Abstract
With no sharp cure, breast cancer still be the major and the most serious life-threatening disease worldwide. Colorectal is the third most commonly occurring cancer in men and the second most commonly occurring cancer in women. In the present investigation, colon cancer cells (CaCo-2) and breast cancer cells (MCF-7) were treated with elevated doses of metformin (MET) for 48h. Cell count was assessed using trypan blue test, and the cytotoxicity was evaluated using MTT assay. Methylation-specific PCR was performed on the bisulfite-treated DNA against two tumor suppressor genes; RASSF1A and RB. Results indicated that: in breast cancer, the cell count was decreased significantly (P>0.005) after being treated with 5, 10, 20, 50, and 100 mM of MET. The elevated concentration had increased reduction percentages on the MCF-7 cells, as 5 mM and 100 mM have yielded 35% and 93.3% reduction in cell viability, respectively. Colon cancer cells have responded to the doses of MET differently, as for the 5 mM and the 100 mM, it gave 88% and 60% reduction in cells viability, respectively. Cytotoxicity assay revealed that 5 mM and 100 mM of MET caused breast cancer cells to loss 61.53% and 85.16% of its viability, respectively, whereas colon cancer cells have responded to the 5 mM and 100 mM of MET by reducing the cells viability with 96.91% and 96.24%, respectively. No RB promoter methylation was detected in colon cells, while RASSF1A was partially methylated. In the MCF-7 breast cancer cells, both RASSF1A and RB were partially methylated.
Keywords: Metformin, breast, colon, cancer, methylation, RASSF1A, RB
Introduction
Breast cancer (BC) incidence is rising worldwide with an increase in aggressive neoplasia in young women (Chajès and Romieu, 2014), with 1 million new cases causing 375, 000 deaths worldwide per year. Breast cancer is the leading cause of cancer death in women in both developing and developed countries, and it is considered a major public health concern with tremendous socioeconomic implications (Ginsburg, 2013; Rivera-Franco and Leon-Rodriguez, 2018). Breast cancer incidence and mortality rates are increasing in the Arab world and the involved women are often diagnosed at advanced stages of the disease (Donnelly et al., 2013; Karim et al., 2015). Meanwhile, the incidence rates of BC being increased during the last 24 years in the Arab region, with high incidence rates in Egypt, Tunisia, Saudi Arabia, Syria, and Palestine, as it constitutes 13-42% of all female cancers (El Saghir et al., 2007; Saggu et al., 2015). While the incidence of breast cancer in the Middle East region is lower than in other western countries, it has substantially increased in the last quarter century (Tarabeia et al., 2007). Furthermore, the diagnosis of breast cancer in this region often occurs at a later stage in the progress of the disease and in a higher proportion of women in their thirties and forties (Tarabeia et al., 2007; Al-Saad et al., 2007; Miller, 2010) than in industrialized nations. Breast cancer that presents at a younger age is generally more aggressive with a possibly poorer prognosis (Cancello et al., 2010; Gnerlich et al., 2009; Kheirelseid et al., 2011). About 50% of the breast cancer cases and 60% of the deaths are estimated to occur in developing countries (Mackay et al., 2006). Nevertheless, there is a large difference in breast cancer incidence among Caucasian, Hispanic, African, and Asian women with Caucasian women being the highest and Asian women being the lowest (Hea and Chenb, 2013).
Colorectal cancer is a major cause of morbidity and mortality throughout the world. It accounts for over 9% of all cancer incidence. It is the third most common cancer worldwide and the fourth most common cause of death (Haggar and Boushey, 2009). Colorectal cancer is one of the most common human malignancies with a high rate of mortality. Most colorectal cancers are due to lifestyle factors and increasing age, with only a small number of cases due to genetic causes. The majority of patients are diagnosed at an advanced stage so that chemotherapy is required, with the incidence and mortality being high among young adults (Grivicich et al., 2007; Marley and Nan, 2016; Bhandari et al., 2017).
Epigenetic alterations are one of the most common molecular alterations in human neoplasia. In particular, aberrant promoter methylation occurs in numerous genes in cancer development and progression (Kim and Paik, 2010). It was suggested that these CpG island methylation of tumor-related genes are an early event in breast cancer progression (Park et al., 2011). Meanwhile, epigenetic profiling represents a promising approach to discover novel disease-specific markers (Esteller, 2007), and might play a key role in most kinds of cancer, both in the early and late stages of disease. One of the most powerful epigenetic mechanisms is DNA hyper-/hypo-methylation (Szyf and Paknesha, 2004) that plays an important role in multi-stages of breast cancer and are considered the main epigenetic modification occurring in the early stages of carcinogenesis (Li et al., 2010).
Metformin (chemically designated as 1,1-Dimethylbiguanide hydrochloride) is a drug of first choice for the treatment of type II diabetes as it functions to and its primary inhibit hepatic gluconeogenesis, but a clear mechanistic understanding of its effects has remained elusive (Hundal et al., 2000; Kirpichnikov et al., 2002; Castillo-Quan and Blackwell, 2016).
Several reports suggested that metformin slows cancer cell growth and protects against multiple human cancers (Pryor and Cabreiro, 2015; Bruno et al., 2015; Rodriguez-Lirio et al., 2015; Wu et al. 2016), although the majority of available clinical data on the anti-cancer potential of metformin are based on observational studies (Gadducci et al., 2016). However, Garcia et al., (2017) observed no statistical significant association between metformin use and overall survival in a matched cohort of 360 ovarian cancer patients.
Metformin regulates mitonuclear communication and modulate the epigenetic landscape in pre-cancerous cells, and this might guide the development of new metabolic-epigenetic strategies for cancer prevention and therapy (Cuyas et al., 2016; Liang et al., 2017). However, the molecular mechanisms underlying the anticancer properties of metformin remain elusive (Zhong et al., 2017).
