Abstract
Background. The Zika virus (ZIKV) outbreak in the Americas has caused international concern due to neurological sequelae linked to the infection, such as microcephaly and Guillain-Barré syndrome (GBS). The World Health Organization stated that there is “sufficient evidence to conclude that Zika virus is a cause of congenital abnormalities and is a trigger of GBS”. This conclusion was based on a systematic review of the evidence published until 30.05.2016. Since then, the body of evidence has grown substantially, leading to this update of that systematic review with new evidence published from 30.05.2016 – 18.01.2017, update 1.
Methods. We review evidence on the causal link between ZIKV infection and adverse congenital outcomes and the causal link between ZIKV infection and GBS or immune-mediated thrombocytopaenia purpura. We also describe the transition of the review into a living systematic review, a review that is continually updated.
Results. Between 30.05.2016 and 18.01.2017, we identified 2413 publications, of which 101 publications were included. The evidence added in this update confirms the conclusion of a causal association between ZIKV and adverse congenital outcomes. New findings expand the evidence base in the dimensions of biological plausibility, strength of association, animal experiments and specificity. For GBS, the body of evidence has grown during the search period for update 1, but only for dimensions that were already populated in the previous version. There is still a limited understanding of the biological pathways that potentially cause the occurrence of autoimmune disease following ZIKV infection.
Conclusions. This systematic review confirms previous conclusions that ZIKV is a cause of congenital abnormalities, including microcephaly, and is a trigger of GBS. The transition to living systematic review techniques and methodology provides a proof of concept for the use of these methods to synthesise evidence about an emerging pathogen such as ZIKV.
Keywords: Zika virus, causality, living systematic review, congenital abnormalities, Guillain-barre syndrome, microcephlay
Introduction
Outbreaks of Zika virus (ZIKV) infection in the Americas have caused international concern owing to the severity of neurological sequelae linked to the infection ( WHO statement IHR 2005). During 2016, the number of countries affected by the ZIKV outbreak had grown from 33 countries ( WHO situation report 05.02.2016) to 75 countries ( WHO situation report 05.01.2017). By March 9, 2017, 31 countries had reported microcephaly or other congenital central nervous system (CNS) abnormalities potentially associated with ZIKV infection and 23 had reported an increase in the incidence of the immune-mediated condition Guillain-Barré syndrome (GBS) or laboratory confirmed ZIKV in persons with GBS ( WHO situation report 10.03.2017). The causal association between ZIKV and adverse neurological outcomes has now been examined in many systematic and non-systematic reviews of research 1, 2. Case reports of other conditions in people with ZIKV infection, including immune-mediated idiopathic thrombocytopaenia purpura (ITP), have also been published 3– 6.
The World Health Organization (WHO) based its assessment, that there is “sufficient evidence to conclude that Zika virus is a cause of congenital abnormalities and is a trigger of GBS” ( WHO Zika causality statement), on a review of systematically identified studies up to May 30, 2016 and nonsystematically identified studies up to July 29, 2016 7. The review addressed specific questions about 10 dimensions of causal associations, based on the work of Bradford Hill 8 and organised as a causality framework ( Supplementary Table 1) that covers: temporality (cause precedes effect); biological plausibility of proposed biological mechanisms; strength of association; exclusion of alternative explanations; cessation (reversal of an effect by experimental removal of, or observed decline in, the exposure); dose-response relationship; experimental evidence from animal studies; analogous cause-and-effect relationships found in other diseases; specificity of the effect; and the consistency of findings across different study types, populations and times. The review included 108 articles about congenital abnormalities or GBS but there was no, or insufficient evidence to answer questions in several dimensions of the causality framework 7. The causality framework included questions about ITP, but the review authors judged the number of published articles to be too low to assess causality. Since the WHO statement and accompanying publication, about 200 scientific publications every month are added to the body of evidence about all aspects of research about ZIKV.
A living systematic review would help to overcome some of the challenges of keeping up to date with the high volume of ZIKV research publications. A living systematic review is a systematic review that is “continually updated, incorporating relevant new evidence as it becomes available” 9, which can help in fields where evidence is emerging rapidly and where new review outcomes might change policy or practice decision 10. Technical solutions are available to facilitate the reviewing process, such as automated searching and deduplication and computer-assisted screening of article titles and abstracts, increase the efficiency and speed of a review team and transform the review into a living document.
This article aims to fulfil two separate objectives. First, we update our systematic review 7 with new evidence published from May 30, 2016 to January 18, 2017 about all 10 dimensions of the causal associations between ZIKV and (a) congenital brain abnormalities, including microcephaly, in the foetuses and offspring of pregnant women and (b) GBS/ITP in any population. Second, we describe the transition of the review into a living systematic review.
Methods
Classic protocol
We performed the review according to the protocol registered in PROSPERO CRD42016036693 ( PROSPERO protocol). The eligibility criteria, information sources and search strategy, study selection and data extraction are the same as reported in the protocol and in the previous publication 7. In brief, the search covers PubMed, Embase and LILACS electronic databases; the Pan American Health Organization (PAHO), WHO, the Centers for Disease Control and Prevention (CDC) and the European Centre for Disease Prevention and Control (ECDC) websites; and several preprint databases (BioRxiv, PeerJ and ArXiv). Search terms included ‘Zika virus’ and ‘ZIKV’ and corresponding MESH terms. Two reviewers screen and select articles for inclusion and extract data independently. We included publications that held information on at least one of the ten dimensions of the causality framework, regardless of the study design 7. We gathered publications systematically from May 30, 2016 to January 18, 2017 for this update. We refer to the previous version of the review as the baseline review 7 and to this current update as update 1. Reporting of the results follows the Preferred Reporting Items of Systematic reviews and Meta-Analyses (PRISMA) statement ( Supplementary File 1) 11.
From systematic review to living systematic review
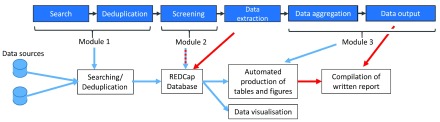
To keep up with the quantity of published research, we developed a living systematic review workflow ( Supplementary File 2). We have identified three modules that could be automated ( Figure 1). As of December 2017, module 1, searching and deduplication, and part of module 3, the output of the report have been automated. Reviewers can be notified daily with a list of new unique search results so that screening can be performed rapidly. Following manual data extraction and synthesis, the output can be updated semi-automatically. We use the online database Research Electronic Data Capture (REDCap) 12 to maintain the references, perform screening and extract data into piloted extraction forms. We plan to update the review twice per year with formal peer reviewed updates ( Figure 2), and continually through a web platform.
Figure 1. Living systematic review automation.
Blue boxes and arrows represent the conceptual steps in a systematic review process. Automation is divided in three modules. Module 1 is the automation of the searching and deduplication of information from different data sources. Module 2 partly automates screening. Module 3 automates the production of tables and figures and outputs the data to a web platform (Data visualisation). Blue arrows represent automated information flows; red arrows represent manual input. The blue-red dashes arrow represents a blended form where reviewers verify automated decisions of the system. The white boxes show the practical implementation of the system and the data flow.
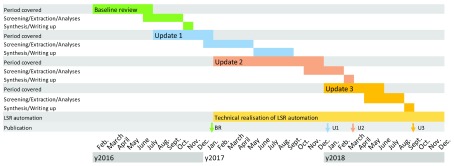
Figure 2. Timeline of review conduct, publication and transition to a living systematic review.
The baseline review (BR, 7) and Update 1 (U1) this version classic, manual systematic review. During 2017 automation of the workflow was conducted resulting in a projected Update 2 (U2) and 3 (U3) with more rapid throughput. LSR, living systematic review.
We synthesised the findings as narrative summaries of the evidence according to causality dimension and outcome, as previously described 7, and compare them with the the baseline review. We use the term ‘confirmation’ to summarise findings of new studies included in update 1 if they report the same findings as those in the baseline review. We use the term ‘expansion’ of evidence if studies included in update 1 provide new findings.
Results
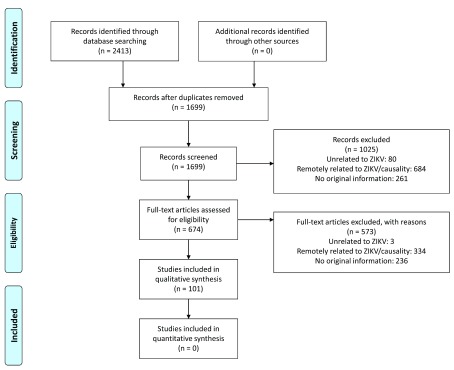
Between May 30, 2016 and January 18, 2017, we identified 2413 publications. After deduplication, we retained 1699 unique records. Based on screening of title and abstract, we discarded 1025 publications, retaining 674 items; after screening of the full text, 101 publications were included. Figure 3 shows the PRISMA flow diagram for this review 11. Seventy-seven publications held information on one or more dimensions of the causality framework on adverse congenital outcomes and 25 on GBS or idiopathic thrombocytopaenia purpura. Table 1 compares the included publications, study types and the causality dimension(s) they address in the baseline review 7 and update 1 of the review.
Figure 3. PRISMA flow diagram of included studies.
Table 1. Summary of included publications by study type and on which causality dimension they provide evidence.
