Abstract
Background:
Due to the possible biomedical potential of nanoparticles, titanium dioxide nanoparticles (TiO2 NPs) have received great attention in cancer research. Although selectivity of cytotoxicity with TiO2 NPs in various cells is clinically significant comparisons of cancer and non-cancer cells have been limited. Therefore, we here studied exposure to TiO2 NPs in colorectal cancer cells (CRCs) and human umbilical vein endothelial cells (HUVECs).
Methods:
After characterization of TiO2 NPs, culture and treatment of cells (HCT116, HT29 and HUVEC), viability was assessed by MTT assay and in terms of morphological features. Acridine orange (AO) and propidium iodide (PI) assays were carried out to estimate the incidence of apoptosis. The RT-PCR method was also employed to evaluate the expression of P53, Bax, Bcl-2 and Caspase 3.
Results:
Exposure to increasing concentrations of TiO2 NPs enhanced overall cell survival of HCT116 cells and reduced the Bcl-2 and Caspase 3 expression while the ratio of Bax/Bcl-2 was down-regulated. TiO2 NPs at 400 and 50 μg/ml concentrations suppressed cell proliferation and induced apoptosis of HT29 cells and also up-regulated P53 and Bax at the mRNA level, enhanced the Bax/Bcl-2 ratio and eventually up-regulated Caspase 3 mRNA. Although, inhibition of cell proliferation in HUVECs was seen at 200 and 400 μg/ml TiO2 NPs, it was not marked.
Conclusion:
TiO2 NPs have selective bio-effects on exposed cells with dose- and cell-dependent influence on viability. Cell proliferation in HCT116 as a metastatic colorectal cancer cell line appeared to be stimulated via multiple signaling pathways, with promotion of apoptosis in less metastatic cells at 50 and 400 μg/ml concentrations. This was associated with elevated P53, Bax and Caspase 3 mRNA and reduced Bcl-2 expression. However, TiO2 NPs did not exert any apparent significant effects on HUVECs as hyperproliferative angiogenic cells.
Keywords: Colorectal cancer cells, HUVEC, TiO2 NPs, cytotoxicity, apoptosis, proliferation
Introduction
Colorectal cancer (CRC) is one of the common malignant cancers in men and women. The prevalence is, unfortunately, rising despite advances in diagnosis and therapy (Moghimi-Dehkordi and Safaee, 2012). CRC is the result of a progressive transformation of epithelial cells in the luminal surface of the intestinal organ to cancer cells. A variety of biomedical and molecular mechanisms are involved in CRC proliferation and metastatic cascade (Fearon and Vogelstein, 1990).
Hence, cancer cells gain the ability to decrease cell death and increase cell proliferation, the main desire in the field of cancer treatments is planning a useful anticancer agent which induces apoptosis as a crucial mechanism in cancer cells (Evan and Vousden, 2001). Apoptosis can be distinguished in cells by modification in morphological, molecular and biochemical properties. In addition, alteration in expression of cell cycle-related genes and dysregulation of apoptosis is thought to contribute to cell growth, differentiation, and carcinogenesis (Hengartner, 2000). P53 as the growth controller plays a role in apoptosis, genomic stability, and inhibition of angiogenesis that have been inactivated in cancer cells (Kang et al., 2008; Park et al., 2008). P53 stimulated apoptosis involves the activation of Bax (pro-apoptotic) and regulation of Bcl-2 (anti-apoptotic) expression (Antonsson and Martinou, 2000). Ultimately, an increase in the ratio of Bax/Bcl-2 expression and release of cytochrome C from the intermembrane space of the mitochondria into the cytoplasm (Chougule et al., 2011; Siddiqui et al., 2013). Cytochrome C, in turn, activates cysteine proteases 3 known as Caspase 3 which is approximately regarded as a mediator of apoptosis (Green and Reed, 1998; Tang et al., 2010).
Nowadays nanoparticles (NPs) are emerging as a novel therapeutic agent for cancer therapy (Davis et al., 2008). Nano-sized material like titanium dioxide nanoparticles (TiO2 NPs) with the dimension of approximately less than 100 nm are positively accepted by the public due to their ability to confer whiteness and opacity (Hood, 2004; Skocaj et al., 2011). Cellular uptake, subcellular localization and cytotoxic effects of TiO2 NPs, as well as penetration into living cells, depend on physicochemical features of NPs such as small size and the large surface area which increase chemical reactivity (Su et al., 2008; Auffan et al., 2009; Kansara et al., 2015). In this way, recent studies indicate that TiO2 NPs can enter the various cells, cause hurt to them and induce apoptosis (Wang et al., 2007; Ramkumar et al., 2012). This cytotoxicity is related to an induction of oxidative stress and mitochondrial injury as well as Caspase 3 overexpression and activation (Petkovic et al., 2011; Tomankova et al., 2015; Wang et al., 2015). In contrast, non-cytotoxic and non-genotoxic effects of TiO2 NPs are displayed in different cultured cells (Jeng and Swanson, 2006; Park et al., 2007), even it has been shown that these NPs increase cell survival and growth (Huang et al., 2009), also tumor-like phenotypes (Botelho et al., 2014).
Several recent studies have investigated the application of TiO2 in biomedical, especially in the field of cancer treatment (Nazir et al., 2014; Pandurangan et al., 2016) as well as in CRC therapy (Fortina et al., 2007). It appears that cell type, the particle concentration, and the surface chemistry determine the cytotoxicity of NPs against different cancer cell lines (Shi et al., 2010; Park et al., 2013; Wang et al., 2015).
The endothelial hyper-proliferation plays a critical role in the process of angiogenesis which is necessary for tumor growth and metastasis (Nishida et al., 2006). Hence, the endothelium is positioned as a barrier between blood and vessel, therefore, it can be injured by anticancer agents (Mailloux et al., 2001). In cancer therapies, NPs may find a way to penetrates through biological barriers and end up in contact with the vascular endothelium and affect their proliferation and apoptosis (Ramos-Godinez Mdel et al., 2013; Ucciferri et al., 2014).
Therefore, the present study was initiated to study how TiO2 NPs affect cytotoxicity and apoptosis in two colorectal cancer cell lines (HCT116 as more metastatic cell line and HT29 which represents less metastatic and more differentiated CRC) and human umbilical vein endothelial cells (HUVECs).
