Abstract
Cell-cell and cell-matrix adhesion are critical processes for the formation and maintenance of tissue patterns during development, as well as control of invasion and metastasis of cancer cells. Although great strides have been made regarding our understanding of the processes that play a role in cell adhesion and cell movement, the precise mechanisms by which diverse signaling events regulate cell and tissue architecture are poorly understood. One group of cell surface molecules, Eph receptor tyrosine kinases, and their membrane-bound ligands, ephrins, are key regulators in these processes. It is the ability of Eph/ephrin signaling pathways to regulate cell-cell adhesion and motility that establishes this family as a formidable system for regulating tissue separation and morphogenesis. Moreover, the de-regulation of this signaling system is linked to the promotion of more aggressive and metastatic tumors in humans.
Keywords: EphA, EphB, ephrin-A, ephrin-B, Adhesion, Repulsion, Metastasis, Morphogenesis, Cell Movement, Review
2. INTRODUCTION
Members of the Eph/ephrin family have been implicated in regulating numerous morphogenetic processes such as axon outgrowth, neural crest and retinal progenitor cell migration, hindbrain segmentation, skeletal patterning, and angiogenesis (1, 2). Interactions between the Eph receptor tyrosine kinases residing on one cell with their membrane bound ligands (ephrins) on another cell results in bi-directional signaling, where both molecules transmit intracellular signals upon cell-cell contact. Although evidence is emerging that both Eph receptors and ligands ultimately affect Rho family signal transduction, various signaling molecules and pathways intersect with Eph receptor or ligand signaling, and further studies are needed to define the Eph/ephrin signal transduction systems. Eph/ephrin signaling emanating from cell-cell contact events during development leads to cell sorting and boundary formation between receptor and ligand bearing cells (3). When motile ligand or receptor-bearing cells come in contact with cells bearing the cognate receptor or ligand, the response is often adhesion or repulsion. Alternative growth factors and signaling pathways can mediate or regulate Eph/ephrin signaling to assist in controlling the movement and positioning of the cognate receptor or ligand-bearing cells (1). These ligands and receptors play important roles in several morphogenetic events during development, but when deregulated can lead to cancer invasion and metastasis. The deregulation of this signaling system is linked to the promotion of more aggressive and metastatic tumor phenotypes in a large variety of human cancers, including breast, lung, prostate, colon, and melanoma (4, 5). Recent data show that members of the Eph/ephrin family mediate cell-cell interactions both in tumor cells and in the tumor microenvironment (ie. stroma and vasculature) (6, 7). Thus, gaining an understanding of the mechanism and pathways that mediate Eph receptor and ephrin signaling is likely to have biomedical importance. In this review we discuss the role of Eph receptors and ligands and how their signaling affects cell adhesion.
3. TWO CLASSES OF RECEPTORS AND LIGANDS EXIST – GPI-LINKED (A) & TRANSMEMBRANE (B)
Eph receptors are transmembrane receptor tyrosine kinases possessing an extracellular domain that includes an N-terminal ligand-binding domain, a cysteine-rich EGF-like domain, and two fibronectin type III motifs. The intracellular domain contains several tyrosine phosphorylation sites, a single kinase domain, and two protein-protein interaction domains: A Sterile Alpha Motif (SAM) and a C-terminal PDZ binding motif (Figure 1 bottom). These receptors are divided into two subclasses (A & B) by sequence similarities and binding specificity towards two subclasses of ligands (A & B) known as ephrins. The ephrins are all membrane-bound proteins with the A subclass being glycosylphosphatidylinositol (GPI)-linked to the membrane, and the B subclass being transmembrane proteins with a short cytoplasmic domain. Generally, the A-type receptors have specificity toward A-type ligands, while B-types bind to their cognate receptors. The exceptions to this rule are EphA4 and EphB2 which can also bind all ephrin-Bs and ephrin-A5, respectively (3, 8).
Figure 1.
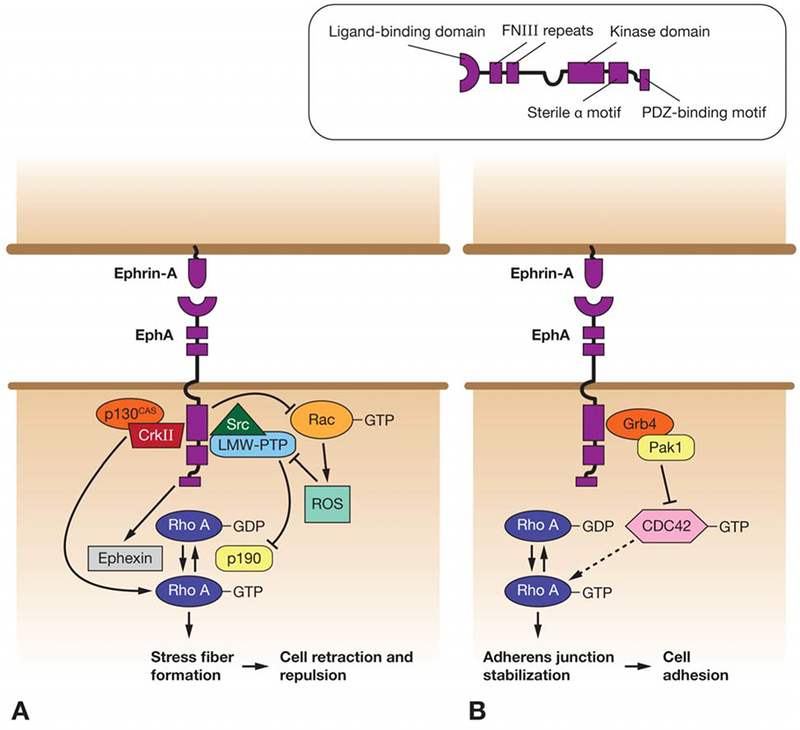
Signaling pathways linking EphA and RhoA in regulation of cell adhesion/repulsion. A) In tumor cell lines and neurons, EphA is thought to stimulate RhoA activation, which causes cell de-adhesion/repulsion via increased stress fiber formation. This can occur via several pathways, including regulation of both Rho GEFs (Ephexin) and GAPs (p190). B) In Xenopus epithelia, RhoA stabilizes pre-existing cell-cell adhesions via interactions with cadherins. EphA forward signaling may antagonize this function via a pathway involving Pak1 kinase and Cdc42, causing loss of adhesion.
4. EPHA FORWARD SIGNALING IN ADHESION
Activation of signal transduction pathways downstream of Eph receptors, following ligand binding, is referred to as ‘forward’ signaling, to distinguish it from ‘reverse’ signaling, which may occur within the ephrin-bearing cell as a result of the same interaction. In common with other receptor tyrosine kinases, ligand binding induces EphA trans-phosphorylation, and this is required for several forward signaling pathways. However, other pathways are phosphorylation-independent. This section will discuss the role of signal transduction downstream of EphA receptors in the regulation of cell-cell and cell-matrix adhesion.
4.1. EphA forward signaling in cell-cell adhesion
The first described function of Eph/ephrin signaling was in the control of axon guidance during nervous system development. Several Eph proteins were found to be expressed on developing axons during their migration (9, 10). Subsequent identification of EphA ligands, the ephrin-As, using soluble receptor affinity methods, showed several of them also to be expressed in the developing nervous system (11, 12). Moreover, ephrin-A5, previously known as RAGS, which is expressed in the optic tectum, was shown to cause growth cone collapse and repulsion of retinal ganglion cells (13). Another article in the same issue of Cell demonstrated the existence of a nasal-to-temporal gradient of EphA3 across the retina, and a complementary gradient of its ligand, ephrin-A2, across the anteroposterior axis of the tectum (14). These and further studies support a role for repulsive EphA/ephrin forward signaling in retinotectal topographic mapping, whereby the combination of levels of EphA receptor in the projecting retinal ganglion cell axons, and of their ephrin-A ligands in the target tissue, the tectum, precisely determines the point to which each axon projects, and thus guides them to their correct position ((15, 16), and references therein). It is now thought that Eph/ephrin repulsive signaling may play a similar role in topographic mapping of other regions of the nervous system, including projections from the retina to lateral geniculate nuclei, hippocampus to lateral septum, thalamus to cortex and from spinal cord motor neurons to muscles ((16) and references therein). In addition, cell-cell repulsion mediated by EphA forward signaling has several effects on cell movement and migration outside the nervous system, as discussed below.
Adhesion between cells is mediated by several types of intercellular adhesion molecules, organized into various different structures on cell surfaces. The best understood and perhaps most critical of these are members of the cadherin family, which form adherens junctions (17, 18). Members of the Rho subfamily of small GTPases, including RhoA, Rac1 and Cdc42, have been shown to play important roles in regulation of the formation and maintenance of cadherin-dependent adhesions. For example, inhibition of Rho using the bacterial toxin C3 transferase causes rapid loss of cadherins from adherens junctions, an effect which is reversible by the addition of C3-resistant RhoA (19). Rac and Cdc42 are thought to act primarily on the actin cytoskeleton, whilst Rho is implicated in regulation of myosin (17). Thus, one major role for RhoA is regulation of stress fiber formation, and consequently of cell retraction, which indirectly influences cell-cell adhesions. However, Rho is also thought to directly regulate adherens junctions, since cadherin removal from junctions upon Rho inhibition occurs prior to cell retraction (18). Rho GTPases have been shown to act as intermediates in the promotion of cell repulsion by EphA forward signaling, in a number of different systems and cellular contexts. Interestingly, it appears that this regulation may occur by a number of different mechanisms. In the context of axon guidance, the first mechanism to be characterized was the regulation of the RhoGEF, ephexin1, in response to EphA signaling in neurons (20, 21). Ephexin can function as a GTP exchange factor (GEF) for Rho, Rac and Cdc42. However, tyrosine phosphorylation of ephexin by activated EphA receptors enhances its activity towards Rho, but does not affect its regulation of Rac or Cdc42, thus shifting the balance of GTPase activities towards RhoA. In this model, it is suggested that this favors repulsion over extension of axons, since RhoA-induced stress fibers stimulate cell contraction, whilst Rac1 and Cdc42 induce formation of membrane protrusions (21). A recent study has shown that the L1 family neural cell adhesion molecules, L1 and CHL1, interact with EphA receptors and are required for growth cone collapse of thalamic axons in response to ephrin-A5 (22).
EphA family members have been shown to be up-regulated in a wide range of tumor types, including breast, prostate, colon, lung, and melanoma, and this up-regulation correlates with increased metastatic potential and poor prognosis (23–26). Recent studies indicate that these effects result from cell-cell repulsion mediated by EphA forward signaling, upon activation by ephrins. A common theme of these studies is the stimulation of RhoA activity downstream of EphA, which is thought to lead to increased cell rounding through stimulation of stress fiber formation and loss of cell-cell contacts, and therefore to increase invasive potential. In particular, Parri et al (2009) describe EphA2 activation as causing a transformation of melanoma cells from a ‘mesenchymal’ type of cell motility, which involves extracellular matrix (ECM) proteolysis and requires inhibition of RhoA and Rac1 activation, to ‘amoeboid’ motility, which conversely requires activation of RhoA, but inhibition of Rac1 (26). However, there is some variation in the pathways suggested to link these various components (summarized in Figure 1A). Whilst in the context of axon guidance Rho activity appears to be regulated via the RhoGEF ephexin, several studies in tumor cell lines have implicated the Rho GTPase Activating Protein, p190RhoGAP, in regulation of RhoA downstream of EphA activation. Two mechanisms have been proposed for p190 regulation. In the first, from a study of prostatic carcinoma cells, generation of reactive oxygen species (ROS) by Rac1 GTPase is inhibited by EphA activation. This leads to derepression of the ROS-sensitive protein tyrosine phosphatase, LMW-PTP, causing dephosphorylation of its substrate, p190RhoGAP, and hence RhoA activation (27). In a separate study of mammary epithelial cells, Fang et al (2008) show that both LMW-PTP and Src kinase interact directly with EphA2. They suggest a model whereby activation of EphA2 causes recruitment of both molecules, promoting phosphorylation of LMW-PTP by Src, and subsequent regulation of p190 and RhoA (28). Src has also been proposed to promote Rho activation by forming a complex with another kinase, FAK, following stimulation of both by activated EphA (29). Finally, a study of melanoma and human kidney cell lines demonstrated recruitment of the adapter protein, CrkII, to activated EphA3 receptors. In turn, CrkII, possibly acting in a complex with p130CAS, is suggested to recruit and activate RhoA, causing cell retraction/repulsion (30).
Developmental studies conducted on Xenopus embryos also demonstrate loss of cell-cell adhesion downstream of EphA forward signaling (31–34). Several of these studies implicate RhoA in this effect. Surprisingly, however, in this context, EphA signaling appears to inhibit RhoA, which in turn is described as a promoter, rather than an inhibitor, of cell-cell adhesion. A detailed model for the pathway linking EphA to RhoA inhibition is proposed by Bisson et al (31) (Figure 1B). In this model, activated EphA acts via the adapter protein Nckbeta (Grb4) to activate Pak1 kinase, by recruiting it to the plasma membrane. Whilst activated Rho, Rac and Cdc42 all rescue the loss of adhesion induced by EphA over-expression, only levels of active RhoA are reduced by EphA or the Pak1 GTPase binding domain. Thus, it is suggested that Pak1 acts by sequestering, rather than reducing levels of, Cdc42. Since Rho family GTPases have been shown to be linked in a cascade (35, 36), this in turn may lead to RhoA inhibition, and therefore, according to these studies, to loss of adhesion. The apparently opposite effects of RhoA activation on the cytoskeleton may be explained by variability between tissue and cell types, in particular in the existence and strength of pre-existing adhesive structures. Whilst studies of axon guidance and tumor cell migration are generally concerned with the generation, or not, of cell-cell contacts between previously unattached cells, studies in Xenopus embryos have focused on epithelial cell types, which already have strong intercellular adhesions. Thus, it may be that the generation of stress fibres by RhoA and subsequent cell rounding in migratory cells is sufficient to prevent the formation of cell-cell adhesions, and thus causes repulsion, whilst pre-existing adhesions, such as those between epithelial cells, are actually strengthened by RhoA-mediated stabilization of cadherin complexes.
T-lymphocytes express both EphA (EphA1 and EphA4) and ephrin-A (ephrin-A1, 2 and 4) family proteins, and ephrin-A1 is also expressed by high endothelial venule (HEV) cells (37, 38). Stimulation of EphA forward signaling in T-lymphocytes was shown to stimulate chemotaxis and reduce adhesion to endothelial cells, as well as to fibronectin, an integrin ligand and ECM component. In addition, increased actin polymerization was observed. Although integrin-mediated adhesion is generally associated with cell-ECM, rather than cell-cell interactions, it has been shown that T-lymphocyte-endothelial cell interactions are at least partially mediated by integrins (39). Thus, in this case, the loss of cell-cell adhesion downstream of EphA activation may be due to effects on integrin-based adhesive structures, rather than on cadherins. Increased phosphorylation of the FAK-related kinase, Pyk2, was also observed (37). Pyk2 has previously been implicated in linking signal transduction to cytoskeletal regulation (40), and thus may mediate the increased actin polymerization observed in these cells. Pyk2 may act via Rho family GTPases, although their role has not been investigated in this system.
As demonstrated by the above examples, EphA forward signaling results in inhibition of cell-cell adhesion in a wide range of cell types, and apparently acting via several different, though related, pathways (Table 1, Figures 1–3). However, one case in which EphA forward signaling appears to actually promote cell-cell adhesion is in the aggregation of blood platelets during thrombus formation (Figure 2). Platelet aggregation is mediated by a beta3 integrin, integrin alphaIIbeta3. On activated platelets, integrin alphaIIbeta3 binds von Willebrand factor, and fibronectin (41). Adhesion via fibronectin occurs indirectly, with fibronectin acting to crosslink alphaIIbeta3-expressing platelets. In addition, von Willebrand factor is expressed on platelet surfaces and promotes cell-cell adhesion through direct interaction with alphaIIbeta3. Platelets also express the receptors EphA4 and EphB1, and their ligand ephrin-B1 (42). Recent studies show that clustering of EphA4 or ephrin-B1 causes increased adhesion of platelets to collagen or fibronectin, as well as cytoskeletal reorganization, an effect which is blocked by inhibition of Eph/ephrin interactions (42, 43). These effects may be mediated via the kinases Fyn and Lyn, and the adhesion molecule, L1, which form complexes with EphA4 in activated platelets. L1 may interact with itself or integrin alphaIIbeta3 on adjacent platelets to stimulate adhesion (Figure 2). In addition, increased levels of the GTPase Rap1B, which has previously been implicated in integrin activation, were observed. A further study demonstrated direct interaction of EphA4 with integrin alphaIIbbeta3 in resting platelets, and showed increased surface expression upon platelet activation (43). Ephrin activation of EphA4 results in phosphorylation of the beta3 cytoplasmic domain of the integrin, promoting its interaction with myosin which is required for clot retraction. Thus, it is suggested that ephrin/Eph forward signaling, stimulated by initial transient contacts following platelet activation, activates downstream pathways involving integrins and cytoskeletal regulation, and this allows the formation of stable platelet aggregates.
