Abstract
Objective:
Cervical carcinoma is the third most common gynecologic cancer, after ovarian and uterine cancers in Iran. The aim of this study was to evaluate the efficacy (response rate) and toxicity of adding Medium Dose Rate (MDR) brachytherapy with concurrent chemotherapy to External Beam Radiotherapy (EBRT) for the treatment of locally advanced uterine cervical carcinoma.
Methods:
This phase I-II study was conducted in 2007-2008 at the cancer institute, Tehran University of Medical Sciences. Patients were treated with pelvic EBRT (50 Gy in 25 Fraction) with concomitant chemotherapy to obtain tumor shrinkage and permit optimal intra-cavitary placement. One week after the completion of EBRT, patients were treated by 12 Gy MDR Intra-cavitary brachytherapy for two periods of one day with a one week interval and concomitant platinum based chemotherapy. Response rate was evaluated by gynecologic physical examination and pelvic MRI +- GD within three months of treatment. Acute and late toxicity were assessed using Radiation Therapy Oncology Group criteria.
Results:
A total of 33 patients with locally advanced cervical cancer were treated according to the above described treatment protocol. The patients mean age was 53.2 (range 31–78) years. Three months after the completion of treatment, the complete clinical, pathologic and radiologic response rate according to WHO-criteria was 81.8% (27 patients). Six cases had a partial response or stable disease. After 48 months, average disease free survival periods were 45.1, 23.0, 33.4 and 8 months for stage IIB, IIIA, IIIB and IVA lesions (according to The International Federation of Gynecology and Obstetrics staging system). The most frequently observed side effects were leukopenia, anemia, proctitis, cystitis, nausea and vomiting (mostly grade 1 and 2).
Conclusion:
Concomitant brachytherapy and chemotherapy with platinum compounds can be well tolerated and is effective in the treatment of locally advanced cervical cancer.
Keywords: Uterine cervical cancer, medium dose rate brachytherapy, chemoradiation
Introduction
Cervical cancer is a major world health problem for women. It is the third most common cancer in women worldwide, with 85% of cases occurring in developing countries, where cervical cancer is the second most frequent cause of cancer death in women (DeSantis et al., 2014). Cervical carcinoma is the third most common gynecologic cancers after ovarian and uterine cancers in Iran (Mousavi et al., 2008). Women with locally advanced cervical cancer (stage IB2 to IVA) have a higher rate of recurrence and worse survival than those with early stage disease (stage IA to IB1). After surgery alone, the rate of relapse is at least 30 percent, and five-year survival rates range from 80 percent for stage IB disease to 30 percent for stage III disease (Rotman et al., 2006). For most women, External Beam Radiation Therapy (EBRT) is delivered to the pelvis using 2D or 3D techniques; vaginal brachytherapy is also administered in an effort to maximize local control. The addition of chemotherapy to RT have improved local tumor control, disease free survival rate, and overall survival in advanced cervical tumors through randomized trials; therefore, nowadays concurrent chemo radiation is considered as standard treatment modality (Yamashita et al., 2010). Brachytherapy, or local application of radiation, represents an integral component of treatment for cervical cancer, because of the close treatment distance and rapid dose fall off, which is governed by the inverse square law, brachytherapy allows for the precise delivery of high-dose radiation to the target tissue and sparing of the surrounding normal tissue, notably, the small bowel, rectum, and bladder. It can often be delivered in a shorter time and in a safer manner than EBRT (Wang et al., 2009). Brachytherapy can be delivered with either a low dose rate (LDR), MDR medium dose rate (MDR) or high dose rate (HDR) system. The International Commission on Radiation Units (ICRU) defines LDR as 0.4 to 2 Gy per hour, whereas HDR is delivered at >12 Gy per hour. The most common LDR and MDR sources used to treat gynecologic malignancies is Cesium-137 and the most common HDR source is Iridium-192 (Kwekkeboom et al., 2009).
Adding a chemotherapeutic agent may cause radio-sensitization of the tumor cells, giving rise to an alteration in the shape of the cell-survival curve after irradiation. This may be due to direct tumor cell cytotoxicity or inhibition of sub-lethal or potentially lethal radiation-induced damage repair. The benefit of chemo radiation for women with locally advanced cervical cancer rather than RT alone was demonstrated in a 2010 meta-analysis. Compared with primary RT, the use of chemo radiation resulted in improving overall and progression free survival (Rydzewska et al., 2010; Pecorelli, 2009).