Materials and Methods
Characterization of TiO2 NPs
The TiO2 NPs powder was purchased from Sigma Company (United States). Nanoparticles were suspended in culture medium or phosphate-buffered saline (PBS) then were sonicated for 5 minutes. The TiO2 NPs structures were studied by transmission electron microscopy (TEM) (Philips Bio Twin, CM100, the Netherlands) and before the observation samples were mounted on copper grids as per standard procedures, respectively. The zeta potential and size distribution of TiO2 NPs were determined by dynamic light scattering (DLS) (Microtrac, Nanotrac Wave).
Cell culture and treatment
Two types of colorectal cancer cells (HCT116 and HT29) and HUVECs were used to evaluate the cytotoxicity against TiO2 NPs exposure. The cells were obtained from National Cell Bank of Iran (NCBI, Tehran, Iran) and were cultured in DMEM/F-12 (HT29 and HUVEC) and RPMI 1,640 (HCT116) medium supplemented with 10% fetal bovine serum, 100 U/ml penicillin and 100 μg/ml streptomycin at 5% CO2 and 37°C to reach 85% confluency. Then cells were trypsinized and sub-cultured according to the selection of experiment. For MTT assay, the cells were seeded in 96-well plates at a density of 1.0× 104 cells per well in 200 μl culture medium. For the other analyses, the cells were seeded in 25 cm2 flasks. The cells were allowed to recover for 24 hours prior to treatment. TiO2 NPs were suspended in cell culture medium by pulse sonication for 5 min to avoid particle agglomeration. A serial dilution (50, 100, 200 and 400 µg/ml) was established by mixing equal volumes of particle suspension and cell culture medium. After cells exposure with following concentrations of TiO2 NPs for 48 hour, cytotoxicity and apoptotic markers were determined. Cells not exposed to TiO2 NPs served as controls in each experiment. The means of at least 3 independent experiments were reported.
Morphology changes of treated cells
Cells were cultured in 25 cm2 flasks (3 × 106), and the cells were allowed for adherence. At the end of exposure with different concentrations of TiO2 NPs (50, 100, 200 and 400 µg/ml) for 48 hours, the morphology of cell were observed by inverted microscope.
Cell viability assay
The viability of HCT116, HT29 and HUVECs were assessed by the MTT assay as described before with some modifications (Mosmann, 1983). Cells in the 96-well plates were treated with culture medium containing different concentrations of TiO2 NPs (50, 100, 200 and 400 µg/ml) for 48 hours. Twenty microliters of a 5 mg/ml solution of MTT in PBS was added to each well, and the plates were incubated at 37 °C in 5% CO2 for 4 hours until a purple-colored formazan product developed. The medium was then carefully removed, and the purple products were dissolved in 100 μl dimethyl sulfoxide (DMSO) and absorbance was measured at 570 nm by a microplate reader. The assay was performed in quintuplet, and the means of at least 3 independent experiments were reported.
Detection of cell death by acridine orange (AO) and propidium iodide (PI)
Cells were cultured in 25 cm2 flasks (3 × 106) and allowed for 24-hour adherence, then were treated with different concentrations of TiO2 NPs (50, 100, 200 and 400 µg/ml) for 48 hours. The suspension was discarded and the cells were washed twice with PBS. Then were stained with 10 μl of AO (Sigma Aldrich. 10 μg/ml) for 15 minutes at room temperature in the dark and immediately before fluorescence invert microscope, 10 μl PI (Sigma Aldrich) was added to the cellular pellet. Apoptotic modification in cells was determined under fluorescent invert microscopy (echo LAB, IMB 600 Fl, and America) and the percentage of cells exhibiting apoptosis was counted.
Total RNA isolation and Reverse-Transcriptase PCR analysis for apoptotic markers
Cells were seeded in 25 cm2 flasks at a density of 3 × 106 and incubated for 24 hours at 37°C and 5% CO2 to attach. Then the cells were treated with different concentrations of TiO2 NPs (50, 100, 200 and 400 µg/ml) for 24 hours. Total RNA was isolated from the control and treated cells by RNeasy Kit (Ampliqon A/S, Denmark) according to the manufacturer’s instructions. The concentration of the extracted RNA was determined using Nanodrop 8000 spectrophotometer (Thermo-Scientific, Wilmington, DE). The cDNA was synthesized from 2 μl of total RNA by using cDNA synthesis Kit (Ampliqon A/S, Denmark) according to the manufacturer’s protocol. RT-PCR was performed using specific primers and three microliters of template cDNA were added to the final volume of 10.5 µl of the reaction mixture. RT-PCR cycle parameters included 6 min at 95 °C followed by 38 cycles involving denaturation at 95 °C for 30 s, annealing (P53: 57 °C, Bax and Bcl-2: 58°C also, Caspase 3 with β-actin: 60 °C) for 30s and elongation at 72°C for 30s then final elongation at 72°C for 5 min. The sequences of the specific sets of primer for P53, Bax, Bcl-2, Caspase 3 and β-actin used in this study are given in Table 1. Expressions of selected genes were normalized to β-actin gene, which was performed as an internal housekeeping control.
Table 1.
Gene Specific Primers Used for Reverse Transcription Polymerase Chain Reaction (RT-PCR)
| Gene Names | Primer Sequence |
|---|---|
| Bax (246bp) | Fwd-5’-TTTGCTTCAGGGTTTCATCC-3’ |
| Rev-5’-CAGTTGAAGTTGCCGTCAGA-3’ | |
| Bcl-2 (134bp) | Fwd-5’-TCGCCCTGTGGATGACTGA -3’ |
| Rev-5’-CAGAGACAGCCAGGAGAAATCA -3’ | |
| P53 (322bp) | Fwd-5’-TCAGTCTACCTCCCGCCATA-3’ |
| Rev-5’-TTACATCTCCCAAACATCCCT-3’ | |
| Caspase 3 (191bp) | Fwd-5’-AGAACTGGACTGTGGCATTGAG-3’ |
| Rev-5’-GCTTGTCGGCATACTGTTTCAG-3’ | |
| B-action (161bp) | Fwd-5’-CTGGAACGGTGAAGGTGACA-3’ |
| Rev-5’-TGGGGTGGCTTTTAGGATGG-3’ |
Statistical analysis
Results of at least three independent experiments are presented as mean ± SD. The comparison between treated and control groups was carried out by ANOVA and Dunnett’s multiple comparison test using Graph Pad Prism 7.0 for Windows (Graph Pad Software, Inc., San Diego, CA, USA). * P <0.05, ** P<0.01, ***P<0.001 and **** P<0.0001 were considered as significance level for all analyses performed. IC50 dose at 50% was calculated by a non-linear regression curve using online IC50 calculator (www.aatbio.com/tools/ic50-calculator/). RT-PCR product were analyzed by Image J.
