Abstract
In the cerebral cortex, GABAergic interneurons have evolved as a highly heterogeneous collection of cell types that are characterized by their unique spatial and temporal capabilities to influence neuronal circuits. Current estimates suggest that up to 50 different types of GABAergic neurons may populate the cerebral cortex, all derived from progenitor cells in the subpallium, the ventral aspect of the embryonic telencephalon. In this review, we provide an overview of the mechanisms underlying the generation of the distinct types of interneuron and their integration in cortical circuits. Interneuron diversity seems to emerge through the implementation of cell-intrinsic genetic programs in progenitor cells, which unfold over a protracted period of time until interneurons acquire mature characteristics. The developmental trajectory of interneurons is also modulated by activity-dependent, non-cell autonomous mechanisms that influence their ability to integrate in nascent circuits and sculpt their final distribution in the adult cerebral cortex.
Introduction
Thirty years is a long time in neuroscience research. At the time when the first issue of Neuron was published in 1988, we thought that excitatory and inhibitory neurons in the cerebral cortex originated from the same progenitor cells in the pallium (Rakic, 1988), the roof of the embryonic telencephalon. Almost ten years later, Anderson and colleagues provided the first direct evidence that, in fact, cortical γ-aminobutyric acid-containing (GABAergic) neurons are born in the same embryonic region of the telencephalon that generates the basal ganglia, the subpallium, from where they migrate tangentially to reach their final destination (Anderson et al., 1997a). Since then, our understanding of the development of cortical interneurons has expanded exponentially (Bartolini et al., 2013; Hu et al., 2017b; Marín and Rubenstein, 2001; Wonders and Anderson, 2006), notwithstanding the difficulties that continue to hamper our ability to classify the enormous diversity of cell types that fall under this umbrella (Ascoli et al., 2008; DeFelipe et al., 2013).
The development of cortical interneurons involves a series of crucial milestones over a protracted period (Figure 1). Interneurons are generated from progenitor cells in the embryonic subpallium. Shortly after becoming postmitotic, they undergo a long tangential migration and reach the pallium via several stereotyped streams. Interneurons continue to disperse throughout the developing cortex using the same migratory routes until they abandon them to adopt their final position within a region and layer of the cortex. Interneurons gradually acquire their biochemical markers during this process, although quite often they do not exhibit their characteristic morphology and connections until relatively late postnatal developmental stages. The long delay that exist between the time when interneurons are born and when they begin to display their mature features has led to very diverging views on the mechanisms controlling the generation of their diversity (Wamsley and Fishell, 2017), although a clearer picture is beginning to emerge from recent studies.
Figure 1. Milestones in the development of cortical interneurons.
(A) Timeline of the development of cortical interneurons in the mouse. The main events have been highlighted in corresponding temporal periods: neurogenesis, tangential migration, laminar allocation (which involves radial migration), wiring (dendritic and axonal morphogenesis and establishment of synapses), programmed cell death and circuit refinement. Interneuron identity is specified at neuronal birth, but it unfolds over a protracted period of time through which the final characteristics of each type of interneuron are acquired.
(B) The development of layer 2/3 SST+ Martinotti cells is used here as an example to illustrate the main developmental milestones in the generation of cortical interneurons in mice. At least a population of SST+ Martinotti cells is generated from progenitor cells in the dorsal aspect of the MGE. SST+ Martinotti cells preferentially migrate to the embryonic cortex through the marginal zone (MZ) stream. During radial migration into the cortical plate (CP), SST+ Martinotti cells leave their trailing neurite in the MZ, which will eventually develop into a characteristic axonal arborization in layer 1. By the end of the first postnatal week, about 30% of interneurons undergo program cell death, including SST+ Martinotti cells. This process depends on the integration of these cells into cortical circuits. The surviving SST+ Martinotti cells remodel their synaptic connections during the second and third week of postnatal development. For example, layer 2/3 SST+ Martinotti cells end up establishing preferential connections with the apical dendrites of pyramidal cells also located in layer 2/3. The yellow thunderbolt symbol indicates processes that depend on neuronal activity.
MGE, medial ganglionic eminence; NCx, neocortex; SVZ, subventricular zone; VZ, ventricular zone.
The purpose of this review is to summarize our current understanding of the mechanisms underlying the generation of interneuron diversity in the cerebral cortex, using the mouse as a model. The emphasis is on those aspects related to the emergence of interneuron diversity in the embryo, and how the dynamic unfolding of transcriptional programs specified early during development leads to the functional diversification of GABAergic neurons during the first few weeks of postnatal development in the cerebral cortex. We also review recent advances on our understanding of non-cell autonomous processes that shape the final configuration of GABAergic interneurons in the adult cerebral cortex. We have purposely centered our attention in the diversity of GABAergic neurons in the isocortex (more commonly known as neocortex), although it is likely that similar principles underlie the generation of interneuron diversity in allocortical (e.g., hippocampus) and mesocortical regions (e.g., entorhinal cortex) of the cerebral cortex.
Interneuron diversity in the neocortex
Modular organization of interneuron features
Cortical interneurons are a highly heterogeneous group of neurons with very diverse morphologies, connectivity, biochemistry and physiological properties. These features are acquired during development through the implementation of specific transcriptional programs that are either intrinsically encoded or driven by interactions with the local microenvironment. Remarkably, each of the main defining features of interneurons seem to exist in a finite number of options (Figure 2A). For example, in spite of the inherently stochastic and noisy nature of developmental processes (Hassan and Hiesinger, 2015), interneurons do not exist as a continuum of neuronal morphologies. Researchers may struggle to consistently identify some of the less characteristic interneuron morphologies (DeFelipe et al., 2013), but each type of interneuron systematically adopts a relatively consistent morphology. Similarly, the different types of interneurons establish stereotyped connections and express specific combinations of channels that endow them with unique electrophysiological properties (Ascoli et al., 2008). It is therefore conceivable that each defining set of features is acquired through dedicated transcriptional programs. For example, recent work has identified transcriptional signatures defining the synaptic communication patterns of different classes of cortical interneurons (Paul et al., 2017), and it is likely that parallel transcriptional programs underlie the acquisition of other defining properties. In this conceptual framework, interneuron diversity can be understood as the outcome of the combinatorial selection of different transcriptional modules during development (Figure 2B and 2C).
Figure 2. Modular organization of cortical interneuron features.
(A) Cortical interneurons are characterized by a combination of morphological, biochemical, intrinsic and connectivity properties. The most prominent morphological characteristics of cortical interneurons are the shape and orientation of dendrites and axons. Biochemical properties define important aspects of synaptic communication, including the co-release of neuropeptides along with GABA. Intrinsic electrophysiological properties are largely determined by the combination of ionic channels they express. Interneurons establish synapses on different subcellular compartments of their targets, which impact their role in neural circuits. (B) The combinatorial organization of cortical interneurons is illustrated for PV+ basket cells (green) and CCK+ basket cells (orange). Both cell types share similar morphology and connectivity principles, but they differ in their intrinsic and biochemical properties. (C) The schema depicts how each set of defining features might be acquired through a combination of partially shared transcriptional programs.
Transcriptional analysis of individual cells from different regions of the brain suggests that neuronal diversity is organized hierarchically, which has led to the suggestion that the relationships that exist among different neuronal types can be considered, in practical terms, analogous to those of species (Zeng and Sanes, 2017). From that perspective, it might be logical to consider the diversification of cortical interneurons as the result of the variation in the transcriptional programs that underlie their fundamental properties. Consistent with this idea, recent work has revealed that the reptilian cortex contains interneurons that exhibit transcriptional similarities with the major classes of cortical interneurons described mammals (Tosches et al., 2018), which suggest that at least these types of interneuron existed in the ancestor of reptiles and mammals. However, there is no evidence for further diversification among GABAergic interneurons in the reptilian cortex (Tosches et al., 2018), which indicates that the generation of new types of interneuron in mammals probably arise through the diversification of the ancestral generegulatory programs. This may explain why certain features are characteristic of certain classes of hierarchically related types of interneuron. For instance, fast-spiking firing properties are common to all cortical interneurons characterized by the expression of the calcium binding protein parvalbumin (PV), which otherwise diverge extensively in their morphology and synaptic connectivity (Tremblay et al., 2016).
A simplified account of the interneuron universe
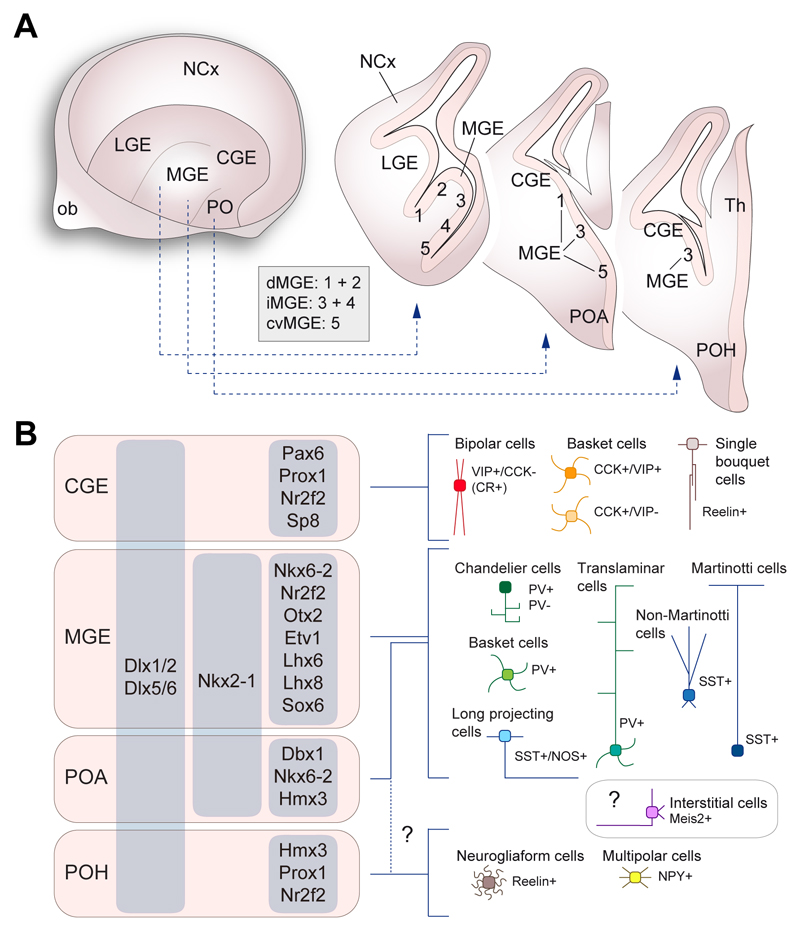
The development of methods to examine gene expression in single cells on a large scale have revolutionized our ability to classify neuronal cell diversity (Zeng and Sanes, 2017). Currently available transcriptomic data suggests that up to 50 distinct transcriptional signatures (final number may vary as sequencing depth and analytical methods improve) can be distinguished among inhibitory neurons in the cerebral cortex (Harris et al., 2018; Tasic et al., 2016; Tasic et al., 2017; Zeisel et al., 2015). However, the identification on inhibitory neurons in the cerebral cortex should be based in a multidimensional space of cellular phenotypes, which requires not only the unequivocal identification of the transcriptional landscape of a cell type, but also its morphological, neurochemical and electrophysiological properties, along with its synaptic partners (Ascoli et al., 2008; DeFelipe et al., 2013). In this review, we refer to them as interneuron types. Over 20 different types of inhibitory neuron have been described in the neocortex and hippocampus with that level of resolution (Klausberger and Somogyi, 2008; Tremblay et al., 2016), most of which probably represent equivalent cell types. Cortical interneurons can be organized into several major classes based on their transcriptional similarities and the expression of selective markers (Figure 3), and some of these classes can also be broken down into subclasses containing similar types of interneuron.
Figure 3. Diversity of GABAergic neurons in the neocortex.
(A) Schemas depicting the main classes of cortical interneuron in the mouse neocortex. Interneurons can be organized into three large classes based on the expression of PV, SST and the serotonin receptor 3A (Htr3a). A small fraction of PV+ basket cells also express SST.
(B) Schematic depicting the general laminar distribution of interneuron types in the neocortex. Some cell types are found in most layers of the cortex (e.g., PV+ basket cells), whereas others seem to have a much more restricted laminar distribution (e.g., Meis2+ cells).
(C) The schematic illustrates the approximate relative frequency of each type of interneuron, color-coded as in (A). It should be noted here are no direct estimates of the frequency of chandelier cells and many other classes of interneuron. In addition, the relative proportion of interneurons likely varies across different cortical areas.
cc, corpus callosum; wm, white matter.
The largest class of cortical interneuron is characterized by the expression of PV. Three major subclasses of PV+ interneurons can be distinguished, all of which exhibit characteristic fast-spiking firing properties: chandelier cells, basket cells and translaminar interneurons. Chandelier or axo-axonic cells have the most stereotypic morphology of all interneurons, due to the characteristic shape of their elaborated axonal arbor that resembles a chandelier light fixture. Chandelier cells forms synapses onto the axon initial segment of pyramidal cells (Somogyi et al., 1982). They are particularly abundant at the border between layers 1 and 2 and in layer 6 (Taniguchi et al., 2013) and their regional distribution is very heterogeneous (Inda et al., 2009). A substantial fraction of chandelier cells in the mouse does not contain detectable levels of PV (Taniguchi et al., 2013). PV+ basket cells are the most abundant type of interneuron in the neocortex. They have highly torturous axons that form synapses on the soma and proximal dendrites of pyramidal cells and other interneurons (Hu et al., 2014). They are distributed through layers 2 to 6 in the neocortex and across all cortical regions. Finally, PV+ translaminar interneurons constitute a relatively rare type of fast-spiking interneurons that is particularly abundant in layers 5 and 6 (Bortone et al., 2014; Buchanan et al., 2012). Fitted with axons that span the thickness of the cortex, these interneurons target pyramidal cells across several layers (Bortone et al., 2014).
A second class of interneurons is characterized by the expression of the neuropeptide somatostatin (SST) and the preferential dendritic targeting of their synapses (Figure 3). There are two major types of SST+ interneurons: Martinotti cells and non-Martinotti cells. SST+ Martinotti cells constitute a transcriptionally heterogeneous group of interneurons characterized by an ascending axon that arborizes profusely in layer 1 (Hilscher et al., 2017; Wang et al., 2004; Xu et al., 2006). Martinotti cells are particularly abundant in layer 5, but they are also common in layers 2 and 3. These cells represent approximately 60% of the SST+ neurons in these layers (Jiang et al., 2015; Nigro et al., 2018). Martinotti cells have relatively heterogenous firing patterns but they often mediate disynaptic inhibition (Silberberg and Markram, 2007). SST+ non-Martinotti cells are found throughout layers 2 to 6 and characteristically lack axons in layer 1. They exhibit lower resting membrane potential and higher firing frequency than Martinotti cells but lower than PV+ basket cells (Nigro et al., 2018; Xu et al., 2006). Non-Martinotti cells are particularly abundant in layer 4 (Ma et al., 2006), where they primarily target PV+ basket cells (Xu et al., 2013). In addition to SST+ interneurons, the neocortex contains a population of long-range GABAergic projection neuron that express SST. These cells are most abundant in deep layers and project to other regions of the neocortex. They have adapting and irregular spiking firing properties, frequently co-express nitric oxide synthase (NOS), chondrolectin (Chodl) and neuropeptide Y (NPY) (He et al., 2016), and active during sleep (Dittrich et al., 2012).
