Abstract
The present study aimed to assess any associations between resistin gene (RETN) polymorphisms and cancer susceptibility by conducting a meta-analysis. A comprehensive literature search was performed with PubMed, Web of Science, Scopus and Google Scholar for relevant studies published before April 2018. For the rs1862513 polymorphism, data from 9 studies covering 1,951 cancer patients and 2,295 healthy controls were included in this meta-analysis. Pooled odds ratios (ORs) with 95% confidence intervals (CIs) were calculated. Our meta-analysis revealed that this RETN polymorphism significantly increased the risk of cancer in codominant (OR=1.23, 95% CI= 1.01-1.50, p=0.04, CG vs CC; and OR=1.25, 95% CI= 1.03-1.53, p=0.03, GG vs CC), dominant (OR=1.19, 95% CI= 1.05-1.35, p=0.006, CG+GG vs CC), and allele (OR=1.14, 95% CI= 1.00-1.30, p=0.04, G vs C) inheritance genetic models. Stratification analysis by cancer type revealed that the rs1862513 variant significantly increased the risk of colorectal and breast cancer, and that cancer overall in Caucasians (OR=1.22, 95% CI= 1.04-1.43, p=0.02, CG+GG vs CC; OR=1.18, 95% CI= 1.04-1.34, p=0.01, G vs C). The data revealed no correlation between the rs3745367 polymorphism and cancer risk. Further well-designed studies with larger sample sizes and different ethnicities are warranted to validate the present findings.
Keywords: Resistin, RETN, cancer, polymorphism, meta-analysis
Introduction
Cancer, a major public health issue, is a leading cause of death worldwide. It has been estimated that more than 14.1 million new cases and 8.2 million cancer-related deaths happened annually (Siegel et al., 2016). Cancer is recognized as a multifactorial disease resulting from the integration between genetic and environmental factors (Lichtenstein et al., 2000). Single-nucleotide polymorphisms (SNPs) and small insertions or deletions (indels) are the most common genetic variations in human genome (Hashemi et al., 2018). Several studies showing the association between functional SNPs in various genes and the risk of developing cancer.
Adipokines, such as resistin, leptin, adiponectin and visfatin, are mainly synthesized in white adipose tissue and have been related to the pathogenesis of autoimmune disease, inflammatory diseases and cancer (John et al., 2006; Salageanu et al., 2010; Riondino et al., 2014; Muppala et al., 2017; Li and Han, 2018).
Resistin is a 12.5-kDa cysteine-rich polypeptide that upregulates the expression of proinflammatory cytokines and helps expand the population of regulatory T cells (Steppan et al., 2001; Bokarewa et al., 2005). The RETN gene encode resistin is mapped to chromosome 9 (19p13.2). Resistin is increased in type 2 diabetes and is closely correlated with insulin resistance and obesity (Shuldiner et al., 2001; Steppan et al., 2001; John et al., 2006). Obesity is well recognized as a risk factor for colorectal cancer development (Joshi et al., 2014; Joshi and Lee, 2014). Resistin may also be involved in the pathogenesis of cancer (Gonullu et al., 2010; Danese et al., 2012; Riondino et al., 2014). The serum levels of resistin have been shown to be higher in colorectal cancer (CRC) (Kumor et al., 2009; Gonullu et al., 2010; Nakajima et al., 2010; Danese et al., 2012; Slomian et al., 2017), and breast cancer (Dalamaga et al., 2013; Assiri et al., 2015; Deshmukh et al., 2015; Assiri and Kamel, 2016; Zeidan et al., 2018) than controls subjects.
Previous studies also demonstrated that the RETN gene variants were associated with the regulation of RETN gene expression and serum levels of resistin (Cho et al., 2004; Osawa et al., 2004).
In the last few years, a number of studies on the association between REST gene polymorphisms and risk of cancer have been published, with controversial results (Wagsater et al., 2008; Pechlivanis et al., 2009; Al-Harithy and Al-Ghafari, 2010; Alharithy, 2014; Mahmoudi et al., 2014; Duzkoylu et al., 2015; Mahmoudi et al., 2016; Hu et al., 2017; Kohan, 2017; Munoz-Palomeque et al., 2018). Therefore, we conducted a meta-analysis to exactly establish the association between RETN rs1862513 C>G (-420 C<G) and rs3745367 (+299 G>A gene polymorphisms and the risk of cancer.