In the present study, we are aiming to investigate the role of metformin in modulating the methylation pattern (s) of two tumor suppressor genes; RASSF1A and RB in colorectal and breast cancer cells.
Materials and Methods
Cell line maintenance
MCF-7 breast cancer cells and CaCo-2 colorectal cancer cells were purchased from the Holding Company for Biological Products and Vaccines (VACSERA), Giza, Egypt. Adherent cells were grown in RPMI 1640 medium (Gibco, USA) supplemented with 10% fetal bovine serum (HyClone, Logan, UT, USA) and 1% penicillin-streptomycin mix (Invitrogen Life Technologies). Cells were seeded in 12-well U-bottom microplates (Nunc, Denmark) and incubated for 24 h at 37 °C in a fully humidified atmosphere of 5% CO2 before being treated with metformin.
Metformin doses
Metformin was kindly provided by Dr. Aya Salem, College of Biotechnology, Misr University for Science and Technology. Cells (1.8×104 cell/mL) were treated with metformin (dissolved in water) in final concentrations of 5, 10, 20, 50, and 100 mM for 48h.
Cell counting
Trypan blue test was employed in the present study to count the cells after being treated with metformin. Briefly, cells were harvested with 0.25% trypsin (Invitrogen, USA) and resuspended again in 1.5 mL fresh RPMI 1640 media. About 50 µL of the cell suspension was mixed with an equal volume of trypan blue dye (Sigma Aldrich, Germany) for 2-4 min. at room temperature. An appropriate volume was loaded on hemocytometer slide, covered with glass coverslip, and read under an inverted microscope. The average of four readings for each sample was taken, and the cell count was calculated according to the following equation:
Number of cells/mL = average cell count x2 x 104.
Cytotoxicity assay
The cytotoxic/cytostatic effects of metformin in vitro on both MCF-7 breast cancer cells and CaCo-2 colon cancer cells was tested with a rapid colorimetric assay using MTT assay and compared with the untreated controls. This assay is based on the metabolic reduction of soluble MTT by mitochondrial enzyme activity of viable tumor cells, into an insoluble colored formazan product, which can be measured spectrophotometrically after dissolving in DMSO (Denizlt and Lang, 1986). To evaluate cell viability, 20 μL of MTT solution (5 mg/mL in PBS) was added to each well and incubated for 3 h. Then the media were replaced with 150 μL of DMSO, and the complete dissolving of formazan crystals was achieved by repeated pipetting of the solution. Optical density was then determined at 540 nm by an ELISA plate reader.
The cytotoxic effect of metformin was expressed as the relative viability (% control). To calculate the percentages of cell viability, the following equation was applied:
Relative viability = Experimental absorbance – background absorbance/absorbance of untreated cells – background absorbance X 100.
DNA extraction
Total DNA was extracted using the Quick-DNA Plus (Zymo research, USA) according to the kit’s instructions. DNA was extracted from treated and untreated cells, and stored in -20°C until being used.
Bisulfite conversion
Bisulfite modification is the most widely used of all the pre-treatment options available for DNA methylation analysis. The extracted DNA was subjected to bisulfite conversion using EZ DNA Methylation Kit (Zymo research, USA). Bisulfite conversion involves the deamination of unmodified cytosines to uracil, leaving the modified bases 5-mC and 5-hmC unconverted. Treatment of denatured DNA with sodium bisulfite leads to deamination of unmethylated cytosine residues to uracil, leaving 5-mC or 5-hmC intact. The uracils are amplified in subsequent PCR reaction as thymines, whereas 5-mC or 5-hmC residues are amplified as cytosines. We followed the kit’s instructions with minor modification in terms of the time needed for incubation of DNA.
Methylation-specific PCR
Methylation-specific PCR was performed to detect the methylation status of two tumor suppressor genes; RASSF1A and RB. The reaction was performed in StepOne Plus (ABI). The primer sequences used in this study are presented in Table 1.
Table 1.
The Methylated and Unmethylated Primer Sequences of RASSF1A and RB used in the Present Study
| Gene name | Primer status | Primer sequence (5’ to 3’) | Ref. |
|---|---|---|---|
| RASSF1A | Unmethylated Forward | GGTTGTATTTGGTTGGAGTG | Matthaios et al., 2016 |
| Unmethylated Reverse | CTACAAACCTTTACACACAACA | ||
| Methylated Forward | GTTGGTATTCGTTGGGCGC | ||
| Methylated Reverse | GCACCACGTATACGTAACG | ||
| RB | Unmethylated Forward | GGGAGTTTTGTGGATGTGAT | |
| Unmethylated Reverse | ACATCAAAACACACCCCA | Liu et al., 2012 | |
| Methylated Forward | GGGAGTTTCGCGGACGTGAC | ||
| Methylated Reverse | ACGTCGAAACACGCCCCG |
The thermal cycling conditions used for the two genes were as follows: For RB, 1 cycle at 95°C for 5 min, followed by 39 cycles of 95°C for 45 sec, 63°C for 60 sec and 72°C for 60 sec, with a final extension cycle of 72°C for 10 min. For RASSF1A, 1 cycle at 95°C for 5 min, followed by 39 cycles of 95°C for 30 sec, 58°C for 45 sec and 72°C for 45 sec, with a final extension cycle of 72°C for 5 min. MSP products for methylated and unmethylated promoters were separated on 2% agarose gels after being stained with ethidium bromide.
Statistical analysis
T-test was used in the present study to identify whether the differences between treated and untreated cell counts significant. Comparisons with p-values less than 0.05 were considered significant.
