Abstract
Background:
Hepatocellular Carcinoma (HCC) is the primary liver cancer with high incidence and mortality rates. Currently one of the major etiologies for liver disease, HCC and liver transplantation is nonalcoholic fatty liver disease (NAFLD). The aim of the present study was to evaluate the epidemiological, histopathological and clinical aspects of HCC transplant patients, with emphasis on NAFLD etiology.
Methods:
This study included all HCC patients submitted to liver transplantation from 2010 to 2016 of the University Reference Center. The analyzed variables were age, gender, ethnicity, causes that led to liver transplantation, alpha-fetoprotein (AFP) dosage, histological aspects, recurrence, survival and NAFLD.
Results:
A total of 60 patients were included in the study being 80% men with a mean age of 58.3 ± 10.6 years. All patients were cirrhotic. The causes that led to the transplantation were the presence of the hepatitis C virus (HCV) (56.6% of the patients), an association of the virus with alcohol (20%), the presence of the hepatitis B virus (HBV) (20%), alcoholic liver disease (ALD) (50.9%) and NAFLD (25%). Of the latter, eight were diagnosed pre-transplantation and seven were NAFLD carriers without a previous diagnosis. Regarding the Edmondson-Steiner histological classification, 58.5% of the patients were classified as grade ≤ II.
Conclusions:
There is predominance of male patients with a mean age of 58.3 years. Degree ≤ II is the most frequent to the Edmondson-Steiner histological classification in the evaluated casuistic. HCV, ALD and NAFLD is the most common etiological agents found in the study. The (high) underestimated prevalence of NAFLD in the pre-transplanted patients is due to the fact that all patients presented cirrhosis, masking NAFLD signals.
Keywords: Liver cancer, non-alcoholic fatty liver disease, liver transplantation, epidemiology, risk factors
Introduction
Hepatocellular carcinoma (HCC) is the primary liver cancer and the 5th most diagnosed type of cancer in men worldwide (Jemal et al., 2011; El-Serag, 2012). In 2012, approximately 78,000 new cases of liver cancer and 745,000 deaths were reported worldwide. The highest rates HCC are reported in East Asia and the Sub-Saharan Africa (Ferlay et al., 2015).
HCC carcinogenesis is due to the interaction between environmental and genetic factors, among others, like cirrhosis, hepatitis B (HBV) or C (HCV) infection, alcohol consumption, aflatoxin-contaminated food and non-alcoholic fatty liver disease (NAFLD) (Figure 1). The latter has been one of the most common causes of liver disease and the third leading cause of liver transplantation in the United States, behind only HCV and cirrhosis related to alcoholic liver disease (ALD) (Charlton et al., 2011).
Figure 1.
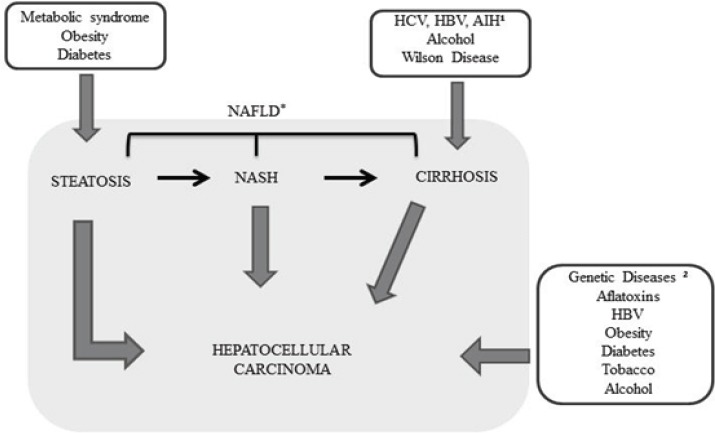
Risk Factors for the Development Stage of Hepatocellular Carcinoma (Tunissiolli et al., 2017). HCV (Hepatitis C virus), HBV (Hepatitis B virus), 1AIH (Autoimmune Hepatitis), 2Genetic Diseases: Alpha-1-antitrypsin deficiency, Hemochromatosis, Porphyria and Tyrosinemia. *The Spectrum of NAFLD: Steatosis, NASH and Cirrhosis.
NAFLD is known to be the hepatic manifestation of metabolic syndrome (MS), defined as presenting three or more of the following factors: BMI ≥ 25 cm/m2, triglycerides >150 mg/dL, low HDL cholesterol levels (<40mg/dL in men and <50 mg/dL in women), high blood pressure and diabetes (Torres et al., 2012; Hassan et al., 2014).
Early HCC diagnosis is important for patient survival. In cirrhotic patients, the diagnosis is non-invasive, based on magnetic resonance imaging and computed tomography with contrast. When the findings are not conclusive, histopathological diagnoses (biopsies) are necessary (EASL-EORTC Clinical practice guidelines: management of hepatocellular carcinoma, 2012). Histopathological evaluation characterizes and differentiates liver biopsies as malignant or benign lesions. Among these evaluations is the Edmondson-Steiner classification, which classifies HCC according to the degree of cellular differentiation, corresponding to grades I, II, III and IV (Edmondson and Steiner, 1954; Gonzalez and Keeffe, 2011).
Currently different HCC treatments are available, and their choice takes into account cancer stage, liver function, patient comorbidities and available resources (Maida et al., 2014). The Barcelona Clinic Liver Cancer (BCLC) classification categorizes patients as very early, early, intermediate, advanced and terminal stages. In the early stages, liver transplantation, resection and ablation are considered curative options for patients. Chemoembolization is indicated for the intermediate stage, while advanced patients may be treated with multikinase inhibitors, such as Sorafenib and Regorafenib. End-stage patients undergo palliative care to improve quality of life (Maida et al., 2014; Yang and Roberts, 2010; Forner et al., 2014; Mazzanti et al., 2016; Bruix et al., 2017).