Literature search
Literature searching in the databases such as PubMed, Web of Science, Scopus, and Google Scholar was performed for all articles describing an association between resistin polymorphisms and cancer risk published up to April 2018. Comprehensive search strategies involved the Mesh term and (‘resistin’ or ‘RETN’), (‘polymorphism’ or ‘variant’ or ‘genotype’ or ‘SNP’ or ‘mutations’), (‘cancer’ or ‘tumor’). Relevant studies which were eligible for the meta-analysis must meet the following criteria: 1) Original case-control studies of the correlation between the RETN polymorphisms and cancer 2) studies provided sufficient information of the genotype frequencies of RETN polymorphisms in both cases and controls. The criteria for exclusion were: 1) the articles have described case reports, reviews, overlapped data, animal or mechanism studies for RETN polymorphisms and cancer; 2) no genotype frequency or genotype information were provided for RETN polymorphism and cancer.
Data extraction
The papers were reviewed by two independent researchers. The following data were collected from each study such as the first author’s last name, publication year, ethnicity, the sample size, and the genotype and allele frequencies of cases and controls.
Statistical analysis
Meta-analysis was carried out using Revman 5.3 software (Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014) and STATA 14.1 software (Stata Corporation, College Station, TX, USA). Hardy-Weinberg Equilibrium (HWE) in the control group was tested by χ2 test. Odds ratios (ORs) and 95% confidence intervals (CIs) were pooled using forest-plots graphs to evaluate the association between RETN polymorphisms and cancer. The significance of the pooled OR was determined with the Z-test, and P-values < 0.05 were considered statistically significant. Heterogeneity among studies was assessed using the I2 statistic and the χ2 test-based Q statistic. A p< 0.10 and an I2 > 50% indicated significant heterogeneity. Once heterogeneity existed among studies, a random-effect model was applied; otherwise a fixed-effect model was used.
Publication bias was assessed by funnel plot. The degree of asymmetry was measured using Egger’s linear regression test; p < 0.05 was considered significant publication bias.
Sensitivity analysis
Sensitivity analysis was achieved using the method of eliminating studies one by one to verify whether our results were influenced by each included study or not.
Results
Study characteristics
10 studies met all the inclusion criteria and were included in this meta-analysis. Characteristics of the eligible studies are summarized in Table 1.
Table 1.
Characteristics of the Included Studies on RETN rs1862513 and rs3745367 Polymorphisms and Risk of Cancer
| First Author | Year | Country | Ethnicity | Cancer type | Source of control | Genotyping Method | Case/ controls | Genotype and allele distribution of cases and controls | HWE (p) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cases | controls | |||||||||||||||||
| rs1862513 | CC | CG | GG | C | G | CC | CG | GG | C | G | ||||||||
| Al-Harithy | 2010 | Saudi Arabia | Asian | Colon cancer | HB | PCR- RFLP | 60/6 | 16 | 33 | 11 | 65 | 55 | 24 | 20 | 16 | 68 | 52 | 0.013 |
| Alharithy | 2014 | Saudi Arabia | Asian | Colon cancer | HB | PCR- RFLP | 60/6 | 15 | 33 | 12 | 63 | 57 | 24 | 20 | 16 | 68 | 52 | 0.013 |
| Duzkoylu | 2015 | Turkey | Caucasian | Colorectal cancer | HB | PCR- RFLP | 123/79 | 53 | 61 | 9 | 167 | 79 | 31 | 36 | 12 | 98 | 60 | 0.771 |