Results
Cell count
In the present study, the role of metformin as an anticancer agent was evaluated. Colon and breast cancer cells were treated with elevated doses of metformin for 48 h before being harvested. Cell count was performed using the trypan blue assay, and the obtained results indicated that, for breast cancer cells, the higher the concentration of metformin the lower count of viable cells (Figure 1). Metformin induced the apoptotic machinery (Saber et al., 2016), as it demonstrated an anti-proliferative activity in MCF-7 cells that was both time- and concentration-dependent (Queiroz et al., 2014). Metformin inhibited 70%, of MCF-7 cell viability at a final concentration of 25 mM (Queiroz et al., 2014; Lee et al., 2014). The present study revealed 35, 50, 91.6, 91.6, and 93.3% inhibition of the viability of cells for the concentrations 5, 10, 20, 50, and 100 mM metformin, respectively. These results were in accordance with several researches (Zhuang and Miskimins, 2011; Ganjali and Ganjali, 2013; Ariaans et al., 2017), where the metformin was found to inhibit CRC, breast, hepatic cancer cells growth.
Figure 1.
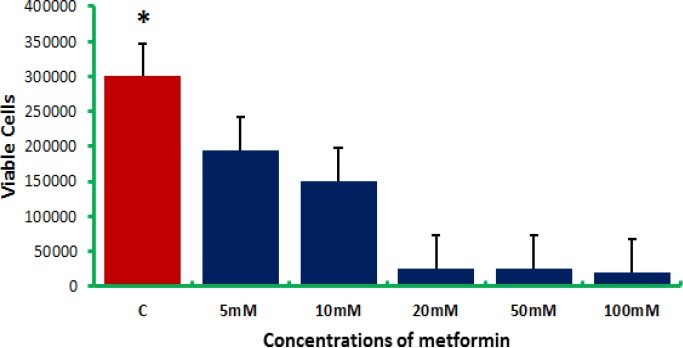
Breast Cancer Cell (MCF-7) Counts after being Treated with Elevated Doses of Metformin
For colon cancer cells, metformin also induced cell death as indicated by trypan blue assay (Figure 2). In CaCo-2 colon cancer cells, the most effective metformin dose was, surprisingly, 5 mM, as it resulted in 95% inhibition of the cell viability compared to control. Metformin is a potent growth inhibitor (Ma et al., 2011), as it was indicated that 10 mM of metformin have resulted in 80% reduction in HCT-116 colorectal cancer cells (du Potet et al., 2009). Our results showed that the reduction in the cell viability was dose-dependent up to a final concentration of 20 mM. The same profile was reported by Kim et al., (2018), who indicated that 5 mM, 10 mM, and 20 mM of metformin have resulted in 78.3%, 63.5%, and 41.9% reduction in colon cancer cell viability, respectively. For that, metformin could be a useful adjuvant agent, with the greatest benefits seen in colorectal cancer (Coyle et al., 2016).
Figure 2.
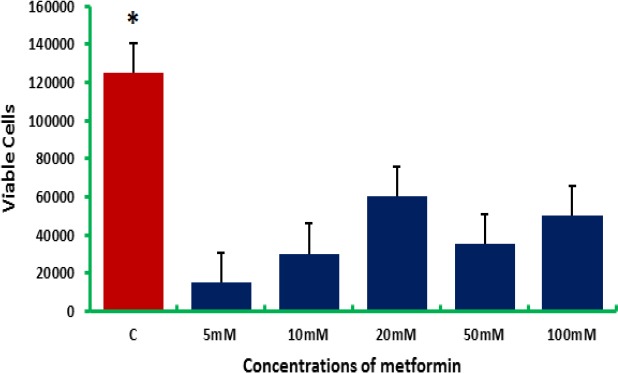
Colon Cancer Cell (CaCo-2) Counts after being Treated with Elevated Doses of Metformin
Discussion
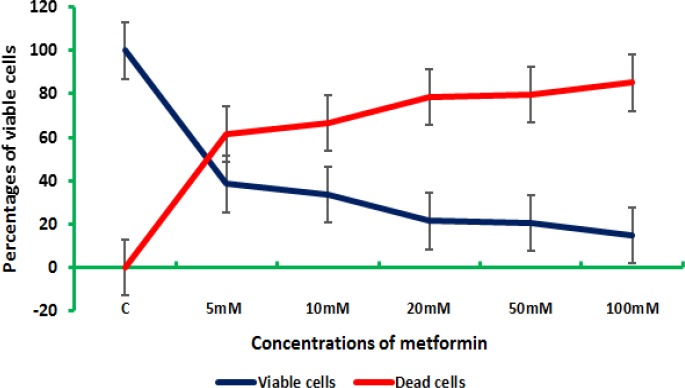
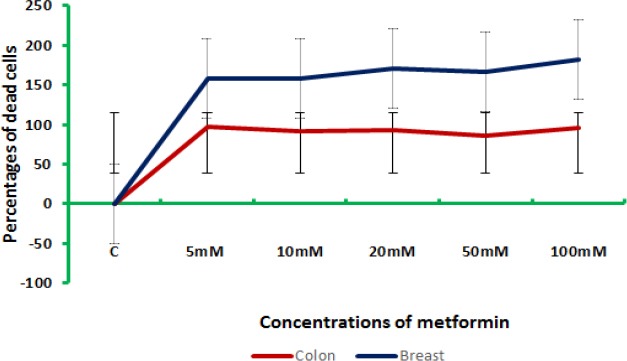
Meanwhile, different concentrations of metformin had different effects on the viability of both colon and breast cancer cells. Figure (3) represents the effect of the elevated concentrations of metformin on colon and breast cells in vitro.
Figure 3.
Colon Cancer Cell (CaCo-2) Counts after being Treated with Elevated Doses of Metformin
For the metformin concentrations 5 and 10 mM, the highest mortality was reported in colon cancer cells, while in 20, 50, and 100 mM, the highest mortality was reported in breast cancer cells. This might indicate that colon cancer cells were more sensitive to the lower concentrations of metformin compared to breast cancer cells. Metformin benefits are a controversial issue, as some researches indicated that there are no sufficient data available to conduct analyses on the impact of metformin dose and duration (Coyle et al., 2016), while others reported its potential effect on breast cancer in vitro (Hadad et al., 2011; Niraula et al., 2012) and in vivo (Soffer et al., 2014) and in colorectal cancer (Hosono et al., 2010).