One publication can address multiple causality dimensions. Comparison between the current (U1) and the baseline review (BR, 7) stratified by outcome. GBS/ITP, adverse autoimmune outcomes (Guillain Barré syndrome/idiopathic thrombocytopaenia purpura). NA, not applicable; evidence about analogous conditions was not searched systematically; the dimension of consistency used information in items included for all other causality dimensions.
| Condition and version | Adverse
congenital outcomes |
GBS/ITP | ||
|---|---|---|---|---|
| BR, N | U1, N | BR, N | U1, N | |
| Study type | ||||
| Case report | 9 | 13 | 9 | 5 |
| Case series | 22 | 12 | 5 | 11 |
| Case-control study | 0 | 3 | 1 | 1 |
| Cohort study | 1 | 8 | 0 | 0 |
| Cross-sectional study | 2 | 1 | 0 | 1 |
| Controlled trials | 0 | 0 | 0 | 0 |
| Ecological study/outbreak report | 5 | 4 | 19 | 7 |
| Modelling study | 2 | 0 | 0 | 0 |
| Animal experiment | 18 | 8 | 0 | 0 |
| In vitro experiment | 10 | 22 | 0 | 0 |
| Sequencing and phylogenetics | 3 | 3 | 2 | 0 |
| Biochemical/protein structure studies | NA | 3 | NA | 0 |
| Total: | 72 | 77 | 36 | 25 |
| Causality dimensions | ||||
| Temporality | 21 | 21 | 26 | 21 |
| Biological plausibility | 25 | 42 | 4 | 0 |
| Strength of association | 3 | 5 | 2 | 4 |
| Alternative explanation | 18 | 23 | 6 | 11 |
| Cessation | 2 | 0 | 6 | 2 |
| Dose-response relationship | 0 | 0 | 0 | 0 |
| Experiment | 20 | 11 | 0 | 0 |
| Analogy | NA | NA | NA | NA |
| Specificity | 0 | 1 | 0 | 0 |
| Consistency | NA | NA | NA | NA |
Adverse congenital outcomes
A detailed overview of the new evidence is provided in Table 2 and Supplementary Table 2. In the search period for review update 1, an additional 548 cases of adverse congenital outcomes were described in 32 studies 12– 43. Adverse congenital outcomes described were: clinical microcephaly 12– 17, 20– 24, 26– 31, 33, 35, 37, 40– 42, imaging confirmed brain abnormalities 12, 15, 17, 19– 24, 26– 31, 35, 37, 38, 40, 42, intrauterine growth restriction 15, 17, 31, 38, 40, 42, ocular disorders 12, 17, 27– 29, 31, 38, 40 and auditory disorders 12, 18, 29.
Table 2. Summary of the evidence on the relation between ZIKV infection and adverse congenital outcomes.
Evidence is displayed for each dimension and for each question of the causality framework. Zika virus (ZIKV); Dengue virus (DENV); West Nile virus (WNV); Chikungunya virus (CHIKV); Toxoplasmosis, Other [Syphilis, Varicella-zoster, Parvovirus B19], Rubella, Cytomegalovirus, and Herpes infections (TORCH); Central Nervous System (CNS). NA, not applicable; evidence about analogous conditions was not searched systematically; the dimension of consistency used information in items included for all other causality dimensions. the baseline review (BR), update 1 (U1).
| Question | BR, N | U1, N | Summary |
|---|---|---|---|
| Temporality | |||
| 1.1a | 18 | 19 | Confirmation. Sufficient information to conclude that ZIKV infection precedes the development of congenital
abnormalities in individuals 12, 15– 18, 26– 31, 35– 38, 40, 42, 44, 45. |
| 1.1b | 2 | 1 | The peak of adverse congenital outcomes in Colombia was 24 weeks after infection
45 (similar to Brazil, 34 and
30 weeks 7). |
| 1.2 | 18 | 19 | Confirmation. Most mothers of infants with adverse outcomes were exposed to ZIKV during the first or the
second trimester of their pregnancy 34, 94.Third trimester exposure can lead to brain malformations as well 19. |
| Biological plausibility | |||
| 2.1 | 1 | 6 | Confirmation of the role of viral entry factors (receptor-ligand interaction) 47– 52. |
| 2.2 | 1 | 4 | Substantial expansion of the evidence on which cells express the receptors responsible for cell entry of
ZIKV 47, 50– 52. |
| 2.3 | 11 | 11 | Expansion of evidence, sufficient information to conclude that ZIKV particles can be found in the umbilical
cord blood and/or amniotic fluid of previously or currently infected mothers 14, 23, 24, 32– 36, 38, 39, 42. |
| 2.4 | 0 | 7 | The evidence that ZIKV particles found in tissue of the offspring are capable of replication was inconclusive in
the previous version. In this update we found that in vitro evidence strongly indicates these ZIKV particles are capable of replication 47, 50, 53– 55. Ex vivo experiments demonstrate ZIKV capable of replication as well 33, 36. |
| 2.5 | 6 | 7 | Expansion of evidence, sufficient information to conclude that particles can be found in the brain and other
tissues of cases with congenital abnormalities 14, 17, 23, 24, 33, 34, 56. |
| 2.6 | 7 | 6 | Confirmation. ZIKV particles found in the brain are capable of replication 33, 56– 60. |
| 2.7 | 9 | 22 | Strong expansion of evidence; Expansion of the understanding of how ZIKV causes congenital
anomalies 49, 52, 54, 57, 58, 60– 76. |
| Strength of association | |||
| 3.1 | 2 | 5 | Expansion of evidence on the strength of association at an individual level
21,
22,
31,
40,
41. However, the estimation of
the effect size remains imprecise. |
| 3.2 | 1 | 0 | At a population level, confirmation lacks on the strength of association. However, 29 countries reported a
relative increase in microcephaly cases during the ZIKV outbreak ( WHO situation report 05.01.2017). |
| Exclusion of alternatives | |||
| 4.1 | 18 | 23 | Confirmation. In many epidemiological studies TORCH infections are assessed 12, 14, 17– 19, 21– 28, 30, 31, 34, 36– 38, 40, 42, 45, 77. |
| 4.2 | 4 | 5 | Confirmation. Exposure to toxic chemicals has been excluded 12, 14, 18, 23, 28. |
| 4.3 | 0 | 0 | No exclusion of alternative explanation: maternal/foetal malnutrition. |
| 4.4 | 0 | 0 | No exclusion of alternative explanation: hypoxic-ischaemic lesions. |
| 4.5 | 3 | 7 | Confirmation of evidence where the role of genetic conditions was excluded 12, 18, 23, 28, 30, 36, 42. |
| 4.6 | 0 | 0 | No exclusion of alternative explanation: radiation. |
| Cessation | |||
| 5.1 | 0 | 0 | No publication with evidence that intentional removal of ZIKV infection in individuals leads to a reduction in
congenital abnormalities. |
| 5.2 | 0 | 0 | No publication with evidence that intentional removal of ZIKV infection at population-level leads to a reduction
of cases of congenital anomalies |
| 5.3 | 2 | 0 | Natural removal (end of epidemic) leads to a reduction in microcephaly cases in Brazil; Other countries have
shown a decrease in reported microcephaly cases as the cumulative ZIKV incidence plateaued ( http://www. paho.org/hq/index.php?option=com_content&view=article&id=12390&Itemid=42090&lang=en). |
| Dose-response | |||
| 6.1 | 0 | 0 | No publication with evidence that the risk of adverse congenital outcomes is associated with the viral load in
the mother. |
| 6.2 | 0 | 0 | No publication with evidence that the clinical severity of the infection of the mother determines the severity of
the congenital anomalies. In one cohort study, symptoms in the mother did not influence the outcome 32. |
| Animal experiments | |||
| 7.1 | 3 | 3 | Expansion of the evidence that the inoculation of pregnant female animals (mice and macaques) with ZIKV
causes congenital anomalies in the offspring 78, 84, 85. |
| 7.2 | 10 | 3 | Confirmation of the evidence that the intracerebral inoculation of newborn mice with ZIKV leads to ZIKV
replication in the CNS 81, 82, 86. |
| 7.3 | 8 | 3 | Expansion of the evidence that other routes of inoculation of newborn animals with ZIKV leads to ZIKV
replication in the CNS (intravaginal infection of adult mice, subcutaneous infection of newborn mice) 79, 80, 84. |
| 7.4 | 1 | 8 | Expansion of the evidence that other experiments with animals or animal-derived cells support the association
of ZIKV infection and congenital anomalies 63, 71, 78– 83. |
| Analogy | |||
| 8.1 | NA | NA | CHIKV was shown to be vertically transmissible and lead to adverse congenital outcomes 88. |
| 8.2 | NA | NA | Confirmation. Congenital ZIKV analogous to other TORCH infections 87. |
| 8.3 | NA | NA | For most analogous pathogens, infections earlier in the pregnancy have a higher risk of adverse outcomes. |
| Specificity | |||
| 9.1 | 0 | 1 | Expansion of evidence for distinct congenital Zika syndrome. Unique pattern of five features suggested:
severe microcephaly with overlapping cranial structures, subcortical location of brain calcifications, macular scarring and retinal mottling, congenital contractures and early pyramidal and extrapyramidal symptoms 89. |
| Consistency | |||
| 10.1 | NA | NA | Confirmation. ZIKV-related adverse congenital outcomes in different regions (South America, Central America,
and the Pacific region). The proportion of cases varies over geographic regions/time. |
| 10.2 | NA | NA | Confirmation. ZIKV exposure and adverse congenital outcome in different populations (people living in ZIKV
endemic areas and travellers. |
| 10.3 | NA | NA | No publication with evidence of consistency across lineages due to circulation of single strain. |
| 10.4 | NA | NA | Confirmation. ZIKV exposure and adverse congenital outcomes found in different study types. |
Temporality. This update confirms the previous conclusion that ZIKV infection precedes the adverse congenital outcomes. We found an additional 21 publications in which ZIKV infection preceded the adverse congenital outcome at an individual level 12, 15– 18, 26- 31, 35- 40, 42, 44, 45 and at a population level 45, 46. Infections in the first and second trimester seemed to be related to the most adverse outcomes 31, 40. Cohort studies of pregnant women from French Guiana and Brazil found a higher proportion of congenital abnormalities in babies born from mothers infected in the first and the second trimester 31, 40.