| Hu | 2017 | China | Asian | Lung cancer | HB | Real- time PCR | 371/451 | 164 | 157 | 50 | 485 | 257 | 182 | 203 | 66 | 567 | 335 | 0.444 |
| Kohan | 2017 | Iran | Asian | Breast cancer | HB | PCR- RFLP | 150/15 | 50 | 63 | 37 | 163 | 137 | 63 | 63 | 24 | 189 | 111 | 0.225 |
| Mahmoudi | 2014 | Iran | Asian | Colorectal cancer | HB | PCR- RFLP | 197/217 | 38 | 83 | 76 | 159 | 235 | 56 | 85 | 76 | 197 | 237 | 0.002 |
| Munoz- Palomeque | 2018 | Mexico | Caucasian | Breast cancer | PB | PCR- RFLP | 100/308 | 53 | 42 | 5 | 148 | 52 | 199 | 102 | 7 | 500 | 116 | 0.144 |
| Pechlivanis | 2009 | Czech Republic | Caucasian | Colorectal cancer | HB | PCR- RFLP | 642/714 | 317 | 262 | 63 | 896 | 388 | 393 | 265 | 56 | 1051 | 377 | 0.230 |
| Wagsater | 2008 | Sweden | Caucasian | Colorectal cancer | HB | Taqman | 248/256 | 127 | 95 | 26 | 349 | 147 | 137 | 103 | 16 | 377 | 135 | 0.563 |
| rs3745367 | GG | GA | AA | G | A | GG | AG | AA | G | A | HWE (p) | |||||||
| Alharithy | 2014 | Saudi Arabia | Asian | Colon cancer | HB | PCR- RFLP | 60/6 | 3 | 51 | 6 | 57 | 63 | 15 | 39 | 6 | 69 | 51 | 0.011 |
| Hu | 2017 | China | Asian | Lung cancer | HB | Real- timePCR | 371/451 | 164 | 164 | 43 | 492 | 250 | 190 | 194 | 67 | 574 | 328 | 0.134 |
| Mahmoudi | 2016 | Iran | Asian | Colorectal cancer | HB | PCR- RFLP | 312/438 | 65 | 72 | 35 | 202 | 142 | 78 | 86 | 26 | 242 | 138 | 0.767 |
For rs1862513 polymorphism, data from 9 studies including 1951 cancer patients and 2,295 healthy controls were included in this meta-analysis. Regarding rs3745367 polymorphism, data from 3 studies containing 603 cases and 701 controls were included in this meta-analysis.
Quantitative synthesis
All the calculated results were summarized in Table 2. Our meta-analysis revealed that rs1862513 polymorphism of RETN significantly increased the risk of cancer in codominant (OR=1.23, 95%CI= 1.01-1.50, p=0.04, CG vs CC; and OR=1.25, 95%CI= 1.03-1.53, p=0.03, GG vs CC), dominant (OR=1.19, 95%CI= 1.05-1.35, p=0.006, CG+GG vs CC), and allele (OR=1.14, 95%CI= 1.00-1.30, p=0.04, G vs C) inheritance genetic models (Figure 1 and Table 2).
Table 2.
The Pooled ORs and 95%CIs for the Association between RETN Polymorphisms and Cancer Susceptibility
| Polymorphism | Association test | Heterogeneity test | Egger’s test P | Begg’s test P | ||||
|---|---|---|---|---|---|---|---|---|
| OR (95%CI) | Z | p | χ2 | I2 (%) | p | |||
| rs1862513 C>G | ||||||||
| CG vs CC | 1.23 (1.01-1.50) | 2.05 | 0.04 | 14.00 | 43 | 0.08 | 0.891 | 0.532 |
| GG vs CC | 1.25 (1.03-1.53) | 2.21 | 0.03 | 13.10 | 39 | 0.11 | 0.607 | 0.621 |
| CG+GG vs CC | 1.19 (1.05-1.35) | 2.74 | 0.006 | 13.24 | 40 | 0.10 | 0.451 | 0.211 |
| GG vs CG+CC | 1.11 (0.85-1.35) | 0.75 | 0.45 | 14.01 | 43 | 0.08 | 0.926 | 0.118 |
| CG vs GG+CC | 1.17 (0.97-1.40 | 1.68 | 0.09 | 13.86 | 42 | 0.09 | 0.153 | 0.466 |
| G vs C | 1.14 (1.00-1.30) | 2.01 | 0.04 | 13.58 | 41 | 0.09 | 0.520 | 0.532 |
| rs3745367 G>A | ||||||||
| AG vs GG | 1.32 (0.72-2.45) | 0.90 | 0.37 | 7.80 | 74 | 0.002 | 0.407 | 0.602 |
| AA vs GG | 1.38 (0.60-3.17) | 0.76 | 0.44 | 7.67 | 74 | 0.02 | 0.883 | 0.117 |
| AG+GG vs AA | 1.34 (0.73-2.46) | 0.96 | 0.34 | 8.43 | 76 | 0.01 | 0.368 | 0.602 |
| AA vs AG+GG | 1.05 (0.60-1.84) | 0.18 | 0.92 | 4.69 | 57 | 0.10 | 0.193 | 0.117 |
| AG vs AA+GG | 1.19 (0.74-1.93) | 0.73 | 0.47 | 6.34 | 68 | 0.04 | 0.679 | 0.602 |
| A vs G | 1.11 (0.83-1.50) | 0.71 | 0.48 | 5.43 | 63 | 0.07 | 0.187 | 0.117 |
Table 3.