Cell viability assay
The viability of both colon and breast cancer cells were assessed using MTT assay as a sensitive and accurate way to assess cell viability in vitro (Hundie et al., 2016).
For breast cancer cells (Figure 4), the elevated doses of metformin have resulted in a significant (P>0.005) reduction in the cell viability. The most effective dose of metformin was 100 mM as it yielded an 85.1% reduction in the cell viability compared to control (calculated as 100% viability). Several studies indicated the effectiveness of metformin in reducing the overall cell survival by increasing reactive oxygen species, which induce DNA damage and apoptosis (Marinello et al., 2016), or by inducing apoptosis in a concentration- and time- dependent manner via decreasing the ATP production (Gao et al., 2016).
Figure 4.
The Overall Cell Viability of Breast Cancer Cells (MCF-7) after being Treated with Elevated Doses of Metformin as Assessed by MTT Assay
For colon cancer cells, results indicated that 5 mM of metformin resulted in the highest cell mortality (96.91%) followed by the concentration 100 mM (96.24%) (Figure 5). This might indicate that colon cancer cells were sensitive even to lower doses of metformin. It was indicated elsewhere (Safari et al., 2015; Mogavero et al., 2017) that lower concentrations of metformin i.e., 5 and 10 mM were capable to inhibit the cell growth of colorectal cancer cells in vitro.
Figure 5.
The Overall Cell Viability of Colon Cancer Cells (CaCo-2) after being Treated with Elevated Doses of Metformin as Assessed by MTT Assay
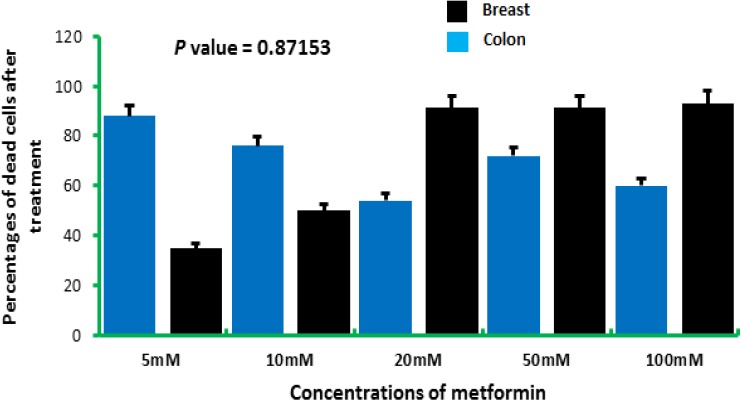
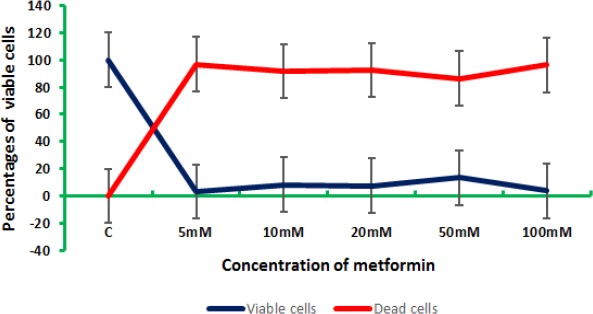
However, colon and breast cells were responding differently to the elevated concentrations of metformin. For the concentration 5 mM, colon cancers cells were severely affected with a rate of reduction of cell viability reached 96.915, while breast cancer cells exhibited a 61.53% reduction of the viability of cells for the same concentration. The concentrations 10, 20, 50, and 100 mM yielded 91.72, 92.55, 86.46, and 96.24%, respectively for colon cells, while breast cells gave 66.84, 78.57, 79.67, and 85.16% for 10, 20, 50, and 100 mM, respectively (Figure 6).
Figure 6.
The Percentages of Dead and Viable Colon and Breast Cells after being Treated with Elevated Doses of Metformin
Methylation detection via MSP
Promoter methylation is an important regulator of gene transcription, and its role in carcinogenesis has been a topic of considerable interest in the last few years (Ohtani-Fujita et al., 1997; Wajed et al., 2001; Das and Singal, 2004; Mamrut et al., 2013). In the present investigation, bisulfite-treated DNA was subjected to methylation-specific PCR to amplify two tumor-suppressor genes; RASSF1A and RB. Results indicated that the methylation patterns of both cancer cells under investigation (MCF- and CaCo-2) exposed to metformin were reshaped (Figure 7).
Figure 7.
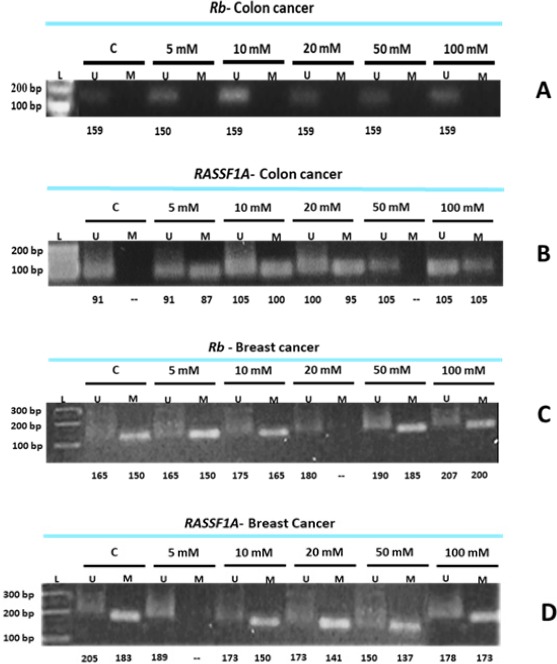
Methylation Detection of RASSF1A and RB via MSP. A, Colon cancer cells (CaCo-2) treated with elevated doses of MET and subjected to MSP to detect RB prompter methylation; B, Colon cancer cells (CaCo-2) treated with elevated doses of MET and subjected to MSP to detect RASSF1A prompter methylation; C, Breast cancer cells (MCF-7) treated with elevated doses of MET and subjected to MSP to detect RB prompter methylation; D, Breast cancer cells (MCF-7) treated with elevated doses of MET and subjected to MSP to detect RASSF1A prompter methylation.
