Abstract
Background:
Cervical cancer is currently the third most common female cancer in Malaysia, with the human papillomavirus (HPV) considered as one of the important contributory factors. This study was conducted to determine HPV prevalence, its genotype distribution, and other potential risk factors among women in Kota Kinabalu, Sabah in order to evaluate the likely efficacy of current HPV vaccines in the local population.
Methods:
A total of 240 cervical samples were collected and subjected to DNA extraction, PCR amplification using the MY09/MY11 primer pair, and restriction fragment length polymorphism (RFLP) for HPV detection and genotyping. Sociodemographic, clinical, and behavioural data were also collected via questionnaires.
Results:
The prevalence of HPV infection was 9.6%. The most common HPVs among 13 genotypes were high-risk HPV-56 (16.7%) and probable high-risk HPV-70 (16.7%) followed by HPV-16, -58, -53, -61, -33, -59, and -66 (in decreasing order of prevalence) including the rare genotypes: HPV-62, -81, -82 and -84. Statistical analyses using logistic regression models showed that HPV infection was significantly associated with employment (OR 4.94; CI 1.58-15.40) and education at secondary/high school level (OR 0.13; CI 0.03-0.62).
Conclusion:
Distribution of HPV genotypes in Sabah indicated a high prevalence of HPV-56 and -70 which are among the rare HPV types in West Malaysia and merit consideration in future strategies for HPV vaccination specifically for local Sabahan women.
Keywords: Cervical cancer, human papillomavirus, prevalence, risk factor, Sabah
Introduction
Cervical cancer is currently the fourth most prevalent female cancer in the world and also ranked as the third among ten prominent cancers in female population of Malaysia (Azizah et al., 2016). The age-standardised incidence rate (ASR) of cervical cancer in Malaysia reported by the national cancer registry was 6.5 per 100,000 in 2011 including a record of high ASR in the population of East Malaysian state of Sabah which indicated 11.5 per 100,000 (Azizah et al., 2016). The incidence rate of cervical cancer among the Malaysian women increased relatively with age whereby more than 25 per 100,000 incidences occurred between 55 and 70 years. Sabah or formerly known as North Borneo, consists largely of 32 officially recognised indigenous ethnic groups with distinct identities, cultures, and languages (The report: Sabah 2011, 2011). According to Sabah cancer registry report 2001-2005 (2009), the largest indigenous group of Kadazan-Dusun was reported with the highest ASR (13.2), followed by Chinese (12.9), Bajau (12.6), Malay (11.4), other minor indigenous groups (11.3), and Murut (5.3).
Human papillomavirus (HPV) is now regarded as one of the crucial factors contributing to the development of cervical carcinoma. There are approximately 198 HPV genotypes currently known and among them, HPV-16 and HPV-18, are the most prevalent types associated with 70% of global cervical cancer incidences (Rajaram et al., 2012, Bzhalava et al., 2015). More than 90% cases of HPV infection typically clear on their own but infection by high-risk HPVs may persist over time and is influenced by various risk factors involving unsafe sexual practices, smoking habit, and long-term use of oral contraceptives (Zitkute and Bumbuliene, 2016).
With the recent introduction of HPV vaccines to the young generation worldwide as a preventive measure against HPV infection, there is an uncertainty of whether the vaccines are effective enough to provide the necessary protection to all various oncogenic HPVs. Geographic distribution data of HPV types across the globe is now considered imperative in order to evaluate the efficiency of HPV vaccines. Information regarding HPV prevalence and distribution in Malaysia mainly revolves around the populations of West Malaysia with little or no relevant data available concerning Sabah which is located on the island of Borneo and separated from West Malaysia by the South China Sea.
In order to evaluate the efficacy of current HPV vaccines on Sabahan women, this study focused on local women residing in Kota Kinabalu, the capital city of Sabah, and conducted to determine the prevalence and distribution of HPV genotypes based on cervical cytology, sociodemographic, clinical, and behavioural factors.
Materials and Methods
Study population and sample collection
Sample size of the study was calculated with 95% confidence interval and 5% precision based on the estimated rate of HPV prevalence in Malaysian women with normal cervical cytology provided by HPV Information Centre which was 13.7% (95% CI: 11.9 – 15.6) (Bruni et al., 2016). Approximately, 195 participants were considered for the minimum sample size. The participants involved were female volunteers who underwent Pap smear screening test in Obstetrics and Gynaecology Clinics at Universiti Malaysia Sabah (UMS) Polyclinic, and Sabah Women and Children Hospital. Sample collection was conducted within a 12-month period from September 2016 to August 2017. Among the eligibility criteria of participants were Malaysian citizens aged 18 to 70 years who were sexually active, not pregnant, and had no history of cervical cancer. Ethical clearance was approved by Medical Research and Ethics Committee (MREC), Ministry of Health, Malaysia (NMRR-16-816-30597 (IIR)) as well as UMS Medical Ethics Committee (JKEtika 2/16 (4)). Informed consent forms were collected from the participants along with questionnaires inquiring their sociodemographic status, medical history, and sexual lifestyle. Results of Pap smear test conducted in the hospital and clinic were later obtained from the gynaecologists in charge.