Stratified Analysis of RETN rs1862513 C>G Polymorphism on Cancer Susceptibility
| Type of cancer | NO. | CG vs CC | GG vs CC | CG+GG vs CC | GG vs CG+CC | CG vs GG+CC | G vs C | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OR (95%CI) | P | OR (95%CI) | P | OR (95%CI) | P | OR (95%CI) | P | OR (95%CI) | P | OR (95%CI) | P | ||
| Cancer type | |||||||||||||
| Colorectal cancer | 6 | 1.25 (1.06-1.47) | 0.009 | 1.31 (1.02-1.68) | 0.04 | 1.25 (1.07-1.46) | 0.005 | 1.01 (0.72-1.43) | 0.93 | 1.19 (1.02-1.39) | 0.03 | 1.16 (1.03-1.30) | 0.01 |
| Breast cancer | 2 | 1.41 (0.99-1.99) | 0.05 | 2.07 (1.18-3.63) | 0.01 | 1.53 (1.10-2.13) | 0.01 | 1.80 (1.07-3.02) | 0.03 | 1.20 (0.87-1.67) | 0.26 | 1.47 (1.15-1.87) | 0.002 |
| Lung cancer | 1 | 0.86 (0.64-1.15) | 0.31 | 0.84 (0.55-1.28) | 0.42 | 0.85 (0.65-1.13) | 0.27 | 0.91 (0.61-1.35) | 0.64 | 0.90 (0.68-1.7) | 0.44 | 0.90 (0.73-1.10) | 0.29 |
| Ethnicities | |||||||||||||
| Asian | 5 | 1.42 (0.94-2.14) | 0.10 | 1.19 (0.91-1.55) | 0.21 | 1.33 (0.94-1.89) | 0.11 | 1.05 (0.83-1.32) | 0.70 | 1.27 (0.89-1.81) | 0.81 | 1.09 (0.95-1.24) | 0.24 |
| Caucasian | 4 | 1.19 (1.00-1.41) | 0.05 | 1.31 (0.75-2.30) | 0.35 | 1.22 (1.04-1.43) | 0.02 | 1.23 (0.71-2.11) | 0.46 | 1.14 (0.97-1.35) | 0.11 | 1.18 (1.04-1.34) | 0.01 |
Figure 1.
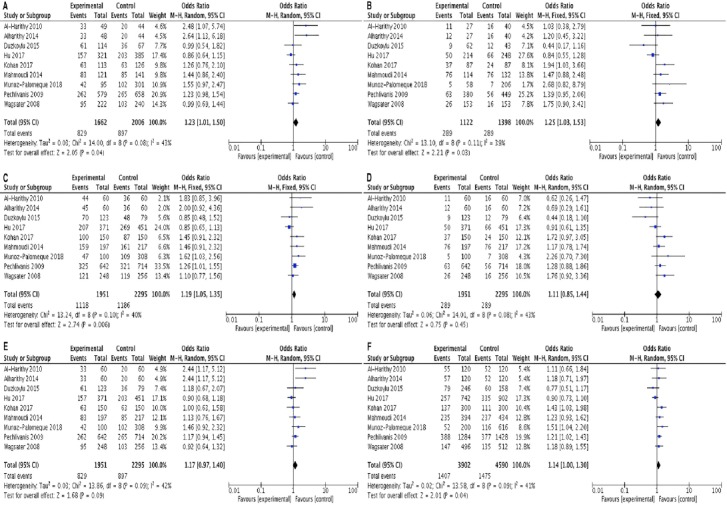
Forest Plot of the Risk of Cancer Associated with RETN rs1862513 Polymorphism under Codominant Heterozygous Model (A), Codominant Homozygous Model (B), Dominant Model (C), Reccesive Model (D), Ovedominanat Model (E), and Allelic Model (F).
Stratification analysis by cancer type showed that rs1862513 variant significantly increased the risk of colorectal cancer as well as breast cancer (Table 2).