Table 1.
Effects of EphA forward signaling on cell-ECM adhesion in different cell types
| Cell type | Ligand/ receptor | Effect of EphA forward signaling on cell-ECM adhesion | Proposed mechanism | Reference |
|---|---|---|---|---|
| Neurons (dendritic spines) | EphA4/ ephrin-A3 | ⇓ | Activation of SPAR (Rap1 GAP), leading to integrin inactivation (Figure 3) | 115, 17, 135 |
| Prostatic carcinoma (PC-3) |
EphA2/ ephrin-A1 | ⇓ |
|
22, 111, 145 |
| Vascular smooth muscle cells (VSMCs) | EphA2/ ephrin-A1 | ⇓ | Inhibition of Rac leading to loss of lamellipodia/reduced integrin recruitment to lamellipodia. | 43 |
| NIH 3T3 cells |
|
⇑ |
|
24 66 |
| Astrocytes | EphA4/ ephrin-A5 | ⇑ |
|
133 |
| Langerhans cells | EphA2, EphA4 & EphA/ ephrin-A3 & ephrin-B1 | ⇑ | Integrin activation. | 41 |
Figure 3.
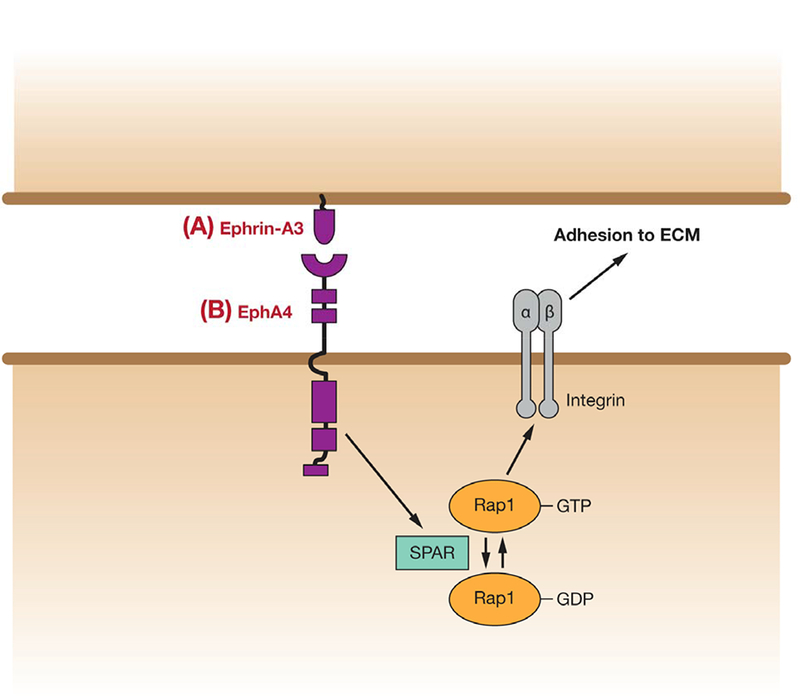
Inhibition of cell-ECM adhesion by EphA4/ephrin-A3 in dendritic spines. EphA4 activation stimulates activity of the Rap GAP, SPAR. Consequent reduction of Rap1-GTP levels inhibits integrin-mediated adhesion to the extracellular matrix, resulting in dendritic spine retraction, and thus regulation of neuron guidance.
Figure 2.
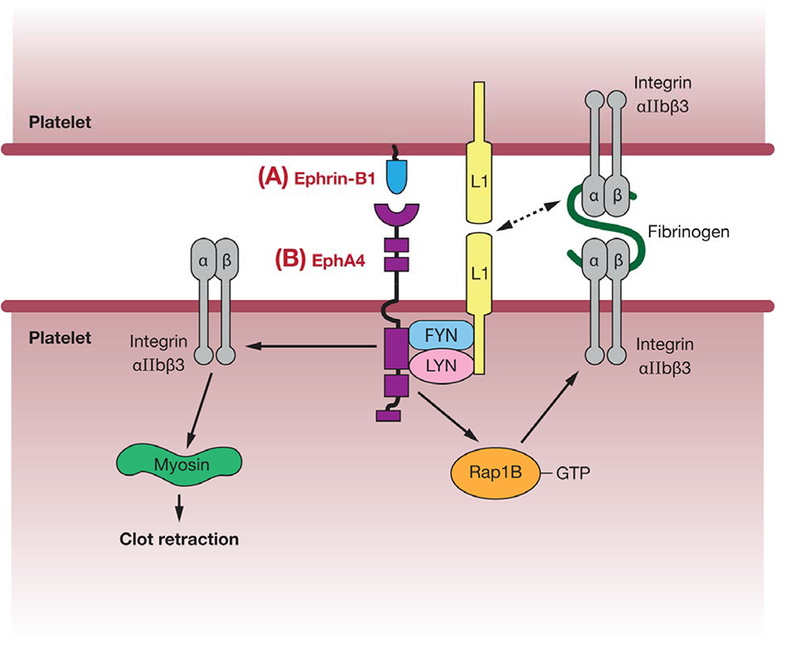
Mechanisms for EphA4-mediated stimulation of platelet aggregation. Activation of EphA4 through binding to its ligand, ephrin-B1, on adjacent platelets may increase their adhesion via several pathways. These include formation of a protein complex leading to activation of the cell adhesion molecule L1; promotion of integrin alphaIIbeta3-mediated adhesion via Rap1B activation; or direct stimulation of integrin alphaIIbeta3 binding to myosin, leading to clot retraction.
4.2. EphA forward signaling in cell-ECM adhesion
Adhesion of cells to the ECM is regulated by members of the integrin family of membrane-bound receptors. Integrins comprise a large family which can be divided into four groups based on ligand binding specificity (44). Three of these groups interact with ECM components: one group binds collagens, one binds laminins and a third group binds ligands containing the RGD recognition motif, such as fibronectin. The fourth group binds leukocyte-specific receptors including the immunoglobulins, ICAMs and VCAMs, and are involved in direct cell-cell interactions between leukocytes and the endothelium, as mentioned above. Several studies have implicated EphA forward signaling in the control of cell-ECM binding, via regulation of integrins. As outlined in the following section, both promotion and inhibition of adhesion have been observed, depending on the cellular context (summarized in Table 1).
In addition to cell-cell adhesion, the guidance and remodeling of neuronal axons and dendrites requires the regulation of cell adhesion to the ECM, and EphA forward signaling has also been implicated in this regulation. In their 2003 paper, Murai et al describe the enriched expression of the EphA4 receptor on the dendritic spines of pyramidal neurons in the mouse hippocampus, and of its receptor, ephrin-A3, in the astrocytes which envelop them (45). They show that EphA4/ephrin-A3 forward signaling is required for correct spine morphology, and activation of this pathway causes spine retraction. Further work by this group investigated the mechanism responsible for this effect (Figure 3) (46). They found that EphA4/ephrin-A3 forward signaling resulted in reduced phosphorylation of several proteins, including Cas, FAK and Pyk2, which are known to be phosphorylated as a result of integrin-mediated adhesion. They further show that inhibition of integrin signaling alone results in similar reduction in length and abnormal morphology of dendritic spines, and that preventing inactivation of integrins can block the effects of EphA4/ephrin-A3 forward signaling on spines. Thus, they suggest that activation of EphA4 in dendritic spines can regulate adhesion to the ECM, and therefore dendrite morphology/remodeling through regulation of integrins. In a further study, SPAR, a GTPase activating protein for the Ras family GTPase, Rap1, was shown to bind EphA4 and to be activated by its stimulation with ephrin-A1 (47). Rap1 is previously known to promote integrin-mediated adhesion in non-neuronal cells (48, 49), and constitutively active Rap1 was shown in this study to block loss of adhesion in response to a soluble form of ephrin-A3 linked to Fc. Thus, one mechanism for the inhibition of integrin-mediated cell-ECM adhesion in neuronal guidance may be via inactivation of Rap1 GTPase, downstream of EphA forward signaling.
Inhibition of integrin-mediated adhesion by EphA forward signaling has also been observed in several other cell types, including prostatic carcinoma (PC-3) cell cultures (27, 50), and in cultures of vascular smooth muscle cells (VSMCs), which express elevated levels of ephrin-A1 and EphA2 during neovascularisation in vivo (51). Two of these studies implicate the small GTPase, Rac, the activation of which is inhibited by treatment with ephrinA1 in both VSMCs and PC-3 cells. Loss of Rac is known to cause loss of lamellipodia, and therefore indirectly inhibit integrin-mediated cell-ECM adhesion (18, 51). However, Rac has also been shown to be involved in the recruitment of activated integrins to lamellipodia, and so Rac inhibition by EphA signaling may also interfere with integrin localization (51, 52). In the case of PC-3 cells, loss of integrin-mediated adhesion was shown to be dependent on EphA kinase activity, although other effects of EphA activation were kinase-independent (53). As mentioned previously, Burricchi et al suggest an additional role for Rac in the generation of reactive oxygen species such as hydrogen peroxide. This is suggested to initiate a pathway leading to RhoA-mediated cell retraction, and is stimulated by integrin signaling but inhibited by active EphA (27). Studies of PC-3 cells also indicate a possible Rac-independent mechanism for EphA inhibition of integrins. Antibodies which lock integrins in an active conformation were shown to block the inhibition of cell adhesion by ephrin-A1-Fc, suggesting that EphA forward signaling may also act through regulation of integrin structure (50). This study also demonstrated de-phosphorylation of FAK and paxillin kinases upon EphA2 activation. This dephosphorylation event was probably accomplished via the protein tyrosine phosphatase, SHP2, which is recruited to activated EphA2. Dissociation of FAK from EphA2 was also observed. These effects probably also contribute to loss of cell-ECM adhesion, in particular focal adhesions, downstream of EphA activation.
In contrast to the work described above, a study using NIH 3T3 cells demonstrated increased phosphorylation of FAK, as well as the adaptor protein, p130cas, and EphA2, concurrent with adhesion to and spreading on ephrinA1-coated surfaces (54). Fibroblasts from FAK or p130cas null mice were found to be defective in ephrinA1-induced cell spreading, indicating that EphA2 forward signaling can promote cell-matrix adhesion through FAK/p130cas phosphorylation and activation (Table 1). This data is supported by a study of astrocytic gliosis, which showed that astrocytes from EphA4 null mice have reduced numbers of focal adhesions and are less adherent, whilst activation of EphA with ephrinA5-Fc increases the number of focal adhesions (55). Astrocytes express EphA4, which is up-regulated upon activation at sites of CNS injury, and has been shown to be involved in inhibiting neuronal regeneration. This probably occurs through promotion of scar tissue formation by astrocytes (astrocytic gliosis), which forms a physical barrier. Thus, increased EphA4 forward signaling may be responsible for the cytoskeletal changes observed in activated astrocytes, which lead to their increased adhesive potential. Reduced phosphorylation of the GTPase activating proteins, Vav2 and Vav3, was also seen in EphA4 null astrocytes, suggesting a possible role for Rho family GTPase regulation (Table 1). Work carried out on Langerhans cells, a subset of antigen-presenting dendritic cells found in epidermal tissues, also demonstrated increased adhesion to fibronectin upon activation of EphA3 forward signaling (56). Langerhans cells express several Eph receptors, including EphA2, EphA4 and EphA7, and epidermal keratinocytes express ephrins, suggesting that Eph/ephrin signaling may be involved in targeting and adhesion of Langerhans cells to their target tissues. Since a peptide containing the RGD integrin recognition motif was found to compete with fibronectin for attachment to these cells, Eph forward signaling is suggested to act via integrin activation in this model (Table 1). Gu and Park (2001) also demonstrate that binding of EphA8 to its ligand promotes integrin-mediated adhesion of NIH 3T3 cells to fibronectin (57). In this case, this effect appears to be independent of EphA8 kinase activity, and instead is due to enhancement of its association with the PI3 kinase isoform, p110gamma. This is suggested to recruit p110gamma to the plasma membrane, where it may influence integrin function indirectly through modification of its lipid substrates.
4.3. Non-canonical EphA-mediated regulation of adhesion
In addition to regulating cell adhesion/repulsion through conventional ephrin-dependent forward signaling, EphA receptors have also been suggested to control adhesion via non-canonical mechanisms in certain contexts. One example of this is the regulation of hindbrain segmentation (discussed in detail later in this review), in which the separation of alternate ephrin-B2- and EphA-expressing rhombomeres appears to be dependent on homotypic affinity between cells expressing the same molecule, rather than repulsion between ephrin- and Eph-expressing cells. Thus, in this instance, adhesion is regulated by an EphA-EphA interaction (58).
There is also limited evidence that Eph-ephrin interactions may sometimes affect adhesion in a manner independent of forward or reverse signaling. An investigation of the role of Eph and ephrins in neural tube fusion suggests a scenario in which interactions between Ephs and ephrins expressed at the tips of the neural folds serve a direct adhesive function, independent of any downstream signal transduction (59). However, the involvement of forward signaling is not ruled out, and the existing literature provides no additional evidence for direct adhesive properties of Eph/ephrin associations.
A final example of regulation of adhesion by non-canonical EphA signaling may be in thrombin-stimulated attachment of leukocytes to the endothelium. In a recent study, thrombin was shown to act through its receptor, PAR-1, and a Src family kinase, to induce tyrosine phosphorylation and activation of EphA2 on endothelial cells (60). This activation was suggested to be independent of ephrin binding, since it was not blocked by exposure to the EphA2 extracellular domain, which inhibits Eph-ephrin associations. EphA2 activation was, in turn, shown to stimulate ICAM-1-mediated leukocyteendothelium adhesion by phosphorylating NFκB, which initiates a pathway leading to increased ICAM-1 expression.
5. EPHB RECEPTOR FORWARD SIGNALING IN ADHESION
There are five B-type Eph receptors, which typically bind to B-type ephrins (61). Activation of Eph may lead to either cell adhesion/attraction or de-adhesion/repulsion in context of cell type and signaling (62). Endocytosis of the Eph/ephrin complex into either Eph- or ephrin-expressing cells is one such mechanism by which cell adhesion is released, allowing them to move to their respective destination. The EphB-ephrin complex is endocytosed in an EphB kinase-dependent manner, preferentially into cells with more adhesive contacts with the substrate and well-developed actin cytoskeleton (63). Loss of cell adhesion initiated by EphB/ephrin-B is observed during developmental processes such as notochord formation where in response to noncanonical Wnt signaling, phosphorylated EphB receptors make a ternary complex with Dvl2 and Daam1, which is transported to the endocytic vesicles in a dynamin-dependent manner. This removal of EphB molecules from the cell surface results in loss of adhesion leading to initiation of convergent extension cell movements (64).