Due to the high prevalence of HCC worldwide, late diagnosis, high mortality rates and increased number of risk factors, we aimed to evaluate clinical, epidemiological and histopathological aspects of HCC patients undergoing liver transplantation. Considering the increasing prevalence of NAFLD as a cause of chronic liver disease, this etiology was emphasized in this study.
Materials and Methods
Study design and patients
A retrospective, descriptive and cross-sectional study was carried out based on data from electronic medical records of HCC patients submitted to liver transplantation from 2010 to 2016, in a University Reference Center and, therefore, encompasses a large region in Brazil. An established form of the Liver Transplantation Service was used to collect epidemiological, clinical and histopathological data. All patients with an HCC diagnosis who underwent liver transplantation consecutively at this institution between 2010 and 2016 were included in the study. Those who did not obey the HCC condition as a diagnosis were excluded.
Clinical, epidemiological and histopathological data were analyzed according to the following variables: gender, age, ethnicity, smoking, diabetes mellitus, presence of cirrhosis and the following etiologies: HBV, HCV, ALD, autoimmune hepatitis (AIH), genetic disease (Table 1), recurrence, survival and NAFLD. Patients diagnosed with NAFLD respected the following criteria: absence or moderate alcohol consumption, absence of exposure to potential hepatotoxins or associated medications and metabolic syndrome (MS: low HDL cholesterol, triglycerides >150mg/dL, hypertension, diabetes, and BMI ≥ 25 kg / m2) (Garvey et al., 2016) (Table 2). These same criteria were sought in patients who were not diagnosed for NAFLD to verify the possibility of undiagnosed NAFLD, since cryptogenic cirrhosis (CC) may mask this diagnosis (Vernon et al., 2011; Chalasani et al., 2012). Pre-transplantation alpha-fetoprotein (AFP) and the Edmondson-Steiner histological classification were also evaluated.
Table 1.
Distribution of Patients According to Clinical and Epidemiological Aspects (n = 60)
| Variables | % | |
|---|---|---|
| Gender (n,%) | ||
| Male | 48 | 80 |
| Female | 12 | 20 |
| Age (mean, standard deviation) | ||
| Male | 57.3 | 10.4 |
| Female | 62 | 10.6 |
| Smoking (n,%) | ||
| Yes | 25 | 55.50 |
| No | 20 | 44.50 |
| Etiology of Chronic Liver Disease (n, %) | ||
| HBV | 12 | 20 |
| HCV | 34 | 56.60 |
| ALD | 26 | 50.90 |
| HCV+ALD | 12 | 20 |
| NAFLD | 15 | 25 |
| AIH | 1 | 1.60 |
| Hemochromatosis | 1 | 1.60 |
| Comorbidity (n,%) | ||
| Diabetes | 24 | 40 |
AIH, autoimmune hepatitis; ALD, alcoholic liver disease; HBV, hepatitis B virus; HCV, hepatitis C virus; NAFLD, nonalcoholic fatty liver disease.
Table 2.
Components Considered for the Classification of Metabolic Syndrome and Used in the Diagnosis of NAFLD (Adapted from ACE Position Statement on the Insulin Resistance Syndrome, 2003; EASL–EASD–EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease, 2016).
| Components | Reference Levels | Patients diagnosed with NAFLD (n=8) mean ± SD | Patients without previous diagnosis of NAFLD (n=7) mean ± SD |
|---|---|---|---|
| BMI | ≥ 25 kg/m2 | 29.3 ± 3.7 | 31.5 ± 3.8 |
| Triglycerides | >150 mg/dL | 76.7 ± 28.4 | 105.8 ± 53.5 |
| HDL Cholesterol | 45.5 ± 13.8 | 47.5 ± 17.8 | |
| Males | <40mg/dL | ||
| Females | <50 mg/dL | ||
| Blood pressure | ≥130mmHg or ≥85mmHg / treatment for SAH | (-) | (-) |
| Glycemia | >100mg/dL / treatment for DM | 131.5 ± 72 | 128.2 ± 46.1 |
BMI, Body mass index; DM, Diabetes mellitus; HDL, High density lipoprotein; NAFLD, non-alcoholic fatty liver disease; SAH, Systemic arterial hypertension; SD, standard deviation.
Abdominal circumference measures were not obtained, because this is a retrospective study and these data are difficult to collect, since they are not included in all medical records. Most MS classification systems use the size of the abdominal circumference as a criterion. Thus, we chose to include the American Association of Clinical Endocrinologists definition in this study, which applies the body mass index (BMI) instead of abdominal circumference (Garvey et al., 2016).
Statistical analysis
Electronic medical record data were analyzed through Microsoft Office Excel 2010 analyses, by means of descriptive statistics and tables containing the percentage calculations of the presented variables. The continuous parameters were expressed average and standard deviation. The survival period was calculated between the surgical intervention and the date of the last consultation, or death. The Kaplan-Meier method was used to calculate the survival rate using the Stats Direct Statistical Software, version 2.0.0 (England: StatsDirect Ltd). Values at p <0.05 were considered significant.
Ethical approval
The present study was conducted in full accordance with the World Medical Association Declaration of Helsinki. The study protocol was reviewed and approved by Sao Jose do Rio Preto Medical School- FAMERP Ethics and Research Committee process no. 6228/2011. The requirement for written informed consent was waived due to the retrospective nature of this study. All patient data were anonymized prior to the analysis.