Biological plausibility. This update includes an additional 42 studies 14, 17, 23, 24, 32– 36, 38, 39, 42, 47– 76, some of which expand the evidence base. Whereas in the baseline review, we found inconclusive evidence of whether ZIKV particles in infants were capable of replication, both in vivo and ex vivo studies now demonstrate that this is the case 33, 36, 47, 50, 53- 55. Furthermore, there was a strong expansion of the evidence clarifying how ZIKV causes adverse congenital outcomes. ZIKV uses receptors from the TAM family to enter cells 47– 52, where the virus induces cell death, primarily in developing neuronal cells 60, 61, 64, 65, 67, 69, 70, 75.
Strength of association. We included five publications that confirm a strong association between ZIKV infection and adverse congenital outcomes 21, 22, 31, 40, 41. The strength of association at an individual level was high but imprecise, owing to small sample sizes. Estimates from cohort studies 31, 40 appeared to be lower than those from case-control studies 21, 22, 41. The definition of the outcomes and the outcomes assessed, varied between studies. The risk of any adverse congenital outcomes was higher and more variable than the risk of microcephaly. The risk ratio for microcephaly between ZIKV unexposed and exposed was 4.4 (95% CI: 0.2-80.8) in a cohort in Brazil 31 and 6.6 (95% CI: 0.8-56.4) in a cohort in French Guiana 40. In the Brazilian cohort 31, the proportion of any adverse congenital outcomes among ZIKV infected women was high (41.9% [49/117]), compared with the uninfected group (5.2% [3/57]). In a prospective case- control study in Brazil, women with laboratory-confirmed ZIKV had 55.5 (95% CI: 8.6-infinity) times the odds of having a baby with microcephaly compared with women without evidence of ZIKV infection 21. A retrospective case- control study in Hawaii found an odds ratio of 11.0 (95% CI: 0.8-147.9) 41. In the latter, however, exposure was assessed retrospectively using serology.
Exclusion of alternatives. We included 23 new studies in this update 12, 14, 17– 19, 21– 28, 30, 31, 34, 36– 38, 40, 42, 45, 77. Many studies included in this review that reported on adverse outcomes of congenital ZIKV excluded TORCH infections 12, 14, 17– 19, 21– 28, 30, 31, 34, 36– 38, 40, 42, 45, 77; exposure to toxic chemicals 12, 14, 18, 23, 28 or genetic conditions 12, 18, 23, 28, 30, 36, 42. Maternal or foetal malnutrition, hypoxic-ischaemic lesions and underlying genetic conditions were not excluded. No single alternative explanation could be given to explain the relation between ZIKV and adverse congenital outcomes.
Cessation. We did not find any new publications for this causality dimension. Evidence is still lacking on the effect of intentional removal due to lack of vaccination or elimination of mosquitoes on a large scale.
Dose-response. There is still no direct evidence about the association between Zika viral load and probability of adverse congenital outcome in observational studies, or of an association between symptomatic status and outcome. In a study in the United States, Honein et al. found similar proportions of adverse congenital outcomes in symptomatic and asymptomatic ZIKV-infected mothers 32.
Animal experiments. This update of the review includes an additional 11 studies 63, 71, 78– 86. These studies confirm a consistent relation between a range of contemporary ZIKV and adverse congenital outcomes, including from Brazil 85, Puerto Rico 79 and Mexico 80, 81. The body of evidence coming from animal studies has grown; both in mice and macaques, congenital anomalies such as intra-uterine growth restriction and signs of microcephaly were observed after ZIKV infection 78, 84, 85.
Analogy. As for the baseline review, evidence for this dimension was not reviewed systematically because our search strategy did not include terms for other infections or conditions. Studies included in this version of the review confirm the analogy between congenital ZIKV and TORCH infections 87. Vertical transmission of West Nile virus and dengue virus were summarised in the baseline review. In update 1, we included a case series from El Salvador that reported Chikungunya virus in 169 newborns of women with symptomatic infection; a minority had CNS infection, but microcephaly was not reported 88. For most analogous pathogens, infections earlier in the pregnancy have a higher risk of adverse outcomes 87.
Specificity. We included one study 89, suggesting an expansion of evidence of a distinct congenital Zika syndrome (CZS) 89. In a review of 34 published reports, the authors suggest five congenital abnormalities that, in conjunction, comprise a pattern that is unique to ZIKV: severe microcephaly with overlapping cranial structures, subcortical location of brain calcifications, macular scarring and retinal mottling, congenital contractures and early pyramidal and extrapyramidal symptoms 89.
Consistency. The studies included in this version of the review confirm the pattern of consistency observed in the baseline review. ZIKV infection in association with adverse congenital outcomes were reported in a range of study designs from different regions ( WHO situation report 05.01.2017), although the proportion of affected infants varies over geographic region and time. ZIKV exposure resulted in adverse congenital outcome in people living in ZIKV endemic areas 12– 19, 21– 34, 40– 42, 44, 45, 77, 90, 91 and in female travellers who returned to non-endemic countries 34– 39, 92, 93. Direct evidence from epidemiological studies comparing different lineages is lacking due to circulation of a single strain.
Conclusion. The evidence added in update 1 of the review confirms the conclusion of a causal association between ZIKV and adverse congenital outcomes. New findings expand the evidence base in the dimensions of biological plausibility, strength of association, animal experiments and specificity. In vitro and in vivo studies elucidate pathways on how these outcomes likely occur. Conclusive evidence on the strength of association is lacking. Studies provide crude overall measures of association, not taking into account potential co-factors.
GBS/ITP
In the search period for update 1 of the review, an additional 154 cases of ZIKV-related GBS 95 and 11 ZIKV-related cases of ITP 3– 6 were described in 18 studies. Table 3 summarises the evidence for specific questions in each of 10 causality dimensions (detailed overview in Supplementary Table 3).
Table 3. Summary of the evidence on the relation between ZIKV infection and adverse autoimmune outcomes.
Evidence is displayed for each dimension of the causality framework and for each question. Zika virus (ZIKV); Dengue virus (DENV); Guillain-Barré syndrome (GBS); immune-mediated idiopathic thrombocytopaenia purpura (ITP). NA, not applicable; evidence about analogous conditions was not searched systematically; the dimension of consistency used information in items included for all other causality dimensions. the baseline review (BR), Update 1 (U1).
| Question | BR, N | U1, N | Summary |
|---|---|---|---|
| Temporality | |||
| 1.1a | 9 | 17 | Expansion of the evidence. Additional case reports and case series were identified that confirmed that
ZIKV infection preceded adverse autoimmune outcomes 3, 5, 6, 95– 102, 103– 108. |
| 1.1b | 9 | 4 | Expansion of the evidence that on the population level ZIKV precedes GBS or ITP 103, 109– 111. |
| 1.2 | 7 | 14 | Expansion of evidence that the interval between exposure to ZIKV and occurrence of symptoms is
typical for para- or post-infectious autoimmune-mediated disorders 5, 6, 95– 102, 103– 106, 112. |
| Biological plausibility | |||
| 2.1 | 3 | 0 | No additional evidence was identified that ZIKV epitopes mimic host antigens (molecular mimicry). |
| 2.2 | 1 | 0 | No additional evidence was identified that ZIKV infection leads to an increased in detectable
autoreactive immune cells or autoreactive antibodies. |
| 2.3 | 0 | 0 | There is no evidence on other biologically plausible mechanisms of ZIKV infection leading to GBS/ITP. |
| Strength of association | |||
| 3.1 | 1 | 0 | No additional evidence was identified on the association between Zika infection and GBS/ITP at the
individual level. |
| 3.2 | 2 | 4 | Expansion of evidence. GBS incidence increased in several regions, during the same time ZIKV was
circulating 103, 109– 111. |
| Exclusion of alternatives | |||
| 4.1 | 7 | 9 | Confirmation of the evidence where other infections were assessed. However, often previous DENV
infection was reported, and not excluded 4– 6, 95, 98, 101, 103, 104, 111. |
| 4.2 | 0 | 1 | Expansion on the evidence where vaccines were excluded 5. |
| 4.3 | 0 | 5 | Expansion on the evidence where other systemic illnesses were excluded 4– 6, 95, 99, 112. |
| 4.4 | 0 | 2 | Expansion on the evidence where medication, drugs or other chemicals was excluded 99, 112. |
| Cessation | |||
| 5.1 | 0 | 0 | No relevant studies identified that intentional removal or prevention of ZIKV infection in individuals
leads to a reduction in cases with GBS/ITP. |
| 5.2 | 0 | 0 | No relevant studies identified that intentional removal or prevention of ZIKV infection at population level
leads to a reduction in cases with GBS/ITP. |
| 5.3 | 6 | 2 | Expansion. Additionally, in Venezuela and the Dominican Republic, it was shown that GBS cases
decreased with a decrease in reported ZIKV cases 103, 111. |
| Dose-response | |||
| 6.1 | 0 | 0 | No relevant studies identified that the risk and the clinical severity of GBS/ITP are associated with viral
titres. |
| Animal experiments | |||
| 7.1 | 0 | 0 | No relevant studies identified where the inoculation of animals with ZIKV leads to an autoimmune
reaction resulting in peripheral neuropathy or thrombocytopenia. |
| 7.2 | 0 | 0 | No relevant studies identified that other animal experiments support the association of ZIKV infection
and GBS/ITP. |
| Analogy | |||
| 8.1 | NA | NA | No additional studies identified that other flaviviruses or arboviruses cause GBS/ITP. |
| 8.2 | NA | NA | No additional studies identified that other pathogens cause GBS/ITP. |
| 8.3 | NA | NA | No additional studies identified that explain which pathogen or host factors facilitate the development
of GBS/ITP. |
| Specificity | |||
| 9.1 | 0 | 0 | No relevant studies identified that pathological findings in cases with GBS/ITP are specific for ZIKV
infection. |
| Question | v1, N | v2, N | Summary |
| Consistency | |||
| 10.1 | NA | NA | Confirmation that the association between ZIKV cases and cases with GBS is consistently found
across different geographical regions. |
| 10.2 | NA | NA | Confirmation that the association between ZIKV cases and cases with GBS is consistently found
across different populations/subpopulations. |
| 10.3 | NA | NA | No additional studies identified that the association between ZIKV cases and cases with GBS/ITP is
consistently found across different ZIKV lineages/strains. |
| 10.4 | NA | NA | Confirmation that the association between ZIKV cases and cases with GBS is consistently found
across different study designs. |
Temporality. We found an additional 17 publications that confirmed that ZIKV infection preceded the GBS or ITP at an individual level 3, 5, 6, 95– 108 or at a population level 103, 109– 111. ZIKV infections seems to be followed by GBS on average between 5 and 10 days. In one case series from Colombia 103, the authors distinguished between rapid onset of GBS symptoms after ZIKV symptoms (para-infectious) and post-infectious onset, with an asymptomatic period after ZIKV symptoms before the start of GBS symptoms.
