Abstract
Background:
Overexpression of human leukocyte antigen G (HLA-G) and increased plasma levels of soluble HLA-G (sHLA-G) have been reported in different human malignancies, and are believed to be involved in tumor immune evasion.
Objectives:
This study was designed to evaluate the expression of HLA-G in tumor tissues and the plasma levels of sHLA-G in patients with gastrointestinal cancer, and to determine their associations with clinicopathological factors. The link between Helicobacter pylori infection and increased HLA-G expression or sHLA-G levels was also investigated in patients with gastric cancer.
Methods:
HLA-G expression was investigated in tumor tissues from 100 patients with gastric and colorectal adenocarcinoma using immunohistochemistry test, and plasma levels of sHLA-G were measured in 82 patients with ELISA method. The presence of H. pylori genome was investigated in tumor tissues from 25 patients with gastric cancer by PCR method.
Results:
HLA-G expression was observed in 43% of colorectal cancers and 34.6% of gastric cancers, and was not related with any of the clinicopathological factors. There was a significant correlation between increased sHLA-G level and stage I tumors. Eight of 25 (32%) gastric cancer specimens were positive for H. pylori, of which 3 samples were positive for HLA-G. Soluble HLA-G levels were above the cut-off value in all H. pylori-positive patients.
Conclusion:
Plasma levels of sHLA-G were significantly increased in our patients with a sensitivity of 89% and a specificity of 62%. Soluble HLA-G level can be considered a useful indicator for the early diagnosis of gastric and colorectal adenocarcinoma.
Keywords: HLA-G, sHLA-G, colorectal cancer, gastric cancer, Helicobacter pylori
Introduction
Human leukocyte antigen (HLA)-G is a nonclassical HLA class Ib antigen with seven spliced isoforms: four membrane-bound (-G1 to -G4) and three soluble forms (-G5 to -G7) (Guo et al., 2013; Alegre et al., 2014). In addition to soluble isoforms, HLA-G may be shed proteolytically from cells (Rizzo et al., 2013). Membrane-bound and soluble forms of HLA-G exert similar inhibitory functions through their interaction with immunoglobulin-like transcript (ILT) receptor-2, ILT4 and killer immunoglobulin-like receptor (KIR) 2DL4, which are differentially expressed by lymphoid cells and myeloid antigen-presenting cells (Alegre et al., 2014).
Primarily, HLA-G is expressed on the placental extravillous cytotrophoblast, which plays a key role in modulating the maternal immune system during pregnancy. Ectopic HLA-G expression has also been detected in several pathological conditions such as transplantation, cancer, viral infection and autoimmune diseases (Amiot et al., 2011; Deschaseaux et al., 2011; Fainardi et al., 2011). Overexpression of HLA-G and increased plasma levels of soluble HLA-G (sHLA-G) have been reported in different human malignancies, and are believed to protect tumor cells from immune surveillance (Amiot et al., 2011). The acquisition of functional HLA-G molecules from antigen-bearing cells by trogocytosis is another mechanism of evasion which can induce immune suppression in the tumor milieu (LeMaoult et al., 2015).
Gastric and colorectal cancers have a high mortality rate and are the most common gastrointestinal cancers in the Iranian general population (Pourhoseingholi et al., 2013). The present study was designed to evaluate the expression of HLA-G in tumor tissues and the plasma levels of sHLA-G in patients with gastric and colon adenocarcinoma, and to determine their relation with clinicopathological factors. The link between the concurrent presence of Helicobacter pylori genome and increased levels of HLA-G and/or sHLA-G in gastric cancer was also investigated.
Materials and Methods
A total of 100 patients with gastric and colon adenocarcinoma who were scheduled for surgery at Faghihie Hospital (affiliated to Shiraz University of Medical Sciences, Iran) from 2013 to 2016 were enrolled in the study after they had provided their written informed consent. Our study protocol was approved by our University Ethics Committee. Blood samples were collected from 82 patients before surgery and plasma was isolated and stored at −20°C until further analysis. The best paraffin-embedded block containing tumor tissue from each patient was selected by an expert pathologist for immunostaining analysis.