Metformin had no effect on the methylation status of RB promotor region in colon cancer cells (Figure 7A), since the loss or inactivation of the RB gene is infrequent in colorectal carcinomas, and the reduced RB expression in these cells is probably due to a cellular regulatory mechanism (Ali et al., 1993). It seems that the function of RB gene in colorectal carcinoma is often preserved (Collard et al., 2012), and this might explain the insensitivity of RB to metformin as an anti-cancer agent. RB methylated primers generated no bands in the colon control cells, and this might indicate that this gene is normally unmethylated in this type of cancer.
The methylation of RASSF1A promoter is frequent in colorectal cancer, although it appears significantly more frequently methylated in metastasis than in the organ itself (Schirosi et al., 2016), and in cancer tissues than in benign, adjacent, and normal tissues (Wang et al., 2014). It seems that the methylation pattern was more editable after treating colon cancer cells with metformin as indicated in the present study and in other studies (Fernandes et al., 2013). However, some studies on CRCs found no RASSF1A promoter methylation (van Engeland et al., 2002).
Breast cancer cells have different methylation profiles in RB and RASSF1A genes after been treated with metformin (Figure 7B). Aberrant methylation is a common feature across many types of cancers, and these hallmarks are shared by almost all solid tumors, but some epigenetic marks most often found in distinct types of tumors, e.g., RB in retinoblastoma (Sakai et al., 1991). RB promoter region appeared to be partially unmethylated in the control cells, while the methylated primers were able to generate a defined band (Figure 7C) although with variable molecular sizes. It is believed that RB promoter methylation is crucial, and seems to facilitate, via multiple mechanisms, the tumorigenesis process (Ertel et al., 2010; Witkiewicz and Knudsen, 2014). However, the unmethylated primers were also able to generate a less intense band, and this might indicate that the promoter region of this gene was partially methylated.
The same profile was obtained in the case of RASSF1A (Figure 7D). Meanwhile, several studies have indicated the correlation between RASSF1A hypermethylation and breast tumorigenesis (Dammann et al., 2001; Jezkova et al., 2017) and tumor progression (Geng and Wu, 2016). Others indicated that RASSF1A could serve as a potential prognostic biomarker (Kioulafa et al., 2009; Xu et al., 2012).
In conclusion the role of metformin (MET) as an anticancer agent was evaluated. Breast cancer cells (MCF-7) and colon cancer cells (CaCo-2) was challenged with different doses of MET i.e., 5, 10, 20, 50, and 100 mM. Trypan blue assay revealed a significant decrease in the cell count of both colon and breast cancer cells. The same was indicated by the cytotoxicity assay (MTT), as it revealed a decrease the cell viability after treating the cell lines with elevated doses of MET. Promoter hypermethylation was assessed in both RASSF1A and RB as two tumor-related genes. RASSF1A was shown to be involved in the apoptosis process, as a level of hypermethylation was detected in colon cancer cells. RB was found to be less responding to the treatment, although the colon cancer cell count decreased upon treatment, but this might be attributed to another mechanism of cell death apart from RB-mediated one. In breast cancer cells, both RB and RASSF1A were proved to be involved in inducing cell death via hypermethylation of these genes, which might be correlated with apoptosis.
Funding
No fund was received for this project, and the study was self-funded.
Author participation
HS: put the idea and write the manuscript
SEA: conducted the practical experiments
OAMS: revised the manuscript and statistics
MAM: participated in the revision of the manuscript
ME: took the final revision of the manuscript
Conflict of interests
The authors declare no conflict of interests.
References
- 1.Ali AA, Marcus JN, Harvey JP, et al. RB1 protein in normal and malignant human colorectal tissue and colon cancer cell lines. FASEB J. 1993;7:931–7. doi: 10.1096/fasebj.7.10.8344490. [DOI] [PubMed] [Google Scholar]
- 2.Al-Saad S, Al-Shinnawi H, Shamsi NM. Risk factors of breast Cancer in Bahrain. Bahrain Med Bull. 2007;31:1–11. [Google Scholar]
- 3.Ariaans G, Jalving M, de Vries EGE, de Jong S. Anti-tumor effects of everolimus and metformin are complementary and glucose-dependent in breast cancer cells. BMC Cancer. 2017;17:232. doi: 10.1186/s12885-017-3230-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bruno S, Ledda B, Tenca C. Metformin inhibits cell cycle progression of Bcell chronic lymphocytic leukemia cells. Oncotarget. 2015;6:22624e22640. doi: 10.18632/oncotarget.4168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cancello G, Maisonneuve P, Rotmensz N, et al. Prognosis and adjuvant treatment effects in selected breast cancer subtypes of very young women (<35 Years) with operable breast cancer. Ann Oncol. 2010;21:1974–81. doi: 10.1093/annonc/mdq072. [DOI] [PubMed] [Google Scholar]
- 6.Castillo-Quan JI, Blackwell K. Metformin: Restraining nucleocytoplasmic shuttling to fight cancer and aging. Cell. 2016;167:1670–1. doi: 10.1016/j.cell.2016.11.058. [DOI] [PubMed] [Google Scholar]