Biological plausibility. We did not find any publications about the biological plausibility of ZIKV as a cause of GBS or ITP.
Strength of association. We did not find any comparative observational studies during the search period for update 1. Several surveillance studies confirmed an increase in notified GBS cases during ZIKV outbreaks at the population level 111. Rate ratios were significantly higher for Brazil, Colombia, the Dominican Republic, El Salvador, Honduras, Suriname and Venezuela when comparing pre-ZIKV GBS incidence and the incidence during the outbreak 111; this ratio ranged from 2.0 (95% CI: 1.6-2.6) to 9.8 (95% CI: 7.6-12.5).
Exclusion of alternatives. We included 11 publications 4– 6, 95, 98, 99, 101, 103, 104, 111, 112 that expanded the list of alternative causes for autoimmune disease that were excluded, such as infections, vaccines, other system illnesses and medication, drugs or other chemicals. Many GBS cases in these publications had serological evidence of previous exposure to DENV, as seen in the baseline review. It remains unclear how large the potential role of co-factors such as antibody dependent enhancement are.
Cessation. We did not identify any publications with evidence about the effect of intentional removal/elimination/prevention of ZIKV on either GBS or ITP. An additional publication confirmed evidence that the natural removal of ZIKV resulted in a decrease in GBS cases in Brazil, Colombia, Dominican Republic, El Salvador, Honduras, Suriname and Venezuela 104, 111.
Dose-response. We did not identify any publications about this dimension for either GBS or ITP.
Animal experiments. No additional evidence from animal experiments was identified that support the association between ZIKV infection and GBS/ITP development.
Analogy. As for the baseline review, evidence for this dimension was not reviewed systematically because our search strategy did not include terms for other infections or conditions. We did not identify any new publications addressing this dimension for either GBS or ITP.
Specificity. We did not identify any new publications addressing this dimension for either GBS or ITP.
Consistency. Studies included in update 1 confirmed the consistency of the evidence for 3 of 4 questions about the association between ZIKV and GBS. By geographical region, ZIKV transmission has been associated with the occurrence of GBS in 2 of 4 regions; increased GBS incidence has been reported in the WHO regions of the Americas and the Western Pacific region, but not in the African or Southeast Asian region, despite recent ZIKV circulation 113. By study design, the association between ZIKV infection and GBS has been found at individual and population level and with different study designs. By population, ZIKV infection has been linked to GBS in ZIKV endemic regions 4– 6, 95, 96, 98– 101, 103– 105, 109, 111, 114 and travellers from non-affected countries who were exposed in these endemic regions 3, 97, 102, 106, 112. There was insufficient evidence to examine the consistency of evidence about ZIKV and ITP.
Conclusion. The body of evidence has grown during the search period for update 1 but only for dimensions that were already populated in the original publication for GBS. There is still a limited understanding of the biological pathways that potentially cause the occurrence of autoimmune disease following ZIKV infection. Additionally, prospective comparative epidemiological studies are still lacking. It remains unclear how co-factors such as age and previous exposure to flaviviruses influences the risk of developing GBS. The evidence supports a temporal association between ZIKV and ITP but there is an absence of evidence for other dimensions of causality.
Search results from January 19, 2017 to January 05, 2018
Automated search and deduplication processes identified 2410 publications about any aspect of ZIKV infection. The next update of this review will address causality dimensions in the realm of epidemiological studies; strength of association, dose-response relationship, specificity and consistency.
Discussion
Statement of principal findings. This systematic review confirms evidence of a causal association between ZIKV and adverse congenital outcomes and between ZIKV and GBS, although evidence about biological plausibility is still lacking. We assessed evidence about an association between ZIKV and ITP but found that this only addressed the dimension of temporality. The review is transitioning from classic systematic review methods to those of a living systematic review.
Strengths and limitations of the study. The strengths of this study are the systematic approach to the identification, selection and extraction of data following a causality framework that provides a structure for the consideration of heterogeneous sources of evidence and a large set of review questions. Automation of the review output allows rapid updating of tables of results. We have also developed methods to automate search and deduplication of search results to make the transition to a living systematic review that will allow continual updating of results. The main limitation of the classic systematic review of such a complex topic is the high workload and time required to maintain it. Another limitation, resulting from the large number of review questions, is the time taken to resolve inter-reviewer differences in interpretation of eligibility criteria. This could have resulted in subjectivity over decisions about inclusion in the review. Although a second reviewer checked all extractions, changes in the review team could introduce inconsistency. As in the baseline review, we used case definitions as authors described them in individual publications. This potential source of information bias is likely to decrease over time as standardised case definitions and protocols are adopted 115. As in the previous version, we did not systematically apply quality assessment tools to individual studies. Because much of the technical infrastructure was built as the evidence emerged, output was delayed. As much of the LSR methodology was novel, it took time to find a balance between speed and efficiency.
Strengths and weaknesses in relation to other publications. Our systematic review differs from most standard reviews because of the number of questions within the dimensions of the causality framework and the number of outcomes. Other recent examples of living systematic reviews only distinguish between two study types (RCT and non-RCT) 116 and are guided by only a small set of review questions 117, 118. Our review conclusion, confirming evidence for a causal association between ZIKV and GBS differs from that of a review 119 of the findings of four case reports 104, 120– 122 and one case-control study 123. The authors found insufficient evidence to confirm the presence of an acute motor axonal neuropathy variant of GBS. They did not, however, suggest an alternative explanation for the increase in incidence of GBS in the countries that experienced ZIKV outbreaks. The two versions of our review included 64 publications about ZIKV and GBS across ten dimensions of causality.
Meaning of the study: possible mechanisms and implications for basic researchers, clinicians or policymakers. The conclusions on the causal relation between ZIKV and adverse congenital outcomes and ZIKV and GBS did not change with this update. We found insufficient evidence about the association between ZIKV and ITP to state with certainty that there is a causal association. The total volume of evidence about the association between ZIKV and GBS is less than for the association with adverse congenital outcomes. There is, in particular a lack of published research to elucidate biological mechanisms for direct neuronal or autoimmune damage in GBS 124. The descriptive data about the numbers and types of different studies over time illustrates how evidence about a new, or re-emerging, infection emerges over time. The evidence from many regions that were affected by the ZIKV outbreak remains limited to anecdotal evidence of adverse outcomes, in the form of case reports or case series. The slowing of ZIKV transmission in 2017 means that fewer people are being affected by ZIKV and its complications and fewer people are being enrolled into prospective studies. Further progress in epidemiological research will rely more heavily on research consortia who are contributing to joint analyses of data from existing studies.
Unanswered questions and future research. As the volume and complexity of the evidence in different causality dimensions accumulates, the need for expert input and interpretation of the findings of this systematic review increases. The focus of research on ZIKV and causal associations with different types of adverse outcomes is also changing. For congenital abnormalities resulting from ZIKV vertical transmission, epidemiological research should examine CZS in comparative studies, quantify the strength of association with ZIKV, clarify associations with gestational age, symptomatology and viral load and further investigate potential co-factors such as previous dengue infection and flavivirus vaccination. WHO standardised study protocols provide suggestions for exclusion of alternative explanations and exploration of co-factors ( Harmonization of ZIKV Research Protocols). For GBS, epidemiological studies are needed to quantify the association with ZIKV more precisely, but also to determine whether there are distinct phenotypes resulting from autoimmune mechanisms or direct neuronal involvement. For ITP, additional evidence across all causality dimensions is needed.