As shown in Table 2, the rs1862513 variant significantly increased the risk of cancer in Caucasian in dominant (OR=1.22, 95% CI= 1.04-1.43, p=0.02, CG+GG vs CC) and allele (OR=1.18, 95% CI= 1.04-1.34, p=0.01, G vs C) genetics model.
Regarding rs3745367 variant, the finding showed no significant association between the variant and cancer risk (Table 2).
Publication bias
The potential publication bias was assessed using a Begg’s funnel plot (Figure 2) and Egger’s test (Table 2). Begg’s and Egger’s tests proposed no evident publication bias in codominant, dominant recessive, overdominant, and allele inheritance models.
Figure 2.
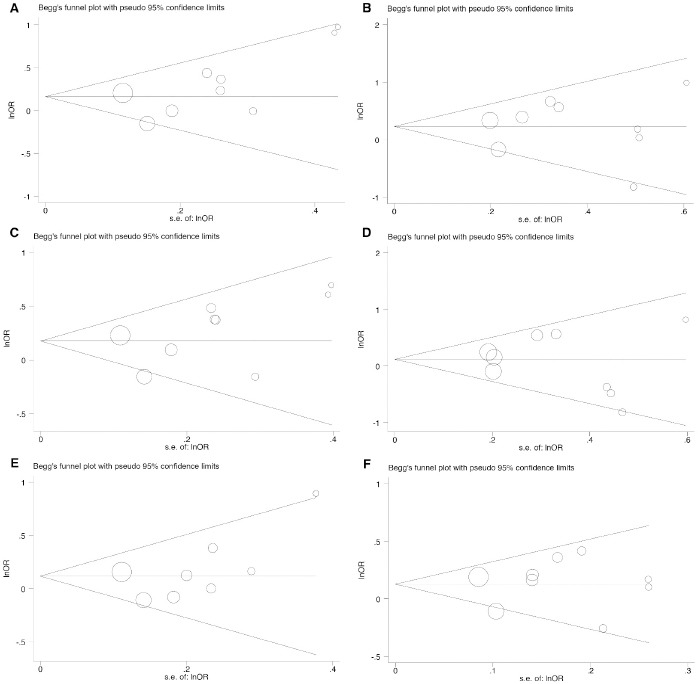
Begg’s Funnel Plot for Publication Bias Test for RETN rs1862513 Polymorphism. Each point represents a separate study for the indicated association. (A), heterozygous model; (B), codominant homozygous model; (C), dominant model; (D), reccesive model; (E), ovedominanat model; (F), allelic model.
Sensitivity analysis
To verify the outcome of our analyses, we conducted a sensitivity analysis by excluding studies one by one, and then calculating the pooled estimate for the remaining studies (Figure 3). The sensitivity analysis proposed that certain studies significantly affect the association between RETN polymorphism and risk of cancer. We believe that the small number of studies included in our meta-analysis may contribute to the influence of the abovementioned studies; if more studies had been included, the influence of any one study would be decreased.
Figure 3.
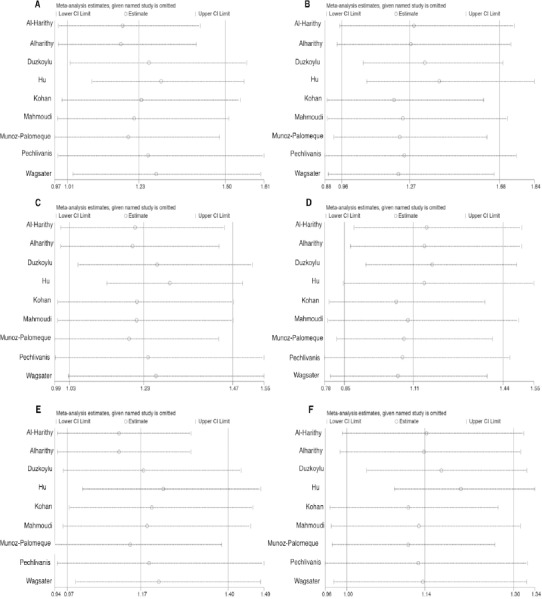
Results of Sensitivity Analysis of the Entire Database under Codominant Heterozygous Model (A), Codominant Homozygous Model (B), Dominant Model (C), Reccesive Model (D), Ovedominanat Model (E), and Allelic Model (F).