In this phase II-study, patients with locally advanced cervical carcinoma were administered cisplatin concomitantly and exclusively during two brachytherapy insertions, with attention to known radio-sensitizing effects of cisplatin agent. The primary goal of this phase II-study was to evaluate the efficacy (response rate) and toxicity of this new treatment approach for the treatment of locally advanced uterine cervical carcinoma; secondary objectives were to assess disease-free and overall survival.
Materials and Methods
Thirty–three patients were included in this phase I-II study between October 2007 to May 2008 at the Cancer Institute, Tehran University of Medical Sciences. The initial evaluation included chest radiography, computerized tomography (CT) scan of the abdomen and pelvis, a complete blood count and biochemistry. All Patients had biopsy-proven uterine cervical carcinoma, stage IB2 bulky up to stage IVA according to The International Federation of Gynecology and Obstetrics staging criteria (Table 1). To begin the treatment, patients were required to have an Eastern Cooperative Oncology Group performance status of 0–2.
Table 1.
Tumor Characteristics
| Number (%) | |
|---|---|
| Stage (FIGO) | |
| IIB | 24 (73) |
| IIIA | 3 (9) |
| IIIB | 5 (15) |
| IVA | 1 (3) |
| Histologic subtype | |
| SCC | 29 (88) |
| ADS | 2 (6) |
| ASC | 1 (3) |
| Undiff | 1 (3) |
| Involvement | |
| Vaginal | 12 (36) |
| Parametrical | 12 (36) |
| Lymph Node | 3 (9) |
FIGO, the International Federation of Gynecology and Obstetrics; SCC, Squamous Cell Carcinoma; ADC, Adenocarcinoma; ASC, Adeno Squamous Carcinoma; Undiff, Undifferentiated.
Radiotherapy treatment: After CT simulation and treatment planning, EBRT was delivered using linear accelerator with 18- MV photons. Patients were treated with a combination of 4-field (2 opposed AP-PA and 2 lateral fields without midline shield) box technique of irradiation and received 25 fractions of external radiotherapy with a daily dose of irradiation of 2 Gy to obtain tumor shrinkage and permit optimal intra-cavitary placements. The volume of EBRT covered the gross disease, parametrium, uterosacral ligaments, sufficient vaginal margin from the gross disease (at least 3 cm), presacral nodes, and other nodal volumes at risk. For patients with negative nodes on surgical or radiologic imaging, the radiation volume covered the entirety of the external iliac, internal iliac, and obturator nodal basins. For patients presumed at higher risk of lymph node involvement (e.g. bulkier tumors; suspected or confirmed nodes confined to the low true pelvis) the radiation volume increased to cover the common iliac as well. Boost dose to the parametrium was added for patients who had distal parametrial involvement to achieve 60 Gy to PTV.
Chemotherapy protocol: The chemotherapy consisted of cisplatin at a dose of 35 mg/m2 (one Hour infusion) in weekly schedule during EBRT and in the day of each intra-cavitary brachytherapy (IC-BT) insertion. Preventive medication for nausea and vomiting was routinely provided as well.
Brachytherapy protocol: One week after the completion of external radiotherapy, IC-BT was administered. After dilation of the uterine cervix under general or spinal anesthesia, intrauterine tandem with appropriate size and angle was inserted into the uterus; two colpostats were placed in the vaginal vault and packed with iodoformed gauze. After insertion of applicators, X-Ray simulation of the pelvis (AP, LAT) and treatment planning with Plato software (Nucletron® B.V, Veenendaal, Netherlands) was done. We delivered 12 Gy IC-BT with the dose rate of 2-2.4Gy/h for a period of two days with a one week interval through the use of after-loading MDR Selectron machine (Nucletron® B.V. Veenendaal, Netherlands).
With MDR intracavitary systems, total doses from brachytherapy and external-beam radiation to point A (a reference location 2 cm superior and 2 cm lateral to the central cervical os) of at least 80 Gy was given for small tumors, with doses of 85 Gy or higher given to larger tumors. The dose delivered to Point A, bladder, rectum, and parametrial points according to the International Commission on Radiation Units and Measurements (ICRU-38) recommendations were determined. The maximal given dose was limited to 70 Gy to the rectum and 75 Gy to the bladder points.