Results
During the study period, 236 liver transplants were performed of which 60 patients presented HCC (25.4%). Of the total, 48 were men (80%) and 12 were women (20%). Overall mean age was 58.3 ± 10.6 years. For men, mean age was of 57.3 years ± 10.4 years, while women presented a mean age of 62 ± 10.6 years.
Regarding demographic data, the patients reside mostly in the Southeastern region of Brazil (85%), while the rest inhabit the Center-West (5%), Northeastern (5%) and South (5%) regions. For the calculation of the percentage of the analysis of each variable, only patients who did not present all data in their medical records were excluded. Table 1 displays the clinical and demographic characteristics of the patients.
Initially, eight patients were diagnosed with NAFLD. Undergoing a detailed investigation of the MS risk factors, it was possible to classify another seven, previously undiagnosed, patients with NAFLD. These patients had three or more criteria that indicate MS and could, thus, be classified as NAFLD. In non-NAFLD patients, the mean BMI was of 25.1 kg/m2, well below the average presented by the reported or sub-notified NAFLD patients, of 29.3 kg/m2 and 31.5 kg/ m2, respectively (Table 2).
The histopathological diagnoses were described according to the Edmondson-Steiner classification for 53 patients. No classification was possible for seven samples, due to the extensive necrotic characteristics of the tumors, making it impossible to measure the degree of cell differentiation. A total of 58.5% of the patients were characterized as grade ≤ II, while 41.5% were classified as grade ≥ III (Table 3).
Table 3.
Distribution of Patients According to Histological Differentiation and the Edmondson-Steiner Classification (n= 53)
| Edmondson Steiner’s Classification | (n) | Total | (%) |
|---|---|---|---|
| Grade I | 1 | 31 ≤ II | 58.50% |
| Grade I/II | 5 | ||
| Grade II | 25 | ||
| Grade I, II, III | 2 | 22 ≥ III | 41.50% |
| Grade II/III | 17 | ||
| Grade III | 2 | ||
| Grade II/III/IV | 1 |
Of the 42 patients in which alpha-fetoprotein (AFP) was dosed, 37 presented levels below 20 ng/mL (88.09%); two presented levels ranging from 20 ng/mL to 55 ng/mL (4.76%) and three presented AFP levels higher than 150 ng/mL (7.14%).
HCC post-transplant recurrence was observed in two patients (3.3%), and, in both, the tumor recurrence site was the liver. The means between the transplantation period and the tumor recurrence diagnosis was of 14.1 months for one patient and 9.5 months for the other, and both subsequently died.
Regarding survival estimates, the Kaplan-Meier method was applied, with a mean survival estimate of approximately 12 months. The following values were obtained: 66% for NAFLD patients and 69% for non-NAFLD patients (log-rank value p = 0.712). These results were not significant among the analyzed variables and, therefore, did not influence post-transplant survival in HCC patients.
Discussion
Among the transplanted HCC patients, an epidemiological and clinical profile similar to those reported in the literature was identified. Male patients were more frequent (80%), corroborating data from Globocan (Ferlay et al., 2015). This male predominance is probably related to the greater exposure of men to risk factors, such as alcoholism, viral hepatitis and hormonal factors (Mittal and El-Serag, 2013; Keng et al., 2012). In Brazil, Carrilho et al., (2010) conducted a multicenter study with 1405 patients, and reported that 78% were male. Another study, also conducted in Brazil, over a 12-year period, found that 84.2% of HCC patients were men (Raphe et al., 2013). In a recent study in Saudi Arabia, men comprised approximately 3/4 of the HCC sample (n=128; 77.3%) (Qari and Mosli, 2017). Thus, the predominance of males among HCC patients was confirmed.
The mean age of the patients was of 58.3± 10.6 years (57.3 for men and 62 for women). Other Brazilian epidemiological studies report means of 57 and 59 years (Paranagua-Vezozzo et al, 2014; Carrilho et al., 2010). In a multicenter study conducted in Turkey, the mean age of HCC individuals was 62 years old (Alacacioglu et al., 2008). The literature has shown that HCC incidence increases with age. However, hepatocarcinogenesis in regions where HBV prevalence is highest affects age, which ranges between 45 and 60, due to HBV infection epidemics through vertical transmission, an important risk factor for the development of HCC without cirrhosis in younger individuals (Boussouar et al., 2016; El-Serag and Kanwal, 2014).
Studies in the West have pointed to other important risk factors for the development of HCC, such as NAFLD, diabetes mellitus and MS (El-Serag and Kanwal, 2014). NAFLD currently affects about 1/3 of the adult American population and, in conjunction with other co-factors, such as obesity, diabetes mellitus and steatosis, promotes hepatocyte injury, progressing to fibrosis, cirrhosis and, consequently, HCC (Gao and Yao, 2009; Tunissiolli et al., 2017). NAFLD is a clinical and pathological condition with a progressive and spectral character, in which excessive fat accumulation occurs in hepatocytes, while non-alcoholic steatohepatitis (NASH) represents the inflammatory and fibrotic form of progression for cirrhosis (Vernon et al., 2011; Hassan et al., 2014).
Of the 236 transplanted patients in the study period, 60 were transplanted with HCC and eight were diagnosed with NAFLD as one of the causes that led to the transplant, while seven presented undiagnosed NAFLD. This calls attention to a growing number of patients with peculiar metabolic alterations that require specific care implementations, including public health policy aspects. It should be noted that the number of patients with undiagnosed NAFLD increased from 13.3% to 25% of the total number of patients with NAFLD in this HCC transplant sample, pointing to the potential for metabolic changes and their consequences before and after transplantation, which could go unnoticed without this diagnosis.