Detection of HLA-G in tumor tissues from patients with gastrointestinal cancer
The expression of HLA-G was investigated by immunohistochemical (IHC) staining in 4-µm thick paraffin-embedded tissue sections from patients with gastrointestinal cancer. Briefly, after deparaffinization by xylene and hydration in graded ethanol, endogenous peroxidase activity was blocked with 3% H2O2 in methanol for 3 min. Antigen retrieval was performed in Tris buffer at 120°C for 15 min, and tissues were then incubated with goat serum 1:100 in PBS for 20 min. After that, mouse anti-human HLA-G antibody 4H84 (Exbio, Prague, Czech Republic), which detects all HLA-G isoforms, was added (diluted 1:250 in PBS) followed by incubation for 1 h. After washing, bound antibodies were visualized with a detection system (Dako, Glostrup, Denmark) consisting of HRP-conjugated secondary antibody coupled to a dextran polymer backbone and 3,3’diaminobenzidine tetrahydrochloride (DAB) chromogen with H2O2. Sections were then counterstained with hematoxylin, dehydrated with ethanol and mounted. Human placental tissue from the first trimester was used as positive control, and adjacent normal tissue in each section was considered as negative control for IHC.
Tissue staining was scored in a blind manner by two experienced pathologists, based on the intensity of staining and/or the proportion of cells expressing HLA-G. Staining results were considered negative for tumor tissues without staining, 1+ for specimens with <25% of the tumor cells stained and/or weakly stained, 2+ for specimens with 25%–50% of the tumor cells stained and/or moderately stained, and 3+ for specimens with >50% of the tumor cells stained and/or strongly stained (Ye et al., 2007).
Evaluation of sHLA-G plasma levels in patients with gastrointestinal cancer
Plasma levels of sHLA-G including shed HLA-G1 and HLA-G5 were quantified in 82 patients and 45 healthy individuals with an ELISA kit (Exbio) at a detection limit of 3 U/mL based on the manufacturer’s instructions. As the capturing antibody we used 4H84, and as a detecting antibody we used HRP-conjugated anti-human β2-microglobulin. The concentration of sHLA-G in each sample was obtained from a standard curve constructed by plotting the absorbance against corresponding known calibration concentrations ranging from 3.9 to 125 U/mL on a logarithmic scale, using the four-parameter algorithm.
Detection of H. pylori UreC gene in tumor tissues from patients with gastric cancer
DNA was extracted from 3 to 4 (5-μm thick) formalin-fixed, paraffin-embedded sections of gastric cancer tissue using a commercial kit (GeNet Bio, Daejeon, South Korea) after deparaffinization in warm xylene and washing in 100%, 80%, and 60% ethanol. A 294-bp fragment of H. pylori UreC gene was amplified by PCR using forward 5’AAGCTTTTAGGGGTGTTAGGGGTTT3’ and reverse 5’AAGCTTACTTTCTAACACTAACGC3’ primers (Lage et al., 1995), and amplicons were then resolved on 2% agarose gel.
Statistical analyses
The chi-squared test was used to determine the association between HLA-G expression in tumor tissues and each of the clinicopathological parameters. The correlation between HLA-G expression in tumors and sHLA-G serum levels was estimated with the Spearman rho test. The association between plasma sHLA-G levels and each of the clinicopathological parameters was explored with the Mann–Whitney U test. A receiver operating characteristic (ROC) curve was used to evaluate the diagnostic ability of the sHLA-G test to discriminate between patients with gastrointestinal cancer and healthy individuals. All statistical analyses were done with SPSS v. 19, and two-sided P-value less than 0.05 was considered statistically significant.
Results
To investigate the association between HLA-G expression in tumor tissues and plasma sHLA-G levels in patients with gastrointestinal cancer, a total of 100 patients were examined in this study. The demographic and clinicopathological data for these patients are summarized in Table 1.
Table 1.
Demographic and Clinicopathological Characteristics of Patients with Gastrointestinal Cancer
| Age (years) (n=100) | |
| Mean±SD | 56.59±14.79 |
| Range | 26-81 |
| Sex (n=100) | |
| Male | 67 |
| Female | 33 |
| Depth of invasion (n=92) | |
| T1 | 3 |
| T2 | 21 |
| T3 | 50 |
| T4 | 18 |
| Lymph node involvement (n=92) | |
| Yes | 51 |
| No | 41 |
| Tumor stage (n=92) | |
| I | 15 |
| II | 32 |
| III | 42 |
| IV | 3 |
| Tumor cell differentiation (n=100) | |
| Poor | 16 |
| Moderate | 21 |
| Well | 63 |
| Tumor location (n=100) | |
| Gastric | 26 |
| Colorectal | 74 |
The results of IHC staining showed that HLA-G expression was detected in 41% of the samples, of which 13% were 3+, 15% were 2+ and 13% were 1+ (Figure 1).
