Abstract
Objectives:
In the present study, we aimed to identify the anti-proliferative potential of [Cu(L)(2imi)] complex [L = 2-(((5-chloro-2-oxyphenyl)imino)methyl)phenolato) and 2imi = 2-methyl imidazole] against HepG2 cells as an in vitro model of human hepatocellular carcinoma and normal mouse fibroblast L929 cells.
Methods:
The cytotoxic and apoptotic effects of [Cu(L)(2imi)] complex on HepG2 cells and normal fibroblasts (L929) were examined by MTT assay and flow cytometry, respectively.
Results:
Cytotoxicity induced by [Cu(L)(2imi)] complex was time dependent. Also, there was a positive correlation between cytotoxicity and an increase in Cu complex concentration. For HepG2 cells, the cell viability percentage was 50% at 58 μg/mL after 24 h treatment, whereas in the same concentration and conditions, the viability percentage was surprisingly higher (about 100%) for L929 cells. Also, after 48 h treatment, the viability percentage of HepG2 cells at 55 μg/mL concentration was 50% in contrast with 89.3% for L929 cells in the same conditions. Flow cytometry findings suggest that [Cu(L)(2imi)] complex is capable of decreasing cancer cell viability through apoptosis and did not efficiently activate the necrosis process.
Conclusions:
Finally, we found that [Cu(L)(2imi)] complex possess the potential for development as an anti-cancer drug for human hepatocellular carcinoma.
Keywords: Apoptosis, [Cu(L)(2imi)] complex, cytotoxicity, hepatocellular carcinoma, mouse fibroblast L929 cells
Introduction
Cancer is one of the most deadly diseases (Chen and Hu, 2009) and is a major health problem of global concern that afflicts a significant proportion of the world’s population in all generations (Atawodi, 2011). Reports of the American Cancer Society show that Deaths due to cancer and new cancer cases will increase to approximately 13.2 and 21.4 million patients by 2030, respectively (Mi et al., 2013). Liver cancer is the seventh most common cancer in women and the fifth most common cancer in men worldwide (El-Serag, 2012; Hosseini et al., 2017).
Primary liver cancer, especially hepatocellular carcinoma (HCC) is one of the most common and deadly cancers in the world (El–Serag and Rudolph, 2007), and many efforts have been made to treat the disease (Abid-Essefi et al., 2003; Franke et al., 2003). The apoptosis induction is an effective way to kill cancer cells (Karimabad et al., 2017; Ramezani et al., 2017; Sheikhrezaei et al., 2018)
It has been shown in many reports that metal complexes are used as anticancer agents in many drugs (Fricker, 1994). Presumably, the most well-known of these drugs is cisplatin [cis-diamminedichloroplatinum(II)] (Marzano et al., 2002), although cisplatin is applied as an anti-cancer drug to treat many types of cancer. However, severe side effects and resistance induced by long-term treatment with this drug has attracted attention to the development of alternative drugs with the increase in morbidity (Kim et al., 2011).
Several therapeutic approaches have been introduced to treatment of HCC including, chemotherapy, radiotherapy and immunotherapy. Chemotherapy is a famous approach which is used for treatment of several cancers including HCC world-widely. However, the current drugs which are applied for chemotherapy are associated with several side effects which are derived from their effects on the non-cancerous normal cells. Therefore, investigators are trying to find new therapeutic strategies for cancer treatment with the lowest side effects (Zainodini et al., 2018; Bagrezaei et al., 2018). Based on the fact that HCC is a prevalent cancer word-wild, hence, several studies are designed to introduce new chemotherapy strategies to overcome the disease. The use of metallic complexes as anticancer drugs attracted many attentions of researchers in the field of pharmaceutical chemistry (van Rijt and Sadler, 2009; Barry and Sadler, 2013; Santini et al., 2013; Munteanu and Suntharalingam, 2015). Copper-based complexes are one of these compounds that have shown promising anticancer activities (Santini et al., 2013; Mohammadizadeh et al., 2018). Copper is an essential element involved in critical biological functions such as energy metabolism, oxygen transport, enzyme activity, and cell signaling. Moreover, this metal is a necessary cofactor for the tumor angiogenesis (Brem, 1999; Brewer, 2001; Theophanides and Anastassopoulou, 2002; Tisato et al., 2010).
The main aim of this study was to evaluate the anti-cancerous effects of a Cu(II) complex [Cu(L)(2imi)] derived from 2-(((5-chloro-2-oxyphenyl)imino) methyl) phenolato (L) and 2-methylimidazole (2imi) on the HepG2 cell line. On the other hand, due to the various side effects of chemotherapy on the normal cells, another aim of this study was to explore the effects of the [Cu(L)(2imi)] complex on the survival and apoptosis of mouse fibroblast L929 cells, as normal cells. The in vitro anti-cancer activity of [Cu(L)(2imi)] complex was evaluated by MTT (3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide) assay and apoptosis was studied using flow cytometry.
Materials and Methods
Materials and instrumentation
MTT (3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide) and dimethyl sulfoxide (DMSO) were prepared from Roche (Mannheim, Germany). Fetal bovine serum (FBS), RPMI-1640, trypsin enzyme and penicillin–streptomycin were purchased from Gibco-BRL (Grand Island, NY, USA). Annexin V-fluorescein isothiocyanate (FITC) apoptosis detection kit was purchased from Ebioscience (San Diego, CA, USA).
All reagents and solvents for synthesis and analysis were commercially available and purchased from Merck or Sigma and used as received without further purifications. Elemental analyses were performed on a Thermo Finnigan Flash Elemental Analyzer 1112EA. Melting points were measured on an Electrothermal-9100 apparatus and uncorrected. FT-IR spectra were recorded on an FT-IR Tensor 27 infrared spectrophotometer as KBr discs in the range of 400-4,000 cm-1.