- 7.Chajès V, Romieu I. Nutrition and breast cancer. Maturitas. 2014;77:7–11. doi: 10.1016/j.maturitas.2013.10.004. [DOI] [PubMed] [Google Scholar]
- 8.Collard TJ, Urban BC, Patsos HA, et al. The retinoblastoma protein (RB) as an anti-apoptotic factor: expression of RB is required for the antiapoptotic function of BAG-1 protein in colorectal tumour cells. Cell Death Dis. 2012;3:e408. doi: 10.1038/cddis.2012.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coyle C, Cafferty FH, Vale C, Langley RE. Metformin as an adjuvant treatment for cancer: a systematic review and meta-analysis. Ann Oncol. 2016;27:2184–5. doi: 10.1093/annonc/mdw410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cuyasa E, Salvador F, Jorge J, Javier AM. Metformin targets histone acetylation in cancer-prone epithelial cells. Cell Cycle. 2016;24:3355–1. doi: 10.1080/15384101.2016.1249547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dammann R, Yang G, Pfeifer GP. Hypermethylation of the CpG island of Ras association domain family 1A (RASSF1A), a putative tumor suppressor gene from the 3p21.3 locus, occurs in a large percentage of human breast cancers. Cancer Res. 2001;61:3105–9. [PubMed] [Google Scholar]
- 12.Das PM, Singal R. DNA Methylation and Cancer. J Clin Oncol. 2004;22:4632–2. doi: 10.1200/JCO.2004.07.151. [DOI] [PubMed] [Google Scholar]
- 13.Denizlt F, Lang R. Rapid colorimetric assay for cell growth and survival. Modification to the tetrazolium dye procedure giving improved sensitivity and reliability. J Immunol Methods. 1986;89:271–7. doi: 10.1016/0022-1759(86)90368-6. [DOI] [PubMed] [Google Scholar]
- 14.Donnelly TT, Al-Khater A, Al-Bader SB, Al-Ku MG. Arab women's breast cancer screening practices: A literature review. Asian Pac J Cancer Prev. 2013;14:4519–8. doi: 10.7314/apjcp.2013.14.8.4519. [DOI] [PubMed] [Google Scholar]
- 15.du Potet E, Germain C, Harbottle R, et al. Oxaliplatin chemoresistant CaCO-2 cells do not exhibit primitive cancer stem cell-like characteristics. Proc Am Assoc Cancer Res. 2009;10:18–22. [Google Scholar]
- 16.El Saghir NS, Mazen KK, Toufic E, et al. Trends in epidemiology and management of breast cancer in developing Arab countries: A literature and registry analysis. Int J Sur. 2007;5:225–3. doi: 10.1016/j.ijsu.2006.06.015. [DOI] [PubMed] [Google Scholar]
- 17.Ertel A, Dean JL, Rui H, et al. RB-pathway disruption in breast cancer: Differential association with disease subtypes, disease-specific prognosis and therapeutic response. Cell Cycle. 2010;9:4153–3. doi: 10.4161/cc.9.20.13454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Esteller M. Cancer epigenomics: DNA methylomes and histone-modification maps. Nat Rev Genet. 2007;8:286–8. doi: 10.1038/nrg2005. [DOI] [PubMed] [Google Scholar]
- 19.Fernandes MS, Carneiro F, Oliveira C, Seruca R. Colorectal cancer and RASSF family-A special emphasis on RASSF1A. Int J Cancer. 2013;132:251–8. doi: 10.1002/ijc.27696. [DOI] [PubMed] [Google Scholar]
- 20.Gadducci A, Biglia N, Tana R, Cosio S, Gallo M. Metformin use and gynecological cancers: A novel treatment option emerging from drug repositioning. Crit Rev Oncol Hematol. 2016;105:73–83. doi: 10.1016/j.critrevonc.2016.06.006. [DOI] [PubMed] [Google Scholar]
- 21.Ganjali M, Ganjali H. Anticancer effect of metformin, an anti- diabetic drug, on breast cancer cells. J Novel Appl Sci. 2013;2:796–1. [Google Scholar]
- 22.Gao ZY, Liu Z, Bi MH, et al. Metformin induces apoptosis via a mitochondria-mediated pathway in human breast cancer cells in vitro. Exp Ther Med. 2016;11:1700–6. doi: 10.3892/etm.2016.3143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Garciaa C, Aaron Y, Fabian C, Rajesh B, Leigh AC. A SEER-Medicare analysis of the impact of metformin on overall survival in ovarian cancer. Gynecol Oncol. 2017;146:346. doi: 10.1016/j.ygyno.2017.05.006. [DOI] [PubMed] [Google Scholar]
- 24.Geng H, Wu L. The application of RASSF1A promoter methylation as a biomarker in breast cancer: a comprehensive literature review. Int J Clin Exp Med. 2016;9:7729–2. [Google Scholar]
- 25.Gnerlich JL, Deshpande AD, Jeffe DB, et al. Elevated breast cancer mortality in women younger than age 40 years compared with older women is attributed to poorer survival in early-stage disease. J Am Coll Surg. 2009;208:341–7. doi: 10.1016/j.jamcollsurg.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grivicich I, Regner A, Zanoni C, et al. Hsp70 response to 5-fluorouracil treatment in human colon cancer cell lines. Int J Colorectal Dis. 2007;22:1201–8. doi: 10.1007/s00384-007-0307-x. [DOI] [PubMed] [Google Scholar]
- 27.Hadad S, Iwamoto T, Jordan L, et al. Evidence for biological effects of metformin in operable breast cancer: a pre-operative, window-of-opportunity, randomized trial. Breast Cancer Res Treat. 2011;128:783–4. doi: 10.1007/s10549-011-1612-1. [DOI] [PubMed] [Google Scholar]
- 28.Haggar FA, Boushey RP. Colorectal cancer epidemiology: incidence, mortality, survival, and risk factors. Clin Colon Rectal Surg. 2009;22:191–7. doi: 10.1055/s-0029-1242458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hea F, Chenb J. Consumption of soybean, soy foods, soy isoflavones and breast cancerincidence: Differences between Chinese women and women in Westerncountries and possible mechanisms. Food Sci Hum Wellness. 2013;2:146–61. [Google Scholar]