5.1. Role of EphB receptors in cell-matrix adhesion
Cell-matrix adhesion involves interaction between molecules on the surface of cells and components of extracellular matrix. Cell-matrix adhesion regulates the morphology and function of a cell, including cell migration, growth and differentiation and regulates spatiotemporal organization of tissues and organs during embryonic development. Earlier studies have demonstrated the role of EphB receptor-mediated forward signaling, primarily by its kinase activity, in cell matrix adhesion, cytoskeletal organization and activation of MAPK (65). In one such study, Guo et al (2006) examined EphB2 expression in a large series of normal and metastatic colorectal mucosa. They found reduction in EphB2 expression in colon cancer progression. To determine significance of EphB2 in colorectal cancer (CRC) progression, they engineered SW480, human colon adenocarcinoma cell line with low EphB2 expression, to stably express EphB2. Stimulation by ephrinB1-Fc induces cell rounding and inhibits adhesion to fibronectin and laminin. Similar to other cell lines, this ephrinB1-induced inhibition of cell migration is mediated by Rho GTPase as inhibiting Rho effector ROCK, attenuates the effects of ephrinB1-Fc (66). As shown in Figure 4, EphB2 can also mediate cell-matrix interaction by regulating integrin activity through a small GTPase, R-Ras. Cells with activated EphB2 adhere poorly to integrin-coated plates and show phosphorylation of a tyrosine residue in the R-Ras effector domain (67). EphB2 inhibits adhesion by recruiting and phosphorylating R-Ras, which suppresses the ability of R-Ras to support integrin activity (68, 67). Reduced adhesiveness in EphB2 activated cells may facilitate migration and contribute to axonal path finding and tumor cell invasion. Similar to EphB2, stimulation of EphB3 receptors by ephrin-B1 in colorectal epithelial cells inhibits integrin-mediated cell adhesion and induces cell rounding in a kinase-dependent manner. However, EphB3 seems to affect integrin-mediated adhesion by converting integrins to an inactive confirmation, as locking integrin in an active state by beta1-integrin activating antibody 8A2 reversed the effects of EphB3- mediated inhibition of cell adhesion (69). Indeed EphB3 overexpressing HT29 cells are more epithelial than mesenchymal in morphology with cells exhibiting a round morphology and impaired cell-matrix adhesion. These effects seem to be a result of inactivation of Rac1 and Crk, an adaptor protein. Rac1 and Crk are Epithelial-to-Mesenchymal Transition (EMT) effectors and inactivation of Rac-Crk signaling inhibits EMT and promotes Mesenchymal-to-Epithelial Transition (70) (Figure 4).
Figure 4.
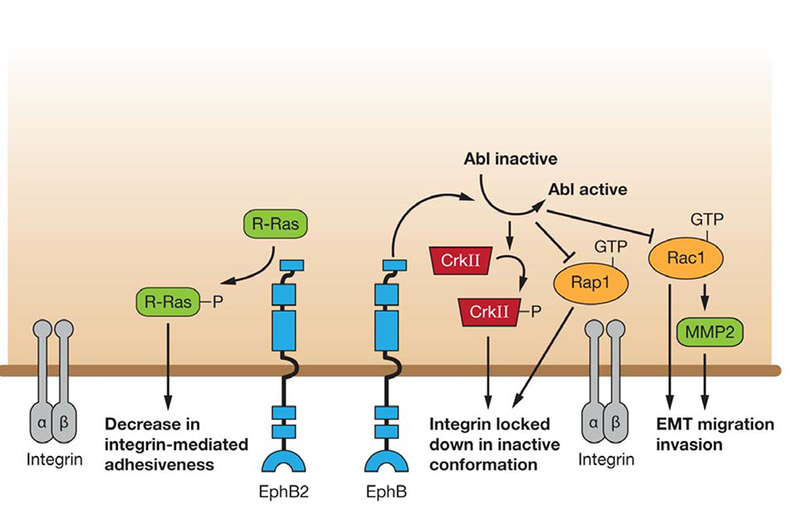
A model of EphB forward signaling regulating cell-ECM adhesion. EphB2 forward signaling phosphorylates R-Ras which results in a decrease in integrin binding to extracellular substrate. EphB also regulates integrin by locking it into an inactive confirmation mediated via the Abl/CrkII pathway and inhibiting Rap1. EphB receptors lead to a mesenchymal to epithelial transition by activating Abl which in turn inhibits Rac1 and MMP2, resulting in inhibition of EMT, migration and invasion.
In breast cancer cell lines (MDA-MB-435c, MDA-MB-231), ephrin-B2 stimulation of EphB4 signaling leads to enhanced phosphorylation of the adaptor protein Crk via Abl-Arg kinase activity, leading to inhibition of Crk-mediated signaling. This reduction in Crk-mediated signaling leads to reduced cell motility, and a corresponding reduction in the MMP-2 metalloprotease levels (71). In a prostate cancer cell line (PC-3), Abl has been shown to down-regulate the small GTPase Rap1, and cause cell rounding and detachment through the Rho-ROCK1 pathway (72) (Figure 4). In addition, EphB– ephrin-B interactions in the HT-29 colon carcinoma cell line promotes a corresponding reduction in Rac1 activity (70), suggesting the possibility that EphB receptors activate Abl, which ultimately inhibits Rap1 and Rac1, leading to a Mesenchymal to Epithelial Transition (MET) and less invasive phenotype (73).
Cell adhesion and migration assays on retinal pigmented epithelium (RPE) show that soluble EphB4, which inhibits EphB4 activation, inhibited cell attachment on fibronectin and basement membrane matrix. These cells show reduced FAK and MAPK phosphorylation which may contribute to the decrease in cell adhesion to extracellular matrix (74,75). Studies using xenografts of a murine model of intestinal tumorigenesis show that inactivation of a single allele of EphB4 results in transcriptional reprogramming of several genes, including those involved in cell proliferation, remodeling of the extracellular matrix, and cell attachment to the basement membrane (76).
In addition, EphB4 may play a role in cell adhesion by a ligand-independent mechanism, as demonstrated in MCF7 and MDA-MB cancer cells, where ephrin-B2, the preferred ligand of EphB4, is minimally expressed. EphB4 knockdown in human breast carcinoma cell lines increases cancer cell adhesion, spreading and migration on fibronectin and collagen, two extracellular matrix ligands for beta1-integrins. Using the EphB4 extracellular domain or a TNYL-RAW antagonistic peptide to compete for an interaction between EphB4 and ephrin-B2, it was shown that activation of EphB4 by ephrin-B2 is not required for this activity. However, kinase activity of EphB4 is required for ligand-independent inhibition of cell attachment and spreading (77).
5.2. EphB in spine morphogenesis and synaptic plasticity
EphB receptors, ephrins, integrins and cadherins are among many cell adhesion molecules located at the surface of dendritic spines. EphB forward signaling is important for normal spine development as inhibition of EphB2 kinase activity by over-expression of a kinase-inactive form of EphB2 or a triple knockout of EphB1/2/3 in cultured hippocampal neurons blocks normal spine formation (78, 79). Conversely, EphB receptor activation induces filopodia retraction and shortening leading to spine formation (78). Although there is mounting evidence that EphBs play an important role in spine development, the downstream pathways are not clear. One of the early studies on dissociated hippocampal neurons showed that EphB2 plays a role in spine morphogenesis by interacting with intersectin, a GEF for Cdc42, activating it in cooperation with N-WASP and consequently activating Cdc42-mediated spine morphogenesis (80) (Figure 5). Using hippocampal neurons, Tolias et al demonstrated the kinase-dependent interaction of EphB2 with Tiam1, a Rac1 guanine nucleotide exchange factor (81). Ephrin-B activation of EphB2 leads to phosphorylation of Tiam1 and recruits it to EphB complexes containing NMDA receptors. Knockdown of Tiam1 by siRNA or a dominant negative mutant blocks ephrin-B induced spine formation (81). Their results suggest that EphB receptors mediate spine development, in part, by recruitment and phosphorylation of Tiam 1, leading to Rac1 dependent actin remodeling essential for spine formation.
Figure 5.
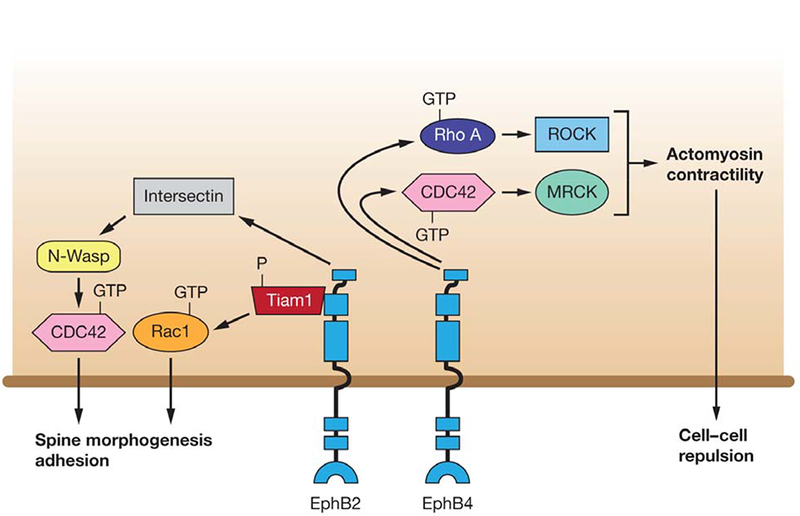
A model of EphB forward signaling regulating cell-cell repulsion and cell architecture. EphB4 forward signaling mediates cell-cell repulsion via both Rho-ROCK and Cdc42-MRCK mediated actomyosin contractility. EphB2 regulates spine morphogenesis by interacting with Intersectin and activating it along with N-Wasp, thereby activating Cdc42. EphB2 stimulation by ephrin-B phosphorylates Tiam1 and recruits it to EphB, which in turn stimulates Rac1 dependent actin remodeling, leading to spine formation.
Moeller et al demonstrated that ephrin-B ligand activation of the EphB receptor tyrosine kinase induces morphogenesis of dendritic filopodia into a mature mushroom-shaped spine, mediated through assembly of a complex containing FAK, Src, Grb2 and Paxillin (82). Activated EphB2 leads to activation of FAK, Src and paxillin, thereby potentially triggering a number of downstream signaling cascades. It was demonstrated that FAK is essential for ephrin-B mediated dendritic spine formation and this involves RhoA, as expression of dominant negative RhoA blocks spine morphogenesis and constitutively active RhoA mimics the ephrin-B phenotype (82).
EphB2 receptors regulate stability of mature dendritic spines by phosphorylating FAK, thus activating the RhoA-ROCK-LIMK-1 pathway to suppress cofilin activity. Reduced cofilin activity is linked to decreased cofilin-mediated dendritic spine remodeling. In addition, the EphB receptor directly phosphorylates cofilin, thus inactivating it, which down-regulates cofilin-induced F-actin disassembly and reorganization (83, 84). EphB2, activated by ephrin-B1, induces growth cone collapse and neurite retraction in NG108 neuronal cells through down-regulation of GTP-bound Ras and consequently of ERK and MAPK pathways. ERK activation induced by attachment of NG108 cells to fibronectin is thus attenuated by its downregulation via activated EphB2. This suggests a contribution of EphB2 to an adhesion response in neuronal growth cones (85).
5.3. EphB receptors in cell-cell adhesion
EphB receptors regulate cell behavior in various developmental processes such as axon guidance, vasculature development, segmentation of sympathetic ganglia, and in diseases such as cancer to restrict/contain tumor cells by eliciting a repulsion response from ligand-bearing normal cells.
EphB receptors elicit repulsion mechanisms to establish spatial-temporal cell positioning in normal developmental mechanisms. For example, EphB receptor functions to restrict neural crest cell migratory streams through the rostral clefts to achieve segmental pattern of neural crest-derived sympathetic ganglia. Eph/ephrin-mediated inhibitory interactions within inter-ganglionic regions, and adhesive cell-cell contacts at ganglia sites mediated by N-cadherin, coordinate to form discrete sympathetic ganglia (86). EphB receptors and their ligand-mediated forward signaling are required for axon terminals to defasiculate, where they migrate to specific topographical positions to form synapses with appropriate target neurons (87). EphB2 and EphB3 receptor signaling regulates the positioning of cell types along the crypt villus axis through repulsive interactions with ephrin-B1 ligands (88).
Mouse knockout studies show that EphB2 and EphB3 and ephrin-B2 play an important role in midline cell-cell adhesion and fusion events that tubularize the urethra and partition urinary and alimentary tracts. Ephrin-B2 or EphB2;EphB3 truncation mutants display a hypospadia phenotype characterized by incomplete urethral tubularization. Consistent with their proposed role in midline fusion, EphB2 and ephrin-B2 are co-expressed at the caudal midline. Interestingly, analysis of Ephrin-B2 or EphB2 cytoplasmic truncation mutants reveals the requirement of both forward and reverse signaling in the same cell for an adhesion response (89).
Nakada et al in a recent study showed that EphB2 increases cell migration and invasion in U251 cells, a glioma cell line expressing ephrin-B2 (90). In migrating glioblastoma cells over-expressing EphB2 in vitro and in vivo, migration and invasion can be inhibited by blocking EphB2. Furthermore, using ephrin-B2 antibody to block phosphorylation of endogenous ephrin-B2 in U87 cells, Nakada et al demonstrated the requirement of ephrin-B2 phosphorylation in EphB2-mediated cell migration and invasion.
EphB3 seems to play a role in cell-cell adhesion via E-cadherin, an epithelial cell adhesion marker. Using CRC cell lines with restored EphB2 or EphB3 expression, Cortina et al demonstrated that EphB receptor-expressing CRC cells do not intermingle with co-cultured ephrin-B-expressing cells, but are excluded by forming large homogenous clusters and acquiring a more compact epithelial shape (91). Analysis of E-cadherin in EphB-expressing CRC cells reveals its redistribution from the cytoplasm to the baso-lateral surface with no change in total protein levels. Furthermore, knocking down E-cadherin by shRNA in CRC cells did not change the level of activated EphB3 receptor, but resulted in actin cytoskeleton remodeling. Such cells exhibited contraction, but fail to cluster and remain as round individual cells (91). This suggests a mechanism where EphB signaling restricts the ability of tumor cells to migrate into ephrin-B positive tissues by inducing E-cadherin adhesion. This was also confirmed in vivo where Apc Min/+ mice, a mouse strain that spontaneously develops intestinal adenomas, engineered to express reduced levels of ephrin-B ligands in the intestinal epithelium, show enhanced growth of colorectal adenomas and impaired cell-cell adhesion and polarization. In a recent study, EphB3 over-expression in the HT-29 colon carcinoma cell line led to cytoskeletal reorganization from a mesenchymal to an epithelial phenotype, as evidenced by cortical actin, E-cadherin, and ZO-1 localization (70). Functionally, the EphB3 over-expressing cells showed decreased transwell migration, and increased calcium-dependent cell-cell adhesion, along with increased epithelial markers and decreased mesenchymal markers. Additionally, these cells displayed tumor suppression in a mouse xenograft system. The authors conclude that the EphB3-ephrin-B interaction promotes MET by re-establishing epithelial cell-cell junctions and thus contributes to EphB3-mediated tumor suppression (70).
EphB4 and its ligand ephrin-B2 play an important role in cell adhesion and migration in various cell types including endothelial cells (EC) and peripheral blood leukocytes (PBLs). EphB4 and ephrin-B2 are specifically expressed on venous and arterial endothelial cells, respectively, and mediate forward or reverse signaling required for either cell adhesion or repulsion, providing guidance for proper spatial organization and development of the vasculature. Induction of EphB4 forward signaling induces de-adhesion and detachment of ECs (92). This observation is supported by studies on a murine brain-derived endothelial cell line, b-End3, in which EphB4 forward signaling inhibits cell adhesion whereas ephrin-B2-mediated reverse signaling does not (93). EphB4-ephrin-B mediated adhesion is switched to repulsion when Eph receptors are locally activated at the sites of contact with ephrin-B ligand expressing cells. This initiates Rac-regulated membrane ruffles at the contact sites, followed by endocytosis of EphB-ephrin-B complex concomitantly with the two cell types retracting from one another (94). Studies in endothelial cells to determine components downstream of EphB receptor signaling that affect actomyosin contractility have revealed that rapid contractility in response to ephrin-B-induced EphB activation is mediated by a combination of Rho-ROCK (Rho Kinase) signaling and Cdc42 and its effector MRCK (Myotonic dystrophy kinase-related Cdc42-binding kinase) (95) (Figure 5).