A third, very heterogeneous, class of cortical interneurons express the serotonin receptor 5HT3aR (Rudy et al., 2011) (Figure 3). Among them, interneurons expressing vasoactive intestinal peptide (VIP) are one of the largest subclasses. They are mainly disinhibitory because they preferentially target SST+ and PV+ interneurons (Jiang et al., 2015). The most characteristic VIP+ interneurons are bipolar cells with vertically oriented axons. They are highly enriched in layers 2 and 3, and often display continuous adapting firing properties (Pronneke et al., 2015) and co-express the calcium binding protein calretinin (CR).
Multipolar VIP+ cells are transcriptionally very different from bipolar interneurons. Indeed, they are basket cells that co-express the neuropeptide cholecystokinin (CCK) and so they are transcriptionally more similar to other CCK+ basket cells that lack the expression of VIP (He et al., 2016; Tasic et al., 2017). CCK+ basket cells that co-express VIP have a small soma and are particularly abundant in supragranular layers of the cortex, whereas CCK+ basket cells lacking VIP are enriched in layers 5 and 6 (He et al., 2016). Both types of CCK+ interneurons express 5HT3aR (Rudy et al., 2011). CCK+ basket cells make synapses on the soma of pyramidal cells and other interneurons and exhibit regular or burst spiking firing (Kawaguchi and Kubota, 1998).
Neurogliaform cells are the most abundant type of interneuron in layer 1, although they are also sparsely distributed through other layers. They have a very dense and characteristic axonal arbor and late spiking firing properties. Neurogliaform cells express 5HT3aR and very often co-express reelin and NPY (Lee et al., 2010). They have been proposed to mediate volumetric GABA transmission (Olah et al., 2007).
Single bouquet cells are also abundant in layer 1 and are transcriptionally similar to neurogliaform cells (Tasic et al., 2017), but in contrast to these they have axonal ramifications that extend to deep layers of the cortex (Jiang et al., 2015). Single bouquet cells express 5HT3aR and reelin. Two additional types of interneuron express 5HT3aR: NPY+ multipolar cells and Meis2+ interstitial GABAergic neurons. NPY+ multipolar interneurons exhibit irregular spiking properties and are particularly abundant at the boundary between layers 1 and 2 (Gelman et al., 2009; Miyoshi et al., 2010). Meis2+ neurons reside in the white matter and extend axons to deep layers of the cortex and striatum (Frazer et al., 2017; von Engelhardt et al., 2011).
Emergence of cortical interneuron diversity
The embryonic subpallium generates all GABAergic neurons in the telencephalon. GABAergic projection neurons are particularly abundant in the striatum, globus pallidus, central amygdala and septum, but long-range projection neurons such as the SST+/NOS+ neuron are also present in the neocortex (He et al., 2016; Tomioka et al., 2005) and other cortical regions (Jinno et al., 2007; Melzer et al., 2012). GABAergic interneurons are distributed through the amygdala, striatum and cerebral cortex. A regulatory network of genes encoding the transcription factors Dlx1, Dlx2, Ascl1, Gsx1 and Gsx2 is required for the specification of all GABAergic neurons in the subpallium (Long et al., 2009; Wang et al., 2013). The emergence of neuronal diversity from this region is linked to the spatial and temporal specification of progenitor cells by additional networks of transcription factors (Box 1), which restrict the potential of these cells to generate only certain classes of GABAergic neurons.
Box 1. Transcriptional regulation of cortical interneuron development.
Our understanding of the molecular mechanisms regulating cortical interneuron specification in the subpallium is still very limited, but details on the genetic cascade of transcription factors controlling the development of interneurons in the MGE have begun to emerge (Figure inset). Two largely parallel gene regulatory networks, with several points of intersection, regulate the development of SST+ and PV+ interneurons.
The DLX family of homeodomain transcription factors is at the core on the gene regulatory network that control general aspects in the development of GABAergic interneurons, not unique to MGE-derived cells. Dlx1 and Dlx2 are initially expressed by VZ progenitor cells, in which they promote the specification of GABAergic lineages by repressing Olig2, a transcription factor required for oligodendrocyte fate specification (Petryniak et al., 2007). As interneurons become postmitotic, Dlx1 and Dlx2 in promote neural differentiation towards a GABAergic fate by inducing the expression of Gad1 and Gad2, which encode the enzymes that convert glutamate to GABA (Le et al., 2017; Stühmer et al., 2002). Dlx1 and Dlx2 function is also required to induce Dlx5 and Dlx6 (Pla et al., 2017), which are in turn important for the migration and differentiation of cortical interneurons (Wang et al., 2010). DLX proteins coordinate the programs of migration and neuronal differentiation. During early interneuron development, Dlx1 and Dlx2 promote migration by restraining neurite growth through the repression of PAK3, a downstream effector of the Rho family of GTPases (Cobos et al., 2007), and by inducing the expression of factors that are essential for the tangential migration of cortical interneurons, such as Arx (Bonneau et al., 2002; Colasante et al., 2008; Kitamura et al., 2002). Subsequently, the same genes are critical for the integration of MGE-derived interneurons in developing cortex, as they generally promote the development of dendrites and axons in these cells (Pla et al., 2017). In the absence of these factors, interneurons struggle to integrate into nascent neural circuits and many of them undergo programed cell death during the critical window of postnatal development in which interneuron numbers are adjusted (see ‘Regulation on interneuron numbers’ for details). Interestingly, Dlx1 and Dlx2 are differentially expressed in postnatal MGE-derived interneurons. Early postmitotic cells that are fated to become PV+ interneurons express both Dlx1 and Dlx2, but postnatal PV+ interneurons only maintain the expression of Dlx2 (Pla et al., 2017). In contrast, SST+ interneurons rely primarily on the function of Dlx1 (Cobos et al., 2005). Consequently, loss of Dlx1 only perturb the survival of SST+ interneurons (Cobos et al., 2005), whereas loss of both Dlx1 and Dlx2 increase cell death in both SST+ and PV+ interneurons (Pla et al., 2017).
The second gene regulatory network is commanded by transcription factor Nkx2-1, which is essential for the specification of SST+ and PV+ interneurons in the MGE (Sussel et al., 1999). Nkx2-1 is required for the initiation of Lhx6 expression (Sandberg et al., 2016; Sussel et al., 1999), a LIM-homeodomain transcription factor critical for the maturation cortical GABAergic interneurons derived from the MGE (Liodis et al., 2007; Vogt et al., 2014). Combinatorial binding of Nkx2-1 and Lhx6 promotes a transcriptionally permissive chromatin state and activates genes required for the migration of interneurons. Downregulation of Nkx2-1 is subsequently required for the migration of MGE-derived interneurons toward the cortex (Nóbrega-Pereira et al., 2008). Nkx2-1 is directly repressed by Zfhx1b and Sp9, two transcription factors that are induced by Dlx1 and Dlx2 and whose expression is maintained in migrating cortical interneurons (Liu et al., 2018; Long et al., 2009; McKinsey et al., 2013). In addition, Sp9 contributes to maintain the expression of Lhx6 independently of Nkx2-1 (Liu et al., 2018), which is no longer required to activate Lhx6 after its initial onset (Sandberg et al., 2016). Lhx6 and Sp9 promote the expression of multiple genes that are subsequently required in the migration and maturation of cortical interneurons, including Arx, Cxcr7, Maf, Mafb, Satb1, Sox6 and St18 (Azim et al., 2009; Batista-Brito et al., 2009; Close et al., 2012; Denaxa et al., 2012; Liu et al., 2018; Sandberg et al., 2016; Vogt et al., 2014).
Spatial specification of interneuron progenitor cells
The subpallium of rodents and primates, including humans, can be subdivided into several progenitor domains each characterized by the expression of a specific combination of transcription factors (Flames et al., 2007; Hansen et al., 2013; Ma et al., 2013). These comprise the lateral, medial and caudal ganglionic eminences (LGE, MGE and CGE, respectively), the preoptic region, which includes the preoptic area (POA) and the adjacent preoptic-hypothalamic (POH) border domain, and the septum (Figure 4A). Cortical interneurons seem to derive primarily from three of these progenitor regions: MGE, CGE and preoptic (Gelman and Marín, 2010).
Figure 4. Developmental origin of cortical interneurons.
(A) Regional organization of the embryonic subpallium. The schematic on the left depicts a telencephalic hemisphere viewed from the midline and from which medial structures have been removed. The drawings on the right illustrate the organization of the subpallium in three coronal sections through the telencephalon. The medial ganglionic eminence (MGE) consists of several progenitor domains (numbered 1 to 5) as described by Flames and colleagues (2007) and can be broadly subdivided in dorsal (dMGE), intermediate (iMGE) and caudoventral (cvMGE) regions.
(B) Regional origin of the main types of cortical interneurons. The preoptic region (PO) comprise two progenitor domains, the preoptic area (POA) and the preoptic-hypothalamic (POH) border domain. The precise origin of neurogliaform and multipolar cells within these domains remain uncertain. The origin of interstitial Meis2+ cells also remains unclear.
CGE, caudal ganglionic eminence; LGE, lateral ganglionic eminence; NCx, neocortex, PO, preoptic region.
The regional identity of the MGE and POA is specified by the homeobox transcription factor Nkx2-1, which is induced in the ventral subpallium prior to the onset of neurogenesis in this region. Expression of Nkx2-1 confers progenitor cells in the MGE and POA with unique properties that distinguish them from the adjacent LGE, CGE and POH regions. Consistently, MGE/POA progenitor cells take on properties of the LGE and CGE in mice lacking Nkx2-1 (Butt et al., 2008; Sussel et al., 1999). The LGE and CGE share many molecular markers, but the specific expression of transcription factors such as Nr2f2 bestow CGE progenitors with the ability to generate interneurons that migrate to the cerebral cortex (Kanatani et al., 2008; Tripodi et al., 2004). The POH, the most caudal progenitor domain in the preoptic region, lacks expression of Nkx2-1 and exhibit molecular similarities with the CGE (Flames et al., 2007). Thus, the preoptic region consists of two adjacent progenitor domains – POA and POH – that exhibit molecular similarities with the MGE and CGE, respectively.
Multiple lines of evidence indicate that the MGE, CGE and preoptic region generate different types of cortical interneuron (Figure 4B). The MGE and adjacent POA generate virtually all interneurons in the SST+ and PV+ classes (Gelman et al., 2011; Lavdas et al., 1999; Wichterle et al., 2001; Xu et al., 2004; Xu et al., 2008), whereas the CGE produces all VIP+ and CCK+ cortical interneurons along with several other less abundant types of interneurons such as single bouquet cells (Nery et al., 2002)}(Butt et al., 2005; Niquille et al., 2018; Xu et al., 2004). Neurogliaform cells and multipolar NPY+ interneurons are born in the preoptic region (Gelman et al., 2009; Niquille et al., 2018). Most of these cells do not derive from Nkx2-1-expressing progenitor cells (Niquille et al., 2018; Torigoe et al., 2016), and so their most likely origin is the POH domain within the preoptic region. The small contingent of neurogliaform cells that derive from Nkx2-1+ progenitor cells, which is particularly abundant in the hippocampus (Tricoire et al., 2010), is probably generated within the adjacent POA (Niquille et al., 2018). These observations suggest that the potential neuronal fate of the progeny of progenitor cells in the subpallium is restricted by the expression of specific molecular programs. In other words, progenitor cell diversity is a major determinant of interneuron diversity.
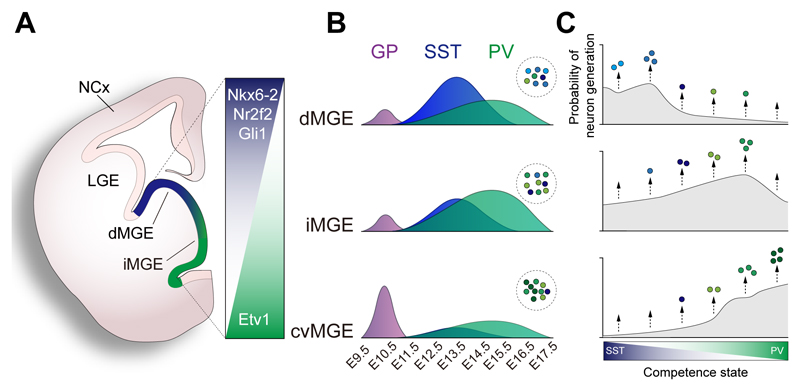
It remains to be clarified how interneuron diversity is generated within each of the main interneuron-producing regions of the subpallium, especially in the MGE and CGE. For example, the MGE is the origin of several types of cortical GABAergic neurons, including SST+ Martinotti cells, SST+ non-Martinotti cells, SST+ long-range neurons, PV+ basket cells, PV+ translaminar interneurons and chandelier cells. One possibility is that each of these regions contains several progenitor pools, each specialized in generating certain types of interneuron. In agreement with this idea, recent unbiased single-cell RNA sequencing (scRNA-seq) analyses that have identified heterogenous pools of progenitor cells in the MGE and CGE (Mi et al., 2018). The ventricular zone (VZ) of the MGE, CGE and preoptic region can be subdivided into multiple domains of progenitor cells that are uniquely defined by the combinatorial expression of several transcription factors (Flames et al., 2007). For instance, the MGE can be further subdivided into dorsal, intermediate and ventrocaudal domains (Figure 5A), although progenitor pools in the subpallium do not seem to segregate as sharply as in the spinal cord (Jessell, 2000). The dorsal MGE is enriched in progenitor cells that express the homeodomain transcription factor Nkx6-2, whereas progenitor cells expressing the ETS transcription factor Etv1 are most abundant in the intermediate and ventrocaudal domains. Consistent with the relative spatial segregation of these progenitor pools, fate mapping and transplantation experiments have shown that the dorsal MGE has a strong bias toward the generation of SST+ interneurons, whereas the ventrocaudal domains produce a larger fraction of PV+ neurons (Flames et al., 2007; Fogarty et al., 2007; He et al., 2016; Inan et al., 2012; Wonders et al., 2008). The intermediate MGE produces both PV+ and SST+ interneurons with a rostral (PV+) to caudal (SST+) bias (Hu et al., 2017a; Inan et al., 2012). Interestingly, the generation of cortical interneurons is largely limited to the dorsal and intermediate domains of the MGE, whereas the ventrocaudal domain specializes in the production of PV+ projection neurons for the globus pallidus (Flandin et al., 2010; Nóbrega-Pereira et al., 2010). These observations emphasize the relevance of the spatial organization of progenitor cells in the generation of cortical interneurons.
Figure 5. Spatiotemporal patterning of interneuron neurogenesis.
(A) Schematic representation of a coronal section through the anterior aspect of the medial ganglionic eminence (MGE). Several transcription factors contribute to the spatial patterning of MGE into different domains: dorsal (dMGE), intermediate (iMGE) and caudoventral (cvMGE).
(B) Spatio-temporal specification of cortical interneurons. Progenitor pools within the MGE generate different numbers of neurons following a temporal sequence. All domains generate an early cohort of projection neurons for the globus pallidus (GP), followed by SST+ and PV+ interneurons in different ratios. The area under each curve represents relative numbers of neurons arising from each MGE domain.