Somboon et al., (2014) in a study performed with 308 HCC patients in Asia found a 2.4% rate of patients presenting with NAFLD. Another study reported 3% of patients with this etiology for HCC (Carrilho et al., 2010). In the present study, 25% of the patients presented NAFLD, highlighting the increase in the percentage of this etiology in the last years. Studies in developed countries, such as the USA, have justified obesity, metabolic syndrome and diabetes epidemics as contributing factors to this increase (Yang et al., 2010; Waller et al., 2015). Studies have also demonstrated changes in the causes that lead to liver transplantation and significant NAFLD increases, as well as among those in HCC patients (Charlton et al., 2011; Wang et al., 2014).
Cirrhosis of any etiology is an important risk factor for hepatocarcinogenesis, associated with approximately 80% to 90% of HCC cases worldwide (Fattovich et al., 2004; El-Serag and Kanwal, 2014; Balogh et al., 2016). Carrilho et al., (2010) reported cirrhosis in 98% of cases when evaluating 1,405 patients in several regions of Brazil. According to Somboon et al., (2014) in Thailand, of a total of 125 HCC patients studied, 90% presented cirrhosis. In the present study, cirrhosis was present in all of the patients, confirming the high incidence of cirrhosis as a risk factor.
Cirrhosis masks the variables linked to MS and, therefore, the diagnosis of pre-transplantation NAFLD may go unnoticed. Malik et al., (2009) found a significant number of patients with cirrhosis due to NAFLD with MS characteristics, namely, significantly more obese patients with a higher incidence of diabetes and pre-transplant hypertension when compared to controls (Malik et al., 2009). Malnutrition, as well as dyslipidemia and MS, also mask obesity (Bonin-Guillaume et al., 2006; Kim et al., 2013). Peripheral vasodilation in the advanced phase of cirrhosis masks arterial hypertension, initially reducing the need for antihypertensive drugs, progressively leading to hypotension (Henriksen and Moller, 2006).
NAFLD rates are underestimated due to the difficulty in diagnosis, since this is an asymptomatic disease implicated as a cause of cirrhosis, due to the disappearance of histological signs and diagnoses performed by clinical-laboratory findings in annual check-ups classified only as MS (Bugianesi et al., 2002; Adam et al., 2005; Sass et al., 2005).
MS is represented by an association of risk factors related to systemic arterial hypertension, central obesity, insulin resistance and dyslipidemia, which were found in 25% of the patients evaluated in the present study. With the increasing upsurge of MS in the USA, a study comprising 3,649 HCC cases and 743 intrahepatic cholangiocarcinoma cases was carried out in order to evaluate the association between MS and the development of these types of liver cancers. The study concluded that MS is indeed an important risk factor for these liver cancers (Wenzel et al., 2011). Thus, the notification of NAFLD cases could contribute to a more effective treatment when this condition is associated with other causes of cirrhosis. We emphasize that there has been an increase in this etiology worldwide, and the need to screen for pre-transplant signs and symptoms is extremely relevant.
Hyperinsulinemia and insulin resistance are major causative factors in the pathogenesis of MS and NAFLD (Reisin and Alpert, 2005; Paschos and Paletas, 2009). Studies have shown that patients with fatty pancreas present higher rates of MS when compared to those without pancreatic steatosis (Lee et al., 2009; Wu and Wang, 2013).
Among the factors involved with MS, diabetes has been found at high rates in NAFLD patients. These patients also show higher mortality secondary to cardiovascular disease when compared to patients with non-diabetic NAFLD (Adams et al., 2010; Torres et al., 2012; Wang et al., 2014). Regarding NAFLD progression, the theory of multiple hits stands out: as an initial condition, first-stage hepatic steatosis develops, followed by a succession of events, such as oxidative stress, intestinal endotoxin production and inflammation (multiple hits). Thus, the steatotic liver becomes vulnerable to multiple hits, resulting in hepatocyte lesions, inflammation, fibrosis and, consequently, HCC (Takaki et al., 2013; Buzzetti et al., 2016).
In addition to the metabolic conditions associated with NAFLD, hereditary genetic-metabolic factors also play a role, such as glycogen deposition diseases, lipodystrophies, tyrosinemia, Weber-Christian disease and Wilson’s disease (Sass et al., 2005; Hassan et al., 2014). Metabolites resulting from drug intake are also risk factors for the development of hepatic steatosis, with methotrexate, amiodarone and tamoxifen among the most hepatotoxic (Sass et al., 2005; Torres et al., 2012). Some surgical procedures are also associated with NAFLD, such as jejunoileal bypass, gastroplasties and total parenteral nutrition (Paschos and Paletas, 2009).
In relation to the etiology of liver disease, alcohol metabolites are responsible for the formation of adducts toxic to hepatocytes, resulting in defects in DNA repair and oxidative stress, becoming an important risk factor for cirrhosis and consequent HCC development (Testino et al., 2014). In the present study, 69.3% of the analyzed individuals were drinkers (consumption ≥ 80g alcohol/ day) and ALD was present in 50.9% of the cases. A multicenter study in Spain found that 30% of the analyzed individuals were alcoholics (Varela et al., 2010). Another epidemiological study conducted in Thailand reported that 26% of patients had ALD as an etiology for HCC (Somboon et al., 2014). The ALD rates found herein were more than two-fold higher when compared to other studies. In England, transplant rates, hospital admissions and mortality have increased, and ALD has made significant contributions to this statistic (Thomson et al., 2008; Green et al., 2017).