Sample processing and DNA extraction
A cervical swab was obtained from both endocervix and ectocervix of each woman by a gynaecologist or a trained nurse using a sterile cytobrush. The head of cytobrush containing collected cervical cells was detached, immersed in phosphate buffered saline (PBS) solution (pH 7), and later transported to the laboratories for HPV testing. The cells were dislodged from cytobrush head via vortex and transferred into falcon tube for centrifugation. Resulted cell pellet was resuspended in 200 µl PBS and either stored at -40°C or proceeded immediately to DNA extraction. Extraction of DNA from the cell samples was performed using GF-1 Viral Nucleic Acid Extraction kit (Vivantis Technologies, Malaysia) according to the protocol provided by the manufacturer. Eluted pure DNA was stored at -40°C prior to polymerase chain reaction (PCR).
Detection of HPV DNA from cervical samples
HPV detection was conducted by amplifying the consensus L1 gene from HPV viral genome via standard PCR method. The primer pair used in this procedure was MY09/MY11 (5’-CGTCCMARRGGAWACTGATC-3’ and 5’-GCMCAGGGWCATAAYAATGG-3’) which targeted a fragment size of 450 bp within L1 open reading frame and capable of detecting more than 40 genotypes of genital HPV (Manos et al., 1989). A separate PCR was conducted to amplify human β-globin reference gene in order to determine the quality of the DNA extract by using forward primer PC03 (5’- ACACAACTGTGTTCACTAGC-3’) and reverse primer PC04 (5’-CAACTTCATCCACGTTCACC-3’), targeting a fragment size of 110 bp. Gene amplification was performed in a total volume of 25 µl PCR mixture comprised of 1X GoTaq® PCR Master Mix (Promega, USA), 0.6 µM of each primer, template DNA, and nuclease-free water. PCR cycle conditions were set up with a cycle of initial denaturation for 4 min at 95°C, 35 cycles of denaturation for 30 sec at 94°C, annealing for 30 sec at 56°C, and extension for 30 sec at 72°C, and lastly, a cycle of final extension for 8 min at 72°C. PCR products were later loaded onto 1.5% agarose gel including 100 bp DNA ladder (Vivantis Technologies, Malaysia), blanks (nuclease-free water), and positive controls for electrophoresis. DNA bands were visualized under ultraviolet light after ethidium bromide staining.
HPV Genotyping
Genotyping of the HPV was performed by using PCR-Restriction Fragment Length Polymorphism (PCR-RFLP) assay according to Nobre et al., (2008). This was done by digesting the PCR products of HPV-positive samples with four restriction endonucleases namely, PstI, HaeIII, DdeI, and RsaI (Promega, USA). The PCR-RFLP profiles, which are specific to each individual HPV genotype, are based on the typing algorithm generated by Nobre et al., (2008). Each restriction endonuclease digestion was conducted separately in a total volume of 20 µl containing 2X recommended RE buffer (Promega, USA), 0.1 mg/ml Acetylated BSA (Promega, USA), 1 µg/µl PCR product, nuclease-free water, and 10 units of restriction endonucleases, respectively, according to the manufacturer’s protocol. The reaction mixtures were incubated at 37°C for 60 min and inactivated at 65°C for 15 min. The digested products were loaded onto 3% agarose gel along with undigested PCR products as controls, ready-to-use VC 50 bp DNA ladder (Vivantis Technologies, Malaysia), and 25 bp DNA step ladder (Promega, USA). The RFLP profiles were later visualized under ultraviolet light after ethidium bromide staining.
Statistical Analyses
The genotypes of HPV were categorized into four distinct groups according to their established oncogenic properties: high-risk (HPV-16, -18, -31, -33, -35, -39, -45, -51, -52, -56, -58, -59), probable high-risk (HPV-26, -30, -34, -53, -66, -67, -68, -69, -70, -73, -82, 85, -97), low-risk (HPV-6, -11, -13, -40, -42, -43, -44, -54, -61, -72, -81, -89), and undetermined-risk (HPV-32, -62, -71, -74, -83, -84, -86, -87, -90, -91) according to Muñoz et al., (2006), Nobre et al., (2008) and Bouvard et al., (2009). The prevalence and distribution of HPV infection were described as numbers and percentages as well as stratified according to age group, ethnicity, and cytological findings. Two types of logistic regression models were applied in the study involving univariate and multivariable analyses. Univariate analysis was performed using binary logistic regression to calculate odds ratio (OR) and 95% confidence interval (CI) in order to evaluate the strength of relationship between each risk factor and HPV infection. Multivariable analysis was further carried out by adding the risk factors with p-value <0.1 from the previous analysis into a model and then simplified by stepwise backward logistic regression with p-value of 0.05 as elimination threshold to determine the risk factors which are significantly associated with HPV infection. All analyses were conducted using SPSS version 19.0 (IBM Inc., NY, USA) and a p-value <0.05 was regarded as statistically significant.