Figure 1.
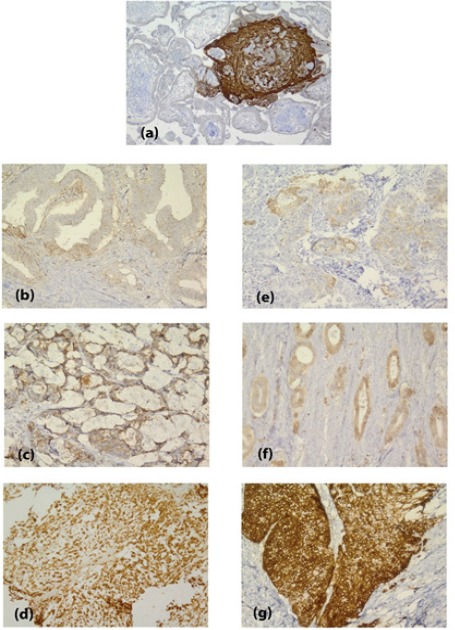
Immunohistochemical Staining for HLA-G in Colorectal Adenocarcinoma (Left) and Gastric Adenocarcinoma (Right); (a), Placenta as Positive Control; (b) and (e), 1+; (c) and (f), 2+; (d) and (g), 3+. Magnification: ×250
HLA-G expression was observed in 43% of colorectal cancers and 34.6% of gastric cancers. We found no correlation between HLA-G expression in tumor cells and any of the clinicopathological characteristics.
The area under the ROC curve for sHLA-G was 75.2% (95% confidence interval 64.8 to 85.5) and the optimal cut-off value was 57.85 U/mL with a sensitivity of 89.2% and a specificity of 62.2% (Figure 2).
Figure 2.
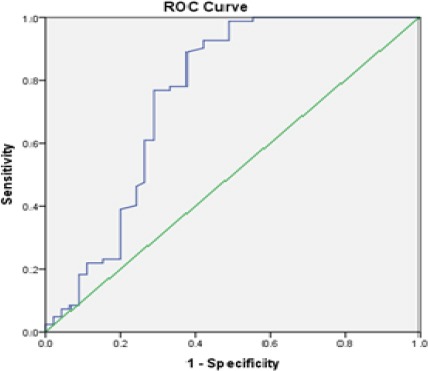
ROC Curve as an Indicator to Distinguish between Patients with Gastrointestinal Cancer and Healthy Individuals based on sHLA-G Positivity
Plasma levels of sHLA-G were significantly higher in patients than in healthy controls (85.37±60.83 U/mL vs. 56.99±48.45 U/mL, P<0.001). There was a significant correlation between increased sHLA-G level and stage I tumors (Figure 3).
Figure 3.
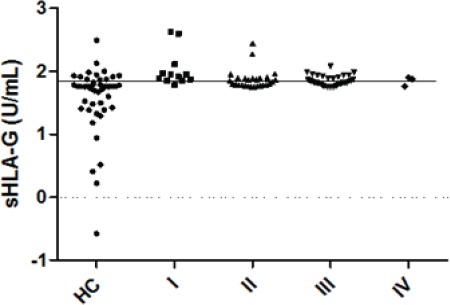
Plasma Levels of sHLA-G in Patients at Different Stages of Gastrointestinal Cancer and Healthy Controls (HC), the Solid Line Indicates the Cut-off Value of 57.85 U/mL
The H. pylori genome was detected in 8 out of 25 (32%) gastric cancer specimens, of which only 3 samples were positive for HLA-G expression (two samples were 2+ and one sample was 3+). In all H. pylori-positive patients, sHLA-G levels were above the cut-off value.