(C) An integrated model of interneuron generation. In this model, MGE progenitors undergo progressive intrinsic changes in their competence to generate different types of neuron during development. For any MGE progenitor, the generation of more or less neurons of a specific class is determined by the probability to generate cells during a given competence state. Color gradients represent progression of a single progenitor cells across different competency states, while the curves represent the probability of cell generation in each state. Progenitors in each MGE region would have different probabilistic rules, which would lead to the differences in the relative number of interneurons of each type generated in each region.
Temporal specification of interneuron progenitor cells
Each progenitor domain in the MGE generates multiple types of cortical interneurons (Flames et al., 2007; Fogarty et al., 2007; He et al., 2016; Inan et al., 2012; Wonders et al., 2008), and this is not just due to the existence of a heterogenous pool of progenitor cells within each domain. Indeed, clonal studies have shown that individual progenitor cells in the MGE generate both SST+ and PV+ interneurons (Brown et al., 2011; Ciceri et al., 2013; Harwell et al., 2015; Mayer et al., 2015). Consistently, genetic fate mapping studies indicate that seemingly homogenous pools of progenitor cells generate distinct types of interneurons at different developmental stages. For example, Nkx6-2+ progenitor cells produce several types of SST+ neurons along with some PV+ basket cells and chandelier cells, and a mixed progeny of interneurons from the SST+ and PV+ classes has also been described for Etv1+ progenitor cells (He et al., 2016). These observations suggest that most (perhaps all) progenitor cells in the VZ of the MGE have the potential to generate distinct types of interneuron – within the limits imposed by the molecular specification of this region –, implying a temporal control of interneuron cell fate that is still poorly understood (Figure 5B).
Temporally-restricted fate mapping experiments suggests that MGE progenitors generate most SST+ interneurons during the first half of the neurogenic period, whereas PV+ interneurons are produced at an almost constant rate throughout neurogenesis (Inan et al., 2012; Miyoshi et al., 2007). This is consistent with the observation that MGE-derived interneurons adopt their laminar distribution following an ‘inside-out’ sequence that correlates with their birthdate, similar to pyramidal cells (Fairén et al., 1986; Miller, 1985; Pla et al., 2006; Rymar and Sadikot, 2007; Valcanis and Tan, 2003). Accordingly, SST+ interneurons are more abundant in infragranular than in supragranular layers of the neocortex, while PV+ basket cells – which constitute the majority of PV+ interneurons – are found throughout all layers with the exception of layer 1. Interestingly, chandelier cells are most frequent in layers 2 and 6 of neocortex although they are preferentially generated toward the end of neurogenesis (Inan et al., 2012; Taniguchi et al., 2013). This indicates that the laminar allocation of cortical interneurons is not entirely dictated by the time of neurogenesis.
There are several additional aspects related to the temporal segregation of progenitor cells in the MGE that are worth considering. First, not all MGE lineages generate cortical interneurons simultaneously. For instance, VZ progenitor cells in the dorsal MGE are already generating supragranular interneurons at E13.5, while at the same stage lineages in the intermediate MGE are producing infragranular layer interneurons (He et al., 2016). Second, individual MGE lineages seem to be biased toward generating infra- or supragranular interneurons. Indeed, genetic labelling of VZ progenitor cells at early stages of neurogenesis identifies many more interneurons in infra- than supragranular layers of the neocortex (Ciceri et al., 2013). This would suggest that lineages might get exhausted after generating a certain number of cortical interneurons, whose laminar distribution would depend on their time of neurogenesis.
An integrated model of interneuron generation
Our current understanding of neurogenesis in the MGE suggests that multipotent progenitor cells in the VZ of this region are able to generate different types of SST+ and PV+ neurons in a temporal sequence. Consequently, an integrated model of interneuron generation should consider both the spatial segregation of progenitor cells and the temporal control of interneuron cell fates. One such a model is based on the idea that progenitor cells in the MGE would undergo progressive intrinsic changes in their competence to produce interneuron cell fates following a defined sequence (Figure 5C), similar to the generation of neuronal diversity in Drosophila (Doe, 2017). In this model, VZ progenitor cells in the MGE would divide to generate intermediate progenitor cells which would be committed to specific cell fates, with globus pallidus projection neurons generated before interneurons and, within the latter group, SST+ cell fates generated earlier than PV+ cell fates. The molecular specification of the VZ would endow progenitor cells with a certain probability to generate more or less neurons during each of the sequential competence states, therefore biasing their output toward the generation of pallidal neurons, PV+ and SST+ interneurons. This is consistent with the observation that molecularly distinct progenitor cells generate different proportions of the same types of interneuron.
Since VZ progenitor cells in the ganglionic eminences are multipotent, the proposed model implies that neuronal specification occurs primarily at the level of intermediate progenitor cells for all neurons generated in the MGE and CGE. This hypothesis is consistent with the observation that intermediate progenitor cells in the MGE and CGE are transcriptionally heterogeneous (Mi et al., 2018; Petryniak et al., 2007), but it contrasts with previous work suggesting that SST+ interneurons are preferentially generated from VZ progenitors through direct neurogenesis (Petros et al., 2015). Work in the LGE indicates that direct neurogenesis might be particularly important at early stages of neurogenesis (Anderson et al., 1997b; Kelly et al., 2018; Sheth and Bhide, 1997), but it is relatively rare after E12.5, when rapid proliferative divisions expanding the pool of intermediate progenitor cells are most characteristic (Pilz et al., 2013). It is presently unclear whether the VZ progenitor cell population previously identified to preferentially generate SST+ interneurons (short neural precursors) corresponds to one of the sequential competence states of VZ progenitor cells in the MGE.
A number of predictions can be made with the proposed model. First and foremost, MGE progenitors should express a cascade transcription factors controlling the timing of the progression from one competence state to the next. These factors should be expressed transiently and influence neuronal identity, as shown for Drosophila neuroblasts (Doe, 2017). Although additional work is required to identify the transcriptional network regulating temporal specification in the MGE, some of these factors have already been identified. For instance, genetic experiments have shown that Nr2f1 and Nr2f2 (Coup-TFI and Coup-TFII, respectively) have similar functions to their Drosophila homolog seven up (svp), which controls temporal specification in several neuroblast lineages (Mlodzik et al., 1990). Loss of Nr2f1 and Nr2f2 in the MGE leads to an abnormal increase in the number of PV+ projection neurons and interneurons at the expense of SST+ neurons, which suggests that these transcription factors normally promote SST+ interneuron cell fates within the temporal sequence of these lineages (Hu et al., 2017a). Interestingly, Nr2f1 and Nr2f2 influence neuronal specification by regulating cell cycle dynamics (Naka et al., 2008), which may explain why factors involved in the regulation of the cell cycle have also been involved in the regulation of interneuron diversity (Glickstein et al., 2007; Petros et al., 2015).
A second prediction from the proposed model is that the generation of neuronal diversity should follow similar temporal sequences of specific cell fates in individual MGE lineages. This sequence would probably involve the generation of projection neurons (e.g., PV+ globus pallidus neurons) followed by interneurons, with several SST+ and PV+ interneuron cell fates in a defined temporal sequence. Although recent clonal analyses have explored the origin of neuronal diversity in the MGE (Brown et al., 2011; Ciceri et al., 2013; Harwell et al., 2015; Mayer et al., 2015), additional work would be required with enhanced temporal resolution to verify this prediction.
Our current understanding of the generation of interneuron diversity in the CGE is more limited than in the MGE, but it is likely that the proposed model applies to progenitor cells in both eminences. Consistently, a temporal sequence has also been described for the generation of neuronal diversity in the CGE (Miyoshi et al., 2010). In sum, the generation of interneuron diversity in the embryonic subpallium seems to involve two patterning mechanisms: the spatial patterning of progenitor identities, which distinguishes VZ progenitor cells in the MGE, CGE, POA and POH, and the temporal patterning of progenitor lineages – at least in the MGE and CGE – to further expand neural diversity.
Timing of interneuron cell specification
Interneurons undergo a protracted period of migration before they reach their final position within the cortex (Bartolini et al., 2013; Marín and Rubenstein, 2001), and it is only at this point that the different types of cortical interneuron become distinguishable from each other. This had led to the suggestion that the fate of interneurons is only restricted into a general class as they become postmitotic (e.g., SST+ neurons), acquiring their definitive identity (e.g., SST+ Martinotti cell) at postnatal stages through activity-dependent interactions with the cortical microenvironment (Kepecs and Fishell, 2014; Wamsley and Fishell, 2017). In other words, interneurons would remain relatively ‘plastic’ to adopt a particular cell fate (within a general class) during migration, as classically proposed for neural crest cells (Harris and Erickson, 2007). Several lines of evidence suggest that this might not be the case for cortical interneurons. First, a recent scRNA-seq study has shown that newborn interneurons are transcriptionally heterogeneous within a few hours of becoming postmitotic in the MGE and CGE (Mi et al., 2018). Some of these early transcriptional signatures seem to correlate with those found in specific types of interneuron in the mature cortex, which would indicate that cell specification in the ganglionic eminences transcend interneuron classes already at early stages. These results, however, should be considered with prudence, since a contemporaneous study based on a different scRNA-seq approach found no evidence for early embryonic specification of interneurons into specific cell types (Mayer et al., 2018). Second, it has been recently shown that different types of interneuron use different migratory streams to reach the cortex (Lim et al., 2018). In the case of SST+ interneurons, SST+ Martinotti cells and SST+ non-Martinotti cells use different migratory routes to disperse through the cortex (Figure 1B). This suggests that cortical interneurons are already fated to become Martinotti or non-Martinotti cells in the embryonic subpallium prior to entering the cortex, and that the choice of a particular migratory route is part of the intrinsic program controlling the development of interneurons. An alternative interpretation of these results would be that SST+ interneurons become Martinotti cells because they interact with the specific microenvironment of their migratory route, and not because they were specified prior to arriving to the cortex. However, experimentally reducing the number of SST+ neurons migrating through their normal route does not affect the ratio between Martinotti and non-Martinotti cells in the mature cortex (although it affects specific features of these cells), which suggests that the specification of at least these two types of SST+ interneuron is independent of – and prior to – the selection of a migratory route (Lim et al., 2018). In sum, cell type-specific genetic programs seem to be established by the time interneurons become postmitotic, or at least by the time they reach the developing cortex.
It is presently unclear whether all defining features of cortical interneuron are specified during embryonic development. For instance, are layer 2/3 and layer 5 basket cells specified by the same genetic program? What about Martinotti cells in both of these layers? It is conceivable that the same transcriptional program specifies PV+ basket cells or SST+ Martinotti cells, independently of their final laminar allocation. Subsequently, the integration of these cells in specific layers of the neocortex would expose them to different influences, which would shape their final features, through activity-dependent mechanisms (Wamsley and Fishell, 2017).
Migration and integration into cortical circuits
Tangential migration and regional distribution
Interneurons from the MGE, CGE and preoptic region use essentially the same cellular mechanisms to translocate to the cortex (Marín et al., 2006; Martini et al., 2009; Yanagida et al., 2012), and it is therefore perhaps not surprising that they exhibit great transcriptional similarity at this stage (Mayer et al., 2018). Irrespectively of their identity, cortical interneurons avoid the striatum and other regions of the subpallium in their way towards the cortex. In the case of MGE-derived interneurons, the molecular mechanisms controlling this process have been identified. Interneurons migrating to the cortex express receptors for chemorepulsive factors of the semaphorin family such as Sema3A and Sema3F, which are very abundant in the striatum (Marín et al., 2001). Interestingly, the expression of these receptors (Nrp1 and Nrp2) is repressed by Nkx2-1, and so the expression (Nkx2-1ON) or downregulation (Nkx2-1OFF) of this transcription factor in postmitotic interneurons born in the MGE determine whether they are targeted to the striatum or the cerebral cortex, respectively (Nóbrega-Pereira et al., 2008). Repression of Nkx2-1 in cortical interneurons derived from the MGE requires the function of the zinc-finger homeobox transcription factor Zfhx1b (McKinsey et al., 2013; van den Berghe et al., 2013).
Interneurons migrate toward the cortex following a gradient of increasing permissivity generated by chemoattractive cues produced in the developing LGE and pallium (Marín et al., 2003; Wichterle et al., 2003). One of such chemoattractive factors is Neuregulin1 (Nrg1), a member of a family of four structurally related proteins that are part of the epidermal growth factor superfamily (Mei and Nave, 2014). The function of Nrg1 is mediated by the tyrosine kinase receptor ErbB4, the main neuregulin receptor expressed by interneurons (Flames et al., 2004; Yau et al., 2003). In the absence of ErbB4, many interneurons fail to reach the cerebral cortex (Flames et al., 2004). Although the expression of ErbB4 is confined to specific types of interneuron in the adult cortex (Fazzari et al., 2010; Neddens and Buonanno, 2010; Vullhorst et al., 2009), ErbB4 is expressed in the majority of cortical interneurons at perinatal stages (Bean et al., 2014).
Interneurons disperse throughout the developing cortex via two large migratory streams, a superficial route that courses through the marginal zone (MZ), and a deep route that largely overlaps with the pallial subventricular zone (SVZ) (Lavdas et al., 1999; Wichterle et al., 2001). A smaller fraction of interneurons migrates through the subplate. During this phase of their development interneurons colonize homogenously the entire cerebral cortex, including the hippocampus, piriform cortex and the neocortex (Marín and Rubenstein, 2001). This process requires that tangentially migrating interneurons actively avoid migrating into the cortical plate, where they will eventually settle. The confinement of interneurons to each of the three routes of tangential migration involves the chemokine Cxcl12, which is expressed by cells located in each of the routes (Li et al., 2008; López-Bendito et al., 2008; Sánchez-Alcaniz et al., 2011; Stumm et al., 2007; Tiveron et al., 2006; Wang et al., 2011).
Several lines of evidence suggest that interneurons do not distribute randomly across the MZ and SVZ streams. For example, the transcriptional profile of interneurons migrating through the MZ and SVZ is not equivalent (Antypa et al., 2011), as it would be expected for a random distribution of interneurons in both routes. In addition, it has been suggested that interneurons generated in the preoptic region – most notably neurogliaform cells (Niquille et al., 2018) – preferentially reach the cortex through the MZ (Zimmer et al., 2011). Recent work has added further support to this idea by showing that SST+ Martinotti and PV+ translaminar interneurons migrate preferentially through the MZ, while non-Martinotti cells disperse via the SVZ (Lim et al., 2018). These findings suggest that at least some components of the transcriptional module controlling the migration of cortical interneurons are not universal, although they might be shared by certain types (e.g., SST+ Martinotti cells and PV+ translaminar interneurons). Interestingly, the migration of SST+ Martinotti cells through the MZ seems to be part of a wider developmental program that controls the development of their axonal arborization in layer 1. SST+ Martinotti cells begin to develop their profuse layer 1 axons at the time they migrate into the cortical plate; preventing the migration of these cells through the MZ perturbs the growth of their axons in layer 1 without apparently affecting any other aspect of their identity (Lim et al., 2018). This indicates that the development of layer 1 axons by SST+ Martinotti cells is facilitated by their interactions with components of the MZ at early neonatal stages, which demonstrates that the cortical microenvironment is crucial for the acquisition of specific features in specific types of interneuron. The fact that not all interneurons migrating through the MZ acquire the morphology of SST+ Martinotti cells reinforces the idea that these features are intrinsically-encoded, although they require the correct local environment – in this case, the MZ – to unfold appropriately.