Other important risk factors for the development of cirrhosis and HCC are chronic HCV and HBV infections, where prevalence varies according to region. Estimates attributed to viral hepatitis are 60% for HCV in Europe and the USA, and 60% for HBV in Africa and Asia (Fassio et al., 2010). A study conducted in Germany found that 20.5% of HCC cases displayed HCV infections (Kirchner et al., 2010). A multicenter study conducted in Turkey reported that 21.3% of patients presented positive HCV serology, with this condition becoming a precursor etiology for HCC in these cases (Alacacioglu et al., 2008). In the present study, 56.6% of the analyzed individuals presented HCV and 20% presented HBV, corroborating data obtained in the study performed by Carrilho and colleagues in which 54% and 16% rates were found for HCV and HBV, respectively, evidencing that the most frequent viral disease in Brazil is HCV (Carrilho et al., 2010).
Currently, several serological markers for HCC detection and monitoring are available, with AFP being the most commonly applied. However, AFP presents greater specificity than sensitivity (Lok et al., 2010; Morimoto et al., 2012). In the present study, 88.09% (n= 37) of the cases presented normal levels of AFP (<20 ng/mL), 4.76% presented rates between 20 and 55 ng/mL and 7.14%, over 150 ng/mL. According to literature data, 29.2% of the analyzed cases of HCC patients had normal levels of AFP (<20 ng/mL), concluding would not be a good diagnostic marker (Raphe et al., 2013). A study conducted in Turkey with HCC patients reported that 38.9% had AFP levels <20 ng/mL and only 21.7% had levels >400 ng/mL, also demonstrating low marker sensitivity in the Turkish population presenting HCC (Alacacioglu et al., 2008). The high AFP normality index observed in the present study reinforces the fact that AFP is a low sensitivity serological marker.
The Edmondson-Steiner classification indicated that the majority of patients (58.5%, n= 31) belonged to the intermediate grade (≤ II). Regarding histological analysis, when present, necrosis and hemorrhage injure hepatic tissue, making classification impossible (Edmondson and Steiner, 1954). Boussouar and colleagues failed to classify four HCC samples from patients on transplant waiting lists due to tumor necrosis (Boussouar et al., 2016). In the present study, due to the widely necrotic tumor area, seven HCC patient samples (11.7%) were not classified. The degree of histological HCC differentiation has clinical implications related to the greater potential for severity and risk of cancer recurrence (Tamura et al., 2001; Cillo et al., 2004). The recurrence of HCC post-transplantation has been reported, with rates ranging from 10 to 20%, being a major clinical concern (Waller et al., 2015). In the present study, 3.33% (n=2) of the patients presented HCC recurrence. It is known that a relationship between proliferative cellular activity in the post-resected cirrhotic liver and the occurrence or recurrence of HCC exists (Chiu et al., 1993).
Because it is one of the malignant tumors with the highest lethality and a short survival, HCC is considered by the World Health Organization (WHO) as an important public health problem (Ferlay et al., 2015). In a recent multicenter study in Brazil, AFP >200 ng/mL and non-chemoembolization were associated with lower survival rates (Bina-Possatto et al., 2017). In Brazil, Franca et al., (2004) found a survival of 84 and 74% in the 1st and 5th years after liver transplantation, respectively, similar data to those found in a study conducted in Europe, where, after a follow-up of 31 months, patient survival rates were was 84%, 74% and 74% in the 1st, 3rd and 5th years, respectively. In the present study, the results regarding survival were not significant among the analyzed variables.
Even with the scientific advances and implementation of actions for early HCC detection, no improvement in the survival rate in the last three decades has occurred, because most patients start to present symptoms only at an advanced stage, limiting treatments (Papaiordanou et al., 2009). Thus, liver cancer studies are important to obtain better knowledge and improvement of risk factors, preventive measures and diagnostic methods.
In conclusion, the prevalence of HCC among transplant recipients is 25.4%, with a mean age of 58.3 years and a predominance of males. The most frequent risk factors are HCV, ALD and NAFLD. NAFLD rates among transplanted HCC patients increased from 13.3% to 25% when considering an NAFLD diagnosis masked by cirrhosis. This data indicates that NAFLD is underestimated when transplants are performed. This reinforces the fact that NAFLD is considered to be the hepatic manifestation of MS, so the monitoring of these components (glycemia, triglycerides and HDL cholesterol levels, obesity and arterial hypertension) is important for the early NAFLD detection. Prospective and multicenter studies are relevant to create new diagnostic methods for MS and NAFLD, which are key to ensure progress in the longevity of liver transplant recipients.
Abbreviations
AFP: alpha-fetoprotein; AIH: autoimmune hepatitis; ALD: alcoholic liver disease; BMI: Body Mass Index; CC: cryptogenic cirrhosis; HBV: hepatitis B virus; HCC: hepatocellular carcinoma; HCV: hepatitis C virus; HDL: High Density Lipoproteins; LT: liver transplantation; MS: metabolic syndrome; NAFLD: nonalcoholic fatty liver disease.
Funding details
This study was conducted without internal or external funding.
Disclosure statement
No potential conflict of interest was reported by the authors.
Authors’ contribution
N.M.T. and M.M.U.C.N.: conception and design of study; N.M.T and M.M.U.C.N.: acquisition of data; N.M.T., M.M.U.C.N., R.C.M.A.S and E.M.G.B.: analysis and/or interpretation of data; N.M.T.: drafting of the manuscript; E.M.G.B., E.C.P., R.C.M.A.S and R.F.S: critically revising the manuscript for important intellectual content. All authors approved the final version of the manuscript for publication.