Synthesis of 6-(((5-chloro-2-hydroxyphenyl)amino)methylene)cyclohexa-2,4-dien-1-one [H2L]
[H2L] was prepared according to our previous report (Takjoo et al., 2014). Briefly, 5 mL ethanol containing 0.3 g (2 mmol) of 2-amino-4-chlorophenol was added to an ethanolic solution (5 mL) of 2-hydroxy-benzaldehyde (0.2 g, 2 mmol). The obtained mixture was vigorously stirred and simultaneously heated for 15 min. After cooling the mixture, an orange precipitate was observed that was separated, washed with cold ethanol and dried in a desiccator over anhydrous CaCl2. Yield:
84%. M.p.: 156 °C. Anal. Calcd for C13H10ClNO2 (247.68 g mol-1): C, 63.04; H, 4.07; N, 5.66. Found: C, 63.07; H, 4.08; N, 5.64%., FT-IR (KBr) cm-1: ν(OH) 3045, ν(N-H) 2097 ν(C=N) 1626, ν(C=Cring) 1494, ν(C–O) 1313, ν(C–Cl) 653 (Figure 1).
Figure 1.
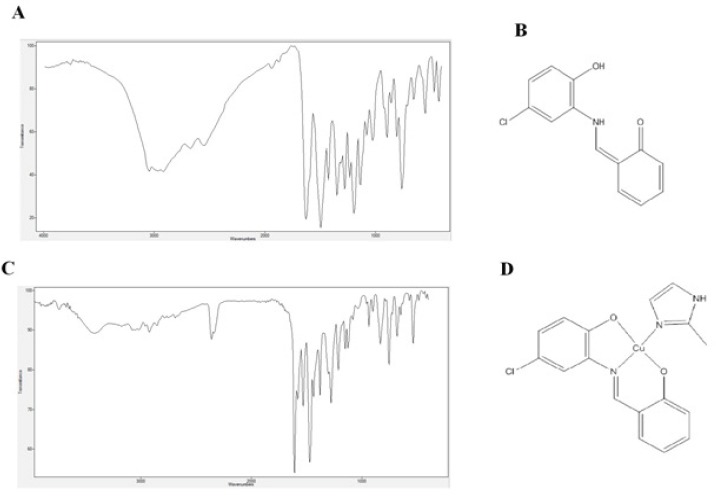
Demonstrates FTIR Spectrum of H2L (A), chemical structure of H2L (B), FTIR spectrum of [Cu(L)(2imi)] (C) and chemical structure of [Cu(L)(2imi)] (D).
Synthesis of (2-Methylimidazole)(2-(((5-chloro-2-oxyphenyl)imino)methyl)phenolato)) copper(II) [Cu(L)(2imi)]
[Cu(L)(2(imi)] was synthesized according to the procedure reported previously (Ebrahimipour et al., 2015). Briefly, a 4-mL solution of Cu(NO3)2•4H2O (0.02 g, 0.1 mmol) in methanol was added to 3 mL methanol containing H2L (0.1 mmol, 0.03 g) and NaOH (0.2 mmol, 0.01 g). The mixture was refluxed in a water for 5 min while stirred at a constant speed. After addition of 4 mL methanolic solution of 2-methyl imidazole (0.3 mmol, 0.02 g) to the reaction mixture, the reflux was continued for 2 h. Then, the solvent of the mixture was slowly evaporated, resulted in the precipitation of red crystals which were separated, dried in air at room temperature and stored in a CaCl2 desiccator. Yield: 58%. M.p.: 206°C. Molar conductance (10-3 M, DMSO) 21.0 Ω-1 cm2 mol-1. Anal. Calcd for C17H14ClCuN3O2 (391.31 g mol-1): C, 52.18; H, 3.61; N, 10.74. Found: C, 52.16; H, 3.49; N, 10.76%. FT-IR (KBr) cm-1: ν(NH) 3420, ν(C=N) 1610, ν(C=Cring) 1471, ν(C–O) 1280, ν(C–Cl) 654 (Figure 1).
Cell culture
Mouse fibroblast L929 cells and Human hepatocellular carcinoma cell line HepG2 were obtained from Pasteur Institute (Tehran, Iran). Cells were maintained in a humidified atmosphere containing 5% CO2 at 37°C in RPMI 1640 medium supplemented with streptomycin (100 µg/mL), Penicillin (100 IU/mL) and 10% fetal bovine serum (FBS). Culture medium was exchanged with fresh medium every 1–2 days, when the cells occupied 70–80% of the flask, the cells were treated with different concentrations (0.03–100 µg/mL) of copper (II) complex [Cu(L)(2imi)] for 24 and 48 hours.
Cytotoxicity evaluation
MTT assay
Both HepG2 and L929 Cells were cultured in 96-well plates in triplicate and incubated at 37 °C and 5% CO2 for 24 h. Then, graded amounts of [Cu(L)(2(imi)] complex were added to the wells in 10 μL of FBS free culture medium and the plates were incubated in a 5% CO2 humidified atmosphere for 24 h and 48 h. Six replica wells were used for controls. The [Cu(L)(2(imi)] complex were tested at concentrations ranging between 0.03 and 100 μM. After that, 0.1 mg of MTT (in 20 μL of PBS) was added to each well, and cells were incubated at 37°C for 4h. The formed formazan crystals were then dissolved in 100 μL of DMSO and the absorbance was read at 570 nm using an Enzyme-linked Immunosorbent Assay (ELISA) reader. The sensitivity of HepG2 and L929 to [Cu(L)(2imi)] complex treatment was expressed in terms of IC50 (drug concentration producing 50% inhibition of cell growth, calculated on the regression line in which absorbance values at 570 nm were plotted against the logarithm of drug concentration).