Increasing evidence indicates that the same types of interneuron are found in neocortical areas (Tasic et al., 2017). Indeed, lineage analysis studies have shown that the same progenitor cell can generate interneurons populating different regions of the telencephalon (Harwell et al., 2015; Mayer et al., 2015). These studies seem to contradict earlier work, which concluded that clonally-related interneurons have a strong tendency to cluster in the mouse neocortex (Brown et al., 2011; Ciceri et al., 2013). These apparently conflicting conclusions might nevertheless be compatible, because the methods used for the detection of clonally-related interneuron clusters did not allow the identification of entire lineages. Consequently, clusters of interneurons found across different regions of the neocortex might derive from the same progenitor cell, with each cluster representing the outcome of one or several intermediate progenitor cells dividing concurrently in the subpallium.
Laminar allocation
The mechanisms that triggers the tangential-to-radial switch in the migration of cortical interneurons and mediates their invasion of the developing cortical plate are likely linked to a loss of responsiveness to Cxcl12 (Li et al., 2008). In addition, recent work suggests that pyramidal cells in the cortical plate express a different member of the neuregulin family, Nrg3, and this molecule facilitates the dispersion of cortical interneurons in the laminar dimension of the cortex (Bartolini et al., 2017). The subsequent sorting of interneurons into specific layers occurs at later stages and depends on largely unidentified factors.
Several lines of evidence suggest that the interneurons adopt their laminar distribution in response to cues provided by pyramidal cells. First, interneurons systematically acquire their laminar position after the corresponding pyramidal cells have begun to differentiate into a particular cortical layer (Hevner et al., 2004; Miyoshi and Fishell, 2011; Pla et al., 2006). Second, disruption of the normal layering of pyramidal cells modifies the normal laminar distribution of interneurons (Lodato et al., 2011; Pla et al., 2006). Importantly, interneurons seem to interact with specific classes of pyramidal cells when acquiring their laminar position (Lodato et al., 2011), which suggests that this interaction may actually predate the formation of specific connections between excitatory and inhibitory neurons in the developing cerebral cortex, at least for certain classes on interneurons (Ye et al., 2015).
Neuronal activity is important for the normal laminar distribution of cortical interneurons. The termination of cortical interneuron migration is linked to the upregulation of the potassium/chloride exchanger KCC2 in interneurons, which halts the motility of interneurons by reducing the frequency of spontaneous intracellular calcium transients in response to GABA (Bortone and Polleux, 2009). Consistently, reducing the neuronal excitability of interneurons during the first week of postnatal development is sufficient to modify their laminar distribution (De Marco García et al., 2011). Upregulation of KCC2 seems to be part of an intrinsic program of maturation that might be common to all classes of interneurons (Inamura et al., 2012). However, the precise laminar distribution of each type of interneuron likely emerge from the interaction of their normal program of maturation with specific signals provided by distinct classes of pyramidal cells.
Integration in transient circuits
Recent work has shown that the integration of interneurons in cortical microcircuits shapes the maturation of the columnar organization of the neocortex. While the connectivity motifs of some types of interneuron remain relatively constant in the juvenile and mature brain, others are recruited during early postnatal development into transient microcircuits that modulate the emergence of functional topography. For example, transient networks involving specific types of interneuron have been described in deep and superficial layers of the barrel cortex, a region of the somatosensory cortex that contains a topographic representation of the whiskers (Petersen, 2007). As discussed earlier, this illustrates how some the most critical features of a particular type of interneuron – for example, its connectivity – evolve during development and consequently can only be used as definitory except in a particular temporal context.
Layer 4 receives the vast majority of thalamocortical input, and so it plays a prominent role in the initial processing of sensory information (Douglas and Martin, 2004). Feedforward inhibition is critical in this process, as it sharpens receptive fields and regulates temporal integration and timing (Gabernet et al., 2005; Wehr and Zador, 2003). In the mature brain, feedforward inhibition in layer 4 is provided by PV+ basket cells. However, the connectivity between these interneurons and excitatory neurons in layer 4 remains relatively weak until the second postnatal week, when PV+ basket cells begin receiving direct inputs from thalamic neurons (Chittajallu and Isaac, 2010; Daw et al., 2007). Interestingly, layer 5 SST+ interneurons receive dense transient thalamic inputs during the first week of postnatal development and innervate both layer 4 excitatory neurons and layer 5 pyramidal cells and immature PV+ interneurons (Marques-Smith et al., 2016; Tuncdemir et al., 2016). This suggests that layer 5 SST+ interneurons transiently control early sensory-evoked activity in layer 4 prior to the emergence of the canonical feedforward inhibitory circuit mediated by PV+ interneurons (Marques-Smith et al., 2016). In addition, the transient connections established by early-born SST+ interneurons are also required for the maturation of PV+ basket cells in deep layers (Tuncdemir et al., 2016). SST+ interneurons therefore emerge as a critical population in the maturation of deep layer intracortical circuits during early postnatal development.
The connectivity of superficial layers of the neocortex is also transiently shaped by interneurons during early postnatal development. The organization of the barrel cortex relies on the segregation of layer 4 to layer 2/3 excitatory connections in distinct barrel and septal circuits. Specifically, layer 2/3 neurons above barrels receive stronger inputs than those immediately above the septa separating the barrels (Shepherd et al., 2003). Columnar refinement begins at the end of the first postnatal week and requires thalamocortical inputs. In sensory deprived mice, layer 2/3 neurons receive stronger inputs from layer 4 neurons in septal regions altered, which suggests that the normal maturation of the columnar organization requires competitive interactions among layer 4 neurons (Shepherd et al., 2003). While experience-dependent plasticity at excitatory synapses is known to contribute to the maturation of neuronal circuits in superficial layers of the cortex (Bender et al., 2006), recent work suggests that a specific population of interneurons is also involved in this process. During the first week of postnatal development, the activity of layer 1 5HT3aR+ interneurons (mostly neurogliaform cells) in the barrel field is highly synchronized by inputs from the somatosensory thalamus (ventroposteromedial thalamic nucleus, VPM), a process that requires NMDAR function (Che et al., 2018). The activity of neurogliaform cells during this period inhibits the response of layer 2/3 pyramidal cells during whisker stimulation. Inhibition is likely more prominent on weakly activated layer 2/3 pyramidal cells immediately above septal regions than in layer 2/3 pyramidal cells above the barrels (where VPM axons concentrate), which would contribute to the formation of barrel columns tuned to individual whiskers (Che et al., 2018). Intriguingly, the activation of layer 1 neurogliaform cells by thalamic input is transient, much like the temporary recruitment of deep layer SST+ interneurons (Che et al., 2018). Consequently, the transient recruitment of specific populations of interneurons by thalamic axons seems to be critical for the maturation of cortical microcircuits during early postnatal development.
Regulation of cortical interneuron numbers
Similar to other neurons in the nervous system, a surplus of GABAergic interneurons is generated during development. In mice, more than 30% of the interneurons generated embryonically in the subpallium are eliminated through programmed cell death between the end of the first and the second postnatal week (Southwell et al., 2012). This process seems to impact all classes of interneurons (Denaxa et al., 2018; Priya et al., 2018; Wong et al., 2018) (Figure 6A). While much emphasis has been made on the mechanisms underlying the generation of cortical interneuron diversity, programmed cell death – particularly if it affects interneurons in different ways across regions and layers – may have very important implications for our understanding of the very diverse patterns of distribution adopted by interneurons in the cerebral cortex.
Figure 6. Activity dependent regulation of interneuron assembly.
(A) Postnatal maturation and assembly of interneurons into cortical circuits. During the first postnatal week, cortical interneurons invade the cortical plate and begin to interact with surrounding pyramidal neurons as well as other local interneurons and thalamic afferents. At early postnatal stages (P0 to P5) these interactions are required for the correct morphological maturation of most types of interneuron. Following that period, about a third of cortical interneurons undergo programmed cell death. Activity-dependent mechanisms are critical for the survival of the remaining cortical interneurons.
(B) Activity dependent regulation of interneuron numbers. Cortical interneurons are programmed to die in the absence of external signals, most of which are likely provided by pyramidal cells. Consistently, reduction of interneuron or pyramidal cell activity decreases interneuron survival, while increase in the number or activity of excitatory neurons promotes the survival of a larger fraction of interneurons.
Activity-dependent regulation of interneuron programmed cell death
Experiments in vitro suggest that cortical interneurons are intrinsically programmed to die in the absence of external influences (Xu et al., 2004), and that they undergo apoptosis when they reach a predetermined cellular age – roughly 12 days after being born in the subpallium (Southwell et al., 2012). This is consistent with the observation that interneuron cell death occurs progressively in vivo, with early-born infragranular layer interneurons disappearing before late-born supragranular layer interneurons (Wong et al., 2018). Consequently, interneurons seem to be programmed to die when reaching a certain stage of maturation. The intriguing question is why then not all interneurons die during normal development. Multiple lines of evidence suggest that the process of programmed cell death is linked to the integration of interneurons into nascent cortical circuits. First, long-term Ca2+ imaging experiments in vivo indicate that activity levels at the onset of the cell death period predict the fate of interneurons (Wong et al., 2018). In other words, interneurons with relatively low levels of activity immediately before the period of interneuron cell death have an increased probability of undergoing apoptosis. Second, cell-autonomous manipulation of the excitability of cortical interneurons influence their survival. For example, increasing the excitability of interneurons expressing depolarizing designer receptors exclusively activated by designer drugs (DREADDs) or the bacterial voltage-gated sodium channel NaChBac cell-autonomously enhance interneuron survival (Denaxa et al., 2018; Priya et al., 2018), while decreasing the excitability of interneurons through the overexpression of the inward-rectifying potassium channel Kir2.1 cell-autonomously diminishes their survival (Close et al., 2012; Priya et al., 2018) (Figure 6B).
Previous studies have shown that the morphological maturation of interneurons depends of their functional integration into nascent cortical circuits. For instance, experimental reduction of the excitability of interneurons leads to a severe reduction in the complexity of their dendritic and axonal arbors (De Marco García et al., 2011). These observations indicate that interneurons require excitatory inputs to mature normally during the first week of postnatal development, and that their ability to integrate into functional circuits influence their probability of survival. The role of activity in interneuron maturation is mediated, at least in part, by transcriptional mechanisms. For instance, the expression of Dlx1, a transcription factor that is critically required for the maturation of some classes of cortical interneurons (Cobos et al., 2005), is modulated by neuronal activity (De Marco García et al., 2011). Dlx1 is in turn required for expression of Elmo1 (Cobos et al., 2007), a gene encoding a Rac activator protein that regulates the migration of some types of interneuron (De Marco García et al., 2011).
Since interneurons receive most of their inputs from local pyramidal cells during the first postnatal week (Anastasiades et al., 2016), it is perhaps not surprising that these cells play a critical role in the survival of cortical interneurons (Figure 6B). Consistent with this idea, increasing or decreasing the excitability of pyramidal cells using DREADDs during the period of interneuron programmed cell death respectively enhances or diminishes the survival of cortical interneurons (Wong et al., 2018). Moreover, preventing programmed cell death in pyramidal cells by genetically ablating the proapoptotic Bcl-2 family members Bax and Bak (Lindsten et al., 2000) from these cells is sufficient to abolish the programmed cell death of cortical interneurons (Wong et al., 2018). Interestingly, the functional maturation of some types of cortical interneurons such as neurogliaform cells seems to be particularly dependent on excitatory inputs from the thalamus (De Marco García et al., 2015). Consequently, it is likely that afferent connections other than those provided by local pyramidal cells influence the survival of cortical interneurons during the normal period of programmed cell death.
The molecular mechanisms regulating programmed cell death in cortical interneuron are only beginning to be elucidated. One of the factors that seem to mediate the intrinsic program of cell death in cortical interneurons is the phosphatase and tensin homologue PTEN. This protein antagonizes the activity of the serine-threonine kinase AKT (Stambolic et al., 1998), which is a critical mediator of neuronal survival (Datta et al., 1997; Dudek et al., 1997). Interestingly, PTEN levels rise in cortical interneurons during the period of programmed cell death in a temporal pattern that coincides with the normal development of interneurons, transiently increasing first in infragranular layer interneurons and, subsequently, in superficial layer interneurons (Wong et al., 2018). Two lines of evidence indicate that PTEN plays an important role in this process. First, genetic deletion of PTEN from postmitotic MGE-derived interneurons dramatically increases their survival (Wong et al., 2018). Second, experimentally increasing the activity of pyramidal cells (which increases interneuron survival) non-cell autonomously represses the expression of PTEN in interneurons (Wong et al., 2018). In sum, one of the possible signaling pathways increasing the survival of interneurons involves the transient repression of PTEN during the period of programmed cell death.
Functional implications
Inhibitory circuits share general organization principles across cortical areas, but increasing evidence suggests that the abundance of specific inhibitory connectivity motifs varies between regions (Katzel et al., 2011), probably reflecting different computational requirements. Recent scRNA-seq studies have shown that specific types of interneurons populating different regions of the neocortex are virtually identical, at least at the transcriptional level (Tasic et al., 2017). However, the density of specific types of interneuron varies substantially across cortical areas (DeFelipe, 1997; He et al., 2016; Whissell et al., 2015). How do these heterogeneous patterns of distribution emerge during development? One possibility would be that cortical interneurons are instructed to settle in specific patterns in each cortical region. Alternatively, interneurons would initially distribute homogeneously, but the process of programmed cell death would then sculpt the various patterns of interneuron distribution that exist across the cerebral cortex. Indeed, the rate of apoptosis in pyramidal cells varies among functionally different neocortical areas and even across layers within the same cortical area (Blanquie et al., 2017). Consequently, the regulation of programmed cell death in interneurons by pyramidal cells (and perhaps also thalamic inputs) might be an important regulator of the functional specialization of cortical areas.
It is presently unclear whether the overproduction of GABAergic interneurons has any functional relevance during the development and assembly of cortical circuits. Are some of the transient interneurons important for shaping early neuronal networks, similar to Cajal-Retzius cells? GABA has been shown to influence neuronal migration in the embryonic cortex (Behar et al., 2000; López-Bendito et al., 2003), the settlement of interneurons in the cortical plate (Bortone and Polleux, 2009), and the formation on inhibitory synapses (Oh et al., 2016), and may also influence the neurogenesis of pyramidal cells (Silva et al., 2018). Consequently, it is possible that developmental processes have adapted to greater numbers of interneurons during embryonic and early postnatal development. In addition, it is conceivable that some of the transient inhibitory connections that have been described in the early postnatal cortex are established by interneurons that are subsequently eliminated by programmed cell death. Although this remains to be investigated thoroughly, interneurons that engage in transient connections during the first week of postnatal development do not seem to be selectively eliminated through programmed cell death (Tuncdemir et al., 2016).
Dynamic tuning of interneuron features
It is generally accepted that neuronal fate is maintained throughout life once the identity of a neuron has been established. However, some of the defining features of interneurons, such as their synaptic connections and intrinsic properties, are known to change reversibly in the adult brain to mediate the adaptation of neural circuits to experience. For example, PV+ basket cells seem to dynamically oscillate between different “states” in response to neuronal activity, a process that is required for learning and memory in the adult brain (Donato et al., 2013; Lagler et al., 2016). These cell states are characterized by specific ratios of synaptic connections and distinctive intrinsic properties, and they are differentially modulated by experience. More generally, the identification of continuous gene expression variation within discrete types of cortical interneurons (Harris et al., 2018; Tasic et al., 2017) suggests that a certain degree of cellular heterogeneity might be relatively common for some interneuron types.