Discussion
HLA-G is one of the major factors in tumor cell escape from immune responses (Amiot et al., 2011). The first report of HLA-G expression by tumor cells was in a study of patients with melanoma published in 1998 (Paul et al., 1998). Since then, different levels of HLA-G expression have been detected in various cancers (Ye et al., 2007; Menier et al., 2009). In this study we found HLA-G expression in 41% of patients with gastrointestinal cancer (43% in colorectal cancer and 34.6% in gastric cancer). In similar studies, Tuncel et al., (2013) identified HLA-G expression in 31% of gastric cancer tissue samples, and Ye et al., (2007) detected HLA-G expression in 65% of colorectal cancer specimens. The atypical expression of HLA-G in malignant lesions may explained by epigenetic changes in tumor cells (Mouillot et al., 2005), although HLA-G production is primarily controlled by inherited alleles as well as polymorphisms in the 5’ upstream regulatory region and the 3’ untranslated region of the gene (Dias et al., 2015).
We found no association between HLA-G expression and any of the clinicopathological factors studied here. In contrast, a correlation between HLA-G expression and tumor stage and/or tumor grade was reported in gastric cancer (Tuncel et al., 2013; Yie et al., 2007a), and in other tumors such as esophageal squamous cell carcinoma (Yie et al., 2007b), hepatocellular carcinoma (Lin et al., 2010) and lung cancer (Yie et al., 2007c). These findings may reflect the role of HLA-G in tumor progression. The inconsistency between our results and those of earlier studies may be due to preoperative chemotherapy and radiotherapy, which 49% of our patients had received. One previous study reported cleavage of HLA-G from the tumor cell and a concomitant increase in its soluble form following radiation therapy (Michelin et al., 2009).
Overexpression of HLA-G has been detected not only in tumor biopsies but also in biological fluids from patients with different malignancies (Cao et al., 2011). In our study, sHLA-G was significantly increased in patients compared to controls, as shown by the area under the ROC curve of 75.2% for the discrimination between patients and healthy individuals. This finding is consistent with the superiority of sHLA-G compared to alpha-fetoprotein for the early diagnosis of malignant hepatocellular carcinoma versus liver cirrhosis (Eldin et al., 2016), and compared to carcinoembryonic antigen in differentiating colorectal cancer from benign colorectal diseases (Zhu et al., 2011). Unlike Jeong et al., (2014), who found higher levels of sHLA-G in patients with advanced stages of breast cancer, our results showed a significant increase in sHLA-G expression in patients with stage I gastrointestinal cancer. We believe that HLA-G overexpression and its shedding by early-stage tumor cells induce immune suppression, which in turn facilitates tumor progression.
We found no correlation between plasma sHLA-G concentration and HLA-G expression in tumor tissues. This is consistent with the results of Lin et al., (2010) in hepatocellular carcinoma. The lack of positive relation between HLA-G and sHLA-G may reflect the existence of other soluble isoforms in addition to those released from tumor cells (Rebmann et al., 2003).
We detected H. pylori DNA in 32% of the gastric tumor samples tested here. Although H. pylori is considered a risk factor for gastric cancer, there is no direct relationship between the incidence of H. pylori infection and the prevalence of gastric cancer (Conteduca et al., 2013). The immunosuppressive effect of HLA-G may play a role in susceptibility to infections (Amiot et al., 2014). In this regard, Oliveira Souza et al. (2016) detected HLA-G expression in 79.6% of H. pylori-positive gastric samples, whereas we detected HLA-G expression in only 3 of 8 H. pylori-bearing gastric tumors. Increased HLA-G production following infection may depend on the host genetic background rather than the type of infectious agent. This possibility is supported by Haddad et al., (2011) who reported an association between the HLA-G 14-bp deletion/deletion genotype and a higher risk of HTLV-1 infection. This genotype is related with greater mRNA stability and increased HLA-G expression. Unexpectedly, Genre et al., (2016) observed a strong association between the insertion/insertion genotype and increased susceptibility to H. pylori infection, but it should be noted that H. pylori uses different strategies to evade immune responses (Lina et al., 2014).
Acknowledgements
This work was performed in partial fulfillment of the requirements for the MSc degree in Immunology (Maryam Tabebordbar), and was supported by a grant from Shiraz University of Medical Sciences (No: 9054). We thank K. Shashok (AuthorAID in the Eastern Mediterranean) for improving the use of English in the manuscript.