Gynecologic physical examination of pelvis under general anesthesia for the evaluation of tumor size and parameters were performed before and after each MDR IC-BT. Response rate was evaluated by gynecologic physical examination and pelvic MRI with and without intravenous gadolinium within three months after treatment. In the follow-up after treatment; Pap smear and gynecologic physical examination were also performed every 3 months within first year, every 4 months within second year and every 6 months thereafter. Any recurrence should be confirmed by tissue biopsy.
Tumor response was defined according to the World Health Organization (WHO) criteria: complete response, disappearance of all clinical and radiologic evidence of tumor plus negative histopathologic examination of a cervical biopsy; partial response, ≥50% shrinkage of the tumor and no evidence of disease progression; no response (stable disease), no change or <50% shrinkage of the tumor or <25% increase in tumor size; and progressive disease, ≥25%increase in tumor size or appearance of new lesions (Morice et al., 2012).
Toxicity: Toxicity was assessed using RTOG criteria. Acute effects were evaluated by laboratory findings including complete blood count (hemoglobin, leukocytes, and platelets) and renal function tests including blood urea and creatinine. Other subjective acute side effects such as abdominal pain, vaginitis, proctitis, and cystitis during the time of ICBT and one month after treatment and late effects such as proctitis, rectal fistula, cystitis, vaginal fibrosis and abdominal pain were assessed in months 3, 6, 9, 12 for the first year and every 6 months thereafter according to RTOG grading system (Chaturvedi et al., 2007). In the case of cystitis, urine analysis and culture were requested to rule out urinary infections. Side effects of treatment that occurred within 90 days of the start of radiotherapy were considered acute effects, and those occurring or persisting more than 90 days after the start of radiotherapy were considered late effects (specially for rectal, bladder vagina and sigmoid).
Statistical Methods
Disease-free survival (DFS) was calculated from the date of diagnosis to the day of any treatment failure; overall survival (OS) was calculated from the date of diagnosis to the date of death or last follows up. SPSS statistical software version 18.0 was used for statistical analysis. DFS and OS were estimated using the Kaplan Meier method and cumulative survival rates were compared using log-rank test with p<0.05 considered significant. The study was approved by the university Ethics Committee. Written informed consent was obtained from all patients before staging work up and treatment.
Results
Between October 2007 and May 2008, a total number of 33 patients with locally advanced cervical cancer were treated according to the above described treatment protocol with external radiotherapy and concomitant chemo-radiobrachytherapy with cisplatin. The patients’ mean age was 53.2 (range 31–78) years. Vast majority of the patients had biopsy-proven squamous cell carcinoma. Tumor characteristics are shown in Table 1 Tumor characteristics. Cervical tumor size before and after EBRT was 4.8 ± 1.4 cm and 1.8 ± 0.95 cm respectively. The mean RT dose was 5020± 433 cGy. All patients received the planned dose of at least 80Gy to point A. Mean rectal point and bladder point dose was 6198 and 6561 cGy respectively. Average duration of completing radiotherapy, both external radiotherapy and brachytherapy, was 54.6 (range 45–72) days.
Three months after the completion of treatment, complete clinical and radiologic response rate according to WHO-criteria was 81.8% (27 patients). Six patients had partial response or stable disease. Two patients had local recurrences after achieving complete response after completion of ERT+ICBT and one patient had distant metastasis. Nine patients died (8 patients from disease progression and 1 patient from other cause). Four years disease free survival in 33 patients was 76%. At the time of this report, the median follow-up was 48 months (ranged 13-54 months); 1, 2 and 4 year OS were 76%, 73%, 73% respectively. Median survival was 39.8 ± 3.6 months. DFS according to FIGO staging were 45.1, 23, 33.4 and 8 months for stage IIB, IIIA, IIIB and: IVA respectively.