Neuronal activity modulates the connectivity and intrinsic properties of cortical interneurons through several distinct mechanisms, including cell type-specific transcriptional regulation. For instance, neuronal activity induces the expression of the early-response transcription factor Npas4 in SST+ interneurons to regulate the formation of excitatory synapses onto these cells (Figure 7A). Npas4 regulates excitatory synapse formation through the induction of a transcriptional program in SST+ interneurons that involves the expression of several synaptic scaffolding proteins and the shaker-like potassium channel Kv1.1 (Spiegel et al., 2014). In contrast, neuronal activity induces a completely different transcriptional response in VIP+ interneurons, promoting the formation of inhibitory synapses on these cells (Mardinly et al., 2016).
Figure 7. Dynamic regulation of interneuron properties.
(A) The expression of the transcription factor Npas4 is regulated by activity in SST+ interneurons. Increased network activity, for example through sensory stimulation, leads to increased levels of Npas4, which in turn regulates the transcription of genes encoding proteins that increase the number of excitatory synapses SST+ interneurons receive.
(B) Activity negatively regulates the expression of the transcription factor Etv1 and the perineuronal net protein Brevican (Bcan) in PV+ basket cells. Etv1 regulates the expression of genes encoding proteins that regulate the intrinsic properties of PV+ basket cells (e.g., Kv1.1) and the number of excitatory synapses these interneurons receive. Bcan levels are also linked to the expression of proteins regulating the intrinsic properties of PV+ basket cells (e.g., Kv3.1) and their synaptic inputs (e.g., AMPAR).
Transcriptional regulation is not the only mechanism through which neuronal activity regulates the specification of neuronal properties. In supragranular PV+ basket cells, neuronal activity modulates the expression and subcellular location (cytoplasmic or nuclear) of the transcription factor Etv1 (Dehorter et al., 2015), which in turn induces the expression of Kv1.1. Since this channel is critical for delayed firing in these cells (Goldberg et al., 2008), this mechanism allows PV+ basket cells to reversibly adapt to changing levels of activity by dynamically tuning their excitability (Figure 7B).
Activity seems to modify the synaptic connections of PV+ basket cells by modulating specific components of the perineuronal net, a specialization of the extracellular matrix that enfold most of these cells and is perfectly placed for gating synaptic modifications. Recent work has revealed that Brevican, one of the most abundant chondroitin sulfate proteoglycans in perineuronal nets, regulates the synaptic connections of PV+ basket cells in response to changes in network activity (Favuzzi et al., 2017) (Figure 7B). The observation that Brevican is required for spatial working and short-term memories (Favuzzi et al., 2017) reinforces the view that the dynamic transition of interneurons through different cell states in the adult brain is one of the mechanisms used by neuronal networks to adapt in response to experience.
A look ahead
It is evident that we have made very significant advances in our understanding of functional diversification of cortical interneurons over the last 30 years. However, multiple aspects of the genetic, epigenetic, biochemical and cellular mechanisms underlying the emergence of this diversity remain unclear. For instance, the precise mechanisms that control the specification of different types of interneurons generated from the same progenitor cells, such as SST+ Martinotti cells and PV+ basket cells, have not been identified. This process may rely on transcription factors yet to be identified, homologous to Drosophila temporal transcription factors (Doe, 2017). Alternatively, transcription factors that are known to influence the development of both types of interneuron may do so by driving different genetic programs due to the unique chromatin landscape of each cell type, as shown for Nkx2-1 in MGE progenitor cells (Sandberg et al., 2016). Consequently, identifying additional sources of variation (epigenetic regulation, long non-coding RNAs, alternative splicing, etc.) in progenitor cells and immature neurons will be necessary to decipher the molecular mechanisms underlying the generation of cortical interneuron diversity.
Development adds a temporal dimension to the classification of cortical interneurons. The developmental trajectory of cortical interneurons might not be currently perceived as an essential dimension in the definition of these cells, but it certainly helps us to understand the mechanisms driving their diversification. In this context, the limited availability of genetic drivers to label cortical interneurons has led to an era of ‘lumping’ in the study of interneurons. Overcoming this limitation would undoubtedly contribute to clarify the role of the specific types of interneuron in cortical circuits. The design of novel tools to identify and modify the activity of specific types of cortical interneuron directly profits from our understanding of the development of these cells (He et al., 2016; Taniguchi et al., 2013).
Most of our knowledge of the development and functional diversification of cortical interneurons derives primarily from studies in mice. The cellular organization of interneuron diversity is remarkably similar between mouse and human, but there are important differences in the morphology, gene expression, laminar distribution and proportions of certain types of interneurons (Boldog et al., 2018; DeFelipe, 1997; Hodge et al., 2018). Recent work has begun to shed light into human cortical interneuron diversity and their developmental origin (Arshad et al., 2016; Hansen et al., 2013; Ma et al., 2013; Paredes et al., 2016), but we are still far from understanding the developmental and evolutionary processes leading to the emergence of species-specific features in cortical interneurons (Sousa et al., 2017).
Characterizing the developmental trajectories of distinct types of cortical interneurons in humans seems increasingly important, especially considering the involvement of interneuron dysfunction in neurodevelopmental disorders (Marín, 2012). In this context, linking genomic findings associated with neuropsychiatric disorders to specific brain cell types is a powerful strategy to identifying the most likely cellular substrates in these conditions (Skene et al., 2018). However, since genomic variation associated to neuropsychiatric disorders such as autism and schizophrenia affects the developing brain, the relevant transcriptional information for this type of analyses would likely need to be developmental.
Acknowledgements
We thank members of the Marín and Rico laboratories for stimulating discussions and ideas. Our research on this topic is supported by grants from the European Research Council (ERC-2017-AdG 787355) and Wellcome Trust (103714MA) to O.M. L.L. was the recipient of an EMBO long-term postdoctoral fellowship.
References
- Anastasiades PG, Marques-Smith A, Lyngholm D, Lickiss T, Raffiq S, Katzel D, Miesenbock G, Butt SJ. GABAergic interneurons form transient layer-specific circuits in early postnatal neocortex. Nat Commun. 2016;7 doi: 10.1038/ncomms10584. 10584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson SA, Eisenstat DD, Shi L, Rubenstein JLR. Interneuron migration from basal forebrain to neocortex: dependence on Dlx genes. Science. 1997a;278:474–476. doi: 10.1126/science.278.5337.474. [DOI] [PubMed] [Google Scholar]
- Anderson SA, Qiu M, Bulfone A, Eisenstat DD, Meneses J, Pedersen R, Rubenstein JLR. Mutations of the homeobox genes Dlx-1 and Dlx-2 disrupt the striatal subventricular zone and differentiation of late born striatal neurons. Neuron. 1997b;19:27–37. doi: 10.1016/s0896-6273(00)80345-1. [DOI] [PubMed] [Google Scholar]
- Antypa M, Faux C, Eichele G, Parnavelas JG, Andrews WD. Differential gene expression in migratory streams of cortical interneurons. Eur J Neurosci. 2011;34:1584–1594. doi: 10.1111/j.1460-9568.2011.07896.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arshad A, Vose LR, Vinukonda G, Hu F, Yoshikawa K, Csiszar A, Brumberg JC, Ballabh P. Extended production of cortical interneurons into the third trimester of human gestation. Cereb Cortex. 2016;26:2242–2256. doi: 10.1093/cercor/bhv074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ascoli GA, Alonso-Nanclares L, Anderson SA, Barrionuevo G, Benavides-Piccione R, Burkhalter A, Buzsaki G, Cauli B, Defelipe J, Fairén A, et al. Petilla terminology: nomenclature of features of GABAergic interneurons of the cerebral cortex. Nat Rev Neurosci. 2008;9:557–568. doi: 10.1038/nrn2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azim E, Jabaudon D, Fame RM, Macklis JD. SOX6 controls dorsal progenitor identity and interneuron diversity during neocortical development. Nat Neurosci. 2009;12:1238–1247. doi: 10.1038/nn.2387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartolini G, Ciceri G, Marín O. Integration of GABAergic interneurons into cortical cell assemblies: lessons from embryos and adults. Neuron. 2013;79:849–864. doi: 10.1016/j.neuron.2013.08.014. [DOI] [PubMed] [Google Scholar]
- Bartolini G, Sanchez-Alcaniz JA, Osorio C, Valiente M, Garcia-Frigola C, Marín O. Neuregulin 3 mediates cortical plate invasion and laminar allocation of GABAergic interneurons. Cell Reports. 2017;18:1157–1170. doi: 10.1016/j.celrep.2016.12.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batista-Brito R, Rossignol E, Hjerling-Leffler J, Denaxa M, Wegner M, Lefebvre V, Pachnis V, Fishell G. The cell-intrinsic requirement of Sox6 for cortical interneuron development. Neuron. 2009;63:466–481. doi: 10.1016/j.neuron.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bean JC, Lin TW, Sathyamurthy A, Liu F, Yin DM, Xiong WC, Mei L. Genetic labeling reveals novel cellular targets of schizophrenia susceptibility gene: distribution of GABA and non-GABA ErbB4-positive cells in adult mouse brain. J Neurosci. 2014;34:13549–13566. doi: 10.1523/JNEUROSCI.2021-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behar TN, Schaffner AE, Scott CA, Greene CL, Barker JL. GABA receptor antagonists modulate postmitotic cell migration in slice cultures of embryonic rat cortex. Cereb Cortex. 2000;10:899–909. doi: 10.1093/cercor/10.9.899. [DOI] [PubMed] [Google Scholar]
- Bender KJ, Allen CB, Bender VA, Feldman DE. Synaptic basis for whisker deprivation-induced synaptic depression in rat somatosensory cortex. J Neurosci. 2006;26:4155–4165. doi: 10.1523/JNEUROSCI.0175-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanquie O, Yang JW, Kilb W, Sharopov S, Sinning A, Luhmann HJ. Electrical activity controls area-specific expression of neuronal apoptosis in the developing mouse cerebral cortex. Elife. 2017;6 doi: 10.7554/eLife.27696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boldog E, Bakken TE, Hodge RD, Novotny M, Aevermann BD, Baka J, Borde S, Close JL, Diez-Fuertes F, Ding SL, et al. Transcriptomic and morphophysiological evidence for a specialized human cortical GABAergic cell type. Nat Neurosci. 2018;21:1185–1195. doi: 10.1038/s41593-018-0205-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonneau D, Toutain A, Laquerriere A, Marret S, Saugier-Veber P, Barthez MA, Radi S, Biran-Mucignat V, Rodriguez D, Gelot A. X-linked lissencephaly with absent corpus callosum and ambiguous genitalia (XLAG): clinical, magnetic resonance imaging, and neuropathological findings. Ann Neurol. 2002;51:340–349. doi: 10.1002/ana.10119. [DOI] [PubMed] [Google Scholar]
- Bortone D, Polleux F. KCC2 expression promotes the termination of cortical interneuron migration in a voltage-sensitive calcium-dependent manner. Neuron. 2009;62:53–71. doi: 10.1016/j.neuron.2009.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bortone DS, Olsen SR, Scanziani M. Translaminar inhibitory cells recruited by layer 6 corticothalamic neurons suppress visual cortex. Neuron. 2014;82:474–485. doi: 10.1016/j.neuron.2014.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown KN, Chen S, Han Z, Lu CH, Tan X, Zhang XJ, Ding L, Lopez-Cruz A, Saur D, Anderson SA, et al. Clonal production and organization of inhibitory interneurons in the neocortex. Science. 2011;334:480–486. doi: 10.1126/science.1208884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan KA, Blackman AV, Moreau AW, Elgar D, Costa RP, Lalanne T, Tudor Jones AA, Oyrer J, Sjostrom PJ. Target-specific expression of presynaptic NMDA receptors in neocortical microcircuits. Neuron. 2012;75:451–466. doi: 10.1016/j.neuron.2012.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butt SJ, Fuccillo M, Nery S, Noctor S, Kriegstein A, Corbin JG, Fishell G. The temporal and spatial origins of cortical interneurons predict their physiological subtype. Neuron. 2005;48:591–604. doi: 10.1016/j.neuron.2005.09.034. [DOI] [PubMed] [Google Scholar]
- Butt SJ, Sousa VH, Fuccillo MV, Hjerling-Leffler J, Miyoshi G, Kimura S, Fishell G. The requirement of Nkx2-1 in the temporal specification of cortical interneuron subtypes. Neuron. 2008;59:722–732. doi: 10.1016/j.neuron.2008.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Che A, Babij R, Iannone AF, Fetcho RN, Ferrer M, Liston C, Fishell G, De Marco Garcia NV. Layer I interneurons sharpen sensory maps during neonatal development. Neuron. 2018;99:98–116 e117. doi: 10.1016/j.neuron.2018.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chittajallu R, Isaac JT. Emergence of cortical inhibition by coordinated sensory-driven plasticity at distinct synaptic loci. Nat Neurosci. 2010;13:1240–1248. doi: 10.1038/nn.2639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciceri G, Dehorter N, Sols I, Huang ZJ, Maravall M, Marín O. Lineage-specific laminar organization of cortical GABAergic interneurons. Nat Neurosci. 2013;16:1199–1210. doi: 10.1038/nn.3485. [DOI] [PubMed] [Google Scholar]
- Close J, Xu H, De Marco Garcia N, Batista-Brito R, Rossignol E, Rudy B, Fishell G. Satb1 is an activity-modulated transcription factor required for the terminal differentiation and connectivity of medial ganglionic eminence-derived cortical interneurons. J Neurosci. 2012;32:17690–17705. doi: 10.1523/JNEUROSCI.3583-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobos I, Borello U, Rubenstein JL. Dlx transcription factors promote migration through repression of axon and dendrite growth. Neuron. 2007;54:873–888. doi: 10.1016/j.neuron.2007.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobos I, Calcagnotto ME, Vilaythong AJ, Thwin MT, Noebels JL, Baraban SC, Rubenstein JL. Mice lacking Dlx1 show subtype-specific loss of interneurons, reduced inhibition and epilepsy. Nat Neurosci. 2005;8:1059–1068. doi: 10.1038/nn1499. [DOI] [PubMed] [Google Scholar]