Monocytes expressing EphB4 adhere strongly to ECs mediated in part, by ephrin-B2 reverse signaling. Experiments examining adhesion between monocytes and ECs show weaker adhesion of monocytes to ECs expressing an ephrin-B2 mutant lacking the cytoplasmic domain than to ECs expressing full-length ephrin-B2 (96). Identification of a role for EphB4 in cell adhesion has led to further studies investigating its potential role in homing endothelial progenitor cells to sites of neovascularization. In a nude mouse model of hind limb ischemia, EphB4 activation with an ephrin-B2-Fc increases the ability of cells to home into a lesion and incorporate into the neocapillaries. EphB4 mediates this role through increased expression of P-selectin glycoprotein ligand-1 (PSGL-1) and adhesion to E-selectin and P-selectin (97). In addition to initiating a downstream signaling pathway, EphB4 receptors bind a cell adhesion molecule, platelet-endothelial cell adhesion molecule-1 (PECAM-1/CD31), to influence cell migration and organization during development of retinal vasculature and angiogenesis (98). It has also been shown that EphB4 and EphB3 activation by ephrin-B2 is necessary for PC-3 cells (a prostate cancer cell line) to migrate toward fibroblasts (99). In this case, knockdown of the Eph receptors restores contact inhibition and therefore blocks migration of these cells. Of particular interest, contact inhibition occurs with homotypic collisions between two cancer cells, and this is mediated by the EphA-Rho pathway, suggesting that the cell context and the combination of Eph/ephrin molecules present will determine the effects on cell adhesion and migration (99).
In contrast to the requirement for EphB4 to interact with its ligand for cells to home and adhere to sites of neovascularization or towards fibroblasts, EphB4 can inhibit integrin-mediated cell adhesion in the absence of ephrin-B2 binding in cancer cells (77). EphB4 over-expression in MCF7 and MDA-MB-435 cancer cells inhibited cell substrate adhesion and reduced beta1-integrin protein levels, and this effect was independent of ephrin-B2 stimulation (77). There is also data supporting a cell-autonomous role for the ligand, where ephrin-B2 deficient or over-expressing cells lacking receptor contact display adhesion and migration defects (100), (101).
6. EPHRIN-A LIGANDS AND REVERSE SIGNALING IN CELL ADHESION
Dramatic congenital malformations can occur from loss of forward or reverse signaling or both in the Eph/ephrin system (Figure 6A, B) (1). In this section we will focus on reverse signaling through the A-type ephrins. Although these molecules are GPI-linked to the membrane, there are data to suggest that they are capable of signaling within their host cell (Figure 6A). However, the mechanism of action is still unresolved. Evidence indicating that A-type ephrins can regulate adhesion by reverse signaling comes from several studies. For example, loss of ephrin-A5 in mice leads to midline fusion in the neural tube. Adhesion may be a result of weakening the activation of the Eph/ephrin signaling that would normally promote cell repulsion (102). One example that supports this concept is that, in tissues where they are highly expressed relative to the wild-type receptor, such as the neural tube, spliced forms of EphA7 lacking kinase activity redirect the cell from a repulsive to adhesive response upon ligand/receptor contact (102). Forward signaling might also be abrogated via phosphatase function (103), and thus lead to increased adhesion, or through ligand cleavage and proteolysis (104).
Figure 6.
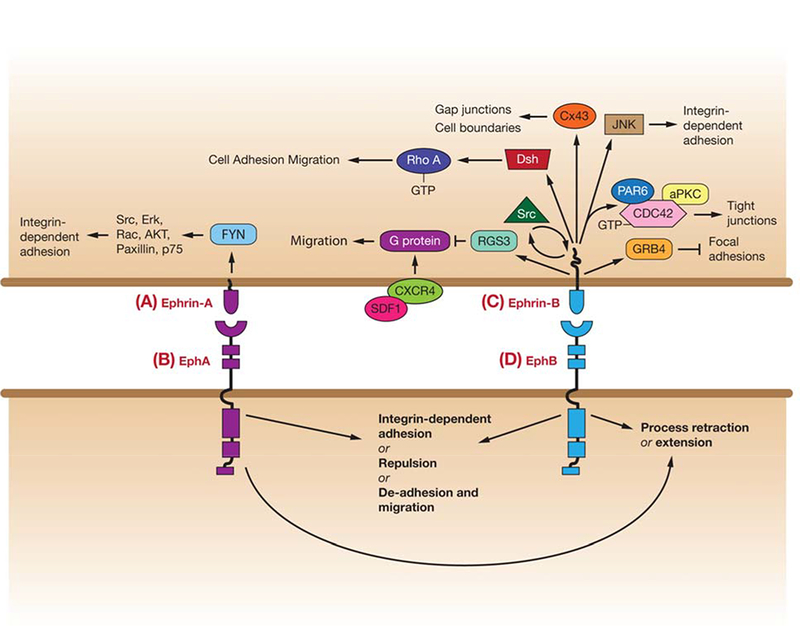
Pathways linking ephrin reverse signaling (phosphorylation-dependent and non-phosphorylation-dependent) to regulation of cell adhesion/repulsion, and movement. (A) Ephrin-A signaling via Fyn to affect downstream players in integrin-dependent adhesion. (B) EphA-mediated responses (for more details see Figure 1). (C) Ephrin-B reverse signaling affects pathways that regulate Src and RhoA. Ephrin-B1 disrupts focal adhesions through GRB4, and can regulate cell boundaries through Connexin 43 (Cx43). Non-phosphorylated ephrin-B1 can bind PAR6 to inhibit atypical protein kinase C (aPKC), and upon phosphorylation inhibits ephrin-B1 binding to PAR6, allowing PAR6 to bind GTP-bound CDC42 and activate aPKC. Ephrin-B also inhibits signaling by CXCR4, a G protein-coupled chemokine receptor, to affect migration. (D) EphB-mediated responses (For more details see Figure 4 & 5).
In contrast to mitigating the forward signal as a strategy for affecting cell adhesion, reverse signals through the ephrin-As can promote attractive effects (105). In the Hek 293 system, it was shown that activation of ephrin-A2 or ephrin-A5 by one of their receptors, EphA3, resulted in a beta1-integrin-dependent increase in adhesion of ephrin-A-expressing cells to laminin (105). It was also found by another group that ephrin-A5 signals within caveola-like domains of the plasma membrane upon engagement with its cognate Eph receptor, leading to increased adhesion of the cells to fibronectin (106). Moreover, this study showed that activation of ephrin-A5 induces an initial change in cell adhesion followed by changes in cell morphology, and both effects appear to be dependent on the activation of beta1 integrin and members of the Src family of protein tyrosine kinases. This work suggested a role for class A ephrins in specifying the affinity of the cells towards various extracellular substrates by regulating integrin function (107). In a mouse blastocyst attachment assay, attachment of blastocysts was delayed in the presence of EphA1/Fc, suggesting that the engagement of ephrin-A1 and/or A3 present on the blastocyst surface utilized reverse signaling to regulate attachment (108). Recently, it was shown that ephrin-A reverse signaling on hematopoietic progenitor cells resulted in augmented adhesion to integrin substrates, suggesting a potential role for EphA/ephrin-A interactions in the anchoring of hematopoietic progenitors within the bone marrow (109). In another study, chronic lymphocytic leukemic cells showed a dramatic impairment in the adhesion to as well as the transmigration through human umbilical vein endothelial cell monolayers, correlating with their higher ephrin-A4 expression. This study provided support for the concept that adhesion and transendothelial migration of normal B cells can be largely dictated by the magnitude of ephrin-A4 reverse signaling into lymphocytes (110). Thus, there are context-dependent circumstances where A-type ephrins signal through their cytoplasmic domains to affect cell adhesion. Although there are several molecules known to be regulated downstream of ephrin-A reverse signaling (ie. Src family members, ERK1/2, Rac, AKT, integrin, paxillin, and p75NTR) (Figure 6A), the mechanism of activation and the pathway utilized is still unclear (105–107, 109, 111–113). This signal may be downregulated through a cleavage mechanism involving the Kuzbanian metalloprotease (ADAM10) (104). ADAM10 constitutively associates with EphA3, but when the Eph receptor complexes with its ligand, it may create a recognition motif that positions the ADAM10 proteinase domain for the effective cleavage of ephrin-A5. Upon activation of the Eph receptor, the kinase domain moves away from the plasma membrane, relieving a steric hindrance that was blocking ADAM10 from aligning properly to cleave the ligand (114). This cleavage occurs with ADAM10 and its substrate being on the membranes of opposing cells (115), thus allowing for a switch from adhesion to repulsion.
7. EPHRIN-B LIGANDS AND REVERSE SIGNALING IN CELL ADHESION
The concept of reverse signaling through the intracellular domain of transmembrane B-type ephrins was introduced over 15 years ago (116, 117). The B-type transmembrane ephrin ligands do not possess any intrinsic catalytic activity for signaling, but rely upon a scaffolding activity that recruits signaling molecules to transmit an effect on cell function (Figure 6C). It has been shown that ephrin-Bs utilize both phosphorylation-dependent and -independent signaling pathways, which may be viewed as different modes of reverse signaling: 1) one mode where tyrosine phosphorylation of the intracellular domain of ephrin-B leads to recruitment of signaling molecules that exert a functional effect; 2) another mode where unphosphorylated ephrin-B associates with a protein complex that transduces a signal, but upon tyrosine phosphosphorylation, the interaction of ephrin-B with the signaling complex is disrupted or modulated (118).
In one form, this signaling occurs upon the contact and clustering of ephrin-Bs in response to the binding and clustering of Eph receptors, leading to activation of a Src family kinase that phosphorylates the intracellular domain of B-type ephrins (116, 117). In another form, an alternative growth factor receptor (ie. FGFR, PDGFR, TIE-2) or cell surface molecule (Claudin) induces this phosphorylation event in cis (116, 117, 119–121).
There are phosphorylation-dependent and -independent signaling molecules and pathways for both ephrin receptors and ligands (118). In this section, we have chosen to focus on signaling through the transmembrane ephrin-B ligand. A limited number of proteins have been shown to interact with ephrin-Bs and mediate a functional effect (Figure 6C). For example, an ephrin-B interaction with PDZ-RGS3, a GTP exchange factor, regulates the migration of cerebellar granule cells (122), and is critical for the maintenance of the neural progenitor cell state (123). Another interacting partner is ZHX2 (a zinc finger homeodmain protein) that also regulates neural progenitor maintenance in the developing murine cerebral cortex (124). Both ephrin-B1 and ephrin-B2 interact with syntenin through their C-terminal PDZ-binding motif and have been shown to function with EphB to mediate presynaptic development (75, 125, 126). It has also been reported that gap junction communication may be regulated by ephrin-B1 through an interaction with Connexin 43 (127). Our laboratory has shown that an interaction with Dishevelled mediates ephrin-B signaling that controls retinal progenitor cell movement into the eye field (128). Although these molecules associate with ephrin-B in a phosphorylation-independent manner, Grb4, an adaptor protein, has been shown to associate with ephrin-B1 in a phosphorylation-dependent manner and mediate functional effects on cell morphology (129, 130). These effects may be mediated through an association of Grb4 with other proteins implicated in cytoskeletal regulation (Figure 6), including Cbl-associated protein (CAP/ponsin), the Abl-interacting protein-1 (Abi-1), dynamin, p21-activated kinase (PAK 1), heterogeneous nuclear ribonucleoprotein K (hnRNPK) and axin (129). Ephrin-B1 has also been shown to regulate dendritic spine morphogenesis through Grb4 and the G protein-coupled receptor kinase-interacting protein (GIT) (131). Our laboratory has recently identified STAT3 as a new member of this group of phosphorylation-dependent ephrin-B-associated signaling molecules (132) (Figure 6C). The recruitment of STAT3 to ephrin-B1, and its resulting Jak2-dependent activation and transcription of reporter targets, reveals a signaling pathway from ephrin-B1 to the nucleus. The in vivo relevance and function of the ephrin-B/STAT3 association is still unclear, however, evidence from a more recent study showing that the STAT3-dependent association is important for ephrin-B2 to contribute to endothelial and mural cell assembly into vascular structures (133).
7.1. Ephrin-B ligands and reverse signaling in cell-cell adhesion
Several years ago, our laboratory provided evidence that over-expression of ephrin-B1 in Xenopus embryos causes the blastomeres of ectodermal tissue to dissociate (134). It is not likely that this effect is merely a result of the adhesive properties of the Eph receptor/ephrin interaction since adhesion was disrupted by over-expressing an ephrin-B1 ligand lacking the receptor-binding domain (134). Genetic evidence clearly shows that the intracellular domain of ephrin-Bs is critical for neural crest movement, and vascular morphogenesis, consistent with a signaling function for this domain(135–138). A particularly interesting aspect of ephrin-B reverse signaling that is beginning to emerge is a role affecting cell-cell junctions.
We recently showed that ephrin-B1 signaling may regulate cell-cell junctions through a cell polarity complex in vivo (139). This study focused on assessing whether ephrin-B1 is a mediator or modulator of cell-cell junction signaling in epithelial cells using the Xenopus system. We presented evidence that the Par polarity complex protein, Par-6, which is a major scaffold protein required for establishing tight junctions, associates with ephrin-B1 and that this results in the loss of tight junctions. Using exogenous expression in the Xenopus system, along with endogenous immunoprecipitation analysis in a human colon carcinoma cell line (HT29), we showed that an interaction exists between ephrin-B1 and Par-6. Par-6 constitutively binds aPKC, and upon binding an active Cdc42-GTP undergoes a conformational change that leads to aPKC activation (Figure 6C, 7). The Par-6/aPKC/Cdc42-GTP complex localizes to the apical cell junctions where it regulates tight junction formation, and tight junction complexes may associate with the actin cytoskeleton, which is reorganized in the formation and maintenance of cell-cell contacts (140).
Figure 7.
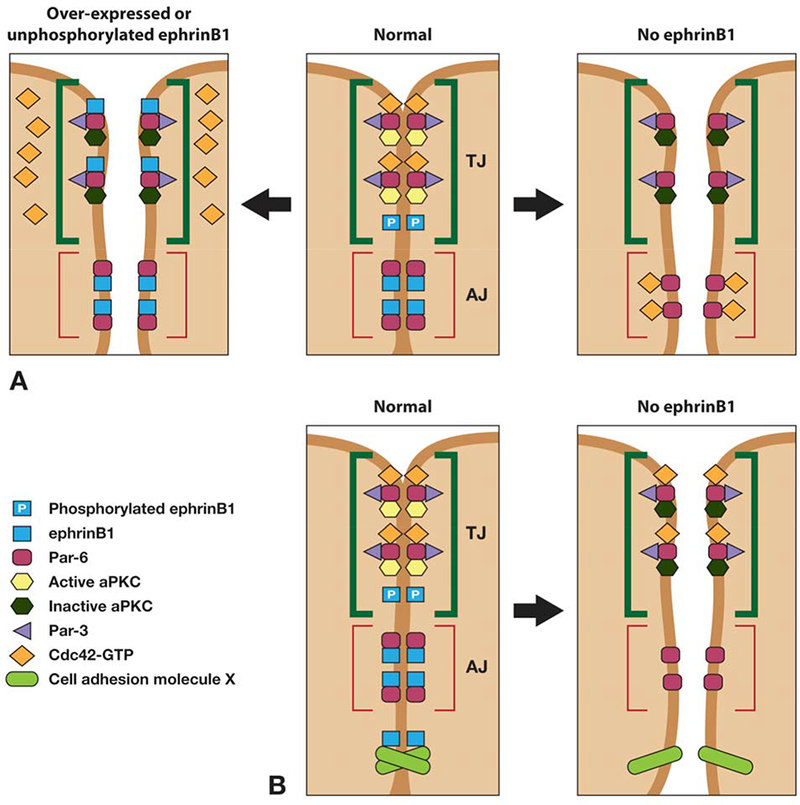
Model for ephrin-B1 regulation of tight junction formation. (A) Unphosphorylated ephrinB1 may compete with Cdc42-GTP for Par-6 binding and inhibit aPKC activation in the Par complex, leading to tight junction disruption. Upon phosphorylation of ephrinB1, an interaction with Par-6 is lost, and Par-6 is now available to interact with Cdc42-GTP and establish tight junctions. Loss of ephrinB1 may allow Par-6 that is localized at adherens junctions and lateral cell borders, to compete with tight junction-associated Par-6 for Cdc42-GTP. The resulting reduction in Cdc42-GTP localized at the apical border may reduce aPKC activity and disrupt tight junctions. (B) An alternative model for loss of ephrin-B1 expression leading to loss of cell-cell adhesion (and TJ dissolution) through loss of interaction with another interacting partner that plays a critical role in adherens junction or gap junction formation or maintenance.