Acknowledgements
Sao Paulo Research Foundation (FAPESP) (Process number No. 2012, CNPq, CAPES and FAMERP/FUNFARME). To Dr. André Rodrigueiro C. P. Oliveira for the research contributions, M.D. Dalísio de Santi Neto, Chief of the Pathology Sector of the Base Hospital of Sao Jose do Rio Preto for his valuable help in the performed analyses and histological classifications; and nurse Helen C.C. Felício for collaboration in data collection.
References
- 1.Adams LA, Lymp JF, St Sauver J, et al. The natural history of nonalcoholic fatty liver disease: a population-based cohort study. Gastroenterology. 2005;129:113–21. doi: 10.1053/j.gastro.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 2.Adams LA, Harmsen S, St Sauver JL, et al. Nonalcoholic fatty liver disease increases risk of death among patients with diabetes: a community-based cohort study. Am J Gastroenterol. 2010;105:1567–73. doi: 10.1038/ajg.2010.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alacacioglu A, Somali I, Simsek I, et al. Epidemiology and survival of hepatocellular carcinoma in Turkey: outcome of multicenter study. Jpn J Clin Oncol. 2008;38:683–8. doi: 10.1093/jjco/hyn082. [DOI] [PubMed] [Google Scholar]
- 4.Balogh J, Victor D, Asham EH, et al. Hepatocellular carcinoma: a review. J Hepatocell Carcinoma. 2016;3:41–53. doi: 10.2147/JHC.S61146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bina-Possatto M, de Ataide EC, Fazzio-Escanhoela CA, et al. Factors related to hepatocellular carcinoma recurrence after liver transplantation-A Brazilian multicenter study. Transplant Proc. 2017;49:863–66. doi: 10.1016/j.transproceed.2017.01.055. [DOI] [PubMed] [Google Scholar]
- 6.Bonin-Guillaume S, Herrmann FR, Boillat D, et al. Insulinemia and leptinemia in geriatric patients: markers of the metabolic syndrome or of undernutrition? Int J Diabetes Metab. 2006;32:236–43. doi: 10.1016/s1262-3636(07)70274-4. [DOI] [PubMed] [Google Scholar]
- 7.Boussouar S, Itti E, Lin SJ, et al. Functional imaging of hepatocellular carcinoma using diffusion-weighted MRI and (18)F-FDG PET/CT in patients on waiting-list for liver transplantation. Cancer Imaging. 2016;16:4. doi: 10.1186/s40644-016-0062-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bruix J, Qin S, Merle P, et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;389:56–66. doi: 10.1016/S0140-6736(16)32453-9. [DOI] [PubMed] [Google Scholar]
- 9.Bugianesi E, Leone N, Vanni E, et al. Expanding the natural history of nonalcoholic steatohepatitis: from cryptogenic cirrhosis to hepatocellular carcinoma. Gastroenterology. 2002;123:134–40. doi: 10.1053/gast.2002.34168. [DOI] [PubMed] [Google Scholar]
- 10.Buzzetti E, Pinzani M, Tsochatzis EA. The multiple-hit pathogenesis of non-alcoholic fatty liver disease (NAFLD) Metabolism. 2016;65:1038–48. doi: 10.1016/j.metabol.2015.12.012. [DOI] [PubMed] [Google Scholar]
- 11.Carrilho FJ, Kikuchi L, Branco F, et al. Clinical and epidemiological aspects of hepatocellular carcinoma in Brazil. Clinics. 2010;65:1285–90. doi: 10.1590/S1807-59322010001200010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chalasani N, Younossi Z, Lavine JE, et al. The diagnosis and management of non-alcoholic fatty liver disease: practice guideline by the American Gastroenterological Association, American Association for the Study of Liver Diseases, and American College of Gastroenterology. Gastroenterology. 2012;142:1592–1609. doi: 10.1053/j.gastro.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 13.Charlton MR, Burns JM, Pedersen RA, et al. Frequency and outcomes of liver transplantation for nonalcoholic steatohepatitis in the United States. Gastroenterology. 2011;141:1249–53. doi: 10.1053/j.gastro.2011.06.061. [DOI] [PubMed] [Google Scholar]
- 14.Chiu JH, Wu LH, Kao HL, et al. Can determination of the proliferative capacity of the nontumor portion predict the risk of tumor recurrence in the liver remnant after resection of human hepatocellular carcinoma? Hepatology. 1993;18:96–102. [PubMed] [Google Scholar]
- 15.Cillo U, Vitale A, Bassanello M, et al. Liver transplantation for the treatment of moderately or well-differentiated hepatocellular carcinoma. Ann Surg. 2004;239:150–9. doi: 10.1097/01.sla.0000109146.72827.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Edmondson HA, Steiner PE. Primary carcinoma of the liver: a study of 100 cases among 48,900 necropsies. Cancer. 1954;7:462–503. doi: 10.1002/1097-0142(195405)7:3<462::aid-cncr2820070308>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 17.El-Serag HB. Hepatocellular carcinoma. N Engl J Med. 2011;365:1118–27. doi: 10.1056/NEJMra1001683. [DOI] [PubMed] [Google Scholar]
- 18.El-Serag HB. Epidemiology of viral hepatitis and hepatocellular carcinoma. Gastroenterology. 2012;142:1264–73. doi: 10.1053/j.gastro.2011.12.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.El-Serag HB, Kanwal F. Epidemiology of hepatocellular carcinoma in the United States: where are we?Where do we go? Hepatology. 2014;60:1767–75. doi: 10.1002/hep.27222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.European Association for the Study of the Liver. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2012;56:908–943. doi: 10.1016/j.jhep.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 21.Fassio E, Diaz S, Santa C, et al. Etiology of hepatocellular carcinoma in Latin America: a prospective, multicenter, international study. Ann Hepatol. 2010;9:63–9. [PubMed] [Google Scholar]