- Colasante G, Collombat P, Raimondi V, Bonanomi D, Ferrai C, Maira M, Yoshikawa K, Mansouri A, Valtorta F, Rubenstein JL, Broccoli V. Arx is a direct target of Dlx2 and thereby contributes to the tangential migration of GABAergic interneurons. J Neurosci. 2008;28:10674–10686. doi: 10.1523/JNEUROSCI.1283-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta SR, Dudek H, Tao X, Masters S, Fu H, Gotoh Y, Greenberg ME. Akt phosphorylation of BAD couples survival signals to the cell-intrinsic death machinery. Cell. 1997;91:231–241. doi: 10.1016/s0092-8674(00)80405-5. [DOI] [PubMed] [Google Scholar]
- Daw MI, Ashby MC, Isaac JT. Coordinated developmental recruitment of latent fast spiking interneurons in layer IV barrel cortex. Nat Neurosci. 2007;10:453–461. doi: 10.1038/nn1866. [DOI] [PubMed] [Google Scholar]
- De Marco García NV, Karayannis T, Fishell G. Neuronal activity is required for the development of specific cortical interneuron subtypes. Nature. 2011;472:351–355. doi: 10.1038/nature09865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Marco García NV, Priya R, Tuncdemir SN, Fishell G, Karayannis T. Sensory inputs control the integration of neurogliaform interneurons into cortical circuits. Nat Neurosci. 2015;18:393–401. doi: 10.1038/nn.3946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFelipe J. Types of neurons, synaptic connections and chemical characteristics of cells immunoreactive for calbindin-D28K, parvalbumin and calretinin in the neocortex. J Chem Neuroanat. 1997;14:1–19. doi: 10.1016/s0891-0618(97)10013-8. [DOI] [PubMed] [Google Scholar]
- DeFelipe J, Lopez-Cruz PL, Benavides-Piccione R, Bielza C, Larranaga P, Anderson S, Burkhalter A, Cauli B, Fairén A, Feldmeyer D, et al. New insights into the classification and nomenclature of cortical GABAergic interneurons. Nat Rev Neurosci. 2013;14:202–216. doi: 10.1038/nrn3444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehorter N, Ciceri G, Bartolini G, Lim L, del Pino I, Marín O. Tuning of fast-spiking interneuron properties by an activity-dependent transcriptional switch. Science. 2015;349:1216–1220. doi: 10.1126/science.aab3415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denaxa M, Kalaitzidou M, Garefalaki A, Achimastou A, Lasrado R, Maes T, Pachnis V. Maturation-promoting activity of SATB1 in MGE-derived cortical interneurons. Cell Reports. 2012;2:1351–1362. doi: 10.1016/j.celrep.2012.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denaxa M, Neves G, Rabinowitz A, Kemlo S, Liodis P, Burrone J, Pachnis V. Modulation of apoptosis controls inhibitory interneuron number in the cortex. Cell Reports. 2018;22:1710–1721. doi: 10.1016/j.celrep.2018.01.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dittrich L, Heiss JE, Warrier DR, Perez XA, Quik M, Kilduff TS. Cortical nNOS neurons co-express the NK1 receptor and are depolarized by Substance P in multiple mammalian species. Front Neural Circuits. 2012;6:31. doi: 10.3389/fncir.2012.00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doe CQ. Temporal patterning in the Drosophila CNS. Annu Rev Cell Dev Biol. 2017;33:219–240. doi: 10.1146/annurev-cellbio-111315-125210. [DOI] [PubMed] [Google Scholar]
- Donato F, Rompani SB, Caroni P. Parvalbumin-expressing basket-cell network plasticity induced by experience regulates adult learning. Nature. 2013;504:272–276. doi: 10.1038/nature12866. [DOI] [PubMed] [Google Scholar]
- Douglas RJ, Martin KA. Neuronal circuits of the neocortex. Annu Rev Neurosci. 2004;27:419–451. doi: 10.1146/annurev.neuro.27.070203.144152. [DOI] [PubMed] [Google Scholar]
- Dudek H, Datta SR, Franke TF, Birnbaum MJ, Yao R, Cooper GM, Segal RA, Kaplan DR, Greenberg ME. Regulation of neuronal survival by the serine-threonine protein kinase Akt. Science. 1997;275:661–665. doi: 10.1126/science.275.5300.661. [DOI] [PubMed] [Google Scholar]
- Fairén A, Cobas A, Fonseca M. Times of generation of glutamic acid decarboxylase immunoreactive neurons in mouse somatosensory cortex. J Comp Neurol. 1986;251:67–83. doi: 10.1002/cne.902510105. [DOI] [PubMed] [Google Scholar]
- Favuzzi E, Marques-Smith A, Deogracias R, Winterflood CM, Sanchez-Aguilera A, Mantoan L, Maeso P, Fernandes C, Ewers H, Rico B. Activity-dependent gating of parvalbumin interneuron function by the perineuronal net protein Brevican. Neuron. 2017;95:639–655. doi: 10.1016/j.neuron.2017.06.028. [DOI] [PubMed] [Google Scholar]
- Fazzari P, Paternain AV, Valiente M, Pla R, Lujan R, Lloyd K, Lerma J, Marín O, Rico B. Control of cortical GABA circuitry development by Nrg1 and ErbB4 signalling. Nature. 2010;464:1376–1380. doi: 10.1038/nature08928. [DOI] [PubMed] [Google Scholar]
- Flames N, Long JE, Garratt AN, Fischer TM, Gassmann M, Birchmeier C, Lai C, Rubenstein JL, Marín O. Short- and long-range attraction of cortical GABAergic interneurons by neuregulin-1. Neuron. 2004;44:251–261. doi: 10.1016/j.neuron.2004.09.028. [DOI] [PubMed] [Google Scholar]
- Flames N, Pla R, Gelman DM, Rubenstein JL, Puelles L, Marín O. Delineation of multiple subpallial progenitor domains by the combinatorial expression of transcriptional codes. J Neurosci. 2007;27:9682–9695. doi: 10.1523/JNEUROSCI.2750-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flandin P, Kimura S, Rubenstein JL. The progenitor zone of the ventral medial ganglionic eminence requires Nkx2-1 to generate most of the globus pallidus but few neocortical interneurons. J Neurosci. 2010;30:2812–2823. doi: 10.1523/JNEUROSCI.4228-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogarty M, Grist M, Gelman D, Marín O, Pachnis V, Kessaris N. Spatial genetic patterning of the embryonic neuroepithelium generates GABAergic interneuron diversity in the adult cortex. J Neurosci. 2007;27:10935–10946. doi: 10.1523/JNEUROSCI.1629-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frazer S, Prados J, Niquille M, Cadilhac C, Markopoulos F, Gomez L, Tomasello U, Telley L, Holtmaat A, Jabaudon D, Dayer A. Transcriptomic and anatomic parcellation of 5-HT3AR expressing cortical interneuron subtypes revealed by single-cell RNA sequencing. Nat Commun. 2017;8 doi: 10.1038/ncomms14219. 14219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabernet L, Jadhav SP, Feldman DE, Carandini M, Scanziani M. Somatosensory integration controlled by dynamic thalamocortical feed-forward inhibition. Neuron. 2005;48:315–327. doi: 10.1016/j.neuron.2005.09.022. [DOI] [PubMed] [Google Scholar]
- Gelman D, Griveau A, Dehorter N, Teissier A, Varela C, Pla R, Pierani A, Marín O. A wide diversity of cortical GABAergic interneurons derives from the embryonic preoptic area. J Neurosci. 2011;31:16570–16580. doi: 10.1523/JNEUROSCI.4068-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelman DM, Marín O. Generation of interneuron diversity in the mouse cerebral cortex. Eur J Neurosci. 2010;31:2136–2141. doi: 10.1111/j.1460-9568.2010.07267.x. [DOI] [PubMed] [Google Scholar]
- Gelman DM, Martini FJ, Nóbrega-Pereira S, Pierani A, Kessaris N, Marín O. The embryonic preoptic area is a novel source of cortical GABAergic interneurons. J Neurosci. 2009;29:9380–9389. doi: 10.1523/JNEUROSCI.0604-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glickstein SB, Moore H, Slowinska B, Racchumi J, Suh M, Chuhma N, Ross ME. Selective cortical interneuron and GABA deficits in cyclin D2-null mice. Development. 2007;134:4083–4093. doi: 10.1242/dev.008524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg EM, Clark BD, Zagha E, Nahmani M, Erisir A, Rudy B. K+ channels at the axon initial segment dampen near-threshold excitability of neocortical fast-spiking GABAergic interneurons. Neuron. 2008;58:387–400. doi: 10.1016/j.neuron.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen DV, Lui JH, Flandin P, Yoshikawa K, Rubenstein JL, Alvarez-Buylla A, Kriegstein AR. Non-epithelial stem cells and cortical interneuron production in the human ganglionic eminences. Nat Neurosci. 2013;16:1576–1587. doi: 10.1038/nn.3541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris KD, Hochgerner H, Skene NG, Magno L, Katona L, Bengtsson Gonzales C, Somogyi P, Kessaris N, Linnarsson S, Hjerling-Leffler J. Classes and continua of hippocampal CA1 inhibitory neurons revealed by single-cell transcriptomics. PLoS Biol. 2018;16:e2006387. doi: 10.1371/journal.pbio.2006387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris ML, Erickson CA. Lineage specification in neural crest cell pathfinding. Dev Dyn. 2007;236:1–19. doi: 10.1002/dvdy.20919. [DOI] [PubMed] [Google Scholar]
- Harwell CC, Fuentealba LC, Gonzalez-Cerrillo A, Parker PR, Gertz CC, Mazzola E, Garcia MT, Alvarez-Buylla A, Cepko CL, Kriegstein AR. Wide dispersion and diversity of clonally related inhibitory interneurons. Neuron. 2015;87:999–1007. doi: 10.1016/j.neuron.2015.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassan BA, Hiesinger PR. Beyond molecular codes: simple rules to wire complex brains. Cell. 2015;163:285–291. doi: 10.1016/j.cell.2015.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He M, Tucciarone J, Lee S, Nigro MJ, Kim Y, Levine JM, Kelly SM, Krugikov I, Wu P, Chen Y, et al. Strategies and tools for combinatorial targeting of GABAergic neurons in mouse cerebral cortex. Neuron. 2016;91:1228–1243. doi: 10.1016/j.neuron.2016.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hevner RF, Daza RA, Englund C, Kohtz J, Fink A. Postnatal shifts of interneuron position in the neocortex of normal and reeler mice: evidence for inward radial migration. Neuroscience. 2004;124:605–618. doi: 10.1016/j.neuroscience.2003.11.033. [DOI] [PubMed] [Google Scholar]
- Hilscher MM, Leao RN, Edwards SJ, Leao KE, Kullander K. Chrna2-Martinotti cells synchronize layer 5 type A pyramidal cells via rebound excitation. PLoS Biol. 2017;15:e2001392. doi: 10.1371/journal.pbio.2001392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodge RD, Bakken TE, Miller JA, Smith KA, Barkan ER, Graybuck LT, Close JL, Long B, Penn O, Yao Z, et al. Conserved cell types with divergent features between human and mouse cortex. bioRxiv. 2018 doi: 10.1038/s41586-019-1506-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu H, Gan J, Jonas P. Fast-spiking, parvalbumin(+) GABAergic interneurons: from cellular design to microcircuit function. Science. 2014;345 doi: 10.1126/science.1255263. 1255263. [DOI] [PubMed] [Google Scholar]
- Hu JS, Vogt D, Lindtner S, Sandberg M, Silberberg SN, Rubenstein JLR. Coup-TF1 and Coup-TF2 control subtype and laminar identity of MGE-derived neocortical interneurons. Development. 2017a;144:2837–2851. doi: 10.1242/dev.150664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu JS, Vogt D, Sandberg M, Rubenstein JL. Cortical interneuron development: a tale of time and space. Development. 2017b;144:3867–3878. doi: 10.1242/dev.132852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inamura N, Kimura T, Tada S, Kurahashi T, Yanagida M, Yanagawa Y, Ikenaka K, Murakami F. Intrinsic and extrinsic mechanisms control the termination of cortical interneuron migration. J Neurosci. 2012;32:6032–6042. doi: 10.1523/JNEUROSCI.3446-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inan M, Welagen J, Anderson SA. Spatial and temporal bias in the mitotic origins of somatostatin- and parvalbumin-expressing interneuron subgroups and the chandelier subtype in the medial ganglionic eminence. Cereb Cortex. 2012;22:820–827. doi: 10.1093/cercor/bhr148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inda MC, DeFelipe J, Munoz A. Morphology and distribution of chandelier cell axon terminals in the mouse cerebral cortex and claustroamygdaloid complex. Cereb Cortex. 2009;19:41–54. doi: 10.1093/cercor/bhn057. [DOI] [PubMed] [Google Scholar]
- Jessell TM. Neuronal specification in the spinal cord: inductive signals and transcriptional codes. Nature Reviews Genetics. 2000;1:20–29. doi: 10.1038/35049541. [DOI] [PubMed] [Google Scholar]
- Jiang X, Shen S, Cadwell CR, Berens P, Sinz F, Ecker AS, Patel S, Tolias AS. Principles of connectivity among morphologically defined cell types in adult neocortex. Science. 2015;350:aac9462. doi: 10.1126/science.aac9462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jinno S, Klausberger T, Marton LF, Dalezios Y, Roberts JD, Fuentealba P, Bushong EA, Henze D, Buzsaki G, Somogyi P. Neuronal diversity in GABAergic long-range projections from the hippocampus. J Neurosci. 2007;27:8790–8804. doi: 10.1523/JNEUROSCI.1847-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanatani S, Yozu M, Tabata H, Nakajima K. COUP-TFII is preferentially expressed in the caudal ganglionic eminence and is involved in the caudal migratory stream. J Neurosci. 2008;28:13582–13591. doi: 10.1523/JNEUROSCI.2132-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katzel D, Zemelman BV, Buetfering C, Wolfel M, Miesenbock G. The columnar and laminar organization of inhibitory connections to neocortical excitatory cells. Nat Neurosci. 2011;14:100–107. doi: 10.1038/nn.2687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaguchi Y, Kubota Y. Neurochemical features and synaptic connections of large physiologically-identified GABAergic cells in the rat frontal cortex. Neuroscience. 1998;85:677–701. doi: 10.1016/s0306-4522(97)00685-4. [DOI] [PubMed] [Google Scholar]
- Kelly SM, Raudales R, He M, Lee JH, Kim Y, Gibb LG, Wu P, Matho K, Osten P, Graybiel AM, Huang ZJ. Radial glial lineage progression and differential intermediate progenitor amplification underlie striatal compartments and circuit organization. Neuron. 2018;99:345–361 e344. doi: 10.1016/j.neuron.2018.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kepecs A, Fishell G. Interneuron cell types are fit to function. Nature. 2014;505:318–326. doi: 10.1038/nature12983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura K, Yanazawa M, Sugiyama N, Miura H, Iizuka-Kogo A, Kusaka M, Omichi K, Suzuki R, Kato-Fukui Y, Kamiirisa K, et al. Mutation of ARX causes abnormal development of forebrain and testes in mice and X-linked lissencephaly with abnormal genitalia in humans. Nat Genet. 2002;32:359–369. doi: 10.1038/ng1009. [DOI] [PubMed] [Google Scholar]