The viability of HepG2 and L929 cells were determined by trypan blue exclusion (TBE) assay and counting in a hemocytometer after 24 h and 48 h treatment with [Cu(L)(2imi)] complex at IC50, concentrations and Morphological changes on cells were examined using an inverted microscope under 400× magnification.
Apoptosis evaluation by flow cytometry
Double staining with Annexin V-FITC and PI for flow cytometry analyses was performed using Annexin V-FITC/PI apoptosis detection kit (Ebioscience, USA). HepG2 cells were incubated with concentrations 0.58 µg/mL and 113µg/mL of [Cu(L)(2imi)] complex for 24 h and 0.55 µg/mL and 113 µg/mL for 48 h. L929 cells were also treated with concentrations 105.3 µg/mL and 250 µg/mL of [Cu(L)(2imi)] complex for 24 h and 0.83 µg/mL and 200 µg/mL for 48 h.
First, 5×105 of treated and/or untreated cells were harvested after 24 h or 48 h and washed twice with sterile and cold PBS (Phosphate saline buffer), then re-suspended in the binding buffer (calcium buffer, 200 µL). Annexin V-FITC (5 µL) was added to the cells and incubated in the dark at room temperature for 10 minutes. After washing the cells with PBS, the remaining cell pellet was dissolved in 190 µL binding buffers. After addition of 10 µL propidium iodide (PI), the samples were then incubated for 5 min in the dark at 4°C and were examined under a Cyflow® space flow cytometer (Partec, Münster, Germany). Flowmax software (Partec) was used for data analysis. The measurements were performed in at least 3 repetitions.
Statistical analysis
SPSS software, version 19 was used for this analysis. Descriptive analysis (mean values and standard error) of cells viability was performed. Whole experiments were carried out for three times in each individual sample and all of obtained results were presented as the mean value of those three. Statistical analysis for compare differences among groups was performed by one-way ANOVA and Dunnett’s procedure for multiple comparisons with a single control group. P value of less than 0.05 was considered statistically significant.
Results
Effect of [Cu(L)(2imi)] complex on cell viability
The MTT method was used to evaluate the inhibitory effects of the [Cu(L)(2imi)] complex on the growth of HepG2 as in vitro model of hepatocellular carcinoma cells and mouse fibroblast L929 as normal cells. HepG2 cells were incubated with different concentrations of [Cu(L)(2imi)] complex (0.03–110 µg/mL) for 24 h and 48 h. The results demonstrated that [Cu(L)(2imi)] complex decreased the viability of HepG2 cells, in a dose- and time-dependent manner (p<0.05) (Figure 2). In the MTT assay, the statistical analysis revealed that [Cu(L)(2imi)] complex significantly inhibited the proliferation of HepG2 cells (p<0.05) (Figure 3). At higher concentrations of [Cu(L)(2imi)], the number of colony-forming cells was reduced in culture when observed under inverted microscope. HepG2 cells stick firmly to the floor of the culture flask and create an overlapping layer of cells, but after treatment with [Cu(L)(2imi)] complex, the morphology of HepG2 cells has changed dramatically. The cells were separated from the culture plates after 24 h of treatment. Also nuclear condensation, apoptotic body formation and cell shrinkage were observed under inverted microscope (Figure 4). The IC50 values obtained for HepG2 cells treated with the Cu(II) complex for 24 h and 48 h were 58 µg/mL and 55 µg/mL, respectively (p <0.05) (Figure 2 and Figure 3). Furthermore, the cytotoxicity of [Cu(L)(2imi)] complex against L929 cells was also investigated using MTT assay (Figure 2 and Figure 3). L929 cells were also incubated with different concentrations of [Cu(L)(2imi)] complex (0.03–110 µg/mL) for 24 h and 48 h (Figure 2 and Figure 3). The IC50 values of [Cu(L)(2imi)] complex against L929 cells were calculated to be 105.3 µg/mL and 83 µg/mL respectively after 24 h and 48 h treatment with the Cu complex (p <0.05) (Figure 2, Table 1).
Figure 2.
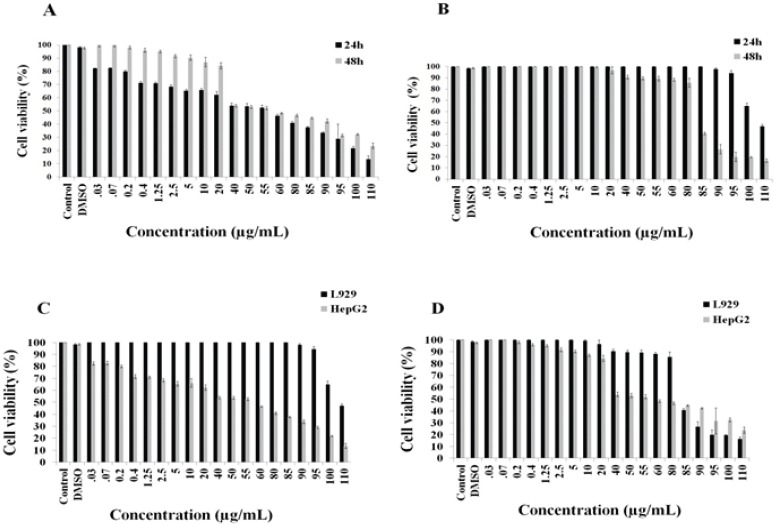
Demonstrates the Changes in Viability of HepG2 Cells Treated with Different Concentrations of [Cu(L)(2imi)] after 24 h and 48 h, Using MTT Method. All changes observed in the cell viability are significant (P < 0.05) except those observed for concentrations 0.03-1.25 µg/mL for 48 h (A). Demonstrates the changes in viability of L929 cells treated with different concentrations of [Cu(L)(2imi)] after 24 h and 48 h, using MTT method. All changes observed in the cell viability are significant (P < 0.05) except those observed for concentrations 0.03-85 µg/mL for 24 h and 0.03-20 µg/mL for 48 h (B). Comparison of the changes in viability of HepG2 with L929 cells treated in different concentrations of [Cu(L)(2imi)] after 24 h (C) Comparison of the changes in viability of HepG2 with L929 cells treated in different concentrations of [Cu(L)(2imi)] after 48 h (D).