Results
We successfully recruited 240 women in this study. The age range of the participants involved were from 21 to 70 years, with an average at 41 years (SD ±10.6 years). All women participated in the study were sorted into five age groups: ≤24 years (2.9%), 25-34 years (27.1%), 35-44 years (33.3%), 45-54 years (25.0%), and ≥55 years (11.7%). Almost half of the participants were Kadazan-Dusuns (46.7%), while the rest were Chinese (10.4%), Bajaus (7.9%), Sino-natives (6.7%), Malays (5.8%), and other minor indigenous ethnic groups (23.3%) namely Murut, Bidayuh, Rungus, Lundayeh, Bisaya, Brunei Malay, Sungai, Kedayan, Bugis, Suluk, Tidong, Iranun, Indian, and Iban.
Distribution of HPV genotypes
Amplification of the β-globin gene resulted in an adequate quality of DNA for all cervical samples collected. Out of 240 samples screened, 23 samples (9.6%) were identified positive for HPV infection. Approximately, 13 genotypes of HPV were detected among the positive samples. High-risk HPVs were found in 43% (10/23) of infected women with HPV-56 as the most prevalent (16.7%) followed by HPV-16 (8.3%), HPV-58 (8.3%), HPV-33 (4.2%), and HPV-59 (4.2%), respectively (Table 1). Another most commonly identified HPV type was HPV-70 (16.7%) from the probable high-risk group. Single infections were identified in most of the samples whereas, only one sample displayed a co-infection of at least two HPV types.
Table 1.
Distribution of Human Papillomavirus (HPV) Genotypes based on Cytological Findings among Malaysian Women Living in Kota Kinabalu, Sabah
| HPV genotype | NILM, n (%) | ASCUS, n (%) | LSIL, n (%) | HSIL, n (%) | Total n (%) |
|---|---|---|---|---|---|
| High-risk HPV | |||||
| 16 | 1 (7.1) | 1 (50.0) | - | - | 2 (8.3) |
| 33 | 1 (7.1) | - | - | - | 1 (4.2) |
| 56 | - | - | 4 (57.1) | - | 4 (16.7) |
| 58 | - | - | 1 (14.3) | 1 (100.0) | 2 (8.3) |
| 59 | 1 (7.1) | - | - | - | 1 (4.2) |
| Probable high-risk HPV | |||||
| 53 | 2 (14.3) | - | - | - | 2 (8.3) |
| 66 | 1 (7.1) | - | - | - | 1 (4.2) |
| 70 | 3 (21.4) | 1 (50.0) | - | - | 4 (16.7) |
| 82 | 1 (7.1) | - | - | - | 1 (4.2) |
| Low-risk HPV | |||||
| 61 | 2 (14.3) | - | - | - | 2 (8.3) |
| 81 | - | - | 1 (14.3) | - | 1 (4.2) |
| Undetermined-risk HPV | |||||
| 62 | 1 (7.1) | - | - | - | 1 (4.2) |
| 84 | 1 (7.1) | - | 1 (14.3) | - | 2 (8.3) |
NILM, Negative for Intraepithelial Lesion or Malignancy; ASCUS, Atypical Squamous Cells of Undetermined Significance; LSIL, Low-grade Squamous Intraepithelial Lesion; HSIL, High-grade Squamous Intraepithelial Lesion
The highest HPV prevalence was observed among women older than 55 years (14.8%), followed by adolescents younger than 24 years (14.3%), and the age group of 45-54 years (11.6%), respectively (Figure 1). In contrast to the other younger age groups, the majority of the elderly participants (three out of four) from the age group of ≥55 years were infected by high-risk HPV types. With respect to ethnicity, the indigenous Sino-natives contributed to the highest prevalence of HPV (18.7%) followed by Kadazan-Dusuns (12.5%) and Malays (7.1%), respectively (Figure 2). All of the HPV-positive women of Sino-native origin suffered from infections by high-risk HPV genotypes. HPV infections were only seen in Rungus (2/7), Lundayeh (1/2), and Sungai (1/4) among the minority groups.
Figure 1.