- Klausberger T, Somogyi P. Neuronal diversity and temporal dynamics: the unity of hippocampal circuit operations. Science. 2008;321:53–57. doi: 10.1126/science.1149381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagler M, Ozdemir AT, Lagoun S, Malagon-Vina H, Borhegyi Z, Hauer R, Jelem A, Klausberger T. Divisions of identified parvalbumin-expressing basket cells during working memory-guided decision making. Neuron. 2016;91:1390–1401. doi: 10.1016/j.neuron.2016.08.010. [DOI] [PubMed] [Google Scholar]
- Lavdas AA, Grigoriou M, Pachnis V, Parnavelas JG. The medial ganglionic eminence gives rise to a population of early neurons in the developing cerebral cortex. J Neurosci. 1999;19:7881–7888. doi: 10.1523/JNEUROSCI.19-18-07881.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le TN, Zhou QP, Cobos I, Zhang S, Zagozewski J, Japoni S, Vriend J, Parkinson T, Du G, Rubenstein JL, Eisenstat DD. GABAergic Interneuron Differentiation in the Basal Forebrain Is Mediated through Direct Regulation of Glutamic Acid Decarboxylase Isoforms by Dlx Homeobox Transcription Factors. J Neurosci. 2017;37:8816–8829. doi: 10.1523/JNEUROSCI.2125-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Hjerling-Leffler J, Zagha E, Fishell G, Rudy B. The largest group of superficial neocortical GABAergic interneurons expresses ionotropic serotonin receptors. J Neurosci. 2010;30:16796–16808. doi: 10.1523/JNEUROSCI.1869-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G, Adesnik H, Li J, Long J, Nicoll RA, Rubenstein JLR, Pleasure SJ. Regional distribution of cortical interneurons and development of inhibitory tone are regulated by Cxcl12/Cxcr4 signaling. J Neurosci. 2008;28:1085–1098. doi: 10.1523/JNEUROSCI.4602-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim L, Pakan JMP, Selten MM, Marques-Smith A, Llorca A, Bae SE, Rochefort NL, Marin O. Optimization of interneuron function by direct coupling of cell migration and axonal targeting. Nat Neurosci. 2018;21:920–931. doi: 10.1038/s41593-018-0162-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsten T, Ross AJ, King A, Zong WX, Rathmell JC, Shiels HA, Ulrich E, Waymire KG, Mahar P, Frauwirth K, et al. The combined functions of proapoptotic Bcl-2 family members bak and bax are essential for normal development of multiple tissues. Mol Cell. 2000;6:1389–1399. doi: 10.1016/s1097-2765(00)00136-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liodis P, Denaxa M, Grigoriou M, Akufo-Addo C, Yanagawa Y, Pachnis V. Lhx6 activity is required for the normal migration and specification of cortical interneuron subtypes. J Neurosci. 2007;27:3078–3089. doi: 10.1523/JNEUROSCI.3055-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Zhang Z, Lindtner S, Li Z, Xu Z, Wei S, Liang Q, Wen Y, Tao G, You Y, et al. Sp9 regulates medial ganglionic eminence-derived cortical interneuron development. Cereb Cortex. 2018 doi: 10.1093/cercor/bhy133. AOP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodato S, Rouaux C, Quast KB, Jantrachotechatchawan C, Studer M, Hensch TK, Arlotta P. Excitatory projection neuron subtypes control the distribution of local inhibitory interneurons in the cerebral cortex. Neuron. 2011;69:763–779. doi: 10.1016/j.neuron.2011.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long JE, Cobos I, Potter GB, Rubenstein JL. Dlx1&2 and Mash1 transcription factors control MGE and CGE patterning and differentiation through parallel and overlapping pathways. Cereb Cortex. 2009;19(Suppl 1):i96–106. doi: 10.1093/cercor/bhp045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Bendito G, Lujan R, Shigemoto R, Ganter P, Paulsen O, Molnar Z. Blockade of GABA(B) receptors alters the tangential migration of cortical neurons. Cereb Cortex. 2003;13:932–942. doi: 10.1093/cercor/13.9.932. [DOI] [PubMed] [Google Scholar]
- López-Bendito G, Sánchez-Alcaniz JA, Pla R, Borrell V, Pico E, Valdeolmillos M, Marín O. Chemokine signaling controls intracortical migration and final distribution of GABAergic interneurons. J Neurosci. 2008;28:1613–1624. doi: 10.1523/JNEUROSCI.4651-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma T, Wang C, Wang L, Zhou X, Tian M, Zhang Q, Zhang Y, Li J, Liu Z, Cai Y. Subcortical origins of human and monkey neocortical interneurons. Nat Neurosci. 2013;16:1588–1597. doi: 10.1038/nn.3536. [DOI] [PubMed] [Google Scholar]
- Ma Y, Hu H, Berrebi AS, Mathers PH, Agmon A. Distinct subtypes of somatostatin-containing neocortical interneurons revealed in transgenic mice. J Neurosci. 2006;26:5069–5082. doi: 10.1523/JNEUROSCI.0661-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mardinly AR, Spiegel I, Patrizi A, Centofante E, Bazinet JE, Tzeng CP, Mandel-Brehm C, Harmin DA, Adesnik H, Fagiolini M, Greenberg ME. Sensory experience regulates cortical inhibition by inducing IGF1 in VIP neurons. Nature. 2016;531:371–375. doi: 10.1038/nature17187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marín O. Interneuron dysfunction in psychiatric disorders. Nat Rev Neurosci. 2012;13:107–120. doi: 10.1038/nrn3155. [DOI] [PubMed] [Google Scholar]
- Marín O, Plump AS, Flames N, Sanchez-Camacho C, Tessier-Lavigne M, Rubenstein JL. Directional guidance of interneuron migration to the cerebral cortex relies on subcortical Slit1/2-independent repulsion and cortical attraction. Development. 2003;130:1889–1901. doi: 10.1242/dev.00417. [DOI] [PubMed] [Google Scholar]
- Marín O, Rubenstein JLR. A long, remarkable journey: tangential migration in the telencephalon. Nat Rev Neurosci. 2001;2:780–790. doi: 10.1038/35097509. [DOI] [PubMed] [Google Scholar]
- Marín O, Valdeolmillos M, Moya F. Neurons in motion: same principles for different shapes? Trends Neurosci. 2006;29:655–661. doi: 10.1016/j.tins.2006.10.001. [DOI] [PubMed] [Google Scholar]
- Marín O, Yaron A, Bagri A, Tessier-Lavigne M, Rubenstein JL. Sorting of striatal and cortical interneurons regulated by semaphorin/neuropilin interactions. Science. 2001;293:872–875. doi: 10.1126/science.1061891. [DOI] [PubMed] [Google Scholar]
- Marques-Smith A, Lyngholm D, Kaufmann AK, Stacey JA, Hoerder-Suabedissen A, Becker EB, Wilson MC, Molnar Z, Butt SJ. A Transient Translaminar GABAergic Interneuron Circuit Connects Thalamocortical Recipient Layers in Neonatal Somatosensory Cortex. Neuron. 2016;89:536–549. doi: 10.1016/j.neuron.2016.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martini FJ, Valiente M, López Bendito G, Szabó G, Moya F, Valdeolmillos M, Marín O. Biased selection of leading process branches mediates chemotaxis during tangential neuronal migration. Development. 2009;136:41–50. doi: 10.1242/dev.025502. [DOI] [PubMed] [Google Scholar]
- Mayer C, Hafemeister C, Bandler RC, Machold R, Batista Brito R, Jaglin X, Allaway K, Butler A, Fishell G, Satija R. Developmental diversification of cortical inhibitory interneurons. Nature. 2018;555:457–462. doi: 10.1038/nature25999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer C, Jaglin XH, Cobbs LV, Bandler RC, Streicher C, Cepko CL, Hippenmeyer S, Fishell G. Clonally related forebrain interneurons disperse broadly across both functional areas and structural boundaries. Neuron. 2015;87:989–998. doi: 10.1016/j.neuron.2015.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinsey GL, Lindtner S, Trzcinski B, Visel A, Pennacchio LA, Huylebroeck D, Higashi Y, Rubenstein JL. Dlx1&2-dependent expression of Zfhx1b (Sip1, Zeb2) regulates the fate switch between cortical and striatal interneurons. Neuron. 2013;77:83–98. doi: 10.1016/j.neuron.2012.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mei L, Nave KA. Neuregulin-ERBB signaling in the nervous system and neuropsychiatric diseases. Neuron. 2014;83:27–49. doi: 10.1016/j.neuron.2014.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melzer S, Michael M, Caputi A, Eliava M, Fuchs EC, Whittington MA, Monyer H. Long-range-projecting GABAergic neurons modulate inhibition in hippocampus and entorhinal cortex. Science. 2012;335:1506–1510. doi: 10.1126/science.1217139. [DOI] [PubMed] [Google Scholar]
- Mi D, Li Z, Lim L, Li M, Moissidis M, Yang Y, Gao T, Hu TX, Pratt T, Price DJ, et al. Early emergence of cortical interneuron diversity in the mouse embryo. Science. 2018;360:81–85. doi: 10.1126/science.aar6821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller MW. Cogeneration of retrogradely labeled corticocortical projection and GABA-immunoreactive local circuit neurons in cerebral cortex. Brain Res. 1985;355:187–192. doi: 10.1016/0165-3806(85)90040-9. [DOI] [PubMed] [Google Scholar]
- Miyoshi G, Butt SJ, Takebayashi H, Fishell G. Physiologically distinct temporal cohorts of cortical interneurons arise from telencephalic Olig2-expressing precursors. J Neurosci. 2007;27:7786–7798. doi: 10.1523/JNEUROSCI.1807-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyoshi G, Fishell G. GABAergic interneuron lineages selectively sort into specific cortical layers during early postnatal development. Cereb Cortex. 2011;21:845–852. doi: 10.1093/cercor/bhq155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyoshi G, Hjerling-Leffler J, Karayannis T, Sousa VH, Butt SJ, Battiste J, Johnson JE, Machold RP, Fishell G. Genetic fate mapping reveals that the caudal ganglionic eminence produces a large and diverse population of superficial cortical interneurons. J Neurosci. 2010;30:1582–1594. doi: 10.1523/JNEUROSCI.4515-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mlodzik M, Hiromi Y, Weber U, Goodman CS, Rubin GM. The Drosophila seven-up gene, a member of the steroid receptor gene superfamily, controls photoreceptor cell fates. Cell. 1990;60:211–224. doi: 10.1016/0092-8674(90)90737-y. [DOI] [PubMed] [Google Scholar]
- Naka H, Nakamura S, Shimazaki T, Okano H. Requirement for COUP-TFI and II in the temporal specification of neural stem cells in CNS development. Nat Neurosci. 2008;11:1014–1023. doi: 10.1038/nn.2168. [DOI] [PubMed] [Google Scholar]
- Neddens J, Buonanno A. Selective populations of hippocampal interneurons express ErbB4 and their number and distribution is altered in ErbB4 knockout mice. Hippocampus. 2010;20:724–744. doi: 10.1002/hipo.20675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nery S, Fishell G, Corbin JG. The caudal ganglionic eminence is a source of distinct cortical and subcortical cell populations. Nat Neurosci. 2002;5:1279–1287. doi: 10.1038/nn971. [DOI] [PubMed] [Google Scholar]
- Nigro MJ, Hashikawa-Yamasaki Y, Rudy B. Diversity and connectivity of layer 5 somatostatin-expressing interneurons in the mouse barrel cortex. J Neurosci. 2018;38:1622–1633. doi: 10.1523/JNEUROSCI.2415-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niquille M, Limoni G, Markopoulos F, Cadilhac C, Prados J, Holtmaat A, Dayer A. Neurogliaform cortical interneurons derive from cells in the preoptic area. Elife. 2018;7 doi: 10.7554/eLife.32017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nóbrega-Pereira S, Gelman D, Bartolini G, Pla R, Pierani A, Marín O. Origin and molecular specification of globus pallidus neurons. J Neurosci. 2010;30:2824–2834. doi: 10.1523/JNEUROSCI.4023-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nóbrega-Pereira S, Kessaris N, Du T, Kimura S, Anderson SA, Marín O. Postmitotic Nkx2-1 controls the migration of telencephalic interneurons by direct repression of guidance receptors. Neuron. 2008;59:733–745. doi: 10.1016/j.neuron.2008.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh WC, Lutzu S, Castillo PE, Kwon HB. De novo synaptogenesis induced by GABA in the developing mouse cortex. Science. 2016;353:1037–1040. doi: 10.1126/science.aaf5206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olah S, Komlosi G, Szabadics J, Varga C, Toth E, Barzo P, Tamas G. Output of neurogliaform cells to various neuron types in the human and rat cerebral cortex. Front Neural Circuits. 2007;1:4. doi: 10.3389/neuro.04.004.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paredes MF, James D, Gil-Perotin S, Kim H, Cotter JA, Ng C, Sandoval K, Rowitch DH, Xu D, McQuillen PS, et al. Extensive migration of young neurons into the infant human frontal lobe. Science. 2016;354 doi: 10.1126/science.aaf7073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul A, Crow M, Raudales R, He M, Gillis J, Huang ZJ. Transcriptional architecture of synaptic communication delineates GABAergic neuron identity. Cell. 2017;171:522–539 e520. doi: 10.1016/j.cell.2017.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen CC. The functional organization of the barrel cortex. Neuron. 2007;56:339–355. doi: 10.1016/j.neuron.2007.09.017. [DOI] [PubMed] [Google Scholar]
- Petros TJ, Bultje RS, Ross ME, Fishell G, Anderson SA. Apical versus basal neurogenesis directs cortical interneuron subclass fate. Cell Reports. 2015;13:1090–1095. doi: 10.1016/j.celrep.2015.09.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petryniak MA, Potter GB, Rowitch DH, Rubenstein JL. Dlx1 and Dlx2 control neuronal versus oligodendroglial cell fate acquisition in the developing forebrain. Neuron. 2007;55:417–433. doi: 10.1016/j.neuron.2007.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilz GA, Shitamukai A, Reillo I, Pacary E, Schwausch J, Stahl R, Ninkovic J, Snippert HJ, Clevers H, Godinho L, et al. Amplification of progenitors in the mammalian telencephalon includes a new radial glial cell type. Nat Commun. 2013;4 doi: 10.1038/ncomms3125. 2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pla R, Borrell V, Flames N, Marín O. Layer acquisition by cortical GABAergic interneurons is independent of Reelin signaling. J Neurosci. 2006;26:6924–6934. doi: 10.1523/JNEUROSCI.0245-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pla R, Stanco A, Howard MA, Rubin AN, Vogt D, Mortimer N, Cobos I, Potter GB, Lindtner S, Price JD, et al. Dlx1 and Dlx2 promote interneuron GABA synthesis, synaptogenesis, and dendritogenesis. Cereb Cortex. 2017:1–19. doi: 10.1093/cercor/bhx241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priya R, Paredes MF, Karayannis T, Yusuf N, Liu X, Jaglin X, Graef I, Alvarez-Buylla A, Fishell G. Activity regulates cell death within cortical interneurons through a Calcineurin-dependent mechanism. Cell Reports. 2018;22:1695–1709. doi: 10.1016/j.celrep.2018.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pronneke A, Scheuer B, Wagener RJ, Mock M, Witte M, Staiger JF. Characterizing VIP neurons in the barrel cortex of VIPcre/tdTomato mice reveals layer-specific differences. Cereb Cortex. 2015;25:4854–4868. doi: 10.1093/cercor/bhv202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakic P. Specification of cerebral cortical areas. Science. 1988;241:170–176. doi: 10.1126/science.3291116. [DOI] [PubMed] [Google Scholar]