The treatment was generally well tolerated and relatively easy to administer. There were no treatment-related deaths. Most frequently observed acute side effects were leukopenia, anemia, proctitis, cystitis, nausea and vomiting (grade 1-2). Grade 1 and 2 leucopenia were seen in 85% and 15% of patients, grade 1 and 2 anemia detected in 95% and 5% of the cases respectively (only one patient needed blood transfusion). A febrile neutropenia was not seen in any of the patients. Thrombocytopenia, renal dysfunction (raised blood urea or creatinine) and grade 3 and 4 hematological toxicity were not seen in any cases neither.
According to the European Organization for Research and Treatment of Cancer (EORTC) late radiation morbidity scoring scheme guidelines most late toxicities were grade 1 and 2: (Figure 1) except one grade 4 rectovaginal fistulas and one grade 4 vesicovaginal fistula.
Figure 1.
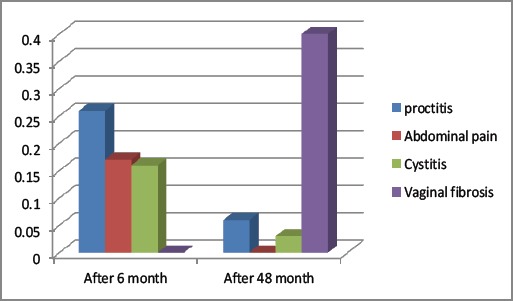
Late Side Effect
Discussion
Concurrent chemo radiation, using cisplatin-based chemotherapy (either cisplatin alone or cisplatin/5-FU), is the treatment of choice for stages IB2, II, III, and IVA disease based on the results of 5 randomized clinical trials. These Five randomized trials have shown that using concurrent chemo radiation results in a 30% to 50% decrease in the risk of death compared with RT alone. Although the optimal concurrent chemotherapy regimen to be used with RT requires further investigation, these 5 trials clearly established a role for concurrent cisplatin-based chemo radiation. Based on these data, the NCI issued an alert stating that strong consideration should be given for using chemo radiation instead of RT alone for invasive cervical cancer.
A large population-based registry analysis in Canada (n=4,069) confirmed that chemo radiation improved outcomes when compared with RT alone. Chemo radiation improves progression-free survival (PFS) and overall survival when compared with RT with (or without) hydroxyurea. Arecent meta-analysis reported that chemo radiation leads to a 6% improvement in 5-year survival (hazard ratio, 0.81; P<.001) (Thomas, 1999; Morris et al., 1999; Peters et al., 2000; Whitney et al., 1999; Rose et al., 1999).
Cisplatin is considered the most effective single agent as systemic therapy in eradicating micro-metastasis and moreover as a radio-sensitizer in uterine cervical carcinoma. Cisplatin when simultaneously administered with radiotherapy, have radio-sensitivity effects by inhibition of DNA synthesis, inhibition of transcription elongation by DNA inter-strand cross-links, inhibition of repair of radiation induced DNA damage and enhancing the sensitivity of hypoxic cells and cell death. Due to its cytotoxic effects, cisplatin reduces tumor bulk, which leads to reoxygenation of the tumor and entry of the tumor cells into a radiation-sensitive phase of their cell cycle (O’Hara et al., 1986).
We tested the hypothesis that local control could be improved without increasing the incidence of early or late radiation-induced toxicities by adding medium-dose rate brachytherapy to external radiotherapy, by which one third of the total radiation dose is given to the tumor with brachytherapy.
We administered chemotherapy during the two MDR brachytherapy insertions with one week separation. It was logical to expect that the best time to apply chemotherapy during the course of radiotherapy will be during the brachytherapy insertions (Rose et al., 2007). There are two explanation for this assumption: the dose of radiation applied during one brachytherapy insertion is 5 times higher than the single dose applied by external radiation; due to that difference we can expect that the effects of the combination of brachytherapy and chemotherapy will be substantially greater; the second reason is that the dose rate of brachytherapy is declining by inverse-square law and thus potentially results in less toxicity to surrounding normal tissues
The first report in concurrent chemotherapy with ICBT was reported by Eifel et al., (2004) (RTOG 90-01) in bulky FIGO stage IB and IIB-IVA that compared extended field pelvic and para-aortic RT versus concurrent Chemo radiation with pelvic RT; patients were treated by IC-BT and in chemotherapy group one of the chemotherapy cycle (cisplatin + 5FU every 3 weeks) was administrated at the time of second LDR intracavitary insertion. The 5 years OS, DFS was significantly greater in chemo radiation group compared to the patients in RT alone group (73% vs 52% and 68% vs 43% respectively) and also 52% risk reduction in death was seen in chemo radiation group. Patients who had positive lymph node also had significantly better outcome if they were treated with chemotherapy. The cumulative incidence of grade 3 or higher late side effects was 14% in both groups. The advantage of using chemotherapy concurrently with RT may be due to administration of the systemic therapy during ICBT.