Results
Characterization of TiO2 NPs
The characteristics of the TiO2 NPs used in this study is illustrated in Figure 1. The UV-Vis spectrum showed a peak at 310 nm. In addition, the zeta potential of TiO2 NPs in PBS and culture medium by DLS was +2.6 mV, respectively. Moreover, the size potential of the most stable form of TiO2 NPs was with a diameter < 20 nm. The apparent average size was confirmed by transmission electron microscopy (TEM).
Figure 1.
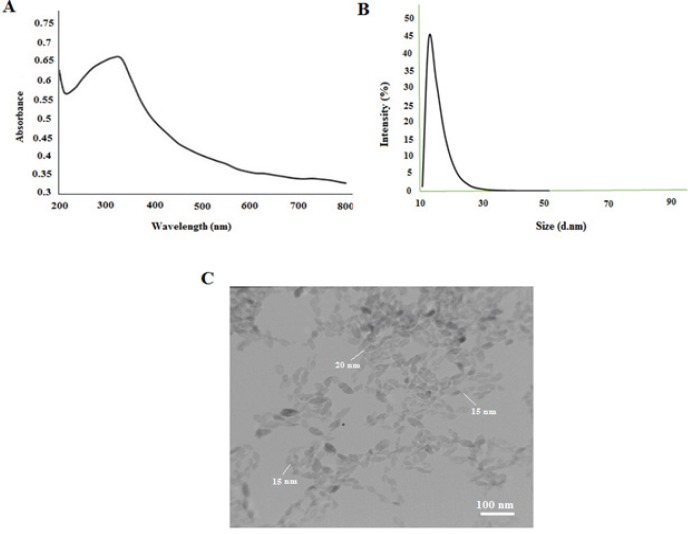
Characterization of TiO2 NPs. A, UV-Vis spectrum; B, zeta sizer; C, TEM images of TiO2 NPs.
Morphology changes of treated cells
The impact of TiO2 NPs on the morphology of HCT116, HT29 and HUVECs at 50-400 μg/ml TiO2 NPs was observed by invert microscopy as are shown in Figure 2.
Figure 2.
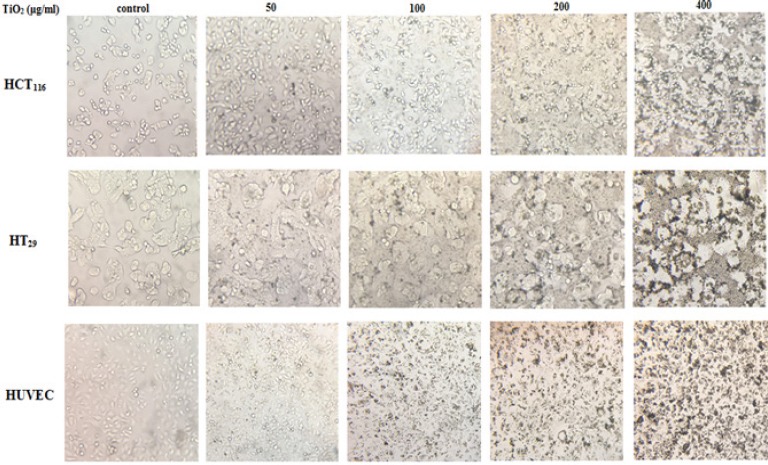
Alternation in the Morphology of HCT116, HT29 and HUVECs after Exposure to a Different Concentration of TiO2 NPs.
Concentration and cell type-dependent cytotoxicity and proliferation in TiO2- exposed cells
To investigate the effect of TiO2 NPs on colorectal cancer cells and HUVECs viability, cells were exposed to increasing concentration of TiO2 NPs for 48 hours (50, 100, 200 and 400 µg/ml), and then, analyzed by MTT assay. We found that TiO2 NPs have different effects on the viability of these three cell lines in a way that HCT116 treated cancer cells proliferated compared to control cells as are shown in Figure 3. A significant increase in survival and proliferation of HCT116 cells was induced (62-68%) by 100 and 400 μg/ml TiO2 NPs (P< 0.0001). However, these results were not seen in HT29 cancer cell lines, respectively. In comparison with the control group, HT29 cells cultured in medium containing 50 and 400 μg/ml TiO2 NPs for 48 hours showed approximately 20-30 % decrease in viability (P< 0.05 and P< 0.01). Interestingly, both enhancement and reduction in viability of HUVECs exposed cells was non-significantly observed. After exposure to 50 and 100 μg/ml TiO2 NPs, growth was observed, but these growth was inhibited at 200 and 400 μg/ml concentrations. This assay showed that increased concentration of TiO2 NPs affect the viability of cells in a dose- and cell-dependent manner for 48 hour. The IC50 was 55.717 μg/ml for HCT116 cell line, and for HT29 it was 198.849 μg/ml, however, it was 158.741 μg/ml for HUVECs.
Figure 3.
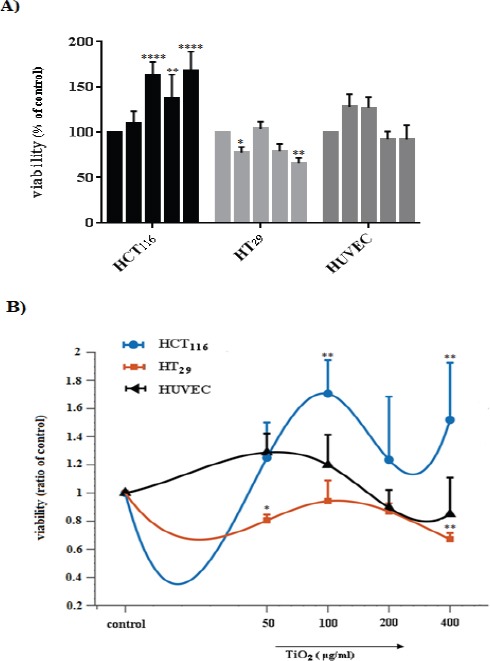
Cytotoxicity and Proliferation in TiO2- exposed Cells (HCT116, HT29, and HUVEC). A) % of control and B) ratio of control. * (p <0.05) and ** (p <0.01) using two-way ANOVA represents a significant difference between control and TiO2-exposed cells.