Discussion
Cancer is a complex disease and it has been proposed that individual genetic variants may only have a modest independent effect on the disease. Adipokines, secreted by the adipose tissue, are convincing candidates for the relationship between obesity and cancer risk (Guadagni et al., 2009; Li et al., 2017; Zhang et al., 2017; Malvi et al., 2018). Obesity leads to insulin resistance and hyperinsulinemia, and insulin levels are positively correlated with colorectal cancer risk (Schoen et al., 1999; Giovannucci, 2007).
Up to now, a number of studies have been carefully designed and investigated the effect of genetic polymorphisms of RETN gene on the risk of cancer. Most of these studies were based on a small sample size and the findings were inconsistent (Wagsater et al., 2008; Pechlivanis et al., 2009; Al-Harithy and Al-Ghafari, 2010; Alharithy, 2014; Mahmoudi et al., 2014; Duzkoylu et al., 2015; Mahmoudi et al., 2016; Hu et al., 2017; Kohan, 2017; Munoz-Palomeque et al., 2018). This is the first meta-analysis conducted to specify the effect of RETN rs1862513 and rs3745367 polymorphisms on susceptibility to cancer. Data from 9 studies indicated that RETN rs1862513 variant significantly increased the risk of cancer in codominant, dominant, and allele inheritance genetic models. We did not find any publication bias, which shows the reliability of the pooled results. Heterogeneity across studies suggests that there is a variation among the outcomes of studies than expected by chance. Sensitivity analysis also revealed an evidence of heterogeneity.
Stratified analyses based on cancer type showed that the rs1862513 variant significantly increased the risk of colorectal cancer as well as breast cancer.
The rs1862513 (-420 C>G) polymorphism is located in the promoter region of RETN and has been shown to be associated with RETN protein expression (Cho et al., 2004; Osawa et al., 2004).
The RETN is a polymorphic and a functional polymorphism at -420 (rs186513) affects promoter activity and increases the expression of resistin. The molecular mechanism by which resistin affect cancer risk is not fully understood.
Regarding rs3745367 variant, data from 3 studies did not support an association between variant and risk of cancer.
A significant deviation from HWE was found in 3 studies included the meta-analysis (Al-Harithy and Al-Ghafari, 2010; Alharithy, 2014). There is no clear clarification for deviation from HWE. The possible cause may be due to genetic drift.
In summary, our metanalysis investigation showed that rs1862513 polymorphism of RETN is a risk factor for cancer development. More studies with larger sample sizes are necessary to clarify the possible roles of RETN polymorphisms in cancer.
Conflict of interest
The Authors declare that there is no conflict of interest to disclose.
Acknowledgements
Saeid Ghavami has been supported by Research Manitoba New Investigator Operating grant and CHRIM operating grant.
References
- 1.Al-Harithy RN, Al-Ghafari AB. Resistin in human colon cancer. Increased expression independently of resistin promoter C-180G genotype. Saudi Med J. 2010;31:495–500. [PubMed] [Google Scholar]
- 2.Alharithy RN. Polymorphisms in RETN gene and susceptibility to colon cancer in Saudi patients. Ann Saudi Med. 2014;34:334–9. doi: 10.5144/0256-4947.2014.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Assiri AM, Kamel HF. Evaluation of diagnostic and predictive value of serum adipokines: Leptin, resistin and visfatin in postmenopausal breast cancer. Obes Res Clin Pract. 2016;10:442–53. doi: 10.1016/j.orcp.2015.08.017. [DOI] [PubMed] [Google Scholar]