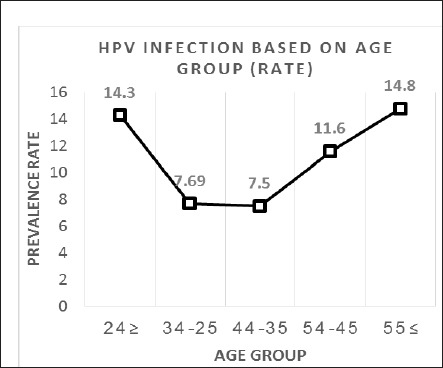
Rate of HPV Infection based on Age Group. Two peaks of infection rate were observed in women in the age groups of ≤24 years and ≥55 years respectively
Figure 2.
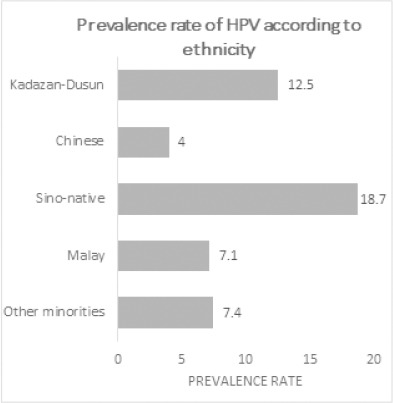
Rate of HPV Infection based on Ethnicity. The highest prevalence rate was recorded in women of Sino native origin followed by Kadazan-Dusuns
HPV positivity according to cytological diagnosis
The majority of HPV infections were detected in women with cytological result of negative for intraepithelial lesion or malignancy (NILM) (58.3%). Two cases (8.3%) were identified with atypical squamous cell of undetermined significance (ASCUS), eight cases (33.3%) with low-grade squamous intraepithelial lesion (LSIL), and one case (4.2%) with high-grade squamous intraepithelial lesion (HSIL), respectively (Table 1). About 70% of high-risk HPV infections occurred in women with abnormal cytological findings (ASCUS, LSIL, and HSIL). All infections by HPV-56 were found in LSIL cases. Most of other genotypes (aside from the high-risk group) were detected in the NILM cases with HPV-70 as the most prevalent (21.4%). There were also a few rare HPV types from low-risk and undetermined-risk groups identified within the LSIL cases namely, HPV-81 and HPV-84, respectively. In addition, HPV-84 was found in one co-infection case with HPV-56.
HPV positivity based on risk factors
The questionnaire provided to the participants was divided into three main categories involving sociodemographic information, medical history, and sexual lifestyle. According to the data collected, the majority of women were married (90.4%), employed (67.9%), and literate (96.3%). High HPV prevalence was noted among participants who ended their studies at primary school level (26%), followed by college and Bachelor’s degree graduates (13%) and illiterates (11.1%). The frequency of infection was also recorded to be higher among unemployed women (16.8%) than employed women (6.1%). HPV prevalence rate was surprisingly high among vaccinated women (16.6%) in which three out of four vaccinated HPV-positive women were infected by high-risk HPVs.
According to the univariate analysis, at least two risk factors namely educational level (p = 0.001) and working status (p = 0.01) were found to have linked with HPV infection (Table 2). After adjustment for age at first sexual intercourse and rate of sexual intercourse in multivariable analysis, significant association was determined between HPV positivity and the two aforementioned risk factors: educational level (secondary/high school, OR: 0.13, 95% CI: 0.03-0.62, p = 0.01) and working status (unemployed, OR: 4.94, 95% CI: 1.58-15.40, p = 0.006) (Table 3).
Table 2.
Prevalence of HPV Infection among Malaysian Women from Kota Kinabalu According to Sociodemographic Status, Clinical History, and Sexual Behaviour (n = 240), and Identification of Factors Associated with HPV Infection based on Univariate Analysis
| Variables | HPV-negative, n (%) | HPV-positive, n (%) | Crude OR (95% CI) | p-value |
|---|---|---|---|---|
| Age (years) | ||||
| ≤24 | 6 (85.7) | 1 (14.3) | 2.05 [0.21-19.9] | 0.53 |
| 25-34 | 60 (92.3) | 5 (7.7) | 1.02 [0.29-3.53] | 0.96 |
| 35-44 | 74 (92.5) | 6 (7.5) | Reference | |
| 45-54 | 53 (88.3) | 7 (11.7) | 1.62 [0.51-5.12] | 0.4 |