- 30.Hosono K, Endo H, Takahashi H, et al. Metformin suppresses colorectal aberrant crypt foci in a short-term clinical trial. Cancer Prev Res (Phila) 2010;3:1077–3. doi: 10.1158/1940-6207.CAPR-10-0186. [DOI] [PubMed] [Google Scholar]
- 31.Hundal RS, Krssak M, Dufour S, et al. Mechanism by which metformin reduces glucose production in type 2 diabetes. Diabetes. 2000;49:2063–9. doi: 10.2337/diabetes.49.12.2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hundie GB, Woldemeskel D, Gessesse A. Evaluation of direct colorimetric MTT assay for rapid detection of rifampicin and isoniazid resistance in mycobacterium tuberculosis. PLoS One. 2017;12:e0171964. doi: 10.1371/journal.pone.0171964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jezkova E, Zubor P, Kajo K, et al. Impact of RASSF1A gene methylation on the metastatic axillary nodal status in breast cancer patients. Oncol Lett. 2017;14:758–6. doi: 10.3892/ol.2017.6204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Karim SAM, Ghalib HHA, Sangar MA, Fattah FHR. The incidence, age at diagnosis of breast cancer in the Iraqi Kurdish population and comparison to some other countries of Middle-East and West. Int J Surg. 2015;13:71–5. doi: 10.1016/j.ijsu.2014.11.029. [DOI] [PubMed] [Google Scholar]
- 35.Kheirelseid EH, Boggs JM, Curran C, et al. Younger age as a prognostic indicator in breast cancer: a cohort study. BMC Cancer. 2011;11:383. doi: 10.1186/1471-2407-11-383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kim C, Paik S. Gene-expression-based prognostic assays for breast cancer. Nat Rev Clin Oncol. 2010;7:340–7. doi: 10.1038/nrclinonc.2010.61. [DOI] [PubMed] [Google Scholar]
- 37.Kim JH, Lee KJ, Seo Y, et al. Effects of metformin on colorectal cancer stem cells depend on alterations in glutamine metabolism. Sci Rep. 2018;8:409. doi: 10.1038/s41598-017-18762-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kioulafa M, Kaklamanis L, Mavroudis D, Georgoulias V, Lianidou ES. Prognostic significance of RASSF1A promoter methylation in operable breast cancer. Clin Biochem. 2009;42:970–5. doi: 10.1016/j.clinbiochem.2009.04.003. [DOI] [PubMed] [Google Scholar]
- 39.Kirpichnikov D, McFarlane SI, Sowers JR. Metformin: an update. Ann Intern Med. 2002;137:25–3. doi: 10.7326/0003-4819-137-1-200207020-00009. [DOI] [PubMed] [Google Scholar]
- 40.Lea MA, Chacko J, Bolikal S, et al. Addition of 2-deoxyglucose enhances growth inhibition but reverses acidification in colon cancer cells treated with phenformin. Anticancer Res. 2011;3(1):42–6. [PubMed] [Google Scholar]
- 41.Lee CP, Irwanto A, Salim A, Yuan JM, Liu J, et al. Breast cancer risk assessment using genetic variants and risk factors in a Singapore Chinese population. Breast Cancer Res. 2014;16:R64. doi: 10.1186/bcr3678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li Y, Zou L, Li Q, et al. Amplification of LAPTM4B and YWHAZ contributes to chemotherapy resistance and recurrence of breast cancer. Nat Med. 2010;16:214–8. doi: 10.1038/nm.2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liang X, Peiyan K, Jin W, et al. Effects of metformin on proliferation and apoptosis of human megakaryoblastic Dami and MEG-01 cells. J Pharmacol Sci. 2017;135:14e21. doi: 10.1016/j.jphs.2017.08.003. [DOI] [PubMed] [Google Scholar]
- 44.Liu X, Tang X, Zhang S, et al. Methylation and expression of retinoblastoma and transforming growth factor-ß1 genes in Epstein-Barr virus-associated and -Negative gastric carcinomas. Gastroenterol Res Pract. 2012;2012:906017. doi: 10.1155/2012/906017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mackay J, Jemal A, Lee NC, Parkin DM. The Cancer Atlas. Atlanta, GA: American Cancer Society; 2006. [Google Scholar]
- 46.Mamrut S, Harony H, Sood R, et al. DNA methylation of specific CpG sites in the promoter region regulates the transcription of the mouse oxytocin receptor. PLoS One. 2013;8:e56869. doi: 10.1371/journal.pone.0056869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Marinello PC, da Silva TN, Panis C, et al. Mechanism of metformin action in MCF-7 and MDA-MB-231 human breast cancer cells involves oxidative stress generation, DNA damage, and transforming growth factor β1 induction. Tumour Biol. 2016;37:5337–6. doi: 10.1007/s13277-015-4395-x. [DOI] [PubMed] [Google Scholar]
- 48.Matthaios D, Balgkouranidou I, Karayiannakis A, et al. Methylation status of the APC and RASSF1A promoter in cell-free circulating DNA and its prognostic role in patients with colorectal cancer. Oncol Lett. 2016;12:748–6. doi: 10.3892/ol.2016.4649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Miller AB. Screening for breast cancer in the Eastern Mediterranean Region. East Mediterr Health J. 2010;16:1022–4. [PubMed] [Google Scholar]
- 50.Mogavero A, Maiorana MV, Zanutto S, et al. Metformin transiently inhibits colorectal cancer cell proliferation as a result of either AMPK activation or increased ROS production. Sci Rep. 2017;7:15992. doi: 10.1038/s41598-017-16149-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Niraula S, Dowling RJO, Ennis M, et al. Metformin in early breast cancer: a prospective window of opportunity neoadjuvant study. Breast Cancer Res Treat. 2012;135:821. doi: 10.1007/s10549-012-2223-1. [DOI] [PubMed] [Google Scholar]