Figure 3.

Demonstrates the Growth Inhibition of HepG2 Cells Treated with Different Concentrations of [Cu(L)(2imi)] after 24, 48 h. All changes observed in the cell inhibition are significant (P < 0.05) except those observed for concentrations 0.03-1.25 µg/mL for 48 h (A). Demonstrates the growth inhibition of L929 cells treated with different concentrations of [Cu(L)(2imi)] after 24 h and 48 h. All changes observed in the inhibition are significant (P < 0.05) except those observed for concentrations 0.03-85 µg/mL for 24 h and 0.03-20 µg/mL for 48 h (B). Demonstrates the growth inhibition of HepG2 cells in comparison with L929 cells treated to different concentrations of [Cu(L)(2imi)] after 24 h (C) and 48 h (D).
Figure 4.
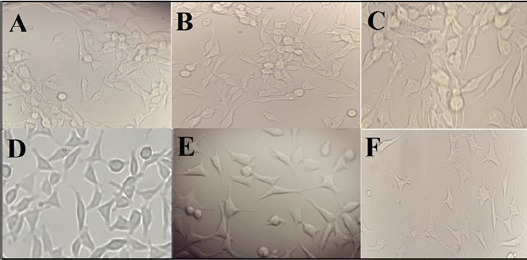
Morphological Changes of HepG2 and L929 Cells after Exposure with [Cu(L)(2imi)] Complex Un-Treated HepG2 Cells (A), HepG2 cells after treated with [Cu(L)(2imi)] complex at ½ IC-50 concentration (29 µg/mL) for 24 h (B), HepG2 cells after treated with [cu(L)(2imi)] complex at IC-50 concentration (58 µg/mL) for 24 h (C), Un-treated L929 cells (D), L929 cells after treated with [Cu(L)(2imi)] complex at ½ IC-50 concentration (52.65 µg/mL) for 24 h (E), L929 cells after treated with [Cu(L)(2imi)] complex at IC-50 concentration (105.3 µg/mL) for 24 h (F) that were observed with an inverted microscope under 400× magnification.
Table 1.
IC50 Values (µg/mL) of [Cu(L)(2imi)] Ccomplex on HepG2 and L929 Cells after 24 h and 48 h treatment
| Cell line | IC50 (µg/mL) for [Cu(L)(2imi)] complex | |
|---|---|---|
| 24 h | 48 h | |
| HepG2 | 58 | 55 |
| L929 | 105.3 | 83 |
The results showed that the [Cu(L)(2imi)] complex inhibited the growth of L929 cells in higher concentration than HepG2 cells and the growth inhibition of both L929 and HepG2 cells had a dose-and time-dependent manner (p <0.05) (Figure 3)
[Cu(L)(2imi)] complex induces apoptosis in HepG2 cells
The HepG2 cells were treated at IC50 concentration of [Cu(L)(2imi)] complex (58 µg/mL) for 24 h and (55 µg/mL) for 48 h. Then, the treated cells were stained with annexin V and propidium iodide and the apoptotic effect of [Cu(L)(2imi)] complex on HepG2 cells were detected by flow cytometry. The results of flow cytometry analysis revealed that the [Cu(L)(2imi)] complex induced apoptosis in treated HepG2 cells compared to control (un-treated cells), indicating that apoptotic cell death is involved in [Cu(L)(2imi)] complex induced toxicity (Figure 5). The percentages of early apoptotic HepG2 cells following treatment at IC50 concentration (58 µg/mL) for 24 h and 55 µg/mL for 48 h were 42.13% and 42.48%, respectively (Figure 5, Table 2). The percentages of necrotic and late apoptotic cells were about 6.96% and 0.60% after 24 h treatment and 4.39% and 3.39% after 48 h treatment, respectively (Figure 5, Table 2).
Figure 5.