Planned updates of a living systematic review. Living systematic review methodology and techniques will continue to develop. Since a chain is only as strong as its weakest link, any processing step has the potential to slow down a living systematic review. Clearly defined protocols that define update frequencies and throughput speed of different actors in the publishing process are vital. The next update of the systematic reviews will use living systematic review methods to assess the evidence for 2017 and early 2018 (update 2, Figure 2). The review will, for the first time, separate evidence from epidemiological study designs from in vitro and in vivo laboratory studies. We will narrow down the inclusion criteria based on study type. Epidemiological evidence will address the causality dimensions ‘strength of association’, ‘dose-response’, ‘specificity’ and ‘consistency’. Several co-factors might play a role in the strength of association. Thus, we will continue to collect information on previous dengue virus infection, yellow fever vaccination status, socioeconomic status, gestational age and others factors that might play a role in the severity of the outcome. We will amend the protocol with a more focused search strategy and inclusion criteria ( Supplementary File 3).
Systematic reviews of questions addressed by laboratory studies are less frequent than those addressing epidemiological research questions. There is still need to update understanding of the causality dimensions ‘biological plausibility’ and ‘animal experiments’, particularly to increase our understanding of biological pathways for ZIKV effects on the peripheral nervous system and the immune system. We encourage and welcome collaboration from scientists with expertise in these fields to update systematic reviews for these causality dimensions.
Conclusion. This systematic review confirms previous conclusions that ZIKV is a cause of congenital abnormalities, including microcephaly and is a trigger of GBS. Evidence suggests an association with idiopathic thrombocytopaenia purpura but is not conclusive. The transition to living systematic review techniques and methodology provides a proof of concept for the use of these methods to synthesise evidence about an emerging pathogen such as ZIKV, ultimately leading to integration in the whole public health information cycle 125. With the infrastructure for living systematic review methods and open source access to the software and outputs, we aim to enhance outbreak preparedness and the study of emerging and re-emerging pathogens.
Data availability
All data underlying the results are available as part of the article and no additional source data are required.
Acknowledgements
We thank the members of the WHO Zika Causality Working Group for their input on the conceptualisation of the causality framework and Anina Häfliger for her assistance on screening publications.
Funding Statement
MJC received salary support from the Swiss National Science Foundation (project grants 320030_170069 and 320030_176233). DEG, YW and MR received salary support from the Swiss National Science Foundation (project grant 320030_170069).
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
[version 1; referees: 2 approved
Supplementary material
Supplementary Table 1 – Bradford Hill’s “viewpoints” of causation.
Supplementary Table 2 – Evidence table adverse congenital outcomes update 1.
Supplementary Table 3 – Evidence table GBS/ITP update 1.
Supplementary File 1 – PRISMA Checklist.
Supplementary File 2 – LSR automation methodology.
Supplementary File 3 – Search strategy update 2.
References
- 1. Broutet N, Krauer F, Riesen M, et al. : Zika Virus as a Cause of Neurologic Disorders. N Engl J Med. 2016;374(16):1506–9. 10.1056/NEJMp1602708 [DOI] [PubMed] [Google Scholar]
- 2. Rasmussen SA, Jamieson DJ, Honein MA, et al. : Zika Virus and Birth Defects--Reviewing the Evidence for Causality. N Engl J Med. 2016;374(20):1981–7. 10.1056/NEJMsr1604338 [DOI] [PubMed] [Google Scholar]
- 3. de Laval F, Matheus S, Maquart M, et al. : Prospective Zika virus disease cohort: systematic screening. Lancet. 2016;388(10047):868. 10.1016/S0140-6736(16)31429-5 [DOI] [PubMed] [Google Scholar]
- 4. Chraïbi S, Najioullah F, Bourdin C, et al. : Two cases of thrombocytopenic purpura at onset of Zika virus infection. J Clin Virol. 2016;83(34):61–2. 10.1016/j.jcv.2016.08.299 [DOI] [PubMed] [Google Scholar]
- 5. Boyer Chammard T, Schepers K, Breurec S, et al. : Severe Thrombocytopenia after Zika Virus Infection, Guadeloupe, 2016. Emerg Infect Dis. 2017;23(4):696–8. 10.3201/eid2304.161967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zea-Vera AF, Parra B: Zika virus (ZIKV) infection related with immune thrombocytopenic purpura (ITP) exacerbation and antinuclear antibody positivity. Lupus. 2017;26(8):890–2. 10.1177/0961203316671816 [DOI] [PubMed] [Google Scholar]
- 7. Krauer F, Riesen M, Reveiz L, et al. : Zika Virus Infection as a Cause of Congenital Brain Abnormalities and Guillain-Barré Syndrome: Systematic Review. PLoS Med. 2017;14(1):e1002203. 10.1371/journal.pmed.1002203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hill AB: The Environment and Disease: Association or Causation? Proc R Soc Med. 1965;58(5):295–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Elliott JH, Turner T, Clavisi O, et al. : Living systematic reviews: an emerging opportunity to narrow the evidence-practice gap. PLoS Med. 2014;11(2):e1001603. 10.1371/journal.pmed.1001603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Elliott JH, Synnot A, Turner T, et al. : Living systematic review: 1. Introduction-the why, what, when, and how. J Clin Epidemiol. 2017;91:23–30. 10.1016/j.jclinepi.2017.08.010 [DOI] [PubMed] [Google Scholar]
- 11. Moher D, Liberati A, Tetzlaff J, et al. : Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Leal MC, Muniz LF, Caldas Neto SD, et al. : Sensorineural hearing loss in a case of congenital Zika virus. Braz J Otorhinolaryngol. 2016; pii: S1808-8694(16)30127-6. 10.1016/j.bjorl.2016.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. França GV, Schuler-Faccini L, Oliveira WK, et al. : Congenital Zika virus syndrome in Brazil: a case series of the first 1501 livebirths with complete investigation. Lancet. 2016;388(10047):891–7. 10.1016/S0140-6736(16)30902-3 [DOI] [PubMed] [Google Scholar]
- 14. Martines RB, Bhatnagar J, de Oliveira Ramos AM, et al. : Pathology of congenital Zika syndrome in Brazil: a case series. Lancet. 2016;388(10047):898–904. 10.1016/S0140-6736(16)30883-2 [DOI] [PubMed] [Google Scholar]
- 15. Carvalho FH, Cordeiro KM, Peixoto AB, et al. : Associated ultrasonographic findings in fetuses with microcephaly because of suspected Zika virus (ZIKV) infection during pregnancy. Prenat Diagn. 2016;36(9):882–7. 10.1002/pd.4882 [DOI] [PubMed] [Google Scholar]
- 16. Bhadelia N: Prospective cohort study of pregnant Brazilian women elucidates link between Zika virus infection and fetal abnormalities. Evid Based Med. 2016;21(5):193. 10.1136/ebmed-2016-110476 [DOI] [PubMed] [Google Scholar]
- 17. van der Linden V, Filho EL, Lins OG, et al. : Congenital Zika syndrome with arthrogryposis: retrospective case series study. BMJ. 2016;354(8):i3899. 10.1136/bmj.i3899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Leal MC, Muniz LF, Ferreira TS, et al. : Hearing Loss in Infants with Microcephaly and Evidence of Congenital Zika Virus Infection - Brazil, November 2015-May 2016. MMWR Morb Mortal Wkly Rep. 2016;65(34):917–9. 10.15585/mmwr.mm6534e3 [DOI] [PubMed] [Google Scholar]
- 19. Soares de Souza A, Moraes Dias C, Braga FD, et al. : Fetal Infection by Zika Virus in the Third Trimester: Report of 2 Cases. Clin Infect Dis. 2016;63(12):1622–5. 10.1093/cid/ciw613 [DOI] [PubMed] [Google Scholar]
- 20. De Paiva Cavalcante T, De Campos JJG, De Freire MRM, et al. : Congenital neural tube defects evaluation in newborns with prenatal Zika virus infection. Child's Nervous System. 2016;32(5):933 10.1007/s00381-016-3044-z [DOI] [Google Scholar]