In a phase I-II trial; Kuske et al., used 5FU+cisplatin concurrently with RT in the first week of treatment and with ICBT in 23 patients with advanced or recurrent cervical carcinomas and stage II and III uterine cancers (Kuske et al., 1989). In another study conducted by Stumpf et al. fifty seven cases with cervical cancer were treated by concurrent 5FU and ICBT (Stumpf et al., 1988). The results of both studies showed that concurrent chemotherapy and ICBT had better outcome comparing to ICBT alone.
Koumantakis et al., (1998) in Greece treated 36 cases with stage IIA/B-IIIA cervical cancers by EBRT and then concurrent Cisplatin or Carboplatin and MDR-ICBT. The dose to point A was 85-90 Gy. Radical hysterectomy and pelvic lymphadenectomy was performed when possible. In 31 patients who underwent surgery after radiotherapy, 83% had pathologic complete response. The most common acute side effects were hematological toxicities as grade 3 or 4 thrombocytopenia in 2 cases, and grade 1, 2 anemia and leucopenia in three patients. Local abdominal pain and grade 1, 2 cystitis were noted in six patients. There was no renal dysfunction. In the aforementioned study, the patients had surgery by hysterectomy and lymphadenectomy in addition to radiotherapy in comparison to our trial which had one treatment modality. Acute hematological toxicities were less common in our study, but other acute effects were similar. Koumantakis did not consider late effects in his study. Another study was conducted by Vrdoljak et al., (2006) in Croatia; sixty two patients with stage IB2-IVA uterine cervical carcinomas were treated with pelvic RT to tumor dose of 50 Gy and then concurrent ifosfamide + cisplatin and LDR-ICBT to total dose of 85Gy. Adjuvant chemotherapy every three weeks was given for three cycles. 48 months recurrence-free and overall survival rates were 88.7%, The incidence of grade 1 and 2 nausea and vomiting was, grade 3, 4 anemia, thrombocytopenia, and leucopenia was 70%, 10%, 8% and 25% respectively. Blood transfusion was prescribed in 60 % of patients. Late effects in rectum and bladder were observed in 16% of cases. In comparison to our study, the treatment outcome in this study was better due to the use of two chemotherapeutic agents and consolidative adjuvant Chemotherapy after chemo radiation; but, the acute and late effects were more common than our study.
Strauss et al., (2002) treated 27 patients with stage IIB-IIIB cervical cancers with concurrent cisplatin and HDR-ICBT in Germany. Complete response rate was 92.3%, and 80% of the patients were disease free after 20 months follow-up. Acute side effects including grade 3 hematological toxicities and late effects were seen in 29.6% and 7.4% of cases, respectively. These toxicities were more common in comparison with our trial.
In a study of 784 patients with stage IB disease, Eifel et al., (1995) reported an overall actuarial risk of major complications of 7.7% at 5 years. Although the actuarial risk was greater during the first 3 years of follow-up, there was a continuing risk to surviving patients of approximately 0.3% per year, resulting in an overall actuarial risk of 14% at 20 years. In the first 3 years after treatment, rectal complications were more common and include bleeding, stricture, ulceration, and fistula. Major gastrointestinal complications were rare in 3 years, but a constant low risk of urinary tract complications persisted for many years. The actuarial risk of developing fistula of any type was 1.7% at 5 years. In the study of Hashemi et al., (2008) in the same center in 2006 with the same protocol of EBRT and ICBT as our study but without concurrent chemo-brachytherapy; The 15 month’s local control was 81% and overall failure (40% of them with distant failure) was 17.5%. Bladder and rectal morbidity was observed in 9% and 8% of patients respectively (2 patients with grade 3 rectal complication). Although duration of follow up in our study is longer than the Hashemi report but our study has the same results in the terms of DFS and complications but the distant failure was lower.