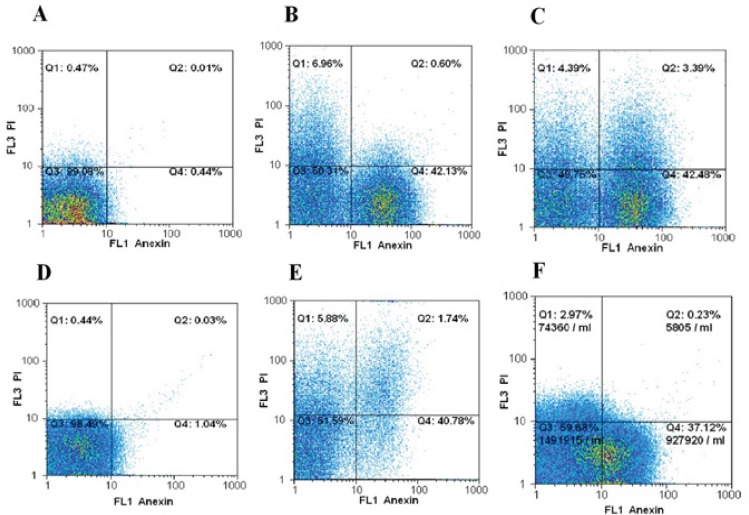
Flow Cytometry Analysis of HepG2 and L929 Cells Treated with [cu(L)(2imi)] Complex. Un-treated HepG2 cells (A), HepG2 cells treated at IC50 concentration (58µg/mL) of [Cu(L)(2imi)] for 24 h (B), HepG2 cells treated at IC50 concentration (55µg/mL) of [Cu(L)(2imi)] for 48 h (C), Un-treated L929 cells (D) L929 cells treated at IC50 concentration (105.3 µg/mL) of [Cu(L)(2imi)] for 24 h (E) and L929 cells treated at IC50 concentration (83 µg/mL) of [Cu(L)(2imi)] for 48 h (F) stained with Annexin V-fluorescein isothiocyanate (FITC) and propidium iodide (PI). Subsequently, apoptotic and necrotic cells were quantified by flow cytometry. The different subpopulations were defined as Q1 Annexin V-negative but PI-positive, i.e. necrotic cells; Q2 Annexin V/PIdouble positive, i.e. late apoptotic cells; Q3 Annexin V/PI-double negative, i.e. normal live cells; and Q4 Annexin V-positive but PInegative, i.e. early apoptotic cells.
Table 2.
Apoptosis Rates in HepG2 and L929 Cells after 24 h and 48 h Treatment with [Cu(L)(2imi)] Complex at IC50 Concentrations
| Apoptosis rates | Apoptosis percentage at IC50 concentration of [Cu(L)(2imi)] complex (µg/mL) | |||
|---|---|---|---|---|
| HepG2 | L929 | |||
| 24 h (58 µg/mL) | 48 h (55 µg/mL) | 24 h (105.3 µg/mL) | 48 h (83 µg/mL) | |
| Normal live cells | 50.31% | 49.75% | 51.59% | 56.68% |
| Early apoptosis cells | 42.13% | 42.48% | 40.78% | 37.12% |
| Late apoptosis cells | 0.60% | 3.39% | 1.74% | 0.23% |
| Necrosis cells | 6.96% | 4.39% | 5.88% | 2.97% |
The test for L929 was also performed as normal cells with concentration of IC50, 105.3 µg/mL for 24 h and 83 µg/mL for 48 h. The percentages of early apoptotic L929 cells following treatment with IC50 concentrations for 24 h and 48 h (105.3 µg/mL and 83 µg/mL) were 40.78% and 37.12%, respectively (Figure 5, Table 2). The percentages of necrotic and late apoptotic cells were about 5.88%,1.74% after 24 h and 2.97%, 0.23%, after 48 h treatment with [Cu(L)(2imi)] complex, respectively (Figure 5, Table 2).
Normal living cells were observed in Q3 region (Figure 5). The early apoptotic cells were determined by the sum of the cells in Q4 and late apoptosis and necrosis cells appeared mainly in the Q2 and Q1 regions, respectively (Figure 5)
Discussion
[Cu(L)(2imi)] was synthesized according to the procedure reported previously (Ebrahimipour et al., 2015) and was characterized. The primary objective of this study was to identify the anti-cancer properties of the [Cu(L)(2imi)] complex. We also attempted to determine whether this complex could selectively kill human cancer cells. This is the first study on the biological activity of this complex as a anti-cancer agent.
To evaluate the anti-cancer potential of the [Cu(L)(2imi)] complex synthesized, the compound was initially tested for cytotoxicity against HepG2 and L929 cells. The cytotoxic activities, expressed as IC50 values, were determined by the MTT assay. This experiment is based on MTT conversion into formazan with water insoluble crystals via dehydrogenases in the mitochondria of alive cells with a dose-dependent fashion (Van Tonder et al., 2015). In previous studies have been reported that copper (II) complexes have anti-cancer activities against several human cancer cell lines (González et al., 2013; Hajrezaie et al., 2014; Ma et al., 2015; Gan et al., 2016; Yu et al., 2016). Thati et al., 2007 reported that a copper (II) complex ([Cu(4-Mecdoa)(phen)2]) had a dose-and time-dependent anti-proliferative effect on non-neoplastic hepatic (CHANG) and HepG2 cells. However, it should be noted that the complex was more effective against HepG2 cells compared to CHANG cells (Thati et al., 2007).
Additionally, copper (II) complexes have been found to inhibit cell growth in HepG2 cell line. For example, a copper (II) complex of anthracenyl terpyridine showed cytotoxicity towards different cancer cell lines such as HepG2 (Kumar et al., 2011).
The IC50 values of [Cu(L)(2imi)] complex in the HepG2 cell line after 24 h and 48 h treatment were compared with the IC50 values of this complex against L929 fibroblast mouse cells, as normal cells. The results were displayed that in HepG2 cells, the IC50 value after 24 h treatment (58 µg/mL) was very close to this observed after 48 h treatment (55 µg/mL). Similarly, in L929 cells, the IC50 values after 24 h and 48 h treatment with the complex were not close to each other about 105.3 µg/mL and 83 µg/mL, respectively (p < 0.05). Altogether data indicate that the IC50 values for L929 cells were about 2-fold over those observed in HepG2 cells after 24 h treatment. On the other hand, surprisingly, in the concentration of 58 µg/mL (IC50 obtained for HepG2 cells after 24 h treatment) and 55 µg/mL (IC50 obtained for HepG2 cells after 48 h treatment) approximately 100 % and 89.3% of L929 cells are alive, respectively. As one can see, the IC50 of the Cu (II) complex against HepG2 cell line was higher than that against L929 cell line. It can be concluded that hepatocellular carcinoma cells are much more sensitive to the complex compared with L929 normal cells. On the other words, [Cu(L)(2imi)] can selectively inhibit the growth of the HepG2 cancerous cells. The results of the flow cytometry measurements also demonstrated that the [Cu(L)(2imi)] complex induced apoptosis in vitro. Several mechanisms exist in cells to promote cell death. Necrosis and Apoptosis are two redundant and distinct forms of cell death occurring in response to physical and chemical stresses.