Quantification of apoptosis and Comparison of the fluorescence images to detect apoptosis
To determine apoptosis, treated cells were double stained and observed by fluorescence invert microscopy. In cell populations, viable cell possesses a uniform bright green nucleus with diffused chromatin (exclude of PI), whereas early apoptotic cells show bright green areas of condensed chromatin in the nucleus, late apoptotic cells in orange color losing their penetrance and necrotic cells in a uniform bright red nucleus with non-condensed chromatin (include of PI). Cells at late stage fluoresce bright red because, PI penetrates through the cell membrane and binds to nucleic acids (Figure 4). The fluorescence method is applied to detect and quantify apoptosis. Cell death may occur by two distinct mechanisms, either apoptosis or necrosis. Various images of apoptotic cells stained with AO/PI were compared in figure 5 to illustrate the detection of apoptosis and describe the morphological patterns. Hundred cells in several fields of each type were randomly selected and counted for each concentration. Then, the percentage of apoptotic cells were calculated from the following formula. Apoptotic cells (%): (Number of apoptotic cells/total number of cells) * 100. The results showed that the apoptosis of HCT116 treated cells was slightly decreased (Figure 6). The increased number of apoptotic cells was related to HT29 cells treated with 50 and 400 μg/ml TiO2 NPs whereas, no significant changes in the percentage of apoptosis in HUVECs was detected.
Figure 4.
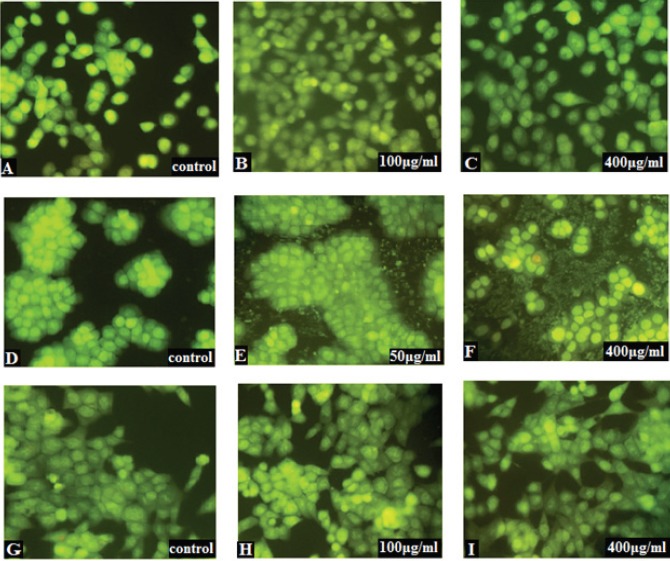
Fluorescence Inverts Microscopic Images of Control and TiO2- Exposed Cells (HCT116, HT29, and HUVEC) Stained by AO/PI. A, B and C define HCT116: A) control, B) 100 µg/ml and C) 400 µg/ml. HT29 is shown at D, E and F: D) control, E) 50 µg/ml and F) 400 µg/ml. G, H and I are related to HUVEC: G) control, H) 100 µg/ml and I) 400 µg/ml. No significant apoptosis and necrosis were observed in HCT116 and HUVEC. The green cells are viable, green with condensed chromatin are early apoptotic and orange cells (penetration of PI through the nuclear membrane) are late apoptotic. Early apoptotic cells are shown by white arrows, the late apoptotic cell is represented by black arrow whereas other cells are seemed healthy, similar size and green color of the intact nucleus which display viable cells, respectively.
Figure 5.
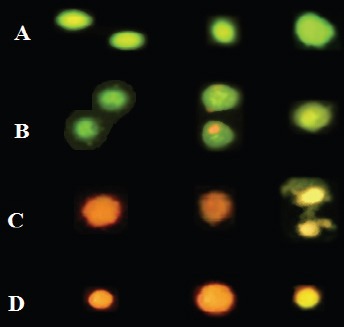
Multiple Images of Cell Death Stained with AO/PI to Compare Their Features. A) Normal viable cells are bright green. Various pictures of apoptotic cells selected from cultures are given in B & C. B) The viable apoptotic cells in earliest stage possess a fragmented and condensed nucleus with circumnuclear chromatin condensation that emerges as a bright green area and even cells may have apoptotic bodies around cytoplasm as are shown. Yellow/orange and condensed nuclei are apoptotic cells which enter the late stage. C) Dead cells with condensed and shrieked nuclei are late apoptotic orange cells. D) Necrotic cells have orange/red nuclei with an organized structure.
Figure 6.
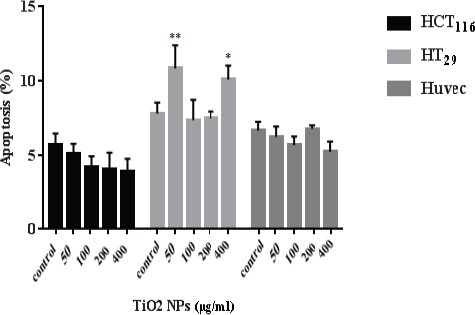
Determination of Apoptosis in HCT116, HT29, and HUVECs by TiO2 NPs Over 48 hours and Observation by AO/PI Staining. The number of apoptotic cells and a total number of cells were calculated, then, the percentage of apoptosis was estimated. Significant differences are statistically representing by * and ** (p <0.05 & P< 0.01) as compared with control. The comparison between treated and control groups was carried out by two-way ANOVA.
Effect of TiO2 NPs on Apoptosis-associated mRNA Expression
The mRNA level of apoptotic markers (P53, Bax, Bcl-2, Caspase 3, and β-actin) by RT-PCR was measured in cells (HCT116, HT29, and HUVEC) exposed to an increased concentration of TiO2 NPs as are shown in Figure 7. The mRNA expression of P53 in HCT116 was downregulated at 50 μg/ml TiO2 NPs (P<0.01). The level of Bax mRNA was downregulated in 100 μg/ml (P< 0.01) and Bcl-2 expression was significantly upregulated at 200 and 400 μg/ml TiO2 NPs treated cells. While the expression of Caspase 3 was downregulated in overall concentrations. In particular, the relative ratio of Bax/Bcl-2 was downregulated at 100 and 200 concentrations (Figure 8). However, exposure to TiO2 NPs in HT29 cells upregulated the expression of P53 in 50, 200 and 400 μg/ml (P< 0.05), moreover, the Bax expression was upregulated at 100, 200 and 400 μg/ml TiO2 NPs (P< 0.0001). The relative ratio of Bax/Bcl-2 in HT29 treated cells was upregulated in 200 and 400 μg/ml (P< 0.05), and even, the expression of Caspase 3 mRNA was upregulated at 200 and 400 μg/ml TiO2 NPs as compared to controls. In HUVECs, the P53, Bax, and Bcl-2 expression were significantly upregulated, although the Caspase 3 mRNA was downregulated at 50 μg/ml (P< 0.001) and upregulated at 200 as well as 400 μg/ml TiO2 NPs.