| ≥55 | 24 (85.7) | 4 (14.3) | 2.05 [0.53-7.90] | 0.29 |
| Ethnicity | ||||
| Kadazan-Dusun | 98 (87.5) | 14 (12.5) | Reference | |
| Chinese | 24 (96.0) | 1 (4.0) | 0.29 [0.03-2.32] | 0.24 |
| Bajau | 19 (100.0) | 0 (0.0) | 0.00[0.00-] | - |
| Sino-native | 13 (81.2) | 3 (18.8) | 1.61 [0.40-6.38] | 0.49 |
| Malay | 13 (92.9) | 1 (7.1) | 0.53 [0.06-4.44] | 0.56 |
| Other ethnics | 50 (92.6) | 4 (7.4) | 0.56 [0.17-1.79] | 0.32 |
| Marital status | ||||
| Married | 196 (90.3) | 21 (9.7) | Reference | |
| Single + divorced + widowed | 21 (91.3) | 2 (8.7) | 0.88 [0.19-4.05] | 0.87 |
| Educational level | ||||
| Primary school | 17 (73.9) | 6 (26.1) | Reference | |
| Secondary / high school | 100 (97.1) | 3 (2.9) | 0.08 [0.02-0.37] | 0 |
| College / university | 87 (87.0) | 13 (13.0) | 0.42 [0.14-1.27] | 0.12 |
| Graduate school | 5 (100.0) | 0 (0.0) | 0.00 [0.00-] | - |
| Illiterate | 8 (88.9) | 1 (11.1) | 0.35 [0.03-3.45] | 0.37 |
| Working status | ||||
| Employed | 153 (93.9) | 10 (6.1) | Reference | |
| Unemployed | 64 (83.1) | 13 (16.9) | 3.10 [1.29-7.45] | 0.01 |
| History of diseases self | ||||
| Cancer | 5 (100.0) | 0 (0.0) | 0.00 [0.00-] | - |
| Diabetes | 11 (78.6) | 3 (21.4) | 2.84 [0.72-11.15] | 0.13 |
| Other diseases | 13 (86.7) | 2 (13.3) | 1.60 [0.33-7.68] | 0.55 |
| None | 188 (91.3) | 18 (8.7) | Reference | |
| Father | ||||
| Cancer | 21 (95.5) | 1 (4.5) | 0.43 [0.05-3.43] | 0.43 |
| Diabetes | 22 (91.7) | 2 (8.3) | 0.83 [0.18-3.83] | 0.81 |
| Other diseases | 9 (81.8) | 2 (18.2) | 2.03 [0.40-10.16] | 0.38 |
| None | 165 (90.2) | 18 (9.8) | Reference | |
| Mother | ||||
| Cancer | 16 (88.9) | 2 (11.1) | 1.13 [0.24-5.33] | 0.87 |
| Diabetes | 17 (89.5) | 2 (10.5) | 1.07 [0.23-4.99] | 0.93 |
| Other diseases | 11 (100.0) | 0 (0.0) | 0.00 [0.00-] | - |
| None | 173 (90.1) | 19 (9.9) | Reference | |
| Age at first sexual intercourse | ||||
| ≤18 | 29 (90.6) | 3 (9.4) | 2.09 [0.44-9.92] | 0.35 |
| 19-24 | 107 (87.0) | 16 (13.0) | 3.02 [0.97-9.40] | 0.05 |
| ≥25 | 81 (95.3) | 4 (4.7) | Reference | |
| Number of lifetime partner(s) | ||||
| One | 186 (90.3) | 20 (9.7) | Reference | |
| Multiple | 31 (91.2) | 3 (8.8) | 0.90 [0.25-3.21] | 0.87 |
| Variables | HPV-negative, n (%) | HPV-positive, n (%) | Crude OR (95% CI) | p-value |
| Rate of sexual intercourse | ||||
| More than once per week | 61 (96.8) | 2 (3.2) | 0.20[0.04-1.04] | 0.05 |
| Once a week | 44 (86.3) | 7 (13.7) | Reference | |
| Once or twice per month | 66 (90.4) | 7 (9.6) | 0.66[0.21-2.03] | 0.47 |
| Once or twice in 3 months | 16 (84.2) | 3 (15.8) | 1.17[0.27-5.11] | 0.82 |
| Once or twice in 6 months | 30 (88.2) | 4 (11.8) | 0.83[0.22-3.11] | 0.79 |
| Rate of condom usage | ||||
| Always | 12 (85.7) | 2 (14.3) | 1.46[0.30-7.15] | 0.63 |
| Occasionally | 64 (92.8) | 5 (7.2) | 0.68[0.24-1.96] | 0.48 |
| Never | 141 (89.8) | 16 (10.2) | Reference | |
| Recent oral contraceptive usage | ||||
| Yes | 33 (91.7) | 3 (8.3) | 0.83[0.23-2.97] | 0.78 |
| No | 184 (90.2) | 20 (9.8) | Reference | |
| History of smoking | ||||
| Yes | 11 (100.0) | 0 (0.0) | 0.00[0.00-] | - |
| No | 206 (90.0) | 23 (10.0) | Reference | |
| HPV vaccine uptake | ||||
| Yes | 20 (83.3) | 4 (16.7) | 2.07[0.64-6.69] | 0.22 |
| No | 197 (91.2) | 19 (8.8) | Reference |
HPV, Human papillomavirus; OR, odd ratio; CI, confidence interval
Table 3.
