. doi: 10.1016/j.addbeh.2018.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schane RE, Glantz SA, Ling PM. Nondaily and social smoking: an increasingly prevalent pattern. Arch Intern Med. 2009;169:1742–1744. doi: 10.1001/archinternmed.2009.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Formagini TDB, Gomide HP, Perales J, Colugnati FAB. Prevalence and correlates of light and non-daily smoking in Brazil: results from a nationwide representative survey. Drug Alcohol Depend. 2017;178:15–19. doi: 10.1016/j.drugalcdep.2017.04.018. [DOI] [PubMed] [Google Scholar]