Figure 7.
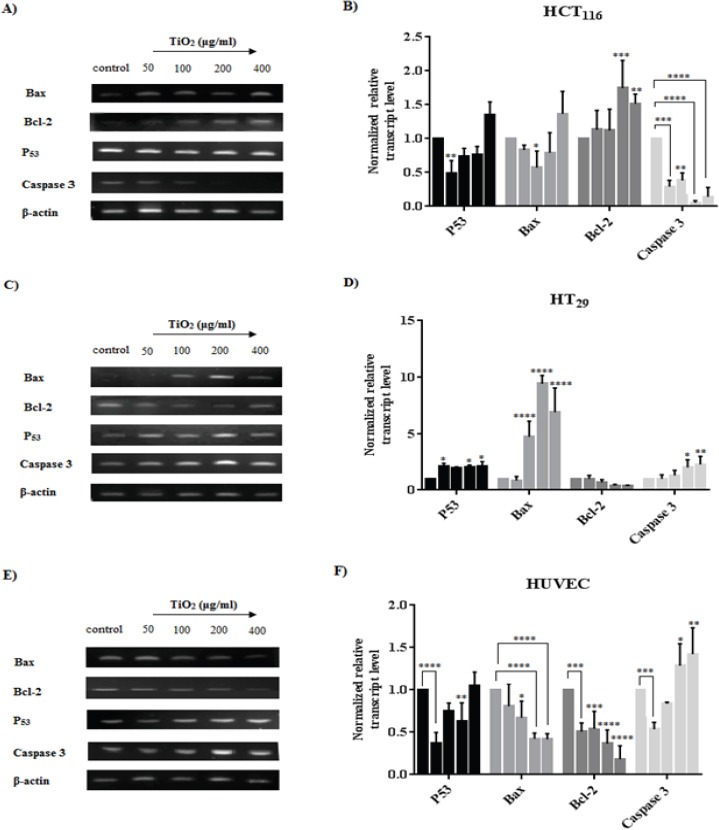
RT-PCR Analysis of the Expression Changes in mRNA Level of P53, Bax, Bcl-2, Caspase 3 after Exposure to TiO2 NPs for HCT116 (A and B), HT29 (C and D) and HUVECs (E and F). A, C and E show the effect of TiO2 NPs on mRNA expression and B, D and F represent an analysis of the fluorescent intensity of each band which is normalized fold change mRNA expression. β-actin was used as housekeeping and control for loading respectively. Band density of the specific gene expression was analyzed with Image J and the results were normalized to β-actin. Graphs represent the normalized grade of fluorescent to assess the gene expression level of the cell lines. Bars represent the mean ± SD of three independent experiments. *P<0.05, **P<0.01, ***P<0.001, ****P<0.0001 vs control.
Figure 8.
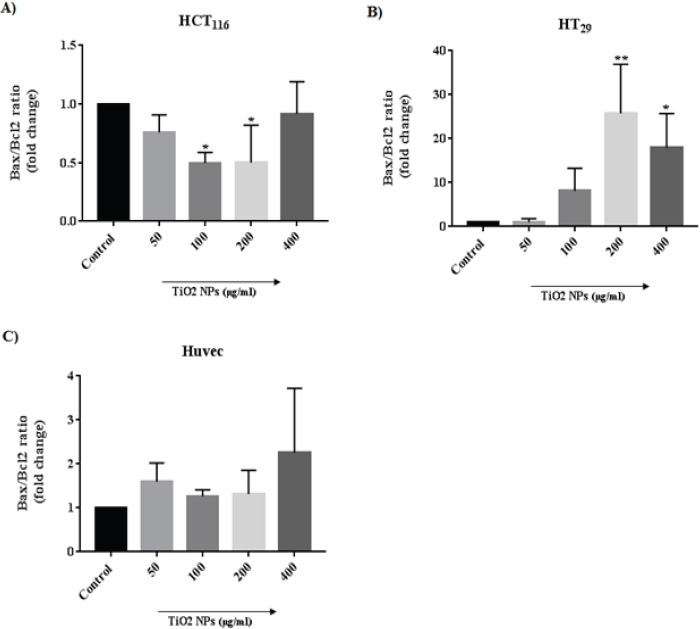
Alternation in the Relative Ratio of Bax/Bcl-2 mRNA. The Bcl-2 and Bax proteins are respectively known for an anti-apoptotic and pro-apoptotic activity. Any increase in Bax/Bcl-2 ratio induces apoptosis. Bars represent the mean ± SD of three independent experiments. *P<0.05, **P<0.01 vs control.
Discussion
TiO2 NPs are considered as natural materials and are widely used in industry and medicine. Biological effects of NPs relate to oxidative stress induction that is responsible for mitochondrial injury subsequently activation of a series apoptotic genes (Petkovic et al., 2011). At a molecular level it has been described that the exposure to TiO2 NPs causes an increase in expression of tumor suppressor protein P53 mRNA and its downstream DNA damage responses (Kang et al., 2008). The P53, activates or represses the transcription of Bax and Bcl-2 and increases the Bax/Bcl-2 ratio, which results in the release of cytochrome C from the intermembrane space of mitochondria (Nemajerova et al., 2005). Cytochrome C in turn, activates caspase cascade (Watson, 2004). Previous studies indicated that the effect of TiO2 NPs on proliferation and apoptosis of cells depends on cellular types and concentration of these nano sized particles (Huang et al., 2009; Valdiglesias et al., 2013; Botelho et al., 2014; Pandurangan et al., 2016). CRC is one of common malignant cancers with high prevalence, thus, the induction of apoptosis in CRC is a crucial mechanism of the useful anti-cancer agent. Nanoparticles like TiO2 NPs are emerging as a novel therapeutic agent for cancer therapy. In addition, it has been illustrated NPs whenever penetrate from entry ports, came contact with endothelial cells and affect their proliferations that have critical role in tumor growth and spread by angiogenesis (Ucciferri et al., 2014). By considering the activation of endothelial cell proliferation and resistance to apoptosis that are vital characteristics of angiogenesis, induction of cell apoptosis may be a promising treatment strategy for cancer. Therefore, in the present study we tried to evaluate the effect of an exposure to TiO2 NPs, in HCT116 and HT29 as two colorectal cancer cell lines as well as HUVEC.