Multivariable Analysis* of Factors Associated with HPV Infection Using Stepwise Backward Logistic Regression
| Variables | Adjusted OR(95% CI) | p-value |
|---|---|---|
| Educational level | ||
| Primary school | Reference | |
| Secondary / high school | 0.13[0.03-0.62] | 0.01 |
| College / university | 1.21[0.31-4.68] | 0.77 |
| Graduate school | 0.00[0.00-] | - |
| Illiterate | 0.30[0.03-3.05] | 0.31 |
| Working status | ||
| Employed | Reference | |
| Unemployed | 4.94[1.58-15.40] | 0.006 |
OR, odd ratio; CI, confidence interval;
All variables with p-value <0.1 by univariate analysis were included in the model.
Discussion
This study was the first to report the prevalence and distribution of HPV genotypes among multi-ethnic female population living in Kota Kinabalu, Sabah. According to the latest national cancer registry of Malaysia, the state was ranked as the second highest among all states after Sarawak in term of cervical cancer age-standardized incidence rate (Azizah et al., 2016). However, the result of HPV prevalence found was unexpectedly low (9.6%) considering Kota Kinabalu is the most populous city in Sabah. Similar prevalence rate was also recorded in the neighbouring country of the Philippines (9.2%) (Domingo and Dy Echo, 2009). In comparison to a few studies targeting women in West Malaysia, high HPV prevalence was observed in southern Selangor (46.7%) (Chong et al., 2010) while another study conducted by Othman and Othman (2014) reported a significantly low frequency of HPV infection (4.4%) in several states of the north-eastern region. The incidence rate of cervical cancer for Selangor was reported low (ASR = 5.7) despite high HPV infection rate in its southern part whereas wide study coverage of the two north-eastern states of Kelantan and Terengganu indicated comparable results for both HPV prevalence rate and cervical cancer incidences. Disparity of HPV prevalence may rise from the sensitivity of each detection method used but the size of target region should also be considered in order for the prevalence of HPV to be able to estimate the whole cervical cancer burden of a state and consecutively, an entire country. Furthermore, there are also other factors which have significant impacts on cervical cancer development that should be reflected such as persistent infection by high-risk types, establishment of abnormal cervical cytology, and other various elements involving sexual behaviour, health management, genetic factors, and even awareness are necessary for the progression of cervical cancer.
In this study, among the most prevalent HPV genotypes discovered were HPV-56 of high-risk group and HPV-70 of probable high-risk followed by HPV-16, -58, -53, and -61. Our finding reveals unique profiles which differ remarkably from the studies conducted in West Malaysia where HPV-16 and HPV-18 were the commonest types found whereas both HPV-56 and HPV-70 were the least prevalent (Sharifah et al., 2009; Sharifa Ezat et al., 2010; Cheah et al., 2011; Raub et al., 2014). The study outcome showed that all infections by HPV-56 were associated with LSIL cases, suggesting that this genotype is capable of promoting low-grade neoplasia in Sabahan women and should be a public health concern for the locals. HPV-70 is considered as potentially carcinogenic yet research studies concerning this genotype are limited. A study by Kim et al., (2014) discovered high prevalence of HPV-70 in LSIL cases, hinting its potential association in precancerous lesions. There were several rare HPV genotypes detected such as HPV-62, -81, -82, and -84. Two of the genotypes (HPV-81 and -84) were identified in abnormal cervices (LSIL) which were uncommon occurrences. Their oncogenic attribute and connection to cancer are yet to be understood but some studies demonstrated that HPV-81 was highly prevalent in HIV-infected women and significantly associated with precancerous lesions (Tornesello et al., 2008; Al-Awadhi et al., 2013) while there was a vital correlation found between HPV-84 and HSIL or worse lesions (Choi et al., 2009).
The current prophylactic vaccines introduced in Malaysia are bivalent Cervarix, quadrivalent Gardasil, and nonavalent Gardasil 9 which protect against HPV-16, -18, -6, -11, -31, -33, -45, -52, -58. These vaccines are known to provide cross-protection to other HPV genotypes (non-vaccine) but their efficacy may also be questionable to a certain extent. The findings of this study showed that three out of four vaccinated HPV-positive women were infected by oncogenic HPV-56, -16, and -66. A study by Guo et al. (2015) demonstrated that vaccinated women had higher tendency to acquire infection by high-risk non-vaccine types than unvaccinated women. Since HPV-56 is highly prevalent among Sabahan women, regular Pap smear screening is strongly advised in order to monitor the cytological changes in infected women and the next generation HPV vaccine targeting type 56 may be necessary in the future.
Prevalence of HPV based on age displayed two distinct peaks of infection which was consistent to several international studies observed among African, American, European, Japanese, and Chinese populations who resided in Hong Kong (De Sanjosé et al., 2007; Chan et al., 2009; Takehara et al., 2011). The first peak was observed among women younger than 24 years while the second peak occurred among women at ≥55 years. The majority of the latter (70%) had abnormal lesions and infected by high-risk types. Frequent and transient HPV infections commonly occur in sexually-active adolescents aged 15-25 years whereas the second peak may indicate emerging persistent infection due to the changes in hormonal and immune responses as well as sexual behaviour of old women (De Sanjosé et al., 2007).