- 4.Assiri AM, Kamel HF, Hassanien MF. Resistin, visfatin, adiponectin, and leptin: risk of breast cancer in pre- and postmenopausal saudi females and their possible diagnostic and predictive implications as novel biomarkers. Dis Markers. 2015;2015:253519. doi: 10.1155/2015/253519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bokarewa M, Nagaev I, Dahlberg L, et al. Resistin, an adipokine with potent proinflammatory properties. J Immunol. 2005;174:5789–95. doi: 10.4049/jimmunol.174.9.5789. [DOI] [PubMed] [Google Scholar]
- 6.Cho YM, Youn BS, Chung SS, et al. Common genetic polymorphisms in the promoter of resistin gene are major determinants of plasma resistin concentrations in humans. Diabetologia. 2004;47:559–65. doi: 10.1007/s00125-003-1319-x. [DOI] [PubMed] [Google Scholar]
- 7.Dalamaga M, Sotiropoulos G, Karmaniolas K, et al. Serum resistin: a biomarker of breast cancer in postmenopausal women? Association with clinicopathological characteristics, tumor markers, inflammatory and metabolic parameters. Clin Biochem. 2013;46:584–90. doi: 10.1016/j.clinbiochem.2013.01.001. [DOI] [PubMed] [Google Scholar]
- 8.Danese E, Montagnana M, Minicozzi AM, et al. The role of resistin in colorectal cancer. Clin Chim Acta. 2012;413:760–4. doi: 10.1016/j.cca.2012.01.019. [DOI] [PubMed] [Google Scholar]
- 9.Deshmukh SK, Srivastava SK, Bhardwaj A, et al. Resistin and interleukin-6 exhibit racially-disparate expression in breast cancer patients, display molecular association and promote growth and aggressiveness of tumor cells through STAT3 activation. Oncotarget. 2015;6:11231–41. doi: 10.18632/oncotarget.3591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Duzkoylu Y, Arikan S, Turan S, et al. Possible relationship between the resistin gene C-420G polymorphism and colorectal cancer in a Turkish population. Turk J Gastroenterol. 2015;26:392–6. doi: 10.5152/tjg.2015.0188. [DOI] [PubMed] [Google Scholar]
- 11.Giovannucci E. Metabolic syndrome, hyperinsulinemia, and colon cancer: a review. Am J Clin Nutr. 2007;86:836–42. doi: 10.1093/ajcn/86.3.836S. [DOI] [PubMed] [Google Scholar]
- 12.Gonullu G, Kahraman H, Bedir A, et al. Association between adiponectin, resistin, insulin resistance, and colorectal tumors. Int J Colorectal Dis. 2010;25:205–12. doi: 10.1007/s00384-009-0828-6. [DOI] [PubMed] [Google Scholar]
- 13.Guadagni F, Roselli M, Martini F, et al. Prognostic significance of serum adipokine levels in colorectal cancer patients. Anticancer Res. 2009;29:3321–7. [PubMed] [Google Scholar]
- 14.Hashemi M, Bahari G, Sarhadi S, et al. 4-bp insertion/deletion (rs3783553) polymorphism within the 3'UTR of IL1A contributes to the risk of prostate cancer in a sample of Iranian population. J Cell Biochem. 2018;119:2627–35. doi: 10.1002/jcb.26427. [DOI] [PubMed] [Google Scholar]
- 15.Hu WW, Tang CH, Sun Y, et al. Correlation between resistin gene polymorphism and clinical aspects of lung cancer. Medicine (Baltimore) 2017;96:e9485. doi: 10.1097/MD.0000000000009485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.John BJ, Irukulla S, Abulafi AM, et al. Systematic review: adipose tissue, obesity and gastrointestinal diseases. Aliment Pharmacol Ther. 2006;23:1511–23. doi: 10.1111/j.1365-2036.2006.02915.x. [DOI] [PubMed] [Google Scholar]
- 17.Joshi RK, Kim WJ, Lee SA. Association between obesity-related adipokines and colorectal cancer: a case-control study and meta-analysis. World J Gastroenterol. 2014;20:7941–9. doi: 10.3748/wjg.v20.i24.7941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Joshi RK, Lee SA. Obesity related adipokines and colorectal cancer: a review and meta-analysis. Asian Pac J Cancer Prev. 2014;15:397–405. doi: 10.7314/apjcp.2014.15.1.397. [DOI] [PubMed] [Google Scholar]
- 19.Kohan L. Investigating the association of rs1↱13 genetic variant in resistin gene with susceptibility to breast cancer. J Fasa Univ Med Sci. 2017;7:217–22. [Google Scholar]
- 20.Kumor A, Daniel P, Pietruczuk M, et al. Serum leptin, adiponectin, and resistin concentration in colorectal adenoma and carcinoma (CC) patients. Int J Colorectal Dis. 2009;24:275–81. doi: 10.1007/s00384-008-0605-y. [DOI] [PubMed] [Google Scholar]