Over-expression of ephrin-B1 in embryonic ectoderm causes the loss of tight junctions, as evidenced by ultrastructural analysis and localization of tight junction proteins (ZO-1 and Cingulin). Expression and immunoprecipitation analysis in Xenopus oocytes indicated that ephrin-B1 competes with the small GTPase Cdc42 for association with the Par-6 protein. We tested and confirmed this competition model (Figure 7) in vivo, where tight junction formation was rescued in ectoderm over-expressing ephrin-B1 when an active form of Cdc42 was also expressed at the appropriate level (139).
Ephrin-B1 is known to be tyrosine phosphorylated (through a Src family kinase) upon interacting with the extracellular domain of its cognate EphB receptor (116, 117), and phosphorylated in cis by an active FGF receptor or an interaction with claudin (121, 141). Immunoprecipitation analysis in the Xenopus oocyte system, as well as the HT29 human colon carcinoma cell line, demonstrates that tyrosine phosphorylation of the intracellular domain of ephrin-B1 disrupts the interaction with Par-6. Furthermore, phosphorylation of ephrin-B1 rescues the interaction between active Cdc42 and Par-6, supporting a model where unphosphorylated ephrin-B1 and active Cdc42 compete for Par-6 binding (Figure 7A). Moreover, it was demonstrated that phosphorylation on tyrosine 310 rescues tight junction formation in embryonic ectoderm that is over-expressing ephrin-B1 (139). In vivo evidence that this phosphorylation event disrupts the ephrin-B1/Par-6 complex and thus maintains tight junctions during normal ectoderm development comes from ephrin-B1 replacement experiments. In these studies, translation of endogenous ephrin-B1 was blocked by ephrin-B1 antisense morpholino oligonucleotides (MOs), and wild type or tyrosine 310 mutant ephrin-B1 RNAs that are resistant to the MOs were introduced at carefully titrated concentrations (139).
Although wild-type ephrin-B1 was able to rescue the localization of the tight junction-associated protein ZO-1 in the presence of the ephrin-B1 MO, expression of the ephrin-B1Y310F mutant in the presence of ephrin-B1 MO failed to restore appropriate localization of ZO-1. This data is consistent with the observed enrichment of ephrin-B1 tyrosine phosphorylation at the apical lateral domain of cell junctions (121, 127, 139). These experiments provide in vitro and in vivo evidence for a mechanistic model (Figure 7A) of how ephrin-B1 controls tight junction formation (139). We proposed and tested a model where unphosphorylated ephrin-B1 possesses a competitive advantage for binding to Par-6, thus displacing or preventing Cdc42-GTP from interacting with Par-6 at apical lateral borders. Since the Cdc42/Par-6 interaction is inhibited, aPKC activity is reduced and tight junctions are disrupted. In contrast, upon cell-cell contact, a cognate Eph receptor (or an active FGF receptor or possibly Claudin) induces phosphorylation of ephrin-B1 at the apical junctions, and dissociates ephrin-B1 from Par-6. Thus, Cdc42-GTP is free from competition with ephrin-B1 and can now bind to Par-6, inducing aPKC activation and establishing tight junctions (139).
Eph/ephrin signaling also is able to affect cell-cell junctions through a role in Gap junction interactions. Studies in zebrafish have shown that expression of Eph receptors and ephrins in ectodermal explant cells blocked Gap junction communication at the boundary between both cell populations(142). Gap junctions are specialized intercellular connections between various cell-types that directly connect the cytoplasm of two cells, and allow various molecules and ions to pass freely between cells. During vertebrate development, regions of Gap junction communication overlap with distinct compartments and tissue boundaries, such as rhombomeric and skeletal boundaries (127). Evidence from one study shows that Gap junction communication is inhibited at ectopic ephrin boundaries and that ephrin-B1 physically interacts with Connexin 43 (gap junction protein) and influences its distribution (Figure 6). Moreover, regulation of Gap junction communication was shown to correlate with cell sorting in response to Eph/ephrin interaction (127).
In a study using MDCK cells, it was shown that ephrin-B1 interacts with claudins on the same cell surface in cis (121). Claudins are important components of tight junctions which establish the paracellular barrier that controls the flow of molecules in the intercellular space between the cells of an epithelium. They possess four transmembrane domains, but the N-terminus and the C-terminus are located in the cytoplasm. Tanaka and colleagues found that tyrosine phosphorylation of the cytoplasmic region of ephrin-B1 was significantly enhanced by cell–cell contacts in a claudin-dependent manner. The phosphorylated ephrin-B1 stimulated the paracellular permeability in MDCK cells. These data provide further evidence that ephrin-B1 is able to regulate tight junctions (121).
7.2. Ephrin-Bs and reverse signaling in tissue boundaries
Ephrin-Bs and reverse signaling have been shown to play a role in tissue boundary formation which is a cell-cell interaction event that depends upon differences in adhesive action (3). One example of this is found in hindbrain segmentation using zebrafish and Xenopus models. In the hindbrain, Eph receptors and ephrins are expressed in alternating rhombomeres. An early study by the Wilkinson laboratory showed that hindbrain segmentation was disrupted when a truncated form of EphA4 lacking the kinase domain was expressed (143). Subsequently they showed that Eph receptors and ephrins are required for the proper sorting of cells at rhombomere boundaries (144). The authors reported that cells expressing ephrin-B2 were excluded from the Eph receptor-expressing rhombomeres (144). However, using ectodermal explants, it was shown that bidirectional, rather than unidirectional signaling restricted cell intermingling between adjacent cell populations (142). Thus, it was proposed that bi-directional signaling leads to cell repulsion (142). In a later study using mosaic analysis in zebrafish, it was determined that EphA4-deficient rhombomere cells sort out from cells where EphA4 is expressed and ephrin-B2a is absent. This data suggested that EphA4 plays an adhesive role in rhombomeres independent of ephrin-B2a (58). However, in a more recent study the authors indicate the existence of a corresponding EphA4-independent requirement for the ephrin-B2a ligand in regulating cell affinity between cells within a specific rhombomere. They conclude that EphA4 and ephrin-B2a are specifically and individually required to facilitate normal integration of newborn progenitors into the neural keel (145).
Another example of Eph/ephrin involvement in boundary formation is found in somites, where an Eph-expressing cell is adjacent to an ephrin-expressing cell. These cells repel each other, leading to either tissue segregation or boundary formation (3, 146). Somites are the segmented primordia of the skeletal muscle and vertebral column. During somite border morphogenesis, boundary cells undergo a mesenchymal-to epithelial transition (147, 148). During normal segmentation of somites, the border separating activity is attributable to an intracellular signal from ephrin-B2 (called a ‘reverse signal’), acting in anterior cells adjacent to EphA4 expressing cells (149). Ephrin-B2 also promotes epithelialization or a mesenchymal to epithelial transition of segmenting somitic cells in chick and zebrafish embryos, and reverse signaling is required for this event (150, 151). Recent evidence has given some insight into the signaling pathway affected by ephrin-B2 during this process. Ephrin-B2 was found to transduce an intracellular signal that suppressed Cdc42 activity, leading to the coordination of boundary formation and cell epithelialization (151). This finding in somitic epithelialization is in contrast to in vitro or in vivo studies in epithelial cells, where activation of Cdc42 activity is required for epithelial contacts and TJ formation (139, 152–154). In addition there is evidence indicating that reverse signaling by Ephrin-B2a is sufficient to initiate integrin alpha5 clustering and induce fibronectin matrix assembly, which is critical for proper somitic boundary formation (149, 155). As mentioned earlier, both forward and reverse signaling through ephrin-B2 and its receptors, EphB2 and EphB3, play a major role in another cell-cell adhesion event, the tubularization of the urethra and partitioning of the urinary and alimentary tracts. Animals lacking ephrin-B2 reverse signaling displayed a failure in cloacal septation, resulting in severe anorectal malformations characterized by an absence of the rectum (89).
7.3. Ephrin-B regulation and the switch from adhesion to repulsion
EphB/ephrin-B activities in cell adhesion are regulated by their endocytosis, which has been shown to terminate adhesion allowing contact mediated repulsion (63). In this study, the authors show that cell contact-induced EphB–ephrin-B complexes are endocytosed during the retraction of cells and neuronal growth cones. The observed endocytosis, which is sufficient to promote cell detachment, occurs in a bi-directional manner and involves full-length receptor and ligand complexes. Some insight regarding the possible involvement of small GTPases has been gleaned from the work of Marston et al. where the authors show endocytosis of activated Eph receptors and their bound, full-length ephrin-B ligands in heterologous, non-neuronal cells. They also observe that both the internalization of the receptor–ligand complexes and the subsequent cell retraction events are dependent on actin polymerization, which in turn is dependent on Rac signaling within the receptor-expressing cells (94). More recently, it was shown that over-expression of ephrin-B2 in endothelial cells in the absence of receptor, can increase motility and trigger repeated cycles of actomyosin-dependent cell contraction and spreading in isolated cells. Upon contact with the soluble recombinant EphB4 cell shape changes still occurred, but non-repetitively, and were terminated by ligand internalization (101). Although the C-terminal PDZ domain was shown to be required for the cell morphology changes, the precise binding partners involved in downstream signaling are not clearly understood. However, it was found that cell retraction and membrane blebbing induced by ephrin-B2 reverse signaling involved ROCK activation (101). This is consistent with other studies in Xenopus which suggest that Rho and ROCK are activated downstream of ephrin-B1 (128, 156), and probably mediated by Dishevelled for either the sorting of EphB2- and ephrin-B1-expressing cell populations in an in vitro re-aggregation assay (156), or for retinal progenitor cell movement in vivo (128, 157) (Figure 6C).
Ephrin-B reverse signaling also affects cell-substrate adhesion (Figure 6C). For example, an early study showed that EphB1/Fc induced endothelial ephrin-B1 tyrosine phosphorylation, migration and integrin-mediated attachment and promoted neovascularization, in vivo, in a mouse corneal micropocket assay (158). Activation of ephrin-B1 by EphB1/Fc induced phosphorylation of JNK, but a mutant ephrin-B1 harboring a deletion of four C-terminal amino acids (a PDZ binding domain) failed to activate JNK (158).
Ephrin-B reverse signaling may also occur through cytoplasmic release of the intracellular domain. A study by Tomita et al showed that ephrin-B1 can be sequentially cleaved by MMPs and gamma-secretase. The released C-terminal fragment can re-localize from the cell surface to the nucleus when the proteasome system is inhibited (159), however, the functional significance of this event is still unclear. Another report demonstrated that ephrin-B2 can also be processed by MMPs and PS1/-gamma secretase to release a C-terminal fragment that binds to the Src kinase, inducing its autophosphorylation. Moreover, the gamma-secretase system is required for EphB-induced ephrin-B2 reverse signaling that regulates endothelial cell sprouting (160). Finally, in vivo evidence for a functional effect of ephrin-B1 cleavage comes from a recent loss-of-function study in Xenopus (161). In this study, it was shown that ADAM13 is expressed in the mesoderm during gastrula stages and cleaves ephrin-B1 and ephrin-B2 in vivo. This cleavage is essential for up-regulation of canonical Wnt signaling and early expression of the neural crest marker snail2 (161). There is evidence that Rho kinase and the Wnt/PCP pathway are activated by ligand-induced activation of EphB receptors via Dishevelled, (156), and receptor-free ephrin-B1 can also activate Wnt/PCP signaling cell autonomously by recruiting Dishevelled to the plasma membrane (157). Since the canonical and noncanonical Wnt pathways are known to inhibit each other (162) it is possible that by cleaving ephrin-B1/B2, ADAM13 shifts Wnt signaling toward Wnt/Beta-catenin signaling rather than the Wnt/PCP pathway. How this event may regulate or effect cell adhesion is still unclear.
8. CLOSING REMARKS
It is clear that EphA family proteins play important roles in the regulation of cell adhesion and repulsion. However, the nature of that role appears to vary considerably, with examples of promotion of both adhesion and repulsion, both between cells and of cells to the extracellular matrix. In addition, several different molecular pathways have been proposed for many of the observed effects. Indeed, it is possible, if not likely, that EphA receptors play several roles within the same cell, and may also act via several different pathways to achieve the same function. In addition, different members of the EphA family may have distinct downstream effectors, and therefore distinct functions. Consequently, the specific EphA family member(s) expressed by each cell may in part, determine variations in effects between cell types. However, there are also examples of differential cellular responses to activation of the same EphA receptor subtype. For example, a comparison of two EphA3-positive tumor cell lines showed that exposure to high levels of ephrin-A5 affected their adhesion to fibronectin in opposite directions: LiBr melanoma cells were dose-dependently repelled from the ephrin/fibronectin-coated surface, whilst adhesion of LK63 pre-B leukemia cells increased with ephrin concentration (163). Further investigation indicated that these distinct responses were due to different endogenous levels of protein tyrosine phosphatase (PTP) activity in these two cell types. In melanoma cells, as well as in other the HEK293T cell line, active EphA forward signaling causes Rho-dependent cytoskeletal retraction and repulsion. In contrast, high levels of PTP in the pre-leukemia cells was suggested to inhibit EphA phosphorylation and forward signaling, in the absence of which a kinase-independent mechanism promotes increased cell-cell contacts and assembly of focal adhesions. In support of this model, a recent study has shown that the phosphatase PTP1B can act directly on EphA3 at the plasma membrane to inhibit both ligand-induced-phosphorylation and recycling (164). Thus, the presence and levels of molecules that can regulate EphA activity, as well as of EphA ligands and effectors, may determine the variations in adhesive/repulsive responses to EphA signaling in different cellular contexts. However, the limitations and possible misleading effects of these studies, in particular those performed on cultured cell lines, should also be acknowledged. It is possible that cells may exhibit responses to EphA signaling in culture that are irrelevant to the normal behavior of similar cell types in vivo. In addition, manipulation of signaling pathways by exogenous expression of wild-type or mutant proteins may have unpredictable outcomes. For example, ephrin-Fc chimeras, in which ephrin monomers are dimerized by fusion to immunoglobulin G (IgG) Fc, are commonly used as exogenous activators of Eph forward signaling. However, in a study of a pancreatic cell line in which over-expression of EphA2 increases cell motility and invasiveness, treatment with ephrin A1-Fc reverses these effects by simulating proteosomal degradation of ligated EphA2 receptors (165). Thus, caution must be used when interpreting the effects of such manipulations.
Similar to EphA, activation of EphB receptors can elicit either adhesion or repulsion depending upon cell type and signaling engagement. Current literature suggests that cell-matrix adhesion mediated by ligand-dependent EphB forward signaling commonly involves regulation of integrin activity. However, EphB receptors may utilize different mechanisms to regulate such activity. For example, activated EphB2 is associated with R-Ras phosphorylation thereby suppressing its ability to support integrin activity, leading to loss of adhesion whereas EphB3 regulates cell-matrix adhesion by converting integrins to an inactive confirmation (67, 69). In addition, Eph receptors may inhibit cell-matrix adhesion by either by de-phosphorylating signaling complexes involved in integrin signaling, such as Fak and p130Cas or regulating their spatio-temporal distribution. On the other hand, a ligand-independent role of EphB receptors in cell-matrix adhesion has been explored only recently. In one such study using cancer cells, Noren et al provides strong evidence of the ligand independent role of EphB4 receptor forward signaling in inhibition of cell-substrate adhesion and migration mediated by regulating beta1 integrin levels although the mechanism of EphB-integrin crosstalk remains to be elucidated (77). Since, many of the studies examining how EphB receptors mediate cell-cell/cell-matrix adhesion, have been predominantly studied using in vitro systems, caution must be used to interpret or extrapolate these observations to in vivo systems. In addition, lack of highly sensitive and specific antibody reagents, due to highly conserved domains within the receptors, increases the difficulty of determining the role of endogenous Eph receptors. Furthermore, although imaging of in vivo cell movement remains a challenge, further studies focusing on 3-dimensional models, either using 3-D cell culture systems or systems-level analysis will be instrumental in understanding the role of EphB-mediated cell adhesion and movement.