Incremental HPV prevalence rate was recorded among Sino-natives in which all of the HPV-positive participants suffered from high-risk HPV infections. This ethnic is composed of racially mixed people between Chinese community and the indigenous residents of Borneo. By referring to the high HPV prevalence rate among the Chinese ethnic in West Malaysia (Sharifa Ezat et al., 2010; Raub et al., 2014), it is suggested that the genetic combination between Chinese and Bornean ethnics may resulted in increasing susceptibility toward high-risk HPV infections. In addition, there was no infection observed among Bajaus and several minor ethnic groups. A strong association was found between HPV infection and two risk factors: education and employment status of women. The odds of obtaining HPV infection was 4.94 times higher among unemployed women than those who were employed. In comparison to the participants with other educational levels and illiterates in the study, women who had only 12 years of education (OR = 0.13) were less likely to suffer from HPV infection. It was observed that most of them were employed and were more likely to be exposed with knowledge and awareness toward HPV and cervical cancer from their working places.
While we would have preferred to obtain a larger sample size to better estimate the prevalence rate for each ethnic group, recruiting participants was a challenge due to social, cultural and religious inhibition and taboos of the local community who frown on sexually active single women. This limitation also led to the absence of important association between HPV infection and typical risk factors such as sexual behaviour, smoking habit, and oral contraceptive usage.
Nevertheless, this study managed to provide baseline information imperative for the locals in Sabah. HPV genotype distribution indicated a high prevalence of HPV-56 and -70 which are rare in West Malaysia. Further studies incorporating larger sample size and covering other districts of Sabah are necessary to obtain more comprehensive data for the state. We note that a new HPV vaccination strategy targeted specifically to Sabahan women may be necessary in the future.
Funding Statement
This study was funded under the research grant UMSGREAT (GUG0067-SKK-2/2016) provided by Universiti Malaysia Sabah.
Statement conflict of Interest
The authors declare that they have no competing interests.
Acknowledgements
The authors would like to thank Dr. Tan Bee Hwai (Director of Sabah Women and Children Hospital) and Datuk Dr. Soon Ruey (Head of the Department of Obstetrics and Gynaecology) for permitting the recruitment of female patients and volunteers at the hospital’s O&G clinic. We thank all gynaecologists, medical officers, and nurses at the clinic for their assistance in sample collection. We also thank Dr. Sophie van Aerde (Director of UMS Polyclinics), UMS medical doctors and nurses who aided in the sample collection at UMS Polyclinics. We thank all volunteers who participated willingly in the study.
References
- 1.Al-Awadhi R, Chehadeh W, Jaragh M, et al. Distribution of human papillomavirus among women with abnormal cervical cytology in Kuwait. Diagn Cytopathol. 2013;41:107–14. doi: 10.1002/dc.21778. [DOI] [PubMed] [Google Scholar]
- 2.Azizah AM, Nor Saleha IT, Noor Hashimah A, Asmah ZA, Mastulu W. Malaysian National Cancer Registry Report 2007-2011. Putrajaya: National Cancer Registry; 2016. [Google Scholar]
- 3.Bzhalava D, Eklund C, Dillner J. International standardization and classification of human papillomavirus types. Virology. 2015;476:341–4. doi: 10.1016/j.virol.2014.12.028. [DOI] [PubMed] [Google Scholar]
- 4.Bouvard V, Baan R, Straif K, et al. A review of human carcinogens-Part B: biological agents. Lancet Oncol. 2009;10:321–2. doi: 10.1016/s1470-2045(09)70096-8. [DOI] [PubMed] [Google Scholar]
- 5.Bruni L, Barrionuevo-Rosas L, Albero G, et al. ICO Information Centre on HPV and Cancer (HPV Information Centre) Human Papillomavirus and Related Diseases in Malaysia. Summary Report 15 December 2016. 2016 [Google Scholar]
- 6.Chan PK, Chang AR, Yu MY, et al. Age distribution of human papillomavirus infection and cervical neoplasia reflects caveats of cervical screening policies. Int J Cancer. 2009;126:297–301. doi: 10.1002/ijc.24731. [DOI] [PubMed] [Google Scholar]