The apoptosis regulates a tightly cell death program and plays an essential role in many biological processes (Jänicke et al., 1998), while in necrosis process the plasma membrane was destructed and cofactors and cytosolic enzymes was released into the external medium (Mignani et al., 2017). The anti-cancer activity reflected the number of apoptotic and necrotic cells after exposure to chemicals. The results of present study revealed that the IC50 concentration of Cu complex reduced the overall number of viable HepG2 cells after 24 h and 48 h (Figure 2). But in the same conditions, this complex acted differently on L929 cells and had low effect on the viability of normal L929 cells in contrast with cancerous HepG2 cells. This means that this complex surprisingly kills the cancerous cells, selectively, therefore its side effects will probably reduce on normal cells. To determine the correlation between anti-proliferative activity and apoptosis induction capability of [Cu(L)(2imi)] complex, the flow cytometry analysis was also carried out. Flow cytometry is a rapid and reliable method to demonstrate the entry of cells into the programmed cell death process. Flow cytometry analysis is based on the use of two fluorescent dyes for intact cells, including annexin-V and propidium iodide (PI). Accordingly to the fluorescence monitored in flow cytometry analysis, cells can be classified as alive or normal, in early apoptosis, in late apoptosis and necrosis or dead cells (Baskić et al., 2006; Hosseini et al., 2017).
Many studies have shown that copper (II) complexes are able to induce apoptosis in HepG2 cells (Pravin et al., 2017). Interestingly, Fan et al., 2016 demonstrated that metam/copper (II) led to cell death by induction of apoptosis, while metam/zinc (II) cell death mode was necrosis (Fan et al., 2016).
As expected DMSO had a negligible effect on the cell viability at 24 and 48 h (Figure 2 and Figure 3), whereas IC50 concentrations of [Cu(L)(2imi)] complex had a considerable increase of cells in early apoptosis at 24 h (42.13%) and 48 h (42.48%), together with a minor number of cells in late apoptosis and necrosis.
For evaluation of the anti-proliferative effect of [Cu(L)(2imi)] complex on HepG2 and L929 cells, initially cells were exposed for 24 h and 48 h to a different concentrations of this complex. As shown in Fig. 5 and Table 2, the number of alive cells was reduced in both cell lines while the percentage of cells in early apoptosis increased and cells remained at the early stage of the apoptotic pathway with only a low percentage of cells reaching the late apoptotic stage. Therefore, it is possible to conclude that [Cu(L)(2imi)] complex induced cell death through the activation of the apoptotic process. Therefore, [Cu(L)(2imi)] complex is a more potent inducer of apoptosis, especially at IC50 concentration.
It was possible to report a dual effect of [Cu(L)(2imi)] complex relative to the range of concentration used in cellular testing. At a low concentration, this complex was toxic and induced apoptosis in HepG2 cells without considerable effects on L929 cells. At higher concentrations, the toxic effect of [Cu(L)(2imi)] complex was observed on normal cells that were not affected in lower concentration. Also, flow cytometry analysis indicates that necrosis does not probably initiate cell death, but rather is a consequence of the activation of the apoptotic process.
In conclusion, the present study confirmed that [Cu(L)(2imi)] complex exerted inhibitory effects on HepG2 cells. Furthermore, the results demonstrated that [Cu(L)(2imi)] complex enhanced apoptosis using flow cytometry measurements. The result of the present study revealed that L929 (normal cells) was less sensitive to the complex. Data was shown that cu complex triggered cell death via apoptosis and did not efficiently activate the necrosis process. Finally, we found that the [Cu(L)(2imi)] complex possess the potential for development as an anti-cancer drug for human hepatocellular carcinoma. Moreover, future studies are required to investigate DNA interaction, cleavage and molecular mechanisms involved in apoptosis induction of [Cu(L)(2imi)] complex.
Abbreviations
DMSO, dimethyl sulfoxide; FBS, fetal bovine serum; FITC, fluorescein isothiocyanate; HCC, hepatocellular carcinoma; HepG2, human hepatocellular liver carcinoma cell line; IC50, the half maximal inhibitory concentration; MTT, 3-(4,5-Dimethyl-2-thiazolyl)-2,5-diphenyl- 2H-tetrazolium bromide; PBS, phosphate buffered saline; PI, propidium iodide; SPSS, statistical package for the social sciences
Conflict of Interest
The authors declare that they have no conflict of interest.
Acknowledgements
The authors would like to thank the Molecular Medicine Research Center (MMRC) in Rafsanjan University of Medical Sciences (RUMS) from of Iran for providing the necessary equipment for this work. This study was supported by the RUMS by the grant number 20.1095 and approved by the RUMS ethical committee by the number of IR.RUMS.REC.1395.110.
This article has been extracted from Miss Azadeh Rezai Thesis, That Was MSc Student in Clinical Biochemistry, Rafsanjan Faculty of Medicine.