The role of ephrin-A and -B reverse signaling in cell adhesive events requires more study. Although more mechanistic information exists regarding ephrin-B signaling, there are still outstanding questions regarding the mechanism and downstream players in ephrin-B regulation of cell-cell junctions. For example, how does loss of ephrin-B1 disrupt tight junction formation? In our previous study, loss of ephrin-B1 expression via the introduction of an ephrin-B1 MO causes a loss of tight junction assembly (139), and in addition, Cortina and colleagues reported that conditional loss of ephrin-B1 in intestinal epithelia of the mouse shows a substantial reduction of tight junctions (91). One possibility within the confines of our model (Figure 7A) is that loss of ephrin-B1 may result in more available Par-6 along the baso-lateral borders of the cell. For example, phosphorylated ephrin-B1 appears to be enriched in apical junctions (121, 127, 139) while more unphosphorylated ephrin-B1 appears to reside along the lateral borders and adherens junctions (139). Thus, it may be possible that loss of ephrin-B1 allows Par-6 at these locations to compete for an interaction with Cdc42-GTP, effectively displacing a portion of the Cdc42-GTP from the apical junction region where aPKC resides and is required for tight junction formation. Alternatively, ephrinB1 may regulate cell-cell adhesion independently from the Par complex. For example, ephrin-B1 may have another interacting partner that plays a critical role in adherens junction formation or maintenance. Thus, loss of ephrin-B1 may affect adherens junction or even gap junction formation, leading to disruption of cell-cell adhesion (Figure 7B).
Another unresolved question is how ephrin-B1 may regulate adherens junctions (AJs), thus affecting cell-cell adhesion. It is possible that the Par polarity complex mediates ephrin-B1 signaling that affects AJs, which are multi-protein complexes that mediate homotypic cell adhesion (166). Eph signaling has recently been shown to regulate AJ complexes, and several papers have linked Cdc42 to AJ stability. One report by Harris and Tepass identifies the Par complex as an effector for Cdc42 in controlling the endocytosis of apical proteins (e.g. Crumbs) that are critical for stabilizing basolateral AJs (167). Two other studies show that Cdc42 functions with Par-6 and aPKC to regulate E-Cadherin endocytosis through the Arp2/3 complex, and this regulation affects AJ stability (168, 169). Support for this concept also comes from a study in the fly eye epithelium, showing that Rho1 maintained AJs by inhibiting endocytosis and recycling of E-Cadherin in a Cdc42/Par-6-dependent manner (154). However, it is ephrin-B2-induced suppression of Cdc42 activity that drives somitic epithelialization, and it is unclear what cell context parameters and mechanisms account for differential signaling from B-type ligands. Moreover, although endocytosis of ligands and receptors can either send signals by clustering within vesicles or by termination of reverse and/or forward signaling, how might the switch mechanism actually initiate a repulsive signal? What is the mechanism and parameters that determine these effects? Significant questions and avenues of investigation remain to be explored regarding the precise signaling pathways and mechanisms used by ephrin-Bs to affect cell-cell and cell-substrate adhesion.
9. ACKNOWLEDGEMENT
Arvinder Singh, and Emily Winterbottom contributed equally to this work.
10. REFERENCES
- 1.Pasquale EB: Eph-ephrin bidirectional signaling in physiology and disease. Cell, 133(1), 38–52 (2008) [DOI] [PubMed] [Google Scholar]
- 2.Arvanitis D and Davy A: Eph/ephrin signaling: networks. Genes Dev, 22(4), 416–29 (2008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pasquale EB: Eph receptor signalling casts a wide net on cell behaviour. Nat Rev Mol Cell Biol, 6(6), 462–75 (2005) [DOI] [PubMed] [Google Scholar]
- 4.Heroult M, Schaffner F and Augustin HG: Eph receptor and ephrin ligand-mediated interactions during angiogenesis and tumor progression. Exp Cell Res, 312(5), 642–50 (2006) [DOI] [PubMed] [Google Scholar]
- 5.Merlos-Suarez A and Batlle E: Eph-ephrin signalling in adult tissues and cancer. Curr Opin Cell Biol, 20(2), 194–200 (2008) [DOI] [PubMed] [Google Scholar]
- 6.Castano J, Davalos V, Schwartz S Jr. and Arango D: EPH receptors in cancer. Histol Histopathol, 23(8), 1011–23 (2008) [DOI] [PubMed] [Google Scholar]
- 7.Vaught D, Chen J and Brantley-Sieders DM: Regulation of mammary gland branching morphogenesis by EphA2 receptor tyrosine kinase. Mol Biol Cell, 20(10), 2572–81 (2009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Poliakov A, Cotrina M and Wilkinson DG: Diverse roles of eph receptors and ephrins in the regulation of cell migration and tissue assembly. Dev Cell, 7(4), 465–80 (2004) [DOI] [PubMed] [Google Scholar]
- 9.Henkemeyer M, Marengere LE, McGlade J, Olivier JP, Conlon RA, Holmyard DP, Letwin K and Pawson T: Immunolocalization of the Nuk receptor tyrosine kinase suggests roles in segmental patterning of the brain and axonogenesis. Oncogene, 9(4), 1001–14 (1994) [PubMed] [Google Scholar]
- 10.Pasquale EB, Deerinck TJ, Singer SJ and Ellisman MH: Cek5, a membrane receptor-type tyrosine kinase, is in neurons of the embryonic and postnatal avian brain. J Neurosci, 12(10), 3956–67 (1992) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cheng HJ and Flanagan JG: Identification and cloning of ELF-1, a developmentally expressed ligand for the Mek4 and Sek receptor tyrosine kinases. Cell, 79(1), 157–68 (1994) [DOI] [PubMed] [Google Scholar]
- 12.Winslow JW, Moran P, Valverde J, Shih A, Yuan JQ, Wong SC, Tsai SP, Goddard A, Henzel WJ, Hefti F and et al. : Cloning of AL-1, a ligand for an Eph-related tyrosine kinase receptor involved in axon bundle formation. Neuron, 14(5), 973–81 (1995) [DOI] [PubMed] [Google Scholar]
- 13.Drescher U, Kremoser C, Handwerker C, Loschinger J, Noda M and Bonhoeffer F: In vitro guidance of retinal ganglion cell axons by RAGS, a 25 kDa tectal protein related to ligands for Eph receptor tyrosine kinases. Cell, 82(3), 359–70 (1995) [DOI] [PubMed] [Google Scholar]
- 14.Cheng HJ, Nakamoto M, Bergemann AD and Flanagan JG: Complementary gradients in expression and binding of ELF-1 and Mek4 in development of the topographic retinotectal projection map. Cell, 82(3), 371–81 (1995) [DOI] [PubMed] [Google Scholar]
- 15.Flanagan JG and Vanderhaeghen P: The ephrins and Eph receptors in neural development. Annu Rev Neurosci, 21, 309–45 (1998) [DOI] [PubMed] [Google Scholar]
- 16.Wilkinson DG: Multiple roles of EPH receptors and ephrins in neural development. Nat Rev Neurosci, 2(3), 155–64 (2001) [DOI] [PubMed] [Google Scholar]
- 17.Borghi N and James Nelson W: Intercellular adhesion in morphogenesis: molecular and biophysical considerations. Curr Top Dev Biol, 89, 1–32 (2009) [DOI] [PubMed] [Google Scholar]
- 18.Braga V: Epithelial cell shape: cadherins and small GTPases. Exp Cell Res, 261(1), 83–90 (2000) [DOI] [PubMed] [Google Scholar]
- 19.Braga VM, Machesky LM, Hall A and Hotchin NA: The small GTPases Rho and Rac are required for the establishment of cadherin-dependent cell-cell contacts. J Cell Biol, 137(6), 1421–31 (1997) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sahin M, Greer PL, Lin MZ, Poucher H, Eberhart J, Schmidt S, Wright TM, Shamah SM, O’Connell S, Cowan CW, Hu L, Goldberg JL, Debant A, Corfas G, Krull CE and Greenberg ME: Eph-dependent tyrosine phosphorylation of ephexin1 modulates growth cone collapse. Neuron, 46(2), 191–204 (2005) [DOI] [PubMed] [Google Scholar]
- 21.Shamah SM, Lin MZ, Goldberg JL, Estrach S, Sahin M, Hu L, Bazalakova M, Neve RL, Corfas G, Debant A and Greenberg ME: EphA receptors regulate growth cone dynamics through the novel guanine nucleotide exchange factor ephexin. Cell, 105(2), 233–44 (2001) [DOI] [PubMed] [Google Scholar]
- 22.Demyanenko GP, Siesser PF, Wright AG, Brennaman LH, Bartsch U, Schachner M and Maness PF: L1 and CHL1 Cooperate in Thalamocortical Axon Targeting. Cereb Cortex (2010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kinch MS and Carles-Kinch K: Overexpression and functional alterations of the EphA2 tyrosine kinase in cancer. Clin Exp Metastasis, 20(1), 59–68 (2003) [DOI] [PubMed] [Google Scholar]
- 24.Dodelet VC and Pasquale EB: Eph receptors and ephrin ligands: embryogenesis to tumorigenesis. Oncogene, 19(49), 5614–9 (2000) [DOI] [PubMed] [Google Scholar]
- 25.Easty DJ, Hill SP, Hsu MY, Fallowfield ME, Florenes VA, Herlyn M and Bennett DC: Up-regulation of ephrin-A1 during melanoma progression. Int J Cancer, 84(5), 494–501 (1999) [DOI] [PubMed] [Google Scholar]
- 26.Parri M, Taddei ML, Bianchini F, Calorini L and Chiarugi P: EphA2 reexpression prompts invasion of melanoma cells shifting from mesenchymal to amoeboid-like motility style. Cancer Res, 69(5), 2072–81 (2009) [DOI] [PubMed] [Google Scholar]
- 27.Buricchi F, Giannoni E, Grimaldi G, Parri M, Raugei G, Ramponi G and Chiarugi P: Redox regulation of ephrin/integrin cross-talk. Cell Adh Migr, 1(1), 33–42 (2007) [PMC free article] [PubMed] [Google Scholar]
- 28.Fang WB, Ireton RC, Zhuang G, Takahashi T, Reynolds A and Chen J: Overexpression of EPHA2 receptor destabilizes adherens junctions via a RhoA-dependent mechanism. J Cell Sci, 121(Pt 3), 358–68 (2008) [DOI] [PubMed] [Google Scholar]
- 29.Parri M, Buricchi F, Giannoni E, Grimaldi G, Mello T, Raugei G, Ramponi G and Chiarugi P: EphrinA1 activates a Src/focal adhesion kinase-mediated motility response leading to rho-dependent actino/myosin contractility. J Biol Chem, 282(27), 19619–28 (2007) [DOI] [PubMed] [Google Scholar]
- 30.Lawrenson ID, Wimmer-Kleikamp SH, Lock P, Schoenwaelder SM, Down M, Boyd AW, Alewood PF and Lackmann M: Ephrin-A5 induces rounding, blebbing and de-adhesion of EphA3-expressing 293T and melanoma cells by CrkII and Rho-mediated signalling. J Cell Sci, 115(Pt 5), 1059–72 (2002) [DOI] [PubMed] [Google Scholar]
- 31.Bisson N, Poitras L, Mikryukov A, Tremblay M and Moss T: EphA4 signaling regulates blastomere adhesion in the Xenopus embryo by recruiting Pak1 to suppress Cdc42 function. Mol Biol Cell, 18(3), 1030–43 (2007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Winning RS, Ward EK, Scales JB and Walker GK: EphA4 catalytic activity causes inhibition of RhoA GTPase in Xenopus laevis embryos. Differentiation, 70(1), 46–55 (2002) [DOI] [PubMed] [Google Scholar]
- 33.Winning RS, Wyman TL and Walker GK: EphA4 activity causes cell shape change and a loss of cell polarity in Xenopus laevis embryos. Differentiation, 68(2–3), 126–32 (2001) [DOI] [PubMed] [Google Scholar]
- 34.Park EK, Warner N, Bong YS, Stapleton D, Maeda R, Pawson T and Daar IO: Ectopic EphA4 receptor induces posterior protrusions via FGF signaling in Xenopus embryos. Mol Biol Cell, 15(4), 1647–55 (2004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ridley AJ and Hall A: The small GTP-binding protein rho regulates the assembly of focal adhesions and actin stress fibers in response to growth factors. Cell, 70(3), 389–99 (1992) [DOI] [PubMed] [Google Scholar]
- 36.Nobes CD and Hall A: Rho, rac, and cdc42 GTPases regulate the assembly of multimolecular focal complexes associated with actin stress fibers, lamellipodia, and filopodia. Cell, 81(1), 53–62 (1995) [DOI] [PubMed] [Google Scholar]
- 37.Aasheim HC, Delabie J and Finne EF: Ephrin-A1 binding to CD4+ T lymphocytes stimulates migration and induces tyrosine phosphorylation of PYK2. Blood, 105(7), 2869–76 (2005) [DOI] [PubMed] [Google Scholar]
- 38.Sharfe N, Nikolic M, Cimpeon L, Van De Kratts A, Freywald A and Roifman CM: EphA and ephrin-A proteins regulate integrin-mediated T lymphocyte interactions. Mol Immunol, 45(5), 1208–20 (2008) [DOI] [PubMed] [Google Scholar]
- 39.Faveeuw C, Di Mauro ME, Price AA and Ager A: Roles of alpha(4) integrins/VCAM-1 and LFA-1/ICAM-1 in the binding and transendothelial migration of T lymphocytes and T lymphoblasts across high endothelial venules. Int Immunol, 12(3), 241–51 (2000) [DOI] [PubMed] [Google Scholar]
- 40.Ganju RK, Hatch WC, Avraham H, Ona MA, Druker B, Avraham S and Groopman JE: RAFTK, a novel member of the focal adhesion kinase family, is phosphorylated and associates with signaling molecules upon activation of mature T lymphocytes. J Exp Med, 185(6), 1055–63 (1997) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bennett JS, Berger BW and Billings PC: The structure and function of platelet integrins. J Thromb Haemost, 7 Suppl 1, 200–5 (2009) [DOI] [PubMed] [Google Scholar]
- 42.Prevost N, Woulfe D, Tanaka T and Brass LF: Interactions between Eph kinases and ephrins provide a mechanism to support platelet aggregation once cell-to-cell contact has occurred. Proc Natl Acad Sci U S A, 99(14), 9219–24 (2002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Prevost N, Woulfe DS, Jiang H, Stalker TJ, Marchese P, Ruggeri ZM and Brass LF: Eph kinases and ephrins support thrombus growth and stability by regulating integrin outside-in signaling in platelets. Proc Natl Acad Sci U S A, 102(28), 9820–5 (2005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Barczyk M, Carracedo S and Gullberg D: Integrins. Cell Tissue Res, 339(1), 269–80 (2010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Murai KK, Nguyen LN, Irie F, Yamaguchi Y and Pasquale EB: Control of hippocampal dendritic spine morphology through ephrin-A3/EphA4 signaling. Nat Neurosci, 6(2), 153–60 (2003) [DOI] [PubMed] [Google Scholar]
- 46.Bourgin C, Murai KK, Richter M and Pasquale EB: The EphA4 receptor regulates dendritic spine remodeling by affecting beta1-integrin signaling pathways. J Cell Biol, 178(7), 1295–307 (2007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Richter M, Murai KK, Bourgin C, Pak DT and Pasquale EB: The EphA4 receptor regulates neuronal morphology through SPAR-mediated inactivation of Rap GTPases. J Neurosci, 27(51), 14205–15 (2007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bos JL: Linking Rap to cell adhesion. Curr Opin Cell Biol, 17(2), 123–8 (2005) [DOI] [PubMed] [Google Scholar]
- 49.Caron E: Cellular functions of the Rap1 GTP-binding protein: a pattern emerges. J Cell Sci, 116(Pt 3), 435–40 (2003) [DOI] [PubMed] [Google Scholar]