Hence, any changes in physicochemical features of TiO2 NPs such as particle shape, size, surface area, purity, crystal structure, surface charge and agglomeration rate can cause an effect on cellular responses upon exposure, we first well investigated the characterization of these NPs. The most stable form of TiO2 for NPs was with a diameter < 20 nm.
Our cell viability assay by MTT in exposed cells indicated a concentration and cell type dependent cytotoxicity and proliferation. These findings show that treatment of HCT116 as a more metastatic CRC, with TiO2 NPs, exhibited significant survival and growth which is in agreement with recent report that indicated TiO2 NPs promote cell viability and enhance cell proliferation in NIH 3T3 cells by time- and dose-dependent manner (Huang et al., 2009). Furthermore, consequence of treatment with nano-TiO2 suggests an increase in overall survival of AGS gastric cells in vitro (Botelho et al., 2014). Accordingly, the growth levels of HCT116 showed that treated cells proliferated significantly faster and more than control cells. In comparison, TiO2 NPs revealed a cytotoxic potential in more differentiated and less metastatic HT29 colorectal cancer cells as are seen in several evidences (Ramkumar et al., 2012; Wang et al., 2015; Murugan et al., 2016). Moreover, HUVEC (as a hyper-proliferated and angiogenic cell line) treated cells displayed a slight both induction and reduction in cell viability of different TiO2 NPs concentration. It seems that an exposure to TiO2 NPs may not effectively influence HUVECs because, as the concentration of TiO2 NPs increased, the viability of HUVEC cultured for 48 hour was not greatly altered. This result was in accordance with L929 mouse fibroblast cell lines from 3 to 600 μg/ml of TiO2 NPs with no significant cytotoxicity (Jin et al., 2008). Based on these findings, cell type and NP concentration determine the cytotoxicity of TiO2 NPs against different cell lines.
Apoptotic cells are characterized by altered morphologic and biochemical features. For early apoptotic study, the morphological observation is required since, DNA fragments cannot be seen during initiation of apoptosis (Baskic et al., 2006). The morphological observation was carried out to determine whether the cytotoxic effect of TiO2 NPs was correlated with the apoptotic process. Quantification of apoptosis have been reported in TiO2-exposed cells via fluorescence staining techniques. AO/PI double staining study represented condense chromatin and orange color also reduction in cell volume in HT29 treated cells. While viable cells with similar size, healthy and green color of intact nucleus were seen in majority of HCT116 and HUVEC (Hajiaghaalipour et al., 2015). Results showed an increasing number of apoptotic cells per field in HT29 treated cells compared to control with 50 and 400 μg/ml of TiO2 NPs. As an assay on human cervical carcinoma cells found that the percentages of the apoptotic cell were 35, 54, and 59 %, respectively, in 2, 4, and 8 mg/ml TiO2 NP-treated samples (Pandurangan et al., 2016).
For detecting the impact of apoptotic regulators such as P53, Bax, Bcl-2 and Caspase 3 in survival or cytotoxicity of HCT116, HT29 and HUVEC treated cells, we determined the mRNA expression. In HCT116 cell line, the level of Bcl-2 and Caspase 3 expression seems to be a determinant marker in the response to TiO2 NPs. It appears that HCT116 (more metastatic cell line) in response to TiO2 NPs, upregulate Bcl-2 expression and increase Bax/Bcl-2 ratio which result in downregulation of Caspase 3 to induce its growth. Whereas TiO2 NPs have been able to enhance cell death in HT29 by promoting the expression of P53 and Bax as well as downregulation of Bcl-2 which respectively, led to raising in the Bax/Bcl-2 ratio expression and activation of Caspase 3. This result is in agree with the study which showed, the over expression of P53 and Bax mRNA in TiO2 treated human cervical carcinoma cells (Pandurangan et al., 2016) and also, in accordance with another study that suggested TiO2 NPs induce apoptosis via caspase dependent pathway by over expression of Caspase 3 mRNA (Wang et al., 2015). These results revealed that TiO2 NPs have genotoxic effects in HT29 (more differentiated colorectal cancer cell line) and induce apoptosis typically by intrinsic mitochondrial pathway. Bax is a pro-apoptotic protein which can inhibit the Bcl-2 protein function, therefore in TiO2-exposed HT29 cells, the mRNA level of Bax was increased to prevent the inhibition function. A recent report over the measuring expression of Bcl-2 and Survivin in Ag NP treated colorectal cancer cells, recorded downregulation of Bcl-2 expression, inhibited colon cancer proliferation (El-Deeb et al., 2015). HUVECs downregulated the mRNA level of P53 and Bax in response to TiO2 NPs to promote their survival but it was not remarkable. It appears that the downregulation of P53 and Bax was not sufficient to enhance significant cell growth of HUVECs. Because, reduction in expression of Bcl-2, typically, led to non-significant change in Bax/Bcl-2 ratio expression as a study which provided evidence that HUVECs in confluent cultures seem to be in an equilibrium of resistance to apoptosis related to Bcl-2 expression, moreover lack of P53 and Bax in quiescent cells contributes to resistance of endothelial cells to DNA damaging agents (Mailloux et al., 2001). Indeed, reduction in level of Bcl-2 mRNA cause non-significant changes in hyper-proliferative, angiogenic cells viability in response to downregulation of P53 and Bax mRNA. Hence, the activation of vascular endothelial cell proliferation and resistance to apoptosis are vital characteristics of angiogenesis, inhibition of cell proliferation and induction of cell apoptosis which is seen non-significantly at 200 and 400 μg/ml TiO2 NPs may get further insights in treatment strategy for cancer as are seen in a recent study that fluvastatin suppressed cell proliferation and induced apoptosis of HUVECs in hypoxia by upregulation of Caspase 3, P53, P27 and a decrease of Bcl-2, Survivin levels, enhancing Bax/Bcl-2 ratio and activation of Caspase 3 (Li et al., 2015). In addition, another study showed that doxorubicin-induced apoptosis was caspase-dependent in HUVECs and the ovarian cancer cell lines A2780 (Bruynzeel et al., 2007).
In conclusion, our results indicate that TiO2 NPs have unexpected effects on cell survival and cytotoxicity in a way that stimulatory effect of TiO2 NPs in cell survival was cell line specific and TiO2 NPs concentration peculiar. These may suggest a selective bio-effect of TiO2 NPs on exposed cells via modifications in mRNA level of apoptotic markers.