References
- 1.Abid-Essefi S, Baudrimont I, Hassen W, et al. DNA fragmentation, apoptosis and cell cycle arrest induced by zearalenone in cultured DOK, Vero and Caco-2 cells: prevention by Vitamin E. Toxicology. 2003;192:237–48. doi: 10.1016/s0300-483x(03)00329-9. [DOI] [PubMed] [Google Scholar]
- 2.Atawodi SE. Nigerian foodstuffs with prostate cancer chemopreventive polyphenols. Infect Agent Cancer. 2011;6:S9. doi: 10.1186/1750-9378-6-S2-S9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bagrezaei F, Hassanshahi G, Mahmoodi M, Falahati-Pour SK, Mirzaei MR. Expression of inhibitor of apoptosis gene family members in bladder cancer tissues and the 5637Tumor Cell Line. Asian Pac J Cancer Prev. 2018;19:529–32. doi: 10.22034/APJCP.2018.19.2.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barry NP, Sadler PJ. Exploration of the medical periodic table: towards new targets. Chem Commun (Camb) 2013;49:5106–31. doi: 10.1039/c3cc41143e. [DOI] [PubMed] [Google Scholar]
- 5.Baskić D, Popović S, Ristić P, et al. Analysis of cycloheximide-induced apoptosis in human leukocytes: fluorescence microscopy using annexin V/propidium iodide versus acridin orange/ethidium bromide. Cell Biol Int. 2006;30:924–32. doi: 10.1016/j.cellbi.2006.06.016. [DOI] [PubMed] [Google Scholar]
- 6.Brem S. Angiogenesis and cancer control: from concept to therapeutic trial. Cancer Control. 1999;6:438–48. [PubMed] [Google Scholar]
- 7.Brewer GJ. Copper control as an antiangiogenic anticancer therapy: lessons from treating Wilson's disease. Exp Biol Med (Maywood) 2001;226:665–73. doi: 10.1177/153537020222600712. [DOI] [PubMed] [Google Scholar]
- 8.Chen Y, Hu L. Design of anticancer prodrugs for reductive activation. Med Res Rev. 2009;29:29–64. doi: 10.1002/med.20137. [DOI] [PubMed] [Google Scholar]
- 9.Ebrahimipour SY, Mohamadi M, Castro J, et al. Syntheses, characterizations, crystal structures, and biological activities of two new mixed ligand Ni (II) and Cu (II) Schiff base complexes. J Coord Chem. 2015;68:632–49. [Google Scholar]
- 10.El-Serag HB. Epidemiology of viral hepatitis and hepatocellular carcinoma. Gastroenterology. 2012;142:1264–73. doi: 10.1053/j.gastro.2011.12.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.El–Serag HB, Rudolph KL. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology. 2007;132:2557–76. doi: 10.1053/j.gastro.2007.04.061. [DOI] [PubMed] [Google Scholar]
- 12.Fan Rm, Zhu Bz, Huang Cp, et al. Different modes of synergistic toxicities between metam/copper (II) and metam/zinc (II) in HepG2 cells: apoptosis vs. necrosis. Environ Toxicol. 2016;31:1964–73. doi: 10.1002/tox.22197. [DOI] [PubMed] [Google Scholar]
- 13.Franke TF, Hornik CP, Segev L, et al. PI3K/Akt and apoptosis: size matters. Oncogene. 2003;22:8983–98. doi: 10.1038/sj.onc.1207115. [DOI] [PubMed] [Google Scholar]
- 14.Gan Q, Fu X, Chen W, et al. Synthesis, DNA/HSA interaction spectroscopic studies and in vitro cytotoxicity of a new mixed ligand Cu (II) complex. J Fluoresc. 2016;26:905–18. doi: 10.1007/s10895-016-1779-2. [DOI] [PubMed] [Google Scholar]
- 15.González SEF, Anguiano EA, Herrera AM, et al. Cytotoxic, pro-apoptotic, pro-oxidant, and non-genotoxic activities of a novel copper (II) complex against human cervical cancer. Toxicology. 2013;314:155–65. doi: 10.1016/j.tox.2013.08.018. [DOI] [PubMed] [Google Scholar]
- 16.Hajrezaie M, Paydar M, Zorofchian Moghadamtousi S, et al. A Schiff Base-derived copper (II) complex is a potent inducer of apoptosis in colon cancer cells by activating the intrinsic pathway. Sci World J. 2014;2014:12. doi: 10.1155/2014/540463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hosseini FS, Falahati-pour SK, Hajizadeh MR, et al. Persian shallot, Allium hirtifolium Boiss, induced apoptosis in human hepatocellular carcinoma cells. Cytotechnology. 2017;69:551–63. doi: 10.1007/s10616-017-0093-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jänicke RU, Sprengart ML, Wati MR, et al. Caspase-3 is required for DNA fragmentation and morphological changes associated with apoptosis. J Biol Chem. 1998;273:9357–60. doi: 10.1074/jbc.273.16.9357. [DOI] [PubMed] [Google Scholar]
- 19.Karimabad MN, Mahmoodi M, Jafarzadeh A, et al. The novel Indole-3-formaldehyde (2-AITFEI-3-F) is involved in processes of apoptosis induction? Life Sci. 2017;181:31–44. doi: 10.1016/j.lfs.2017.05.026. [DOI] [PubMed] [Google Scholar]
- 20.Kim HK, Choi IJ, Kim CG, et al. A gene expression signature of acquired chemoresistance to cisplatin and fluorouracil combination chemotherapy in gastric cancer patients. PLoS One. 2011;6:e16694. doi: 10.1371/journal.pone.0016694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kumar A, Chinta JP, Ajay AK, et al. Synthesis, characterization, plasmid cleavage and cytotoxicity of cancer cells by a copper (II) complex of anthracenyl-terpyridine. Dalton Trans. 2011;40:10865–72. doi: 10.1039/c1dt10201j. [DOI] [PubMed] [Google Scholar]