- 21. Thalia Velho Barreto A, Laura CR, Ricardo AX, et al. : Microcephaly and Zika infection: Preliminary Report of a Case-Control Study. Lancet. 2016;16(12):1356–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. de Araújo TVB, Rodrigues LC, de Alencar Ximenes RA, et al. : Association between Zika virus infection and microcephaly in Brazil, January to May, 2016: preliminary report of a case-control study. Lancet Infect Dis. 2016;16(12):1356–63. 10.1016/S1473-3099(16)30318-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Melo AS, Aguiar RS, Amorim MM, et al. : Congenital Zika Virus Infection: Beyond Neonatal Microcephaly. JAMA Neurol. 2016;73(12):1407–16. 10.1001/jamaneurol.2016.3720 [DOI] [PubMed] [Google Scholar]
- 24. van der Linden V, Pessoa A, Dobyns W, et al. : Description of 13 Infants Born During October 2015-January 2016 With Congenital Zika Virus Infection Without Microcephaly at Birth - Brazil. MMWR Morb Mortal Wkly Rep. 2016;65(47):1343–8. 10.15585/mmwr.mm6547e2 [DOI] [PubMed] [Google Scholar]
- 25. João EC, Gouvea MI, Teixeira ML, et al. : Zika Virus Infection Associated With Congenital Birth Defects in a HIV-infected Pregnant Woman. Pediatr Infect Dis J. 2017;36(5):500–1. 10.1097/INF.0000000000001482 [DOI] [PubMed] [Google Scholar]
- 26. Moura da Silva AA, Ganz JS, Sousa PD, et al. : Early Growth and Neurologic Outcomes of Infants with Probable Congenital Zika Virus Syndrome. Emerg Infect Dis. 2016;22(11):1953–6. 10.3201/eid2211.160956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. de Paula Freitas B, Ko AI, Khouri R, et al. : Glaucoma and Congenital Zika Syndrome. Ophthalmology. 2017;124(3):407–8. 10.1016/j.ophtha.2016.10.004 [DOI] [PubMed] [Google Scholar]
- 28. Campos AG, Lira RP, Arantes TE: Optical coherence tomography of macular atrophy associated with microcephaly and presumed intrauterine Zika virus infection. Arq Bras Oftalmol. 2016;79(6):400–1. 10.5935/0004-2749.20160112 [DOI] [PubMed] [Google Scholar]
- 29. Vargas A, Saad E, Dimech GS, et al. : Characteristics of the first cases of microcephaly possibly related to Zika virus reported in the Metropolitan Region of Recife, Pernambuco State, Brazil. Epidemiol Serv Saude. 2016;25(4):691–700. 10.5123/S1679-49742016000400003 [DOI] [PubMed] [Google Scholar]
- 30. Werner H, Fazecas T, Guedes B, et al. : Intrauterine Zika virus infection and microcephaly: correlation of perinatal imaging and three-dimensional virtual physical models. Ultrasound Obstet Gynecol. 2016;47(5):657–60. 10.1002/uog.15901 [DOI] [PubMed] [Google Scholar]
- 31. Brasil P, Pereira JP, Jr, Moreira ME, et al. : Zika Virus Infection in Pregnant Women in Rio de Janeiro. N Engl J Med. 2016;375(24):2321–34. 10.1056/NEJMoa1602412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Honein MA, Dawson AL, Petersen EE, et al. : Birth Defects Among Fetuses and Infants of US Women With Evidence of Possible Zika Virus Infection During Pregnancy. JAMA. 2017;317(1):59–68. 10.1001/jama.2016.19006 [DOI] [PubMed] [Google Scholar]
- 33. Bhatnagar J, Rabeneck DB, Martines RB, et al. : Zika Virus RNA Replication and Persistence in Brain and Placental Tissue. Emerg Infect Dis. 2017;23(3):405–14. 10.3201/eid2303.161499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Adams L, Bello-Pagan M, Lozier M, et al. : Update: Ongoing Zika Virus Transmission - Puerto Rico, November 1, 2015-July 7, 2016. MMWR Morb Mortal Wkly Rep. 2016;65(30):774–9. 10.15585/mmwr.mm6530e1 [DOI] [PubMed] [Google Scholar]
- 35. Meaney-Delman D, Oduyebo T, Polen KN, et al. : Prolonged Detection of Zika Virus RNA in Pregnant Women. Obstet Gynecol. 2016;128(4):724–30. 10.1097/AOG.0000000000001625 [DOI] [PubMed] [Google Scholar]
- 36. van der Eijk AA, van Genderen PJ, Verdijk RM, et al. : Miscarriage Associated with Zika Virus Infection. N Engl J Med. 2016;375(10):1002–4. 10.1056/NEJMc1605898 [DOI] [PubMed] [Google Scholar]
- 37. Suy A, Sulleiro E, Rodó C, et al. : Prolonged Zika Virus Viremia during Pregnancy. N Engl J Med. 2016;375(26):2611–3. 10.1056/NEJMc1607580 [DOI] [PubMed] [Google Scholar]
- 38. Ventura CV, Fernandez MP, Gonzalez IA, et al. : First Travel-Associated Congenital Zika Syndrome in the US: Ocular and Neurological Findings in the Absence of Microcephaly. Ophthalmic Surg Lasers Imaging Retina. 2016;47(10):952–5. 10.3928/23258160-20161004-09 [DOI] [PubMed] [Google Scholar]
- 39. Herrera K, Bernasko J, Garry D, et al. : Vertical transmission of Zika virus (ZIKV) in early pregnancy: two cases, two different courses. Case Reports in Perinatal Medicine. 2016;5(2):131–3. 10.1515/crpm-2016-0027 [DOI] [Google Scholar]
- 40. Pomar L, Malinger G, Benoist G, et al. : Association between Zika virus and fetopathy: a prospective cohort study in French Guiana. Ultrasound Obstet Gynecol. 2017;49(6):729–36. 10.1002/uog.17404 [DOI] [PubMed] [Google Scholar]
- 41. Kumar M, Ching L, Astern J, et al. : Prevalence of Antibodies to Zika Virus in Mothers from Hawaii Who Delivered Babies with and without Microcephaly between 2009–2012. PLoS Negl Trop Dis. 2016;10(12):e0005262. 10.1371/journal.pntd.0005262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Schaub B, Monthieux A, Najioullah F, et al. : Persistent maternal Zika viremia: a marker of fetal infection. Ultrasound Obstet Gynecol. 2017;49(5):658–60. 10.1002/uog.17210 [DOI] [PubMed] [Google Scholar]
- 43. Villamil-Gómez WE, Rodriguez-Morales AJ, Uribe-Garcia AM, et al. : Zika, dengue, and chikungunya co-infection in a pregnant woman from Colombia. Int J Infect Dis. 2016;51:135–8. 10.1016/j.ijid.2016.07.017 [DOI] [PubMed] [Google Scholar]
- 44. Sanin-Blair JCA, Gutierrez-Marin J, Campo-Campo M, et al. : Fetal ultrasound findings in zika infection. Preliminary report in a Colombian selected population. Journal of Maternal-Fetal and Neonatal Medicine. 2016;29(2):163–4.25572878 [Google Scholar]
- 45. Cuevas EL, Tong VT, Rozo N, et al. : Preliminary Report of Microcephaly Potentially Associated with Zika Virus Infection During Pregnancy - Colombia, January-November 2016. MMWR Morb Mortal Wkly Rep. 2016;65(49):1409–13. 10.15585/mmwr.mm6549e1 [DOI] [PubMed] [Google Scholar]
- 46. Magalhães-Barbosa MC, Prata-Barbosa A, Robaina JR, et al. : Trends of the microcephaly and Zika virus outbreak in Brazil, January-July 2016. Travel Med Infect Dis. 2016;14(5):458–63. 10.1016/j.tmaid.2016.09.006 [DOI] [PubMed] [Google Scholar]
- 47. Tabata T, Petitt M, Puerta-Guardo H, et al. : Zika Virus Targets Different Primary Human Placental Cells, Suggesting Two Routes for Vertical Transmission. Cell Host Microbe. 2016;20(2):155–66. 10.1016/j.chom.2016.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Onorati M, Li Z, Liu F, et al. : Zika Virus Disrupts Phospho-TBK1 Localization and Mitosis in Human Neuroepithelial Stem Cells and Radial Glia. Cell Rep. 2016;16(10):2576–92. 10.1016/j.celrep.2016.08.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Sironi M, Forni D, Clerici M, et al. : Nonstructural Proteins Are Preferential Positive Selection Targets in Zika Virus and Related Flaviviruses. PLoS Negl Trop Dis. 2016;10(9):e0004978. 10.1371/journal.pntd.0004978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Retallack H, Di Lullo E, Arias C, et al. : Zika Virus in the Human Placenta and Developing Brain: Cell Tropism and Drug Inhibition. bioRxiv. 2016; 058883. 10.1101/058883 [DOI] [Google Scholar]
- 51. Retallack H, Di Lullo E, Arias C, et al. : Zika virus cell tropism in the developing human brain and inhibition by azithromycin. Proc Natl Acad Sci U S A. 2016;113(50):14408–13. 10.1073/pnas.1618029113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Liu S, DeLalio LJ, Isakson BE, et al. : AXL-Mediated Productive Infection of Human Endothelial Cells by Zika Virus. Circ Res. 2016;119(11):1183–9. 10.1161/CIRCRESAHA.116.309866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Tsetsarkin KA, Kenney H, Chen R, et al. : A Full-Length Infectious cDNA Clone of Zika Virus from the 2015 Epidemic in Brazil as a Genetic Platform for Studies of Virus-Host Interactions and Vaccine Development. mBio. 2016;7(4):e01114–16. 10.1128/mBio.01114-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Jurado KA, Simoni MK, Tang Z, et al. : Zika virus productively infects primary human placenta-specific macrophages. JCI Insight. 2016;1(13): pii: e88461. 10.1172/jci.insight.88461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. El Costa H, Gouilly J, Mansuy JM, et al. : ZIKA virus reveals broad tissue and cell tropism during the first trimester of pregnancy. Sci Rep. 2016;6: 35296. 10.1038/srep35296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Hanners NW, Eitson JL, Usui N, et al. : Western Zika Virus in Human Fetal Neural Progenitors Persists Long Term with Partial Cytopathic and Limited Immunogenic Effects. Cell Rep. 2016;15(11):2315–22. 10.1016/j.celrep.2016.05.075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Liang Q, Luo Z, Zeng J, et al. : Zika Virus NS4A and NS4B Proteins Deregulate Akt-mTOR Signaling in Human Fetal Neural Stem Cells to Inhibit Neurogenesis and Induce Autophagy. Cell Stem Cell. 2016;19(5):663–71. 10.1016/j.stem.2016.07.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Garcez PP, Nascimento JM, Mota de Vasconcelos J, et al. : Combined proteome and transcriptome analyses reveal that Zika virus circulating in Brazil alters cell cycle and neurogenic programmes in human neurospheres. PeerJ Preprints. 2016;4:e2033v1 10.7287/peerj.preprints.2033v1 [DOI] [Google Scholar]