The present study showed that concomitant brachytherapy and chemotherapy with platinum compounds can be well tolerated and is effective in the treatment of locally advanced cervical cancer. It is recommended to conduct phase II or III clinical trials using intensive dose of chemotherapy or a combining chemotherapy regimen concurrently specially with High Dose rate brachytherapy.
Statement conflict of Interest
The authors whose names are listed above declare that there is no conflict of interest regarding the publication of this article.
References
- 1.Chaturvedi A, Engels E, Gilbert E. Second cancers among 104,760 survivors of cervical cancer: evaluation of long-term risk. J Natl Cancer Inst. 2007;99:1634–43. doi: 10.1093/jnci/djm201. [DOI] [PubMed] [Google Scholar]
- 2.DeSantis C, Ma J, Bryan L, et al. Breast cancer statistics 2013. CA Cancer J Clin. 2014;64:52–62. doi: 10.3322/caac.21203. [DOI] [PubMed] [Google Scholar]
- 3.Eifel P, Levenback C, Wharton J. Time course and incidence of late complications in patients treated with radiation therapy for FIGO stage IB carcinoma of the uterine cervix. Int J Radiat Oncol Biol Phys. 1995;32:1289–1300. doi: 10.1016/0360-3016(95)00118-I. [DOI] [PubMed] [Google Scholar]
- 4.Eifel P, Winter K, Morris M, et al. Pelvic irradiation with concurrent chemotherapy versus pelvic and para-aortic irradiation for high-risk cervical cancer: an update of radiation therapy oncology group. J Clin Oncol. 2004;22:872–80. doi: 10.1200/JCO.2004.07.197. [DOI] [PubMed] [Google Scholar]
- 5.Hashemi F, Kalagchi B, Aghili M. Medium-dose-rate brachytherapy for cancer of the cervix: Preliminary results of a designed schedule. Asia Pac J Clin Oncol. 2008;4:244–7. [Google Scholar]
- 6.Koumantakis E, Haralambakis Z, Koukourakis M, et al. A pilot study on concurrent platinum chemotherapy and intracavitary brachytherapy for locally advanced cancer of the uterine cervix. Br J Radiol. 1998;71:552–7. doi: 10.1259/bjr.71.845.9691902. [DOI] [PubMed] [Google Scholar]
- 7.Kuske R, Perez C, Grigsby P. Phase I/II study of definitive radiotherapy and chemotherapy (Cisplatin and 5-Fluorouracil) for advanced or recurrent gynecologic malignancies: Preliminary report. Am J Clin Oncol. 1989;12:467–73. doi: 10.1097/00000421-198912000-00002. [DOI] [PubMed] [Google Scholar]
- 8.Kwekkeboom KL, Dendaas NR, Straub M, et al. Patterns of pain and distress during high-dose-rate intracavity brachytherapy for cervical cancer. J Support Oncol. 2009;7:108–14. [PubMed] [Google Scholar]
- 9.Morice P, Rouanet P, Rey A, et al. Results of the GYNECO 02 study, an FNCLCC phase III trial comparing hysterectomy with no hysterectomy in patients with a (clinical and radiological) complete. Oncologist. 2012;17:64–71. doi: 10.1634/theoncologist.2011-0276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Morris M, Eifel P, Lu J, et al. Pelvic radiation with concurrent chemotherapy compared with pelvic and para-aortic radiation for high-risk cervical cancer. N Engl J Med. 1999;340:1137–43. doi: 10.1056/NEJM199904153401501. [DOI] [PubMed] [Google Scholar]
- 11.Mousavi S, Gouya M, Ramazani R. Cancer incidence and mortality in Iran. Ann Oncol. 2008;20:556–63. doi: 10.1093/annonc/mdn642. [DOI] [PubMed] [Google Scholar]
- 12.O'Hara J, Douple E, Richmond R. Enhancement of radiation-induced cell kill by platinum complexes (carboplatin and iproplatin) in V79 cells. Int J Radiat Oncol Biol Phys. 1986;12:1419–22. doi: 10.1016/0360-3016(86)90185-9. [DOI] [PubMed] [Google Scholar]