References
- 1.Alegre E, Rizzo R, Bortolotti D, et al. Some basic aspects of HLA-G biology. J Immunol Res. 2014;2014:657625. doi: 10.1155/2014/657625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amiot L, Ferrone S, Grosse-Wilde H, Seliger B. Biology of HLA-G in cancer: a candidate molecule for therapeutic intervention? Cell Mol Life Sci. 2011;68:417–31. doi: 10.1007/s00018-010-0583-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Amiot L, Vu N, Samson M. Immunomodulatory properties of HLA-G in infectious diseases. J Immunol Res. 2014;2014:298569. doi: 10.1155/2014/298569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cao M, Yie SM, Liu J, et al. Plasma soluble HLA-G is a potential biomarker for diagnosis of colorectal, gastric, esophageal and lung cancer. Tissue Antigens. 2011;78:120–8. doi: 10.1111/j.1399-0039.2011.01716.x. [DOI] [PubMed] [Google Scholar]
- 5.Conteduca V, Sansonno D, Lauletta G, et al. H. pylori infection and gastric cancer: state of the art (review) Int J Oncol. 2013;42:5–18. doi: 10.3892/ijo.2012.1701. [DOI] [PubMed] [Google Scholar]
- 6.Deschaseaux F, Delgado D, Pistoia V, et al. HLA-G in organ transplantation: towards clinical applications. Cell Mol Life Sci. 2011;68:397–404. doi: 10.1007/s00018-010-0581-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dias FC, Castelli EC, Collares CV, Moreau P, Donadi EA. The role of HLA-G molecule and HLA-G gene polymorphisms in tumors, viral hepatitis, and parasitic diseases. Front Immunol. 2015;6:9. doi: 10.3389/fimmu.2015.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eldin AMK, Saber MM, Higazi A, et al. Evaluation of the diagnostic performance of soluble human leukocyte antigen-G versus α-fetoprotein for hepatitis C virus-induced hepatocellular carcinoma. J Clin Cell Immunol. 2016;7:433. [Google Scholar]
- 9.Fainardi E, Castellazzi M, Stignani M, et al. Emerging topics and new perspectives on HLA-G. Cell Mol Life Sci. 2011;68:433–51. doi: 10.1007/s00018-010-0584-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Genre J, Reginaldo FP, Andrade JM, et al. HLA-G 14 bp Ins/Ins genotype in patients Harbouring Helicobacter pylori Infection: Potential risk factor? Scand J Immunol. 2016;83:52–7. doi: 10.1111/sji.12390. [DOI] [PubMed] [Google Scholar]
- 11.Guo Y, Lee C-L, So K-H, et al. Soluble human leukocyte antigen-g5 activates extracellular signal-regulated protein kinase signaling and stimulates trophoblast invasion. PLoS One. 2013;8:e76023. doi: 10.1371/journal.pone.0076023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haddad R, Cilião Alves DC, Rocha-Junior MC, et al. HLA-G 14-bp insertion/deletion polymorphism is a risk factor for HTLV-1 infection. AIDS Res Hum Retroviruses. 2011;27:283–8. doi: 10.1089/aid.2010.0165. [DOI] [PubMed] [Google Scholar]
- 13.Jeong S, Park S, Park BW, et al. Human leukocyte antigen-G (HLA-G) polymorphism and expression in breast cancer patients. PLoS One. 2014;9:e98284. doi: 10.1371/journal.pone.0098284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lage AP, Godfroid E, Fauconnier A, et al. Diagnosis of Helicobacter pylori infection by PCR: comparison with other invasive techniques and detection of cagA gene in gastric biopsy specimens. J Clin Microbiol. 1995;33:2752–6. doi: 10.1128/jcm.33.10.2752-2756.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.LeMaoult J, Caumartin J, Daouya M, et al. Trogocytic intercellular membrane exchanges among hematological tumors. J Hematol Oncol. 2015;8:24. doi: 10.1186/s13045-015-0114-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lin A, Chen HX, Zhu CC, et al. Aberrant human leucocyte antigen-G expression and its clinical relevance in hepatocellular carcinoma. J Cell Mol Med. 2010;14:2162–71. doi: 10.1111/j.1582-4934.2009.00917.