- 7.Cheah PL, Looi LM, Sivanesaratnam V. Human papillomavirus in cervical cancers of Malaysians. J Obstet Gynaecol Re. 2011;37:489–95. doi: 10.1111/j.1447-0756.2010.01386.x. [DOI] [PubMed] [Google Scholar]
- 8.Choi YD, Han WC, Chung WJ, et al. Analysis of HPV-other samples by performing HPV DNA sequencing. Korean J Pathol. 2009;43:250–3. [Google Scholar]
- 9.Chong PP, Asyikin N, Rusinahayati M, et al. High prevalence of human papillomavirus DNA detected in cervical swabs from women in southern Selangor, Malaysia. Asian Pac J Cancer Prev. 2010;11:1645–51. [PubMed] [Google Scholar]
- 10.De Sanjosé S, Diaz M, Castellsagué X, et al. Worldwide prevalence and genotype distribution of cervical human papillomavirus DNA in women with normal cytology: a meta-analysis. Lancet Infect Dis. 2007;7:453–9. doi: 10.1016/S1473-3099(07)70158-5. [DOI] [PubMed] [Google Scholar]
- 11.Domingo EJ, Dy Echo AVV. Epidemiology, prevention and treatment of cervical cancer in the Philippines. J Gynecol Oncol. 2009;20:11–6. doi: 10.3802/jgo.2009.20.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guo F, Hirth JM, Berenson AB. Comparison of HPV prevalence between HPV-vaccinated and non-vaccinated young adult women (20–26 years) Hum Vacc Immunother. 2015;11:2337–44. doi: 10.1080/21645515.2015.1066948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim NR, Kang M, Lee SP, et al. Uncommon and rare human papillomavirus genotypes relating to cervical carcinomas. Korean J Pathol. 2014;48:43. doi: 10.4132/KoreanJPathol.2014.48.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Manos MM, Ting Y, Wright DK, et al. The use of polymerase chain reaction amplification for the detection of genital human papillomaviruses. Cancer Cell. 1989;7:209–14. [Google Scholar]
- 15.Muñoz N, Castellsagué X, de González AB, Gissmann L. HPV in the etiology of human cancer. Vaccine. 2006;24:1–10. doi: 10.1016/j.vaccine.2006.05.115. [DOI] [PubMed] [Google Scholar]
- 16.Nobre RJ, de Almeida LP, Martins TC. Complete genotyping of mucosal human papillomavirus using a restriction fragment length polymorphism analysis and an original typing algorithm. J Clin Virol. 2008;42:13–21. doi: 10.1016/j.jcv.2007.11.021. [DOI] [PubMed] [Google Scholar]
- 17.Othman N, Othman NH. Detection of human papillomavirus DNA in routine cervical scraping samples: use for a national cervical cancer screening program in a developing nation. Asian Pac J Cancer Prev. 2014;15:2245–9. doi: 10.7314/apjcp.2014.15.5.2245. [DOI] [PubMed] [Google Scholar]
- 18.Raub SHA, Isa NM, Zailani HA, et al. Distribution of HPV genotypes in cervical cancer in multi-ethnic Malaysia. Asian Pac J Cancer Prev. 2014;15:651–6. doi: 10.7314/apjcp.2014.15.2.651. [DOI] [PubMed] [Google Scholar]
- 19.Rajaram S, Chitrathara K, Maheshwari A. In ‘HPV Carcinogenesis, Clinical Applications and Biomarkers in Cancer Cervix’. New Delhi: Jaypee Brothers Medical Publishers (P) Ltd; 2012. Cervical Cancer: Contemporary Management; pp. 36–44. [Google Scholar]
- 20.Sabah cancer registry report 2001-2005. Public Health Division, Sabah State Health Department. 2009 [Google Scholar]
- 21.Sharifa Ezat WP, Sharifah NA, Sayyidi Hamzi AR, et al. Prevalence of human papillomavirus genotypes in preinvasive and invasive cervical cancer –a UKM study. Med Health. 2010;5:66–76. [Google Scholar]
- 22.Sharifah NA, Seeni A, Nurismah MI, et al. Prevalence of human papillomavirus in abnormal cervical smears in Malaysian patients. Asian Pac J Cancer Prev. 2009;10:303–6. [PubMed] [Google Scholar]
- 23.Takehara K, Toda T, Nishimura T, et al. Human papillomavirus types 52 and 58 are prevalent in uterine cervical squamous lesions from Japanese women. Patholog Res Int. 2011;2011:1–7. doi: 10.4061/2011/246936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.The report: Sabah 2011. In ‘Profile’. London: Oxford Business Group; 2011. pp. 8–11. [Google Scholar]
- 25.Tornesello ML, Duraturo ML, Giorgi-Rossi P, et al. Human papillomavirus (HPV) genotypes and HPV16 variants in human immunodeficiency virus-positive Italian women. J Gen Virol. 2008;89:1380–9. doi: 10.1099/vir.0.83553-0. [DOI] [PubMed] [Google Scholar]
- 26.Zitkute V, Bumbuliene Z. Risk factors affecting HPV infection, persistence and lesion progression in women and men. Clin Res Infect Dis. 2016;3:1026. [Google Scholar]