References
- 1.Antonsson B, Martinou JC. The Bcl-2 protein family. Exp Cell Res. 2000;256:50–7. doi: 10.1006/excr.2000.4839. [DOI] [PubMed] [Google Scholar]
- 2.Auffan M, Rose J, Bottero JY, et al. Towards a definition of inorganic nanoparticles from an environmental, health and safety perspective. Nat Nanotechnol. 2009;4:634–41. doi: 10.1038/nnano.2009.242. [DOI] [PubMed] [Google Scholar]
- 3.Baskic D, Popovic S, Ristic P, et al. Analysis of cycloheximide-induced apoptosis in human leukocytes: fluorescence microscopy using annexin V/propidium iodide versus acridin orange/ethidium bromide. Cell Biol Int. 2006;30:924–32. doi: 10.1016/j.cellbi.2006.06.016. [DOI] [PubMed] [Google Scholar]
- 4.Botelho MC, Costa C, Silva S, et al. Effects of titanium dioxide nanoparticles in human gastric epithelial cells in vitro. Biomed Pharmacother. 2014;68:59–64. doi: 10.1016/j.biopha.2013.08.006. [DOI] [PubMed] [Google Scholar]
- 5.Bruynzeel AM, Abou El Hassan MA, Torun E, et al. Caspase-dependent and -independent suppression of apoptosis by monoHER in Doxorubicin treated cells. Br J Cancer. 2007;96:450–6. doi: 10.1038/sj.bjc.6603598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chougule M, Patel AR, Sachdeva P, et al. Anticancer activity of Noscapine, an opioid alkaloid in combination with Cisplatin in human non-small cell lung cancer. Lung Cancer. 2011;71:271–82. doi: 10.1016/j.lungcan.2010.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Davis ME, Chen ZG, Shin DM. Nanoparticle therapeutics: an emerging treatment modality for cancer. Nat Rev Drug Discov. 2008;7:771–82. doi: 10.1038/nrd2614. [DOI] [PubMed] [Google Scholar]
- 8.El-Deeb NM, El-Sherbiny IM, El-Aassara MR, et al. Novel trend in colon cancer therapy using silver nanoparticles synthesized by honey bee. J Nanomed Nanotechnol. 2015;6:1. [Google Scholar]
- 9.Evan GI, Vousden KH. Proliferation, cell cycle and apoptosis in cancer. Nature. 2001;411:342–8. doi: 10.1038/35077213. [DOI] [PubMed] [Google Scholar]
- 10.Fearon ER, Vogelstein B. A genetic model for colorectal tumorigenesis. Cell. 1990;61:759–67. doi: 10.1016/0092-8674(90)90186-i. [DOI] [PubMed] [Google Scholar]
- 11.Fortina P, Kricka LJ, Graves DJ, et al. Applications of nanoparticles to diagnostics and therapeutics in colorectal cancer. Trends Biotechnol. 2007;25:145–52. doi: 10.1016/j.tibtech.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 12.Green DR, Reed JC. Mitochondria and apoptosis. Science. 1998;281:1309–12. doi: 10.1126/science.281.5381.1309. [DOI] [PubMed] [Google Scholar]
- 13.Hajiaghaalipour F, Kanthimathi MS, Sanusi J, et al. White tea (Camellia sinensis) inhibits proliferation of the colon cancer cell line, HT-29, activates caspases and protects DNA of normal cells against oxidative damage. Food Chem. 2015;169:401–10. doi: 10.1016/j.foodchem.2014.07.005. [DOI] [PubMed] [Google Scholar]
- 14.Hengartner MO. The biochemistry of apoptosis. Nature. 2000;407:770–6. doi: 10.1038/35037710. [DOI] [PubMed] [Google Scholar]
- 15.Hood E. Nanotechnology: looking as we leap. Environ Health Perspect. 2004;112:740–9. doi: 10.1289/ehp.112-a740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang S, Chueh PJ, Lin YW, et al. Disturbed mitotic progression and genome segregation are involved in cell transformation mediated by nano-TiO2 long-term exposure. Toxicol Appl Pharmacol. 2009;241:182–94. doi: 10.1016/j.taap.2009.08.013. [DOI] [PubMed] [Google Scholar]
- 17.Jeng HA, Swanson J. Toxicity of metal oxide nanoparticles in mammalian cells. J Environ Sci Health A Tox Hazard Subst Environ Eng. 2006;41:2699–711. doi: 10.1080/10934520600966177. [DOI] [PubMed] [Google Scholar]
- 18.Jin CY, Zhu BS, Wang XF, et al. Cytotoxicity of titanium dioxide nanoparticles in mouse fibroblast cells. Chem Res Toxicol. 2008;21:1871–7. doi: 10.1021/tx800179f. [DOI] [PubMed] [Google Scholar]
- 19.Kang SJ, Kim BM, Lee YJ, et al. Titanium dioxide nanoparticles trigger p53-mediated damage response in peripheral blood lymphocytes. Environ Mol Mutagen. 2008;49:399–405. doi: 10.1002/em.20399. [DOI] [PubMed] [Google Scholar]
- 20.Kansara K, Patel P, Shah D, et al. TiO2 nanoparticles induce DNA double strand breaks and cell cycle arrest in human alveolar cells. Environ Mol Mutagen. 2015;56:204–17. doi: 10.1002/em.21925. [DOI] [PubMed] [Google Scholar]
- 21.Li X, Liu X, Xu Y, et al. Expression profile of apoptotic and proliferative proteins in hypoxic HUVEC treated with statins. Int J Oncol. 2015;46:677–84. doi: 10.3892/ijo.2014.2780. [DOI] [PubMed] [Google Scholar]
- 22.Mailloux A, Grenet K, Bruneel A, et al. Anticancer drugs induce necrosis of human endothelial cells involving both oncosis and apoptosis. Eur J Cell Biol. 2001;80:442–9. doi: 10.1078/0171-9335-00171. [DOI] [PubMed] [Google Scholar]
- 23.Moghimi-Dehkordi B, Safaee A. An overview of colorectal cancer survival rates and prognosis in Asia. World J Gastrointest Oncol. 2012;4:71–5. doi: 10.4251/wjgo.v4.i4.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 25.Murugan K, Dinesh D, Kavithaa K, et al. Hydrothermal synthesis of titanium dioxide nanoparticles: mosquitocidal potential and anticancer activity on human breast cancer cells (MCF-7) Parasitol Res. 2016;115:1085–96. doi: 10.1007/s00436-015-4838-8. [DOI] [PubMed] [Google Scholar]