- 50.Miao H, Burnett E, Kinch M, Simon E and Wang B: Activation of EphA2 kinase suppresses integrin function and causes focal-adhesion-kinase dephosphorylation. Nat Cell Biol, 2(2), 62–9 (2000) [DOI] [PubMed] [Google Scholar]
- 51.Deroanne C, Vouret-Craviari V, Wang B and Pouyssegur J: EphrinA1 inactivates integrin-mediated vascular smooth muscle cell spreading via the Rac/PAK pathway. J Cell Sci, 116(Pt 7), 1367–76 (2003) [DOI] [PubMed] [Google Scholar]
- 52.Kiosses WB, Shattil SJ, Pampori N and Schwartz MA: Rac recruits high-affinity integrin alphavbeta3 to lamellipodia in endothelial cell migration. Nat Cell Biol, 3(3), 316–20 (2001) [DOI] [PubMed] [Google Scholar]
- 53.Taddei ML, Parri M, Angelucci A, Onnis B, Bianchini F, Giannoni E, Raugei G, Calorini L, Rucci N, Teti A, Bologna M and Chiarugi P: Kinase-dependent and -independent roles of EphA2 in the regulation of prostate cancer invasion and metastasis. Am J Pathol, 174(4), 1492–503 (2009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Carter N, Nakamoto T, Hirai H and Hunter T: EphrinA1-induced cytoskeletal re-organization requires FAK and p130(cas). Nat Cell Biol, 4(8), 565–73 (2002) [DOI] [PubMed] [Google Scholar]
- 55.Puschmann TB and Turnley AM: Eph receptor tyrosine kinases regulate astrocyte cytoskeletal rearrangement and focal adhesion formation. J Neurochem, 113(4), 881–94 (2010) [DOI] [PubMed] [Google Scholar]
- 56.de Saint-Vis B, Bouchet C, Gautier G, Valladeau J, Caux C and Garrone P: Human dendritic cells express neuronal Eph receptor tyrosine kinases: role of EphA2 in regulating adhesion to fibronectin. Blood, 102(13), 4431–40 (2003) [DOI] [PubMed] [Google Scholar]
- 57.Gu C and Park S: The EphA8 receptor regulates integrin activity through p110gamma phosphatidylinositol-3 kinase in a tyrosine kinase activity-independent manner. Mol Cell Biol, 21(14), 4579–97 (2001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cooke JE, Kemp HA and Moens CB: EphA4 is required for cell adhesion and rhombomere-boundary formation in the zebrafish. Curr Biol, 15(6), 536–42 (2005) [DOI] [PubMed] [Google Scholar]
- 59.Abdul-Aziz NM, Turmaine M, Greene ND and Copp AJ: EphrinA-EphA receptor interactions in mouse spinal neurulation: implications for neural fold fusion. Int J Dev Biol, 53(4), 559–68 (2009) [DOI] [PubMed] [Google Scholar]
- 60.Chan B and Sukhatme VP: Receptor tyrosine kinase EphA2 mediates thrombin-induced upregulation of ICAM-1 in endothelial cells in vitro. Thromb Res, 123(5), 745–52 (2009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kullander K and Klein R: Mechanisms and functions of Eph and ephrin signalling. Nat Rev Mol Cell Biol, 3(7), 475–86 (2002) [DOI] [PubMed] [Google Scholar]
- 62.Wilkinson DG: How attraction turns to repulsion. Nat Cell Biol, 5(10), 851–3 (2003) [DOI] [PubMed] [Google Scholar]
- 63.Zimmer M, Palmer A, Kohler J and Klein R: EphB-ephrinB bi-directional endocytosis terminates adhesion allowing contact mediated repulsion. Nat Cell Biol, 5(10), 869–78 (2003) [DOI] [PubMed] [Google Scholar]
- 64.Kida YS, Sato T, Miyasaka KY, Suto A and Ogura T: Daam1 regulates the endocytosis of EphB during the convergent extension of the zebrafish notochord. Proc Natl Acad Sci U S A, 104(16), 6708–13 (2007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zisch AH, Pazzagli C, Freeman AL, Schneller M, Hadman M, Smith JW, Ruoslahti E and Pasquale EB: Replacing two conserved tyrosines of the EphB2 receptor with glutamic acid prevents binding of SH2 domains without abrogating kinase activity and biological responses. Oncogene, 19(2), 177–87 (2000) [DOI] [PubMed] [Google Scholar]
- 66.Guo DL, Zhang J, Yuen ST, Tsui WY, Chan AS, Ho C, Ji J, Leung SY and Chen X: Reduced expression of EphB2 that parallels invasion and metastasis in colorectal tumours. Carcinogenesis, 27(3), 454–64 (2006) [DOI] [PubMed] [Google Scholar]
- 67.Zou JX, Wang B, Kalo MS, Zisch AH, Pasquale EB and Ruoslahti E: An Eph receptor regulates integrin activity through R-Ras. Proc Natl Acad Sci U S A, 96(24), 13813–8 (1999) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nakada M, Anderson EM, Demuth T, Nakada S, Reavie LB, Drake KL, Hoelzinger DB and Berens ME: The phosphorylation of ephrin-B2 ligand promotes glioma cell migration and invasion. Int J Cancer, 126(5), 1155–65 (2009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Miao H, Strebhardt K, Pasquale EB, Shen TL, Guan JL and Wang B: Inhibition of integrin-mediated cell adhesion but not directional cell migration requires catalytic activity of EphB3 receptor tyrosine kinase. Role of Rho family small GTPases. J Biol Chem, 280(2), 923–32 (2005) [DOI] [PubMed] [Google Scholar]
- 70.Chiu ST, Chang KJ, Ting CH, Shen HC, Li H and Hsieh FJ: Over-expression of EphB3 enhances cell-cell contacts and suppresses tumor growth in HT-29 human colon cancer cells. Carcinogenesis, 30(9), 1475–86 (2009) [DOI] [PubMed] [Google Scholar]
- 71.Noren NK, Foos G, Hauser CA and Pasquale EB: The EphB4 receptor suppresses breast cancer cell tumorigenicity through an Abl-Crk pathway. Nat Cell Biol, 8(8), 815–25 (2006) [DOI] [PubMed] [Google Scholar]
- 72.Huang X, Wu D, Jin H, Stupack D and Wang JY: Induction of cell retraction by the combined actions of Abl-CrkII and Rho-ROCK1 signaling. J Cell Biol, 183(4), 711–23 (2008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Pasquale EB: Eph receptors and ephrins in cancer: bidirectional signalling and beyond. Nat Rev Cancer, 10(3), 165–80 (2010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.He S, Kumar SR, Zhou P, Krasnoperov V, Ryan SJ, Gill PS and Hinton DR: Soluble EphB4 inhibition of PDGF-induced RPE migration in vitro. Invest Ophthalmol Vis Sci, 51(1), 543–52 (2010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.McClelland AC, Sheffler-Collins SI, Kayser MS and Dalva MB: Ephrin-B1 and ephrin-B2 mediate EphB-dependent presynaptic development via syntenin-1. Proc Natl Acad Sci U S A, 106(48), 20487–92 (2009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Dopeso H, Mateo-Lozano S, Mazzolini R, Rodrigues P, Lagares-Tena L, Ceron J, Romero J, Esteves M, Landolfi S, Hernandez-Losa J, Castano J, Wilson AJ, Ramon y Cajal S, Mariadason JM, Schwartz S Jr. and Arango D: The receptor tyrosine kinase EPHB4 has tumor suppressor activities in intestinal tumorigenesis. Cancer Res, 69(18), 7430–8 (2009) [DOI] [PubMed] [Google Scholar]
- 77.Noren NK, Yang NY, Silldorff M, Mutyala R and Pasquale EB: Ephrin-independent regulation of cell substrate adhesion by the EphB4 receptor. Biochem J, 422(3), 433–42 (2009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Henkemeyer M, Itkis OS, Ngo M, Hickmott PW and Ethell IM: Multiple EphB receptor tyrosine kinases shape dendritic spines in the hippocampus. J Cell Biol, 163(6), 1313–26 (2003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ethell IM, Irie F, Kalo MS, Couchman JR, Pasquale EB and Yamaguchi Y: EphB/syndecan-2 signaling in dendritic spine morphogenesis. Neuron, 31(6), 1001–13 (2001) [DOI] [PubMed] [Google Scholar]
- 80.Irie F and Yamaguchi Y: EphB receptors regulate dendritic spine development via intersectin, Cdc42 and N-WASP. Nat Neurosci, 5(11), 1117–8 (2002) [DOI] [PubMed] [Google Scholar]
- 81.Tolias KF, Bikoff JB, Kane CG, Tolias CS, Hu L and Greenberg ME: The Rac1 guanine nucleotide exchange factor Tiam1 mediates EphB receptor-dependent dendritic spine development. Proc Natl Acad Sci U S A, 104(17), 7265–70 (2007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Moeller ML, Shi Y, Reichardt LF and Ethell IM: EphB receptors regulate dendritic spine morphogenesis through the recruitment/phosphorylation of focal adhesion kinase and RhoA activation. J Biol Chem, 281(3), 1587–98 (2006) [DOI] [PubMed] [Google Scholar]
- 83.Shi Y, Pontrello CG, DeFea KA, Reichardt LF and Ethell IM: Focal adhesion kinase acts downstream of EphB receptors to maintain mature dendritic spines by regulating cofilin activity. J Neurosci, 29(25), 8129–42 (2009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bamburg JR: Proteins of the ADF/cofilin family: essential regulators of actin dynamics. Annu Rev Cell Dev Biol, 15, 185–230 (1999) [DOI] [PubMed] [Google Scholar]
- 85.Elowe S, Holland SJ, Kulkarni S and Pawson T: Downregulation of the Ras-mitogen-activated protein kinase pathway by the EphB2 receptor tyrosine kinase is required for ephrin-induced neurite retraction. Mol Cell Biol, 21(21), 7429–41 (2001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kasemeier-Kulesa JC, Bradley R, Pasquale EB, Lefcort F and Kulesa PM: Eph/ephrins and N-cadherin coordinate to control the pattern of sympathetic ganglia. Development, 133(24), 4839–47 (2006) [DOI] [PubMed] [Google Scholar]
- 87.Chen ZY, Sun C, Reuhl K, Bergemann A, Henkemeyer M and Zhou R: Abnormal hippocampal axon bundling in EphB receptor mutant mice. J Neurosci, 24(10), 2366–74 (2004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Batlle E, Henderson JT, Beghtel H, van den Born MM, Sancho E, Huls G, Meeldijk J, Robertson J, van de Wetering M, Pawson T and Clevers H: Beta-catenin and TCF mediate cell positioning in the intestinal epithelium by controlling the expression of EphB/ephrinB. Cell, 111(2), 251–63 (2002) [DOI] [PubMed] [Google Scholar]
- 89.Dravis C, Yokoyama N, Chumley MJ, Cowan CA, Silvany RE, Shay J, Baker LA and Henkemeyer M: Bidirectional signaling mediated by ephrin-B2 and EphB2 controls urorectal development. Dev Biol, 271(2), 272–90 (2004) [DOI] [PubMed] [Google Scholar]
- 90.Nakada M, Niska JA, Miyamori H, McDonough WS, Wu J, Sato H and Berens ME: The phosphorylation of EphB2 receptor regulates migration and invasion of human glioma cells. Cancer Res, 64(9), 3179–85 (2004) [DOI] [PubMed] [Google Scholar]
- 91.Cortina C, Palomo-Ponce S, Iglesias M, Fernandez-Masip JL, Vivancos A, Whissell G, Huma M, Peiro N, Gallego L, Jonkheer S, Davy A, Lloreta J, Sancho E and Batlle E: EphB-ephrin-B interactions suppress colorectal cancer progression by compartmentalizing tumor cells. Nat Genet, 39(11), 1376–83 (2007) [DOI] [PubMed] [Google Scholar]
- 92.Fuller T, Korff T, Kilian A, Dandekar G and Augustin HG: Forward EphB4 signaling in endothelial cells controls cellular repulsion and segregation from ephrinB2 positive cells. J Cell Sci, 116(Pt 12), 2461–70 (2003) [DOI] [PubMed] [Google Scholar]
- 93.Hamada K, Oike Y, Ito Y, Maekawa H, Miyata K, Shimomura T and Suda T: Distinct roles of ephrin-B2 forward and EphB4 reverse signaling in endothelial cells. Arterioscler Thromb Vasc Biol, 23(2), 190–7 (2003) [DOI] [PubMed] [Google Scholar]
- 94.Marston DJ, Dickinson S and Nobes CD: Rac-dependent trans-endocytosis of ephrinBs regulates Eph-ephrin contact repulsion. Nat Cell Biol, 5(10), 879–88 (2003) [DOI] [PubMed] [Google Scholar]
- 95.Groeger G and Nobes CD: Co-operative Cdc42 and Rho signalling mediates ephrinB-triggered endothelial cell retraction. Biochem J, 404(1), 23–9 (2007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Pfaff D, Heroult M, Riedel M, Reiss Y, Kirmse R, Ludwig T, Korff T, Hecker M and Augustin HG: Involvement of endothelial ephrin-B2 in adhesion and transmigration of EphB-receptor-expressing monocytes. J Cell Sci, 121(Pt 22), 3842–50 (2008) [DOI] [PubMed] [Google Scholar]
- 97.Foubert P, Silvestre JS, Souttou B, Barateau V, Martin C, Ebrahimian TG, Lere-Dean C, Contreres JO, Sulpice E, Levy BI, Plouet J, Tobelem G and Le Ricousse-Roussanne S: PSGL-1-mediated activation of EphB4 increases the proangiogenic potential of endothelial progenitor cells. J Clin Invest, 117(6), 1527–37 (2007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Dimaio TA, Wang S, Huang Q, Scheef EA, Sorenson CM and Sheibani N: Attenuation of retinal vascular development and neovascularization in PECAM-1-deficient mice. Dev Biol, 315(1), 72–88 (2008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Astin JW, Batson J, Kadir S, Charlet J, Persad RA, Gillatt D, Oxley JD and Nobes CD: Competition amongst Eph receptors regulates contact inhibition of locomotion and invasiveness in prostate cancer cells. Nat Cell Biol, 12(12), 1194–204 (2010) [DOI] [PubMed] [Google Scholar]
- 100.Foo SS, Turner CJ, Adams S, Compagni A, Aubyn D, Kogata N, Lindblom P, Shani M, Zicha D and Adams RH: Ephrin-B2 controls cell motility and adhesion during blood-vessel-wall assembly. Cell, 124(1), 161–73 (2006) [DOI] [PubMed] [Google Scholar]
- 101.Bochenek ML, Dickinson S, Astin JW, Adams RH and Nobes CD: Ephrin-B2 regulates endothelial cell morphology and motility independently of Eph-receptor binding. J Cell Sci, 123(Pt 8), 1235–46 (2010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Holmberg J, Clarke DL and Frisen J: Regulation of repulsion versus adhesion by different splice forms of an Eph receptor. Nature, 408(6809), 203–6 (2000) [DOI] [PubMed] [Google Scholar]
- 103.Kikawa KD, Vidale DR, Van Etten RL and Kinch MS: Regulation of the EphA2 kinase by the low molecular weight tyrosine phosphatase induces transformation. J Biol Chem, 277(42), 39274–9 (2002) [DOI] [PubMed] [Google Scholar]
- 104.Hattori M, Osterfield M and Flanagan JG: Regulated cleavage of a contact-mediated axon repellent. Science, 289(5483), 1360–5 (2000) [DOI] [PubMed] [Google Scholar]
- 105.Huai J and Drescher U: An ephrin-A-dependent signaling pathway controls integrin function and is linked to the tyrosine phosphorylation of a 120-kDa protein. J Biol Chem, 276(9), 6689–94 (2001) [DOI] [PubMed] [Google Scholar]
- 106.Davy A, Gale NW, Murray EW, Klinghoffer RA, Soriano P, Feuerstein C and Robbins SM: Compartmentalized signaling by GPI-anchored ephrin-A5 requires the Fyn tyrosine kinase to regulate cellular adhesion. Genes Dev, 13(23), 3125–35 (1999) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Davy A and Robbins SM: Ephrin-A5 modulates cell adhesion and morphology in an integrin-dependent manner. EMBO J, 19(20), 5396–405 (2000) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Fujii H, Tatsumi K, Kosaka K, Yoshioka S, Fujiwara H and Fujii S: Eph-ephrin A system regulates murine blastocyst attachment and spreading. Dev Dyn, 235(12), 3250–8 (2006) [DOI] [PubMed] [Google Scholar]