- 59. Bayless NL, Greenberg RS, Swigut T, et al. : Zika Virus Infection Induces Cranial Neural Crest Cells to Produce Cytokines at Levels Detrimental for Neurogenesis. Cell Host Microbe. 2016;20(4):423–8. 10.1016/j.chom.2016.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Barreras P, Pamies D, Kumar A, et al. : 2016 Annual Meetings. Ann Neurol. 2016;80(s20):S1–S432. 10.1002/ana.24759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Hughes BW, Addanki KC, Sriskanda AN, et al. : Infectivity of Immature Neurons to Zika Virus: A Link to Congenital Zika Syndrome. EBioMedicine. 2016;10(10038):65–70. 10.1016/j.ebiom.2016.06.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Kumar A, Singh HN, Pareek V, et al. : A Possible Mechanism of Zika Virus Associated Microcephaly: Imperative Role of Retinoic Acid Response Element (RARE) Consensus Sequence Repeats in the Viral Genome. Front Hum Neurosci. 2016;10:403. 10.3389/fnhum.2016.00403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Chan JF, Yip CC, Tsang JO, et al. : Differential cell line susceptibility to the emerging Zika virus: implications for disease pathogenesis, non-vector-borne human transmission and animal reservoirs. Emerg Microbes Infect. 2016;5:e93. 10.1038/emi.2016.99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Xu M, Lee EM, Wen Z, et al. : Identification of small-molecule inhibitors of Zika virus infection and induced neural cell death via a drug repurposing screen. Nat Med. 2016;22(10):1101–7. 10.1038/nm.4184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Zhang F, Hammack C, Ogden SC, et al. : Molecular signatures associated with ZIKV exposure in human cortical neural progenitors. Nucleic Acids Res. 2016;44(18):8610–20. 10.1093/nar/gkw765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Nogueira FCS, Velasquez E, Melo ASO, et al. : Zika virus may not be alone: proteomics associates a bovine-like viral diarrhea virus to microcephaly. bioRxiv. 2016; 062596. 10.1101/062596 [DOI] [Google Scholar]
- 67. Wang Z, Ma'ayan A: An open RNA-Seq data analysis pipeline tutorial with an example of reprocessing data from a recent Zika virus study [version 1; referees: 3 approved]. F1000Res. 2016;5(1574):1574. 10.12688/f1000research.9110.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Weisblum Y, Oiknine-Djian E, Vorontsov OM, et al. : Zika Virus Infects Early- and Midgestation Human Maternal Decidual Tissues, Inducing Distinct Innate Tissue Responses in the Maternal-Fetal Interface. J Virol. 2017;91(4): pii: e01905-16. 10.1128/JVI.01905-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Li Y, Muffat J, Omer A, et al. : Induction of Expansion and Folding in Human Cerebral Organoids. Cell Stem Cell. 2017;20(3):385–96 e3. 10.1016/j.stem.2016.11.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Souza BS, Sampaio GL, Pereira CS, et al. : Zika virus infection induces mitosis abnormalities and apoptotic cell death of human neural progenitor cells. Sci Rep. 2016;6: 39775. 10.1038/srep39775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Aldo P, You Y, Szigeti K, et al. : HSV-2 enhances ZIKV infection of the placenta and induces apoptosis in first-trimester trophoblast cells. Am J Reprod Immunol. 2016;76(5):348–57. 10.1111/aji.12578 [DOI] [PubMed] [Google Scholar]
- 72. Lucchese G, Kanduc D: Minimal immune determinants connect Zika virus, human Cytomegalovirus, and Toxoplasma gondii to microcephaly-related human proteins. Am J Reprod Immunol. 2017;77(2):e12608. 10.1111/aji.12608 [DOI] [PubMed] [Google Scholar]
- 73. Simonin Y, Loustalot F, Desmetz C, et al. : Zika Virus Strains Potentially Display Different Infectious Profiles in Human Neural Cells. EBioMedicine. 2016;12:161–9. 10.1016/j.ebiom.2016.09.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Kodani AT, Reiter JF, Knopp K, et al. : Zika infection disrupts centriole biogenesis. Mol Biol Cell. 2016;27(25):4182–3. 10.1091/mbc.E16-10-0736 [DOI] [Google Scholar]
- 75. Veilleux C, Eugenin E: Characterization of Zika virus infection in Human Astrocytes. J Neurovirol. 2016;22:S78–S9. 10.1007/s13365-016-0478-8 [DOI] [Google Scholar]
- 76. Yaffe Y, Dellibovi-Ragheb TA, Chen Y, et al. : Board Number: B456 The role of centrosomes in the pathogenesis of Zika virus (ZIKV)-related microcephaly. Mol Biol Cell. 2016;27(25):4919 10.1091/mbc.E16-10-0736 [DOI] [Google Scholar]
- 77. Oliveira DB, Almeida FJ, Durigon EL, et al. : Prolonged Shedding of Zika Virus Associated with Congenital Infection. N Engl J Med. 2016;375(12):1202–4. 10.1056/NEJMc1607583 [DOI] [PubMed] [Google Scholar]
- 78. Adams Waldorf KM, Stencel-Baerenwald JE, Kapur RP, et al. : Fetal brain lesions after subcutaneous inoculation of Zika virus in a pregnant nonhuman primate. Nat Med. 2016;22(11):1256–9. 10.1038/nm.4193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Manangeeswaran M, Ireland DD, Verthelyi D: Zika (PRVABC59) Infection Is Associated with T cell Infiltration and Neurodegeneration in CNS of Immunocompetent Neonatal C57Bl/6 Mice. PLoS Pathog. 2016;12(11):e1006004. 10.1371/journal.ppat.1006004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Goodfellow FT, Tesla B, Simchick G, et al. : Zika Virus Induced Mortality and Microcephaly in Chicken Embryos. Stem Cells Dev. 2016;25(22):1691–7. 10.1089/scd.2016.0231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Shao Q, Herrlinger S, Yang SL, et al. : Zika virus infection disrupts neurovascular development and results in postnatal microcephaly with brain damage. Development. 2016;143(22):4127–36. 10.1242/dev.143768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Huang WC, Abraham R, Shim BS, et al. : Zika virus infection during the period of maximal brain growth causes microcephaly and corticospinal neuron apoptosis in wild type mice. Sci Rep. 2016;6: 34793. 10.1038/srep34793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Doobin DJ, Rosenfeld A, Carabalona A, et al. : Genetic and infectious causes of microcephaly caused by NDE1 mutations and Zika virus. Molecular Biology of the Cell. 2016;27(25):4129–30. [Google Scholar]
- 84. Yockey LJ, Varela L, Rakib T, et al. : Vaginal Exposure to Zika Virus during Pregnancy Leads to Fetal Brain Infection. Cell. 2016;166(5):1247–56 e4. 10.1016/j.cell.2016.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Cugola FR, Fernandes IR, Russo FB, et al. : The Brazilian Zika virus strain causes birth defects in experimental models. Nature. 2016;534(7606):267–71. 10.1038/nature18296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Brault JB, Khou C, Basset J, et al. : Comparative Analysis Between Flaviviruses Reveals Specific Neural Stem Cell Tropism for Zika Virus in the Mouse Developing Neocortex. EBioMedicine. 2016;10(2):71–6. 10.1016/j.ebiom.2016.07.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Panchaud A, Stojanov M, Ammerdorffer A, et al. : Emerging Role of Zika Virus in Adverse Fetal and Neonatal Outcomes. Clin Microbiol Rev. 2016;29(3):659–94. 10.1128/CMR.00014-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Torres JR, Falleiros-Arlant LH, Dueñas L, et al. : Congenital and perinatal complications of chikungunya fever: a Latin American experience. Int J Infect Dis. 2016;51:85–8. 10.1016/j.ijid.2016.09.009 [DOI] [PubMed] [Google Scholar]
- 89. Moore CA, Staples JE, Dobyns WB, et al. : Characterizing the Pattern of Anomalies in Congenital Zika Syndrome for Pediatric Clinicians. JAMA Pediatr. 2017;171(3):288–95. 10.1001/jamapediatrics.2016.3982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Soares de Oliveira-Szejnfeld P, Levine D, Melo AS, et al. : Congenital Brain Abnormalities and Zika Virus: What the Radiologist Can Expect to See Prenatally and Postnatally. Radiology. 2016;281(1):203–18. 10.1148/radiol.2016161584 [DOI] [PubMed] [Google Scholar]