- 22.Fattovich G, Stroffolini T, Zagni I, Donato F. Hepatocellular carcinoma in cirrhosis: incidence and risk factors. Gastroenterology. 2004;127:35–50. doi: 10.1053/j.gastro.2004.09.014. [DOI] [PubMed] [Google Scholar]
- 23.Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in Globocan. Int J Cancer. 2015;136:359–86. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 24.Forner A, Gilabert M, Bruix J, Raoul J. Treatment of intermediate-stage hepatocellular carcinoma. Nat Rev Clin Oncol. 2014;11:525–35. doi: 10.1038/nrclinonc.2014.122. [DOI] [PubMed] [Google Scholar]
- 25.Franca AV, Elias Junior J, Lima BL, Martinelli A, Carrilho F. Diagnosis, staging and treatment of hepatocellular carcinoma. Braz J Med Biol Res. 2004;37:1689–1705. doi: 10.1590/s0100-879x2004001100015. [DOI] [PubMed] [Google Scholar]
- 26.Gao C, Yao SK. Diabetes mellitus: a “true” independent risk factor for hepatocellular carcinoma? Hepatobiliary Pancreat Dis Int. 2009;8:465–73. [PubMed] [Google Scholar]
- 27.Garvey WT, Mechanick JI, Brett EM, et al. American association of clinical endocrinologists and american college of endocrinology comprehensive clinical practice guidelines for medical care of patients with obesity. Endocr Pract. 2016;22:1–203. doi: 10.4158/EP161365.GL. [DOI] [PubMed] [Google Scholar]
- 28.Gomaa AI, Khan SA, Toledano MB, Waked I, Taylor-Robinson SD. Hepatocellular carcinoma: epidemiology, risk factors and pathogenesis. World J Gastroenterol. 2008;14:4300–8. doi: 10.3748/wjg.14.4300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gonzalez SA, Keeffe EB. Diagnosis of hepatocellular carcinoma: role of tumor markers and liver biopsy. Clin Liver Dis. 2011;15:297–306. doi: 10.1016/j.cld.2011.03.012. [DOI] [PubMed] [Google Scholar]
- 30.Green MA, Strong M, Conway L, Maheswaran R. Trends in alcohol-related admissions to hospital by age, sex and socioeconomic deprivation in England 2002/03 to 2013/14. BMC Public Health. 2017;17:412. doi: 10.1186/s12889-017-4265-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hassan K, Bhalla V, El Regal ME, A-Kader H. Nonalcoholic fatty liver disease: a comprehensive review of a growing epidemic. World J Gastroenterol. 2014;20:12082–101. doi: 10.3748/wjg.v20.i34.12082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Henriksen JH, Moller S. Liver cirrhosis and arterial hypertension. World J Gastroenterol. 2006;12:678–85. doi: 10.3748/wjg.v12.i5.678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 34.Keng VW, Largaespada DA, Villanueva A. Why men are at higher risk for hepatocellular carcinoma? J Hepatol. 2012;57:453–4. doi: 10.1016/j.jhep.2012.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim D, Kim WR, Kim HJ, Therneau T. Association between noninvasive fibrosis markers and mortality among adults with nonalcoholic fatty liver disease in the United States. Hepatology. 2013;57:1357–65. doi: 10.1002/hep.26156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kirchner G, Kirovski G, Hebestreit A, et al. Epidemiology and survival of patients with hepatocellular carcinoma in Southern Germany. Int J Clin Exp Med. 2010;3:169–79. [PMC free article] [PubMed] [Google Scholar]
- 37.Lee JS, Kim SH, Jun DW, et al. Clinical implications of fatty pancreas: correlations between fatty pancreas and metabolic syndrome. World J Gastroenterol. 2009;15:1869–75. doi: 10.3748/wjg.15.1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Llovet JM, Bruix J, Fuster J, et al. Liver transplantation for small hepatocellular carcinoma: the tumor-node-metastasis classification does not have prognostic power. Hepatol. 1998;27:1572–77. doi: 10.1002/hep.510270616. [DOI] [PubMed] [Google Scholar]
- 39.Lok AS, Sterling RK, Everhart JE, et al. Des-gamma-carboxy prothrombin and alpha-fetoprotein as biomarkers for the early detection of hepatocellular carcinoma. Gastroenterology. 2010;138:493–502. doi: 10.1053/j.gastro.2009.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Maida M, Orlando E, Camma C, Cabibbo G. Staging systems of hepatocellular carcinoma: a review of literature. World J Gastroenterol. 2014;20:4141–50. doi: 10.3748/wjg.v20.i15.4141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Malik SM, Gupte PA, de Vera ME, Ahmad JE. Liver transplantation in patients with nonalcoholic steatohepatitis-related hepatocellular carcinoma. Clin Gastroenterol Hepatol. 2009;7:800–6. doi: 10.1016/j.cgh.2009.02.025. [DOI] [PubMed] [Google Scholar]