- 52.Ohtani-Fujita N, Dryja TP, Rapaport JM, et al. Hypermethylation in the retinoblastoma gene is associated with unilateral, sporadic retinoblastoma. Cancer Genet Cytogenet. 1997;98:43–9. doi: 10.1016/s0165-4608(96)00395-0. [DOI] [PubMed] [Google Scholar]
- 53.Park SY, Kwon HJ, Lee HE, et al. Promoter CpG island hypermethylation during breast cancer progression. Virchows Arch. 2011;458:73–4. doi: 10.1007/s00428-010-1013-6. [DOI] [PubMed] [Google Scholar]
- 54.Pryor R, Cabreiro F. Repurposing metformin: an old drug with new tricks in its binding pockets. Biochem J. 2015;471:307–2. doi: 10.1042/BJ20150497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Queiroz EAIF, Puukila S, Eichler R, et al. Metformin induces apoptosis and cell cycle arrest mediated by oxidative stress, AMPK and FOXO3a in MCF-7 breast cancer cells. PLoS One. 2014;9:e98207. doi: 10.1371/journal.pone.0098207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rivera-Franco MM, Leon-Rodriguez E. Delays in breast cancer detection and treatment in developing countries. Breast Cancer (Auckl) 2018;12:1–5. doi: 10.1177/1178223417752677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rodriguez-Lirio A, Perez-Yarza G, Fernandez-Suarez MR, et al. Metformin induces cell cycle arrest and apoptosis in drug-resistant leukemia cells. Leuk Res Treat. 2015:516460. doi: 10.1155/2015/516460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Saber MM, Galal MA, Ain-Shoka AA, Shouman SA. Combination of metformin and 5-aminosalicylic acid cooperates to decrease proliferation and induce apoptosis in colorectal cancer cell lines. BMC Cancer. 2016;16:126. doi: 10.1186/s12885-016-2157-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Safari Z, Safaralizadeh R, Seyedzadeh MH, et al. The induction of Metformin inhibitory effects on tumor cell growth in Hypoxic condition. Iran J Allergy Asthma Immunol. 2015;14:605–4. [PubMed] [Google Scholar]
- 60.Saggu S, Rehman H, Abbas ZK, Ansari AA. Recent incidence and descriptive epidemiological survey of breast cancer in Saudi Arabia. Saudi Med J. 2015;36:1176. doi: 10.15537/smj.2015.10.12268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sakai T, Toguchida J, Ohtani N, et al. Allele-specific hypermethylation of the retinoblastoma tumor-suppressor gene. Am J Hum Genet. 1991;48:880–8. [PMC free article] [PubMed] [Google Scholar]
- 62.Schirosi L, Mazzotta A, Opinto G, et al. β-catenin interaction with NHERF1 and RASSF1A methylation in metastatic colorectal cancer patients. Oncotarget. 2016;7:67841. doi: 10.18632/oncotarget.12280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Soffer D, Shi J, Chung J, et al. Metformin and breast and gynecological cancer risk among women with diabetes. BMJ Open Diabetes Res Care. 2014;3:e000049. doi: 10.1136/bmjdrc-2014-000049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Szyf M, Pakneshan P, Rabbani SA. DNA methylation and breast cancer. Biochem Pharmacol. 2004;68:1187–7. doi: 10.1016/j.bcp.2004.04.030. [DOI] [PubMed] [Google Scholar]
- 65.Tarabeia J, Baron-Epel O, Barchana M, et al. A comparison of trends in incidence and mortality rates of breast cancer, incidence to mortality ratio and stage at diagnosis between Arab and Jewish women in Israel 1979–2002. Eur J Cancer Prev. 2007;16:36–2. doi: 10.1097/01.cej.0000228407.91223.85. [DOI] [PubMed] [Google Scholar]
- 66.van Engeland M, Roemen GM, Brink M, et al. K-ras mutations and RASSF1A promoter methylation in colorectal cancer. Oncogene. 2002;21:3792–5. doi: 10.1038/sj.onc.1205466. [DOI] [PubMed] [Google Scholar]
- 67.Wajed SA, Laird PW, DeMeester TR. DNA Methylation: an alternative pathway to cancer. Ann Surg. 2001;234:10–2. doi: 10.1097/00000658-200107000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wang H, Liu P, Zhou P, Zhang Y. Retracted: Promoter Methylation of the RASSF1A gene may contribute to colorectal cancer susceptibility: A meta-analysis of cohort studies. Ann Hum Genet. 2014;3:208–6. doi: 10.1111/ahg.12059. [DOI] [PubMed] [Google Scholar]
- 69.Witkiewicz AK, Knudsen ES. Retinoblastoma tumor suppressor pathway in breast cancer: prognosis, precision medicine, and therapeutic interventions. Breast Cancer Res. 2014;16:207. doi: 10.1186/bcr3652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wu L, Zhou B, Oshiro-Rapley N, et al. An ancient, unified mechanism for Metformin growth inhibition in Celegans and Cancer. Cell. 2016;167:1705–8. doi: 10.1016/j.cell.2016.11.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Xu J, Shetty PB, Feng W, et al. Methylation of HIN-1 RASSF1A RIL and CDH13 in breast cancer is associated with clinical characteristics, but only RASSF1A methylation is associated with outcome. BMC Cancer. 2012;12:243. doi: 10.1186/1471-2407-12-243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhong T, Men Y, Lu L, et al. Metformin alters DNA methylation genome-wide via the H19/SAHH axis. Oncogene. 2017;36:2345–4. doi: 10.1038/onc.2016.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhuang Y, Miskimins WK. Metformin induces both caspase-dependent and Poly(ADP-ribose) Polymerase-dependent cell death in breast cancer cells. Mol Cancer Res. 2011;9:603–5. doi: 10.1158/1541-7786.MCR-10-0343. [DOI] [PMC free article] [PubMed] [Google Scholar]