- 91. Sarno M, Aquino M, Pimentel K, et al. : Progressive lesions of central nervous system in microcephalic fetuses with suspected congenital Zika virus syndrome. Ultrasound Obstet Gynecol. 2017;50(6):717–22. 10.1002/uog.17303 [DOI] [PubMed] [Google Scholar]
- 92. Štrafela P, Vizjak A, Mraz J, et al. : Zika Virus-Associated Micrencephaly: A Thorough Description of Neuropathologic Findings in the Fetal Central Nervous System. Arch Pathol Lab Med. 2017;141(1):73–81. 10.5858/arpa.2016-0341-SA [DOI] [PubMed] [Google Scholar]
- 93. Vesnaver TV, Tul N, Mehrabi S, et al. : Zika virus associated microcephaly/micrencephaly-fetal brain imaging in comparison with neuropathology. BJOG. 2017;124(3):521–5. 10.1111/1471-0528.14423 [DOI] [PubMed] [Google Scholar]
- 94. Acosta-Reyes J, Navarro E, Herrera MJ, et al. : Severe Neurologic Disorders in 2 Fetuses with Zika Virus Infection, Colombia. Emerg Infect Dis. 2017;23(6):982–4. 10.3201/eid2306.161702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Zambrano H, Waggoner JJ, Almeida C, et al. : Zika Virus and Chikungunya Virus CoInfections: A Series of Three Cases from a Single Center in Ecuador. Am J Trop Med Hyg. 2016;95(4):894–6. 10.4269/ajtmh.16-0323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Dirlikov E, Major CG, Mayshack M, et al. : Guillain-Barré Syndrome During Ongoing Zika Virus Transmission - Puerto Rico, January 1-July 31, 2016. MMWR Morb Mortal Wkly Rep. 2016;65(34):910–4. 10.15585/mmwr.mm6534e1 [DOI] [PubMed] [Google Scholar]
- 97. Medina MT, England JD, Lorenzana I, et al. : Zika virus associated with sensory polyneuropathy. J Neurol Sci. 2016;369(30):271–2. 10.1016/j.jns.2016.08.044 [DOI] [PubMed] [Google Scholar]
- 98. Langerak T, Yang H, Baptista M, et al. : Zika Virus Infection and Guillain-Barré Syndrome in Three Patients from Suriname. Front Neurol. 2016;7(11):233. 10.3389/fneur.2016.00233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Arias A, Torres-Tobar L, Hernández G, et al. : Guillain-Barré syndrome in patients with a recent history of Zika in Cúcuta, Colombia: A descriptive case series of 19 patients from December 2015 to March 2016. J Crit Care. 2017;37:19–23. 10.1016/j.jcrc.2016.08.016 [DOI] [PubMed] [Google Scholar]
- 100. Anaya JM, Rodríguez Y, Monsalve DM, et al. : A comprehensive analysis and immunobiology of autoimmune neurological syndromes during the Zika virus outbreak in Cúcuta, Colombia. J Autoimmun. 2017;77:123–38. 10.1016/j.jaut.2016.12.007 [DOI] [PubMed] [Google Scholar]
- 101. do Rosário MS, de Jesus PA, Vasilakis N, et al. : Guillain-Barré Syndrome After Zika Virus Infection in Brazil. Am J Trop Med Hyg. 2016;95(5):1157–60. 10.4269/ajtmh.16-0306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Fabrizius RG, Anderson K, Hendel-Paterson B, et al. : Guillain-Barré Syndrome Associated with Zika Virus Infection in a Traveler Returning from Guyana. Am J Trop Med Hyg. 2016;95(5):1161–5. 10.4269/ajtmh.16-0397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Parra B, Lizarazo J, Jiménez-Arango JA, et al. : Guillain-Barré Syndrome Associated with Zika Virus Infection in Colombia. N Engl J Med. 2016;375(16):1513–23. 10.1056/NEJMoa1605564 [DOI] [PubMed] [Google Scholar]
- 104. Fontes CA, Dos Santos AA, Marchiori E: Magnetic resonance imaging findings in Guillain-Barré syndrome caused by Zika virus infection. Neuroradiology. 2016;58(8):837–8. 10.1007/s00234-016-1687-9 [DOI] [PubMed] [Google Scholar]
- 105. Langerak T, Yang H, Baptista M, et al. : Zika Virus Infection and Guillain-Barré Syndrome in Three Patients from Suriname. Front Neurol. 2016;7:233. 10.3389/fneur.2016.00233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Skrove JL, Salhab J, Colella DM, et al. : Zika virus: A rare case with presenting symptoms of gastroenteritis and transaminitis with subsequent progression to Guillain-Barré syndrome. Am J Gastroenterol. 2016;111:S975–S6. 10.1038/ajg.2016.374 [DOI] [Google Scholar]
- 107. Septfons A, Leparc-Goffart I, Couturier E, et al. : Travel-associated and autochthonous Zika virus infection in mainland France, 1 January to 15 July 2016. Euro Surveill. 2016;21(32). 10.2807/1560-7917.ES.2016.21.32.30315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Walker WL, Lindsey NP, Lehman JA, et al. : Zika Virus Disease Cases - 50 States and the District of Columbia, January 1-July 31, 2016. MMWR Morb Mortal Wkly Rep. 2016;65(36):983–6. 10.15585/mmwr.mm6536e5 [DOI] [PubMed] [Google Scholar]
- 109. Ferreira da Silva IR, Frontera JA, Moreira do Nascimento OJ: News from the battlefront: Zika virus-associated Guillain-Barré syndrome in Brazil. Neurology. 2016;87(15):e180–e1. 10.1212/WNL.0000000000003024 [DOI] [PubMed] [Google Scholar]
- 110. Machado-Alba JE, Machado-Duque ME, Gaviria-Mendoza A, et al. : Diagnosis of neurological disorders and the Zika virus epidemic in Colombia 2014 -2016. Int J Infect Dis. 2016;51:133–4. 10.1016/j.ijid.2016.09.010 [DOI] [PubMed] [Google Scholar]
- 111. Dos Santos T, Rodriguez A, Almiron M, et al. : Zika Virus and the Guillain-Barré Syndrome - Case Series from Seven Countries. N Engl J Med. 2016;375(16):1598–601. 10.1056/NEJMc1609015 [DOI] [PubMed] [Google Scholar]
- 112. Siu R, Bukhari W, Todd A, et al. : Acute Zika infection with concurrent onset of Guillain-Barré Syndrome. Neurology. 2016;87(15):1623–4. 10.1212/WNL.0000000000003038 [DOI] [PubMed] [Google Scholar]
- 113. World Health Organization: WHO | Zika situation report 05-01-2017.2017. Reference Source [Google Scholar]
- 114. Lee CT, Vora NM, Bajwa W, et al. : Zika Virus Surveillance and Preparedness - New York City, 2015-2016. MMWR Morb Mortal Wkly Rep. 2016;65(24):629–35. 10.15585/mmwr.mm6524e3 [DOI] [PubMed] [Google Scholar]
- 115. Van Kerkhove MD, Reveiz L, Souza JP, et al. : Harmonisation of Zika virus research protocols to address key public health concerns. Lancet Glob Health. 2016;4(12):e911–e2. 10.1016/S2214-109X(16)30255-8 [DOI] [PubMed] [Google Scholar]
- 116. Wallace BC, Noel-Storr A, Marshall IJ, et al. : Identifying reports of randomized controlled trials (RCTs) via a hybrid machine learning and crowdsourcing approach. J Am Med Inform Assoc. 2017;24(6):1165–8. 10.1093/jamia/ocx053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Akl EA, Kahale LA, Hakoum MB, et al. : Parenteral anticoagulation in ambulatory patients with cancer. Cochrane Database Syst Rev. 2017;9:Cd006652. 10.1002/14651858.CD006652.pub5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Spurling GK, Del Mar CB, Dooley L, et al. : Delayed antibiotic prescriptions for respiratory infections. Cochrane Database Syst Rev. 2017;9:Cd004417. 10.1002/14651858.CD004417.pub5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Leis AA, Stokic DS: Zika Virus and Guillain-Barre Syndrome: Is There Sufficient Evidence for Causality? Front Neurol. 2016;7(1):170. 10.3389/fneur.2016.00170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Oehler E, Watrin L, Larre P, et al. : Zika virus infection complicated by Guillain-Barre syndrome--case report, French Polynesia, December 2013. Euro Surveill. 2014;19(9): pii: 20720. 10.2807/1560-7917.ES2014.19.9.20720 [DOI] [PubMed] [Google Scholar]
- 121. Rozé B, Najioullah F, Fergé JL, et al. : Zika virus detection in urine from patients with Guillain-Barré syndrome on Martinique, January 2016. Euro Surveill. 2016;21(9):30154. 10.2807/1560-7917.ES.2016.21.9.30154 [DOI] [PubMed] [Google Scholar]
- 122. Thomas DL, Sharp TM, Torres J, et al. : Local Transmission of Zika Virus--Puerto Rico, November 23, 2015-January 28, 2016. MMWR Morb Mortal Wkly Rep. 2016;65(6):154–8. 10.15585/mmwr.mm6506e2 [DOI] [PubMed] [Google Scholar]
- 123. Cao-Lormeau VM, Blake A, Mons S, et al. : Guillain-Barré Syndrome outbreak associated with Zika virus infection in French Polynesia: a case-control study. Lancet. 2016;387(10027):1531–9. 10.1016/S0140-6736(16)00562-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Morrison TE, Diamond MS: Animal Models of Zika Virus Infection, Pathogenesis, and Immunity. J Virol. 2017;91(8): pii: e00009-17. 10.1128/JVI.00009-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Akl EA, Meerpohl JJ, Elliott J, et al. : Living systematic reviews: 4. Living guideline recommendations. J Clin Epidemiol. 2017;91:47–53. 10.1016/j.jclinepi.2017.08.009 [DOI] [PubMed] [Google Scholar]