- 42.Mazzanti R, Arena U, Tassi R. Hepatocellular carcinoma: Where are we? World J Exp Med. 2016;6:21–36. doi: 10.5493/wjem.v6.i1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mittal S, El-Serag HB. Epidemiology of hepatocellular carcinoma: consider the population. J Clin Gastroenterol. 2013;47:2–6. doi: 10.1097/MCG.0b013e3182872f29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Morimoto M, Numata K, Nozaki A, et al. Novel Lens culinaris agglutinin-reactive fraction of alpha-fetoprotein: a biomarker of hepatocellular carcinoma recurrence in patients with low alpha-fetoprotein concentrations. Int J Clin Oncol. 2012;17:373–9. doi: 10.1007/s10147-011-0306-3. [DOI] [PubMed] [Google Scholar]
- 45.Papaiordanou F, Ribeiro M, Saad W. Prevention of hepatocellular carcinoma. Arq Bras Cir Dig. 2009;22:115–9. [Google Scholar]
- 46.Paranagua-Vezozzo DC, Ono SK, Alvarado-Mora MV, et al. Epidemiology of HCC in Brazil: incidence and risk factors in a ten-year cohort. Ann Hepatol. 2014;13:386–93. [PubMed] [Google Scholar]
- 47.Paschos P, Paletas K. Non alcoholic fatty liver disease and metabolic syndrome. Hippokratia. 2009;13:9–19. [PMC free article] [PubMed] [Google Scholar]
- 48.Qari YA, Mosli MH. Epidemiology and clinical features of patients with hepatocellular carcinoma at a tertiary hospital in Jeddah. Niger J Clin Pract. 2017;20:43–7. doi: 10.4103/1119-3077.180062. [DOI] [PubMed] [Google Scholar]
- 49.Raphe R, Duca W, Arroyo P, et al. Hepatocellular carcinoma: risk factors, diagnosis, staging and treatment in a referral centre. J Cancer Therapy. 2013;4:384–393. [Google Scholar]
- 50.Reisin E, Alpert MA. Definition of the metabolic syndrome: current proposals and controversies. Am J Med Sci. 2005;330:269–272. doi: 10.1097/00000441-200512000-00003. [DOI] [PubMed] [Google Scholar]
- 51.Sass DA, Chang P, Chopra KB. Nonalcoholic fatty liver disease: a clinical review. Dig Dis Sci. 2005;50:171–80. doi: 10.1007/s10620-005-1267-z. [DOI] [PubMed] [Google Scholar]
- 52.Somboon K, Siramolpiwat S, Vilaichone RK. Epidemiology and survival of hepatocellular carcinoma in the central region of Thailand. Asian Pac J Cancer Prev. 2014;15:3567–70. doi: 10.7314/apjcp.2014.15.8.3567. [DOI] [PubMed] [Google Scholar]
- 53.Takaki A, Kawai D, Yamamoto K. Multiple hits, including oxidative stress, as pathogenesis and treatment target in non-alcoholic steatohepatitis (NASH) Int J Mol Sci. 2013;14:20704–28. doi: 10.3390/ijms141020704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tamura S, Kato T, Berho M, et al. Impact of histological grade of hepatocellular carcinoma on the outcome of liver transplantation. Arch Surg. 2001;136:25–30. [PubMed] [Google Scholar]
- 55.Testino G, Leone S, Borro P. Alcohol and hepatocellular carcinoma: a review and a point of view. World J Gastroenterol. 2014;20:15943–54. doi: 10.3748/wjg.v20.i43.15943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Thomson SJ, Westlake S, Rahman TM, et al. Chronic liver disease-an increasing problem: a study of hospital admission and mortality rates in England 1979-2005, with particular reference to alcoholic liver disease. Alcohol Alcohol. 2008;43:416–22. doi: 10.1093/alcalc/agn020. [DOI] [PubMed] [Google Scholar]
- 57.Torres DM, Williams CD, Harrison SA. Features, diagnosis, and treatment of nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. 2012;10:837–58. doi: 10.1016/j.cgh.2012.03.011. [DOI] [PubMed] [Google Scholar]
- 58.Tunissiolli NM, Castanhole-Nunes MMU, Biselli-Chicote PM, et al. Hepatocellular carcinoma: a comprehensive review of biomarkers, clinical aspects, and therapy. Asian Pac J Cancer Prev. 2017;18:863–72. doi: 10.22034/APJCP.2017.18.4.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Varela M, Reig M, de la Mata M, et al. Treatment approach of hepatocellular carcinoma in Spain. Analysis of 705 patients from 62 centers. Med Clin (Barc) 2010;134:569–76. doi: 10.1016/j.medcli.2009.10.042. [DOI] [PubMed] [Google Scholar]
- 60.Vernon G, Baranova A, Younossi ZM. Systematic review: the epidemiology and natural history of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in adults. Aliment Pharmacol Ther. 2011;34:274–85. doi: 10.1111/j.1365-2036.2011.04724.x. [DOI] [PubMed] [Google Scholar]
- 61.Waller LP, Deshpande V, Pyrsopoulos N. Hepatocellular carcinoma: A comprehensive review. World J Hepatol. 2015;7:2648–63. doi: 10.4254/wjh.v7.i26.2648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang X, Li J, Riaz DR, et al. Outcomes of liver transplantation for nonalcoholic steatohepatitis: a systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2014;12:394–402. doi: 10.1016/j.cgh.2013.09.023. [DOI] [PubMed] [Google Scholar]
- 63.Welzel TM, Graubard BI, Zeuzem S, et al. Metabolic syndrome increases the risk of primary liver cancer in the United States: a study in the SEER-Medicare database. Hepatol. 2011;54:463–71. doi: 10.1002/hep.24397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wu WC, Wang CY. Association between non-alcoholic fatty pancreatic disease (NAFPD) and the metabolic syndrome: case-control retrospective study. Cardiovasc Diabetol. 2013;12:77. doi: 10.1186/1475-2840-12-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yang JD, Roberts LR. Hepatocellular carcinoma: A global view. Nat Rev Gastroenterol Hepatol. 2010;7:448–58. doi: 10.1038/nrgastro.2010.100. [DOI] [PMC free article] [PubMed] [Google Scholar]