- 26.Nazir S, Hussain T, Ayub A, et al. Nanomaterials in combating cancer: therapeutic applications and developments. Nanomedicine. 2014;10:19–34. doi: 10.1016/j.nano.2013.07.001. [DOI] [PubMed] [Google Scholar]
- 27.Nemajerova A, Wolff S, Petrenko O, et al. Viral and cellular oncogenes induce rapid mitochondrial translocation of p53 in primary epithelial and endothelial cells early in apoptosis. FEBS Lett. 2005;579:6079–83. doi: 10.1016/j.febslet.2005.09.074. [DOI] [PubMed] [Google Scholar]
- 28.Nishida N, Yano H, Nishida T, et al. Angiogenesis in cancer. Vasc Health Risk. 2006;2:213. doi: 10.2147/vhrm.2006.2.3.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pandurangan M, Enkhtaivan G, Young JA, et al. In vitro therapeutic potential of Tio2 nanoparticles against human cervical carcinoma cells. Biol Trace Elem Res. 2016;171:293–300. doi: 10.1007/s12011-015-0551-9. [DOI] [PubMed] [Google Scholar]
- 30.Park EJ, Shim HW, Lee GH, et al. Comparison of toxicity between the different-type TiO(2) nanowires in vivo and in vitro. Arch Toxicol. 2013;87:1219–30. doi: 10.1007/s00204-013-1019-3. [DOI] [PubMed] [Google Scholar]
- 31.Park EJ, Yi J, Chung KH, et al. Oxidative stress and apoptosis induced by titanium dioxide nanoparticles in cultured BEAS-2B cells. Toxicol Lett. 2008;180:222–9. doi: 10.1016/j.toxlet.2008.06.869. [DOI] [PubMed] [Google Scholar]
- 32.Park S, Lee YK, Jung M, et al. Cellular toxicity of various inhalable metal nanoparticles on human alveolar epithelial cells. Inhal Toxicol. 2007;19:59–65. doi: 10.1080/08958370701493282. [DOI] [PubMed] [Google Scholar]
- 33.Petkovic J, Zegura B, Stevanovic M, et al. DNA damage and alterations in expression of DNA damage responsive genes induced by TiO2 nanoparticles in human hepatoma HepG2 cells. Nanotoxicology. 2011;5:341–53. doi: 10.3109/17435390.2010.507316. [DOI] [PubMed] [Google Scholar]
- 34.Ramkumar KM, Manjula C, Gnanakumar G, et al. Oxidative stress-mediated cytotoxicity and apoptosis induction by TiO2 nanofibers in HeLa cells. Eur J Pharm Biopharm. 2012;81:324–33. doi: 10.1016/j.ejpb.2012.02.013. [DOI] [PubMed] [Google Scholar]
- 35.Ramos-Godinez Mdel P, Gonzalez-Gomez BE, Montiel-Davalos A, et al. TiO2 nanoparticles induce endothelial cell activation in a pneumocyte-endothelial co-culture model. Toxicol In Vitro. 2013;27:774–81. doi: 10.1016/j.tiv.2012.12.010. [DOI] [PubMed] [Google Scholar]
- 36.Shi Y, Wang F, He J, et al. Titanium dioxide nanoparticles cause apoptosis in BEAS-2B cells through the caspase 8/t-Bid-independent mitochondrial pathway. Toxicol Lett. 2010;196:21–7. doi: 10.1016/j.toxlet.2010.03.014. [DOI] [PubMed] [Google Scholar]
- 37.Siddiqui MA, Alhadlaq HA, Ahmad J, et al. Copper oxide nanoparticles induced mitochondria mediated apoptosis in human hepatocarcinoma cells. PLoS One. 2013;8:e69534. doi: 10.1371/journal.pone.0069534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Skocaj M, Filipic M, Petkovic J, et al. Titanium dioxide in our everyday life;is it safe? Radiol Oncol. 2011;45:227–47. doi: 10.2478/v10019-011-0037-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Su W, Zhang Y, Li Z, et al. Multivalency iodine doped TiO2: preparation, characterization, theoretical studies, and visible-light photocatalysis. Langmuir. 2008;24:3422–8. doi: 10.1021/la701645y. [DOI] [PubMed] [Google Scholar]
- 40.Tang X, Guo Y, Nakamura K, et al. Nitroalkenes induce rat aortic smooth muscle cell apoptosis via activation of caspase-dependent pathways. Biochem Biophys Res Commun. 2010;397:239–44. doi: 10.1016/j.bbrc.2010.05.091. [DOI] [PubMed] [Google Scholar]
- 41.Tomankova K, Horakova J, Harvanova M, et al. Cytotoxicity, cell uptake and microscopic analysis of titanium dioxide and silver nanoparticles in vitro. Food Chem Toxicol. 2015;82:106–15. doi: 10.1016/j.fct.2015.03.027. [DOI] [PubMed] [Google Scholar]
- 42.Ucciferri N, Collnot EM, Gaiser BK, et al. In vitro toxicological screening of nanoparticles on primary human endothelial cells and the role of flow in modulating cell response. Nanotoxicology. 2014;8:697–708. doi: 10.3109/17435390.2013.831500. [DOI] [PubMed] [Google Scholar]
- 43.Valdiglesias V, Costa C, Sharma V, et al. Comparative study on effects of two different types of titanium dioxide nanoparticles on human neuronal cells. Food Chem Toxicol. 2013;57:352–61. doi: 10.1016/j.fct.2013.04.010. [DOI] [PubMed] [Google Scholar]
- 44.Wang JJ, Sanderson BJ, Wang H. Cyto- and genotoxicity of ultrafine TiO2 particles in cultured human lymphoblastoid cells. Mutat Res. 2007;628:99–106. doi: 10.1016/j.mrgentox.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 45.Wang Y, Cui H, Zhou J, et al. Cytotoxicity, DNA damage, and apoptosis induced by titanium dioxide nanoparticles in human non-small cell lung cancer A549 cells. Environ Sci Pollut Res Int. 2015;22:5519–30. doi: 10.1007/s11356-014-3717-7. [DOI] [PubMed] [Google Scholar]
- 46.Watson AJ. Apoptosis and colorectal cancer. Gut. 2004;53:1701–9. doi: 10.1136/gut.2004.052704. [DOI] [PMC free article] [PubMed] [Google Scholar]