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lina TT, Alzahrani S, Gonzalez J. Immune evasion strategies used by Helicobacter pylori. World J Gastroenterol. 2014;20:12753–66. doi: 10.3748/wjg.v20.i36.12753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Menier C, Rouas-Freiss N, Carosella ED. The HLA-G non-classical MHC class I molecule is expressed in cancer with poor prognosis. Implications in tumour escape from immune system and clinical applications. Atlas Genet Cytogenet Oncol Haematol. 2009;6:879–900. [Google Scholar]
- 19.Michelin S, Gallegos CE, Dubner D, Favier B, Carosella ED. Ionizing radiation modulates the surface expression of human leukocyte antigen-G in a human melanoma cell line. Hum Immunol. 2009;70:1010–5. doi: 10.1016/j.humimm.2009.07.030. [DOI] [PubMed] [Google Scholar]
- 20.Mouillot G, Marcou C, Rousseau P, et al. HLA-G gene activation in tumor cells involves cis-acting epigenetic changes. Int J Cancer. 2005;113:928–36. doi: 10.1002/ijc.20682. [DOI] [PubMed] [Google Scholar]
- 21.Oliveira Souza D, Genre J, Alves Silva T, et al. Upregulation of soluble HLA-G5 and HLA-G6 isoforms in the milder histopathological stages of Helicobacter pylori infection: A role for subverting immune responses? Scand J Immunol. 2016;83:38–43. doi: 10.1111/sji.12385. [DOI] [PubMed] [Google Scholar]
- 22.Paul P, Rouas-Freiss N, Khalil-Daher I, et al. HLA-G expression in melanoma: a way for tumor cells to escape from immunosurveillance. Proc Natl Acad Sci U S A. 1998;95:4510–5. doi: 10.1073/pnas.95.8.4510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pourhoseingholi MA, Fazeli Z, Ashtari S, Bavand-Pour FS. Mortality trends of gastrointestinal cancers in Iranian population. Gastroenterol Hepatol Bed Bench. 2013;6:52–7. [PMC free article] [PubMed] [Google Scholar]
- 24.Rebmann V, Regel J, Stolke D, Grosse-Wilde H. Secretion of sHLA-G molecules in malignancies. Semin Cancer Biol. 2003;13:371–7. doi: 10.1016/s1044-579x(03)00028-2. [DOI] [PubMed] [Google Scholar]
- 25.Rizzo R, Trentini A, Bortolotti D, et al. Matrix metalloproteinase-2 (MMP-2) generates soluble HLA-G1 by cell surface proteolytic shedding. Mol Cell Biochem. 2013;381:243–55. doi: 10.1007/s11010-013-1708-5. [DOI] [PubMed] [Google Scholar]
- 26.Tuncel T, Karagoz B, Haholu A, et al. Immunoregulatory function of HLA-G in gastric cancer. Asian Pac J Cancer Prev. 2013;14:7681–4. doi: 10.7314/apjcp.2013.14.12.7681. [DOI] [PubMed] [Google Scholar]
- 27.Ye S-r, Yang H, Li K, et al. Human leukocyte antigen G expression: as a significant prognostic indicator for patients with colorectal cancer. Mod Pathol. 2007;20:375–83. doi: 10.1038/modpathol.3800751. [DOI] [PubMed] [Google Scholar]
- 28.Yie S-M, Yang H, Ye S-R, et al. Expression of human leukocyte antigen G (HLA-G) correlates with poor prognosis in gastric carcinoma. Ann Surg Oncol. 2007a;14:2721–9. doi: 10.1245/s10434-007-9464-y. [DOI] [PubMed] [Google Scholar]
- 29.Yie S-M, Yang H, Ye S-R, et al. Expression of HLA-G is associated with prognosis in esophageal squamous cell carcinoma. Am J Clin Pathol. 2007b;128:1002–9. doi: 10.1309/JNCW1QLDFB6AM9WE. [DOI] [PubMed] [Google Scholar]
- 30.Yie S-M, Yang H, Ye S-R, et al. Expression of human leucocyte antigen G (HLA-G) is associated with prognosis in non-small cell lung cancer. Lung Cancer. 2007c;58:267–74. doi: 10.1016/j.lungcan.2007.06.011. [DOI] [PubMed] [Google Scholar]
- 31.Zhu CB, Wang CX, Zhang X, Zhang J, Li W. Serum sHLA-G levels: a useful indicator in distinguishing colorectal cancer from benign colorectal diseases. Int J Cancer. 2011;128:617–22. doi: 10.1002/ijc.25372. [DOI] [PubMed] [Google Scholar]