- 22.Ma T, Xu J, Wang Y, et al. Ternary copper (II) complexes with amino acid chains and heterocyclic bases: DNA binding, cytotoxic and cell apoptosis induction properties. J Inorg Biochem. 2015;144:38–46. doi: 10.1016/j.jinorgbio.2014.12.011. [DOI] [PubMed] [Google Scholar]
- 23.Marzano C, Trevisan A, Giovagnini L, et al. Synthesis of a new platinum (II) complex: anticancer activity and nephrotoxicity in vitro. Toxicol In Vitro. 2002;16:413–9. doi: 10.1016/s0887-2333(02)00022-x. [DOI] [PubMed] [Google Scholar]
- 24.Mi Y, Zhao J, Feng SS. Targeted co-delivery of docetaxel, cisplatin and herceptin by vitamin E TPGS-cisplatin prodrug nanoparticles for multimodality treatment of cancer. J Control Release. 2013;169:185–92. doi: 10.1016/j.jconrel.2013.01.035. [DOI] [PubMed] [Google Scholar]
- 25.Mignani S, El Brahmi N, Eloy L, et al. Anticancer copper (II) phosphorus dendrimers are potent proapoptotic Bax activators. Eur J Med Chem. 2017;132:142–56. doi: 10.1016/j.ejmech.2017.03.035. [DOI] [PubMed] [Google Scholar]
- 26.Mohammadizadeh F, Falahati-pour SK, Rezaei A, et al. The cytotoxicity effects of a novel Cu complex on MCF-7 human breast cancerous cells. Bio Metals. 2018;31:233–42. doi: 10.1007/s10534-018-0079-5. [DOI] [PubMed] [Google Scholar]
- 27.Munteanu CR, Suntharalingam K. Advances in cobalt complexes as anticancer agents. Dalton Trans. 2015;44:13796–808. doi: 10.1039/c5dt02101d. [DOI] [PubMed] [Google Scholar]
- 28.Pravin N, Kumaravel G, Senthilkumar R, et al. Water-soluble Schiff base Cu (II) and Zn (II) complexes: Synthesis, DNA targeting ability and chemotherapeutic potential of Cu (II) complex for hepatocellular carcinoma–in vitro and in vivo approach. Appl Organomet Chem. 2017;31:e3739. [Google Scholar]
- 29.Ramezani M, Ramezani M, Hassanshahi G, et al. Does the novel class of (2R, 4S)-N-(2, 5-Difluorophenyl)-4-Hydroxy-1-(2, 2, 2-Trifluoroacetyl) pyrrolidine-2-carboxamide's have any effect on cell viability and apoptosis of human hepatocellular carcinoma cells? Int J Cancer Manag. 2017;10:e8413. [Google Scholar]
- 30.Santini C, Pellei M, Gandin V, et al. Advances in copper complexes as anticancer agents. Chem Rev. 2013;114:815–62. doi: 10.1021/cr400135x. [DOI] [PubMed] [Google Scholar]
- 31.Sheikhrezaei Z, Heydari P, Farsinezhad A, et al. A new indole derivative decreased SALL4 gene expression in acute Promyelocytic leukemia cell line (NB4) Iran Biomed J. 2018;22:99–106. doi: 10.22034/ibj.22.2.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Takjoo R, Akbari A, Ebrahimipour SY, et al. Synthesis, characterization, X-ray structure and DFT calculation of two Mo (VI) and Ni (II) Schiff-base complexes. C R Chim. 2014;17:1144–53. [Google Scholar]
- 33.Thati B, Noble A, Creaven BS, et al. Apoptotic cell death: A possible key event in mediating the in vitro anti-proliferative effect of a novel copper (II) complex, [Cu (4-Mecdoa)(phen) 2](phen=phenanthroline, 4-Mecdoa=4-methylcoumarin-6, 7-dioxactetate), in human malignant cancer cells. Eur J Pharmacol. 2007;569:16–28. doi: 10.1016/j.ejphar.2007.04.064. [DOI] [PubMed] [Google Scholar]
- 34.Theophanides T, Anastassopoulou J. Copper and carcinogenesis. Crit Rev Oncol Hematol. 2002;42:57–64. doi: 10.1016/s1040-8428(02)00007-0. [DOI] [PubMed] [Google Scholar]
- 35.Tisato F, Marzano C, Porchia M, et al. Copper in diseases and treatments, and copper-based anticancer strategies. Med Res Rev. 2010;30:708–49. doi: 10.1002/med.20174. [DOI] [PubMed] [Google Scholar]
- 36.van Rijt SH, Sadler PJ. Current applications and future potential for bioinorganic chemistry in the development of anticancer drugs. Drug Discov Today. 2009;14:1089–97. doi: 10.1016/j.drudis.2009.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Van Tonder A, Joubert AM, Cromarty AD. Limitations of the 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyl-2H-tetrazolium bromide (MTT) assay when compared to three commonly used cell enumeration assays. BMC Res Notes. 2015;8:47. doi: 10.1186/s13104-015-1000-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yu H, Yang Y, Li Q, et al. Ternary dinuclear copper (II) complexes of a reduced schiff base ligand with diimine coligands: DNA binding, cytotoxic cell apoptosis, and apoptotic mechanism. Chem Biol Drug Des. 2016;87:398–408. doi: 10.1111/cbdd.12669. [DOI] [PubMed] [Google Scholar]
- 39.Zainodini N, Hassanshahi G, Hajizadeh M, et al. Nisin induces cytotoxicity and apoptosis in human asterocytoma cell line (SW1088) Asian Pac J Cancer Prev. 2018;19:2217–22. doi: 10.22034/APJCP.2018.19.8.2217. [DOI] [PMC free article] [PubMed] [Google Scholar]