- 109.Ting MC, Wu NL, Roybal PG, Sun J, Liu L, Yen Y and Maxson RE Jr.: EphA4 as an effector of Twist1 in the guidance of osteogenic precursor cells during calvarial bone growth and in craniosynostosis. Development, 136(5), 855–64 (2009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Trinidad EM, Ballesteros M, Zuloaga J, Zapata A and Alonso-Colmenar LM: An impaired transendothelial migration potential of chronic lymphocytic leukemia (CLL) cells can be linked to ephrin-A4 expression. Blood, 114(24), 5081–90 (2009) [DOI] [PubMed] [Google Scholar]
- 111.Konstantinova I, Nikolova G, Ohara-Imaizumi M, Meda P, Kucera T, Zarbalis K, Wurst W, Nagamatsu S and Lammert E: EphA-Ephrin-A-mediated beta cell communication regulates insulin secretion from pancreatic islets. Cell, 129(2), 359–70 (2007) [DOI] [PubMed] [Google Scholar]
- 112.Holen HL, Shadidi M, Narvhus K, Kjosnes O, Tierens A and Aasheim HC: Signaling through ephrin-A ligand leads to activation of Src-family kinases, Akt phosphorylation, and inhibition of antigen receptor-induced apoptosis. J Leukoc Biol, 84(4), 1183–91 (2008) [DOI] [PubMed] [Google Scholar]
- 113.Lim YS, McLaughlin T, Sung TC, Santiago A, Lee KF and O’Leary DD: p75(NTR) mediates ephrin-A reverse signaling required for axon repulsion and mapping. Neuron, 59(5), 746–58 (2008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Janes PW, Wimmer-Kleikamp SH, Frangakis AS, Treble K, Griesshaber B, Sabet O, Grabenbauer M, Ting AY, Saftig P, Bastiaens PI and Lackmann M: Cytoplasmic relaxation of active Eph controls ephrin shedding by ADAM10. PLoS Biol, 7(10), e1000215(2009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Janes PW, Saha N, Barton WA, Kolev MV, Wimmer-Kleikamp SH, Nievergall E, Blobel CP, Himanen JP, Lackmann M and Nikolov DB: Adam meets Eph: an ADAM substrate recognition module acts as a molecular switch for ephrin cleavage in trans. Cell, 123(2), 291–304 (2005) [DOI] [PubMed] [Google Scholar]
- 116.Holland SJ, Gale NW, Mbamalu G, Yancopoulos GD, Henkemeyer M and Pawson T: Bidirectional signalling through the EPH-family receptor Nuk and its transmembrane ligands. Nature, 383(6602), 722–5 (1996) [DOI] [PubMed] [Google Scholar]
- 117.Bruckner K, Pasquale EB and Klein R: Tyrosine phosphorylation of transmembrane ligands for Eph receptors. Science, 275(5306), 1640–3 (1997) [DOI] [PubMed] [Google Scholar]
- 118.Lee HS and Daar IO: EphrinB reverse signaling in cell-cell adhesion: is it just par for the course? Cell Adh Migr, 3(3), 250–5 (2009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Adams RH, Wilkinson GA, Weiss C, Diella F, Gale NW, Deutsch U, Risau W and Klein R: Roles of ephrinB ligands and EphB receptors in cardiovascular development: demarcation of arterial/venous domains, vascular morphogenesis, and sprouting angiogenesis. Genes Dev, 13(3), 295–306 (1999) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Moore KB, Mood K, Daar IO and Moody SA: Morphogenetic movements underlying eye field formation require interactions between the FGF and ephrinB1signaling pathways. Dev Cell, 6(1), 55–67 (2004) [DOI] [PubMed] [Google Scholar]
- 121.Tanaka M, Kamata R and Sakai R: Phosphorylation of ephrin-B1 via the interaction with claudin following cell-cell contact formation. EMBO J, 24(21), 3700–11 (2005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Lu Q, Sun EE, Klein RS and Flanagan JG: Ephrin-B reverse signaling is mediated by a novel PDZRGS protein and selectively inhibits G protein-coupled chemoattraction. Cell, 105(1), 69–79 (2001) [DOI] [PubMed] [Google Scholar]
- 123.Qiu R, Wang X, Davy A, Wu C, Murai K, Zhang H, Flanagan JG, Soriano P and Lu Q: Regulation of neural progenitor cell state by ephrin-B. J Cell Biol, 181(6), 973–83 (2008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Wu C, Qiu R, Wang J, Zhang H, Murai K and Lu Q: ZHX2 Interacts with Ephrin-B and regulates neural progenitor maintenance in the developing cerebral cortex. J Neurosci, 29(23), 7404–12 (2009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Lin D, Gish GD, Songyang Z and Pawson T: The carboxyl terminus of B class ephrins constitutes a PDZ domain binding motif. J Biol Chem, 274(6), 3726–33 (1999) [DOI] [PubMed] [Google Scholar]
- 126.Bruckner K, Pablo Labrador J, Scheiffele P, Herb A, Seeburg PH and Klein R: EphrinB ligands recruit GRIP family PDZ adaptor proteins into raft membrane microdomains. Neuron, 22(3), 511–24 (1999) [DOI] [PubMed] [Google Scholar]
- 127.Davy A, Bush JO and Soriano P: Inhibition of gap junction communication at ectopic Eph/ephrin boundaries underlies craniofrontonasal syndrome. PLoS Biol, 4(10), e315(2006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Lee HS, Bong YS, Moore KB, Soria K, Moody SA and Daar IO: Dishevelled mediates ephrinB1 signalling in the eye field through the planar cell polarity pathway. Nat Cell Biol, 8(1), 55–63 (2006) [DOI] [PubMed] [Google Scholar]
- 129.Cowan CA and Henkemeyer M: The SH2/SH3 adaptor Grb4 transduces B-ephrin reverse signals. Nature, 413(6852), 174–9 (2001) [DOI] [PubMed] [Google Scholar]
- 130.Bong YS, Park YH, Lee HS, Mood K, Ishimura A and Daar IO: Tyr-298 in ephrinB1 is critical for an interaction with the Grb4 adaptor protein. Biochem J, 377(Pt 2), 499–507 (2004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Segura I, Essmann CL, Weinges S and Acker-Palmer A: Grb4 and GIT1 transduce ephrinB reverse signals modulating spine morphogenesis and synapse formation. Nat Neurosci, 10(3), 301–10 (2007) [DOI] [PubMed] [Google Scholar]
- 132.Bong YS, Lee HS, Carim-Todd L, Mood K, Nishanian TG, Tessarollo L and Daar IO: ephrinB1 signals from the cell surface to the nucleus by recruitment of STAT3. Proc Natl Acad Sci U S A, 104(44), 17305–10 (2007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Salvucci O, Maric D, Economopoulou M, Sakakibara S, Merlin S, Follenzi A and Tosato G: EphrinB reverse signaling contributes to endothelial and mural cell assembly into vascular structures. Blood, 114(8), 1707–16 (2009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Jones TL, Chong LD, Kim J, Xu RH, Kung HF and Daar IO: Loss of cell adhesion in Xenopus laevis embryos mediated by the cytoplasmic domain of XLerk, an erythropoietin-producing hepatocellular ligand. Proc Natl Acad Sci U S A, 95(2), 576–81 (1998) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Davy A, Aubin J and Soriano P: Ephrin-B1 forward and reverse signaling are required during mouse development. Genes Dev, 18(5), 572–83 (2004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Makinen T, Adams RH, Bailey J, Lu Q, Ziemiecki A, Alitalo K, Klein R and Wilkinson GA: PDZ interaction site in ephrinB2 is required for the remodeling of lymphatic vasculature. Genes Dev, 19(3), 397–410 (2005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Adams RH, Diella F, Hennig S, Helmbacher F, Deutsch U and Klein R: The cytoplasmic domain of the ligand ephrinB2 is required for vascular morphogenesis but not cranial neural crest migration. Cell, 104(1), 57–69 (2001) [DOI] [PubMed] [Google Scholar]
- 138.Herbert SP, Huisken J, Kim TN, Feldman ME, Houseman BT, Wang RA, Shokat KM and Stainier DY: Arterial-venous segregation by selective cell sprouting: an alternative mode of blood vessel formation. Science, 326(5950), 294–8 (2009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Lee HS, Nishanian TG, Mood K, Bong YS and Daar IO: EphrinB1 controls cell-cell junctions through the Par polarity complex. Nat Cell Biol, 10(8), 979–86 (2008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Wang Q and Margolis B: Apical junctional complexes and cell polarity. Kidney Int, 72(12), 1448–58 (2007) [DOI] [PubMed] [Google Scholar]
- 141.Chong LD, Park EK, Latimer E, Friesel R and Daar IO: Fibroblast growth factor receptor-mediated rescue of x-ephrin B1-induced cell dissociation in Xenopus embryos. Mol Cell Biol, 20(2), 724–34 (2000) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Mellitzer G, Xu Q and Wilkinson DG: Eph receptors and ephrins restrict cell intermingling and communication. Nature, 400(6739), 77–81 (1999) [DOI] [PubMed] [Google Scholar]
- 143.Xu Q, Alldus G, Holder N and Wilkinson DG: Expression of truncated Sek-1 receptor tyrosine kinase disrupts the segmental restriction of gene expression in the Xenopus and zebrafish hindbrain. Development, 121(12), 4005–16 (1995) [DOI] [PubMed] [Google Scholar]
- 144.Xu Q, Alldus G, Macdonald R, Wilkinson DG and Holder N: Function of the Eph-related kinase rtk1 in patterning of the zebrafish forebrain. Nature, 381(6580), 319–22 (1996) [DOI] [PubMed] [Google Scholar]
- 145.Kemp HA, Cooke JE and Moens CB: EphA4 and EfnB2a maintain rhombomere coherence by independently regulating intercalation of progenitor cells in the zebrafish neural keel. Dev Biol, 327(2), 313–26 (2009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Tepass U, Godt D and Winklbauer R: Cell sorting in animal development: signalling and adhesive mechanisms in the formation of tissue boundaries. Curr Opin Genet Dev, 12(5), 572–82 (2002) [DOI] [PubMed] [Google Scholar]
- 147.Crawford BD, Henry CA, Clason TA, Becker AL and Hille MB: Activity and distribution of paxillin, focal adhesion kinase, and cadherin indicate cooperative roles during zebrafish morphogenesis. Mol Biol Cell, 14(8), 3065–81 (2003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Holley SA: The genetics and embryology of zebrafish metamerism. Dev Dyn, 236(6), 1422–49 (2007) [DOI] [PubMed] [Google Scholar]
- 149.Koshida S, Kishimoto Y, Ustumi H, Shimizu T, Furutani-Seiki M, Kondoh H and Takada S: Integrinalpha5-dependent fibronectin accumulation for maintenance of somite boundaries in zebrafish embryos. Dev Cell, 8(4), 587–98 (2005) [DOI] [PubMed] [Google Scholar]
- 150.Barrios A, Poole RJ, Durbin L, Brennan C, Holder N and Wilson SW: Eph/Ephrin signaling regulates the mesenchymal-to-epithelial transition of the paraxial mesoderm during somite morphogenesis. Curr Biol, 13(18), 1571–82 (2003) [DOI] [PubMed] [Google Scholar]
- 151.Watanabe T, Sato Y, Saito D, Tadokoro R and Takahashi Y: EphrinB2 coordinates the formation of a morphological boundary and cell epithelialization during somite segmentation. Proc Natl Acad Sci U S A, 106(18), 7467–72 (2009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Wu X, Li S, Chrostek-Grashoff A, Czuchra A, Meyer H, Yurchenco PD and Brakebusch C: Cdc42 is crucial for the establishment of epithelial polarity during early mammalian development. Dev Dyn, 236(10), 2767–78 (2007) [DOI] [PubMed] [Google Scholar]
- 153.Samarin S and Nusrat A: Regulation of epithelial apical junctional complex by Rho family GTPases. Front Biosci, 14, 1129–42 (2009) [DOI] [PubMed] [Google Scholar]
- 154.Warner SJ and Longmore GD: Distinct functions for Rho1 in maintaining adherens junctions and apical tension in remodeling epithelia. J Cell Biol, 185(6), 1111–25 (2009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Julich D, Mould AP, Koper E and Holley SA: Control of extracellular matrix assembly along tissue boundaries via Integrin and Eph/Ephrin signaling. Development, 136(17), 2913–21 (2009) [DOI] [PubMed] [Google Scholar]
- 156.Tanaka M, Kamo T, Ota S and Sugimura H: Association of Dishevelled with Eph tyrosine kinase receptor and ephrin mediates cell repulsion. EMBO J, 22(4), 847–58 (2003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Lee HS, Mood K, Battu G, Ji YJ, Singh A and Daar IO: Fibroblast growth factor receptor-induced phosphorylation of ephrinB1 modulates its interaction with Dishevelled. Mol Biol Cell, 20(1), 124–33 (2009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Huynh-Do U, Vindis C, Liu H, Cerretti DP, McGrew JT, Enriquez M, Chen J and Daniel TO: Ephrin-B1 transduces signals to activate integrin-mediated migration, attachment and angiogenesis. J Cell Sci, 115(Pt 15), 3073–81 (2002) [DOI] [PubMed] [Google Scholar]
- 159.Tomita T, Tanaka S, Morohashi Y and Iwatsubo T: Presenilin-dependent intramembrane cleavage of ephrin-B1. Mol Neurodegener, 1, 2(2006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Georgakopoulos A, Litterst C, Ghersi E, Baki L, Xu C, Serban G and Robakis NK: Metalloproteinase/Presenilin1 processing of ephrinB regulates EphB-induced Src phosphorylation and signaling. EMBO J, 25(6), 1242–52 (2006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Wei S, Xu G, Bridges LC, Williams P, White JM and DeSimone DW: ADAM13 induces cranial neural crest by cleaving class B Ephrins and regulating Wnt signaling. Dev Cell, 19(2), 345–52 (2010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Weidinger G and Moon RT: When Wnts antagonize Wnts. J Cell Biol, 162(5), 753–5 (2003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Wimmer-Kleikamp SH, Nievergall E, Gegenbauer K, Adikari S, Mansour M, Yeadon T, Boyd AW, Patani NR and Lackmann M: Elevated protein tyrosine phosphatase activity provokes Eph/ephrin-facilitated adhesion of pre-B leukemia cells. Blood, 112(3), 721–32 (2008) [DOI] [PubMed] [Google Scholar]
- 164.Nievergall E, Janes PW, Stegmayer C, Vail ME, Haj FG, Teng SW, Neel BG, Bastiaens PI and Lackmann M: PTP1B regulates Eph receptor function and trafficking. J Cell Biol, 191 (6), 1189–1203 (2010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Duxbury MS, Ito H, Zinner MJ, Ashley SW and Whang EE: Ligation of EphA2 by Ephrin A1-Fc inhibits pancreatic adenocarcinoma cellular invasiveness. Biochem Biophys Res Commun, 320(4), 1096–102 (2004) [DOI] [PubMed] [Google Scholar]
- 166.Cooper MA, Son AI, Komlos D, Sun Y, Kleiman NJ and Zhou R: Loss of ephrin-A5 function disrupts lens fiber cell packing and leads to cataract. Proc Natl Acad Sci U S A, 105(43), 16620–5 (2008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Harris KP and Tepass U: Cdc42 and Par proteins stabilize dynamic adherens junctions in the Drosophila neuroectoderm through regulation of apical endocytosis. J Cell Biol, 183(6), 1129–43 (2008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Georgiou M, Marinari E, Burden J and Baum B: Cdc42, Par6, and aPKC regulate Arp2/3-mediated endocytosis to control local adherens junction stability. Curr Biol, 18(21), 1631–8 (2008) [DOI] [PubMed] [Google Scholar]
- 169.Leibfried A, Fricke R, Morgan MJ, Bogdan S and Bellaiche Y: Drosophila Cip4 and WASp define a branch of the Cdc42-Par6-aPKC pathway regulating E-cadherin endocytosis. Curr Biol, 18(21), 1639–48 (2008) [DOI] [PubMed] [Google Scholar]