- 13.Pecorelli S. Revised FIGO staging for carcinoma of the vulva, cervix, and endometrium. Int J Gynecol Obstet. 2009;105:103–4. doi: 10.1016/j.ijgo.2009.02.012. [DOI] [PubMed] [Google Scholar]
- 14.Peters WA, Liu PY, Barrett RJ, et al. Concurrent chemotherapy and pelvic radiation therapy compared with pelvic radiation therapy alone as adjuvant therapy after radical surgery in high-risk early-stage cancer of the cervix. J Clin Oncol. 2000;18:1606–13. doi: 10.1200/JCO.2000.18.8.1606. [DOI] [PubMed] [Google Scholar]
- 15.Rose P, Ali S, Watkins E, et al. Long-term follow-up of a randomized trial comparing concurrent single agent cisplatin, cisplatin-based combination chemotherapy, or hydroxyurea during pelvic. J Clin Oncol. 2007;25:2804–10. doi: 10.1200/JCO.2006.09.4532. [DOI] [PubMed] [Google Scholar]
- 16.Rose P, Bundy B, Watkins E. Concurrent cisplatin-based radiotherapy and chemotherapy for locally advanced cervical cancer. N Engl J Med. 1999;340:1144–53. doi: 10.1056/NEJM199904153401502. [DOI] [PubMed] [Google Scholar]
- 17.Rotman M, Sedlis A, Piedmonte MR, et al. A phase III randomized trial of postoperative pelvic irradiation in stage IB cervical carcinoma with poor prognostic features: Follow-up of a gynecologic oncology group study. Int J Radiat Oncol Biol Phys. 2006;65:169–76. doi: 10.1016/j.ijrobp.2005.10.019. [DOI] [PubMed] [Google Scholar]
- 18.Rydzewska L, Tierney J, Vale C, et al. Neoadjuvant chemotherapy plus surgery versus surgery for cervical cancer. Cochrane Database Syst Rev. 2010:1. doi: 10.1002/14651858.CD007406.pub2. [DOI] [PubMed] [Google Scholar]
- 19.Strauss H, Kuhnt T, Laban C, et al. Chemoradiation in cervical cancer with cisplatin and high-dose rate brachytherapy combined with external beam radiotherapy results of a Phase-II study. Strahlenther Onkol. 2002;178:378–85. doi: 10.1007/s00066-002-0956-1. [DOI] [PubMed] [Google Scholar]
- 20.Stumpf J, Nemeth G, Takassi-Wagu L, et al. Intra-arterial versus intravenous administration during intracavitary irradiation of cervical tumors. Mould RE editor Brachytherapy 2. Proceedings of Selectron user's meeting, The Hugue. 1988:324–7. [Google Scholar]
- 21.Thomas G. Improved treatment for cervical cancer-concurrent chemotherapy and radiotherapy. N Engl J Med. 1999;340:1198–1200. doi: 10.1056/NEJM199904153401509. [DOI] [PubMed] [Google Scholar]
- 22.Vrdoljak E, Omrčen T, Novaković Ž, et al. with ifosfamide and cisplatin followed by consolidation chemotherapy for women with locally advanced carcinoma of the uterine cervix-Final results of a prospective phase 2. Gynecol Oncol. 2006;103:494–9. doi: 10.1016/j.ygyno.2006.03.060. [DOI] [PubMed] [Google Scholar]
- 23.Wang B, Kwon A, Zhu Y, et al. Image-guided intracavitary high-dose-rate brachytherapy for cervix cancer: A single institutional experience with three-dimensional CT-based planning. Brachytherapy. 2009;8:240–7. doi: 10.1016/j.brachy.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 24.Whitney C, Sause W, Bundy B. Randomized comparison of fluorouracil plus cisplatin versus hydroxyurea as an adjunct to radiation therapy in stage IIB-IVA carcinoma of the cervix with negative para. J Clin Oncol. 1999;17:1339. doi: 10.1200/JCO.1999.17.5.1339. [DOI] [PubMed] [Google Scholar]
- 25.Yamashita H, Okuma K, Kawana K, et al. Comparison between conventional surgery plus postoperative adjuvant radiotherapy and concurrent chemoradiation for FIGO stage IIB cervical carcinoma. Am J Clin Oncol. 2010;33:583–6. doi: 10.1097/COC.0b013e3181cae5b7. [DOI] [PubMed] [Google Scholar]