- Rudy B, Fishell G, Lee S, Hjerling-Leffler J. Three groups of interneurons account for nearly 100% of neocortical GABAergic neurons. Dev Neurobiol. 2011;71:45–61. doi: 10.1002/dneu.20853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rymar VV, Sadikot AF. Laminar fate of cortical GABAergic interneurons is dependent on both birthdate and phenotype. J Comp Neurol. 2007;501:369–380. doi: 10.1002/cne.21250. [DOI] [PubMed] [Google Scholar]
- Sánchez-Alcaniz JA, Haege S, Mueller W, Pla R, Mackay F, Schulz S, Lopez-Bendito G, Stumm R, Marín O. Cxcr7 controls neuronal migration by regulating chemokine responsiveness. Neuron. 2011;69:77–90. doi: 10.1016/j.neuron.2010.12.006. [DOI] [PubMed] [Google Scholar]
- Sandberg M, Flandin P, Silberberg S, Su-Feher L, Price JD, Hu JS, Kim C, Visel A, Nord AS, Rubenstein JLR. Transcriptional networks controlled by NKX2-1 in the development of forebrain GABAergic neurons. Neuron. 2016;91:1260–1275. doi: 10.1016/j.neuron.2016.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd GM, Pologruto TA, Svoboda K. Circuit analysis of experience-dependent plasticity in the developing rat barrel cortex. Neuron. 2003;38:277–289. doi: 10.1016/s0896-6273(03)00152-1. [DOI] [PubMed] [Google Scholar]
- Sheth AN, Bhide PG. Concurrent cellular output from two proliferative populations in the early embryonic mouse corpus striatum. J Comp Neurol. 1997;383:220–230. [PubMed] [Google Scholar]
- Silberberg G, Markram H. Disynaptic inhibition between neocortical pyramidal cells mediated by Martinotti cells. Neuron. 2007;53:735–746. doi: 10.1016/j.neuron.2007.02.012. [DOI] [PubMed] [Google Scholar]
- Silva CG, Peyre E, Adhikari MH, Tielens S, Tanco S, Van Damme P, Magno L, Krusy N, Agirman G, Magiera MM, et al. Cell-intrinsic control of interneuron migration drives cortical morphogenesis. Cell. 2018;172:1063–1078 e1019. doi: 10.1016/j.cell.2018.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skene NG, Bryois J, Bakken TE, Breen G, Crowley JJ, Gaspar HA, Giusti-Rodriguez P, Hodge RD, Miller JA, Munoz-Manchado AB, et al. Genetic identification of brain cell types underlying schizophrenia. Nat Genet. 2018;50:825–833. doi: 10.1038/s41588-018-0129-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somogyi P, Freund TF, Cowey A. The axo-axonic interneuron in the cerebral cortex of the rat, cat and monkey. Neuroscience. 1982;7:2577–2607. doi: 10.1016/0306-4522(82)90086-0. [DOI] [PubMed] [Google Scholar]
- Sousa AMM, Zhu Y, Raghanti MA, Kitchen RR, Onorati M, Tebbenkamp ATN, Stutz B, Meyer KA, Li M, Kawasawa YI, et al. Molecular and cellular reorganization of neural circuits in the human lineage. Science. 2017;358:1027–1032. doi: 10.1126/science.aan3456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southwell DG, Paredes MF, Galvao RP, Jones DL, Froemke RC, Sebe JY, Alfaro-Cervello C, Tang Y, Garcia-Verdugo JM, Rubenstein JL, et al. Intrinsically determined cell death of developing cortical interneurons. Nature. 2012;491:109–113. doi: 10.1038/nature11523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiegel I, Mardinly AR, Gabel HW, Bazinet JE, Couch CH, Tzeng CP, Harmin DA, Greenberg ME. Npas4 regulates excitatory-inhibitory balance within neural circuits through cell-type-specific gene programs. Cell. 2014;157:1216–1229. doi: 10.1016/j.cell.2014.03.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stambolic V, Suzuki A, de la Pompa JL, Brothers GM, Mirtsos C, Sasaki T, Ruland J, Penninger JM, Siderovski DP, Mak TW. Negative regulation of PKB/Akt-dependent cell survival by the tumor suppressor PTEN. Cell. 1998;95:29–39. doi: 10.1016/s0092-8674(00)81780-8. [DOI] [PubMed] [Google Scholar]
- Stühmer T, Anderson SA, Ekker M, Rubenstein JL. Ectopic expression of the Dlx genes induces glutamic acid decarboxylase and Dlx expression. Development. 2002;129:245–252. doi: 10.1242/dev.129.1.245. [DOI] [PubMed] [Google Scholar]
- Stumm R, Kolodziej A, Schulz S, Kohtz JD, Hollt V. Patterns of SDF-1alpha and SDF-1gamma mRNAs, migration pathways, and phenotypes of CXCR4-expressing neurons in the developing rat telencephalon. J Comp Neurol. 2007;502:382–399. doi: 10.1002/cne.21336. [DOI] [PubMed] [Google Scholar]
- Sussel L, Marín O, Kimura S, Rubenstein JL. Loss of Nkx2.1 homeobox gene function results in a ventral to dorsal molecular respecification within the basal telencephalon: evidence for a transformation of the pallidum into the striatum. Development. 1999;126:3359–3370. doi: 10.1242/dev.126.15.3359. [DOI] [PubMed] [Google Scholar]
- Taniguchi H, Lu J, Huang ZJ. The spatial and temporal origin of chandelier cells in mouse neocortex. Science. 2013;339:70–74. doi: 10.1126/science.1227622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tasic B, Menon V, Nguyen TN, Kim TK, Jarsky T, Yao Z, Levi B, Gray LT, Sorensen SA, Dolbeare T, et al. Adult mouse cortical cell taxonomy revealed by single cell transcriptomics. Nat Neurosci. 2016;19:335–346. doi: 10.1038/nn.4216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tasic B, Yao Z, Smith KA, Graybuck L, Nguyen TN, Bertagnolli D, Goldy J, Garren E, Economo MN, Viswanathan S, et al. Shared and distinct transcriptomic cell types across neocortical areas. bioRxiv. 2017 doi: 10.1038/s41586-018-0654-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiveron MC, Rossel M, Moepps B, Zhang YL, Seidenfaden R, Favor J, Konig N, Cremer H. Molecular interaction between projection neuron precursors and invading interneurons via stromal-derived factor 1 (CXCL12)/CXCR4 signaling in the cortical subventricular zone/intermediate zone. J Neurosci. 2006;26:13273–13278. doi: 10.1523/JNEUROSCI.4162-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomioka R, Okamoto K, Furuta T, Fujiyama F, Iwasato T, Yanagawa Y, Obata K, Kaneko T, Tamamaki N. Demonstration of long-range GABAergic connections distributed throughout the mouse neocortex. Eur J Neurosci. 2005;21:1587–1600. doi: 10.1111/j.1460-9568.2005.03989.x. [DOI] [PubMed] [Google Scholar]
- Torigoe M, Yamauchi K, Kimura T, Uemura Y, Murakami F. Evidence That the Laminar Fate of LGE/CGE-Derived Neocortical Interneurons Is Dependent on Their Progenitor Domains. J Neurosci. 2016;36:2044–2056. doi: 10.1523/JNEUROSCI.3550-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tosches MA, Yamawaki TM, Naumann RK, Jacobi AA, Tushev G, Laurent G. Evolution of pallium, hippocampus, and cortical cell types revealed by single-cell transcriptomics in reptiles. Science. 2018;360:881–888. doi: 10.1126/science.aar4237. [DOI] [PubMed] [Google Scholar]
- Tremblay R, Lee S, Rudy B. GABAergic interneurons in the neocortex: From cellular properties to circuits. Neuron. 2016;91:260–292. doi: 10.1016/j.neuron.2016.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tricoire L, Pelkey KA, Daw MI, Sousa VH, Miyoshi G, Jeffries B, Cauli B, Fishell G, McBain CJ. Common origins of hippocampal Ivy and nitric oxide synthase expressing neurogliaform cells. J Neurosci. 2010;30:2165–2176. doi: 10.1523/JNEUROSCI.5123-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripodi M, Filosa A, Armentano M, Studer M. The COUP-TF nuclear receptors regulate cell migration in the mammalian basal forebrain. Development. 2004;131:6119–6129. doi: 10.1242/dev.01530. [DOI] [PubMed] [Google Scholar]
- Tuncdemir SN, Wamsley B, Stam FJ, Osakada F, Goulding M, Callaway EM, Rudy B, Fishell G. Early somatostatin interneuron connectivity mediates the maturation of deep layer cortical circuits. Neuron. 2016;89:521–535. doi: 10.1016/j.neuron.2015.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valcanis H, Tan SS. Layer specification of transplanted interneurons in developing mouse neocortex. J Neurosci. 2003;23:5113–5122. doi: 10.1523/JNEUROSCI.23-12-05113.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Berghe V, Stappers E, Vandesande B, Dimidschstein J, Kroes R, Francis A, Conidi A, Lesage F, Dries R, Cazzola S, et al. Directed migration of cortical interneurons depends on the cell-autonomous action of sip1. Neuron. 2013;77:70–82. doi: 10.1016/j.neuron.2012.11.009. [DOI] [PubMed] [Google Scholar]
- Vogt D, Hunt RF, Mandal S, Sandberg M, Silberberg SN, Nagasawa T, Yang Z, Baraban SC, Rubenstein JL. Lhx6 directly regulates Arx and CXCR7 to determine cortical interneuron fate and laminar position. Neuron. 2014;82:350–364. doi: 10.1016/j.neuron.2014.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Engelhardt J, Khrulev S, Eliava M, Wahlster S, Monyer H. 5-HT(3A) receptor-bearing white matter interstitial GABAergic interneurons are functionally integrated into cortical and subcortical networks. J Neurosci. 2011;31:16844–16854. doi: 10.1523/JNEUROSCI.0310-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vullhorst D, Neddens J, Karavanova I, Tricoire L, Petralia RS, McBain CJ, Buonanno A. Selective expression of ErbB4 in interneurons, but not pyramidal cells, of the rodent hippocampus. J Neurosci. 2009;29:12255–12264. doi: 10.1523/JNEUROSCI.2454-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wamsley B, Fishell G. Genetic and activity-dependent mechanisms underlying interneuron diversity. Nat Rev Neurosci. 2017;18:299–309. doi: 10.1038/nrn.2017.30. [DOI] [PubMed] [Google Scholar]
- Wang B, Long JE, Flandin P, Pla R, Waclaw RR, Campbell K, Rubenstein JL. Loss of Gsx1 and Gsx2 function rescues distinct phenotypes in Dlx1/2 mutants. J Comp Neurol. 2013;521:1561–1584. doi: 10.1002/cne.23242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Dye CA, Sohal V, Long JE, Estrada RC, Roztocil T, Lufkin T, Deisseroth K, Baraban SC, Rubenstein JL. Dlx5 and Dlx6 regulate the development of parvalbumin-expressing cortical interneurons. J Neurosci. 2010;30:5334–5345. doi: 10.1523/JNEUROSCI.5963-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Li G, Stanco A, Long JE, Crawford D, Potter GB, Pleasure SJ, Behrens T, Rubenstein JL. CXCR4 and CXCR7 have distinct functions in regulating interneuron migration. Neuron. 2011;69:61–76. doi: 10.1016/j.neuron.2010.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Toledo-Rodriguez M, Gupta A, Wu C, Silberberg G, Luo J, Markram H. Anatomical, physiological and molecular properties of Martinotti cells in the somatosensory cortex of the juvenile rat. Journal of Physiology. 2004;561:65–90. doi: 10.1113/jphysiol.2004.073353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wehr M, Zador AM. Balanced inhibition underlies tuning and sharpens spike timing in auditory cortex. Nature. 2003;426:442–446. doi: 10.1038/nature02116. [DOI] [PubMed] [Google Scholar]
- Whissell PD, Cajanding JD, Fogel N, Kim JC. Comparative density of CCK- and PV-GABA cells within the cortex and hippocampus. Front Neuroanat. 2015;9:124. doi: 10.3389/fnana.2015.00124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wichterle H, Alvarez-Dolado M, Erskine L, Alvarez-Buylla A. Permissive corridor and diffusible gradients direct medial ganglionic eminence cell migration to the neocortex. Proc Natl Acad Sci USA. 2003;100:727–732. doi: 10.1073/pnas.242721899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wichterle H, Turnbull DH, Nery S, Fishell G, Alvarez-Buylla A. In utero fate mapping reveals distinct migratory pathways and fates of neurons born in the mammalian basal forebrain. Development. 2001;128:3759–3771. doi: 10.1242/dev.128.19.3759. [DOI] [PubMed] [Google Scholar]
- Wonders CP, Anderson SA. The origin and specification of cortical interneurons. Nat Rev Neurosci. 2006;7:687–696. doi: 10.1038/nrn1954. [DOI] [PubMed] [Google Scholar]
- Wonders CP, Taylor L, Welagen J, Mbata IC, Xiang JZ, Anderson SA. A spatial bias for the origins of interneuron subgroups within the medial ganglionic eminence. Dev Biol. 2008;314:127–136. doi: 10.1016/j.ydbio.2007.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong FK, Bercsenyi K, Sreenivasan V, Portales A, Fernandez-Otero M, Marin O. Pyramidal cell regulation of interneuron survival sculpts cortical networks. Nature. 2018;557:668–673. doi: 10.1038/s41586-018-0139-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H, Jeong HY, Tremblay R, Rudy B. Neocortical somatostatin-expressing GABAergic interneurons disinhibit the thalamorecipient layer 4. Neuron. 2013;77:155–167. doi: 10.1016/j.neuron.2012.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Q, Cobos I, De La Cruz E, Rubenstein JL, Anderson SA. Origins of cortical interneuron subtypes. J Neurosci. 2004;24:2612–2622. doi: 10.1523/JNEUROSCI.5667-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Q, Tam M, Anderson SA. Fate mapping Nkx2.1-lineage cells in the mouse telencephalon. J Comp Neurol. 2008;506:16–29. doi: 10.1002/cne.21529. [DOI] [PubMed] [Google Scholar]
- Xu X, Roby KD, Callaway EM. Mouse cortical inhibitory neuron type that coexpresses somatostatin and calretinin. J Comp Neurol. 2006;499:144–160. doi: 10.1002/cne.21101. [DOI] [PubMed] [Google Scholar]
- Yanagida M, Miyoshi R, Toyokuni R, Zhu Y, Murakami F. Dynamics of the leading process, nucleus, and Golgi apparatus of migrating cortical interneurons in living mouse embryos. Proc Natl Acad Sci USA. 2012;109:16737–16742. doi: 10.1073/pnas.1209166109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yau HJ, Wang HF, Lai C, Liu FC. Neural development of the neuregulin receptor ErbB4 in the cerebral cortex and the hippocampus: preferential expression by interneurons tangentially migrating from the ganglionic eminences. Cereb Cortex. 2003;13:252–264. doi: 10.1093/cercor/13.3.252. [DOI] [PubMed] [Google Scholar]
- Ye Z, Mostajo-Radji MA, Brown JR, Rouaux C, Tomassy GS, Hensch TK, Arlotta P. Instructing perisomatic inhibition by direct lineage reprogramming of neocortical projection neurons. Neuron. 2015;88:475–483. doi: 10.1016/j.neuron.2015.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeisel A, Munoz-Manchado AB, Codeluppi S, Lonnerberg P, La Manno G, Jureus A, Marques S, Munguba H, He L, Betsholtz C, et al. Cell types in the mouse cortex and hippocampus revealed by single-cell RNA-seq. Science. 2015;347:1138–1142. doi: 10.1126/science.aaa1934. [DOI] [PubMed] [Google Scholar]
- Zeng H, Sanes JR. Neuronal cell-type classification: challenges, opportunities and the path forward. Nat Rev Neurosci. 2017;18:530–546. doi: 10.1038/nrn.2017.85. [DOI] [PubMed] [Google Scholar]
- Zimmer G, Rudolph J, Landmann J, Gerstmann K, Steinecke A, Gampe C, Bolz J. Bidirectional ephrinB3/EphA4 signaling mediates the segregation of medial ganglionic eminence- and preoptic area-derived interneurons in the deep and superficial migratory stream. J Neurosci. 2011;31:18364–18380. doi: 10.1523/JNEUROSCI.4690-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]