- 21.Li J, Han X. Adipocytokines and breast cancer. Curr Probl Cancer. 2018;42:208–14. doi: 10.1016/j.currproblcancer.2018.01.004. [DOI] [PubMed] [Google Scholar]
- 22.Li L, Chen L, Zhang W, et al. Serum cytokine profile in patients with breast cancer. Cytokine. 2017;89:173–8. doi: 10.1016/j.cyto.2015.12.017. [DOI] [PubMed] [Google Scholar]
- 23.Lichtenstein P, Holm NV, Verkasalo PK, et al. Environmental and heritable factors in the causation of cancer--analyses of cohorts of twins from Sweden, Denmark, and Finland. N Engl J Med. 2000;343:78–85. doi: 10.1056/NEJM200007133430201. [DOI] [PubMed] [Google Scholar]
- 24.Mahmoudi T, Karimi K, Arkani M, et al. Resistin -420C>G promoter variant and colorectal cancer risk. Int J Biol Markers. 2014;29:233–8. doi: 10.5301/jbm.5000079. [DOI] [PubMed] [Google Scholar]
- 25.Mahmoudi T, Majidzadeh AK, Karimi K, et al. Gly972Arg variant of insulin receptor substrate 1 gene and colorectal cancer risk in overweight/obese subjects. Int J Biol Markers. 2016;31:68–72. doi: 10.5301/jbm.5000159. [DOI] [PubMed] [Google Scholar]
- 26.Malvi P, Chaube B, Singh SV, et al. Elevated circulatory levels of leptin and resistin impair therapeutic efficacy of dacarbazine in melanoma under obese state. Cancer Metab. 2018;6:2. doi: 10.1186/s40170-018-0176-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Munoz-Palomeque A, Guerrero-Ramirez MA, Rubio-Chavez LA, et al. Association of RETN and CAP1 SNPs, expression and serum resistin levels with breast cancer in Mexican women. Genet Test Mol Biomarkers. 2018;22:209–17. doi: 10.1089/gtmb.2017.0212. [DOI] [PubMed] [Google Scholar]
- 28.Muppala S, Konduru SKP, Merchant N, et al. Adiponectin: Its role in obesity-associated colon and prostate cancers. Crit Rev Oncol Hematol. 2017;116:125–33. doi: 10.1016/j.critrevonc.2017.06.003. [DOI] [PubMed] [Google Scholar]
- 29.Nakajima TE, Yamada Y, Hamano T, et al. Adipocytokines as new promising markers of colorectal tumors: adiponectin for colorectal adenoma, and resistin and visfatin for colorectal cancer. Cancer Sci. 2010;101:1286–91. doi: 10.1111/j.1349-7006.2010.01518.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Osawa H, Yamada K, Onuma H, et al. The G/G genotype of a resistin single-nucleotide polymorphism at -420 increases type 2 diabetes mellitus susceptibility by inducing promoter activity through specific binding of Sp1/3. Am J Hum Genet. 2004;75:678–86. doi: 10.1086/424761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pechlivanis S, Bermejo JL, Pardini B, et al. Genetic variation in adipokine genes and risk of colorectal cancer. Eur J Endocrinol. 2009;160:933–40. doi: 10.1530/EJE-09-0039. [DOI] [PubMed] [Google Scholar]
- 32.Riondino S, Roselli M, Palmirotta R, et al. Obesity and colorectal cancer: role of adipokines in tumor initiation and progression. World J Gastroenterol. 2014;20:5177–90. doi: 10.3748/wjg.v20.i18.5177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Salageanu A, Tucureanu C, Lerescu L, et al. Serum levels of adipokines resistin and leptin in patients with colon cancer. J Med Life. 2010;3:416–20. [PMC free article] [PubMed] [Google Scholar]
- 34.Schoen RE, Tangen CM, Kuller LH, et al. Increased blood glucose and insulin, body size, and incident colorectal cancer. J Natl Cancer Inst. 1999;91:1147–54. doi: 10.1093/jnci/91.13.1147. [DOI] [PubMed] [Google Scholar]
- 35.Shuldiner AR, Yang R, Gong DW. Resistin, obesity, and insulin resistance--the emerging role of the adipocyte as an endocrine organ. N Engl J Med. 2001;345:1345–6. doi: 10.1056/NEJM200111013451814. [DOI] [PubMed] [Google Scholar]
- 36.Siegel RL, Miller KD, Jemal A. Cancer statistics 2016. CA Cancer J Clin. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 37.Slomian G, Swietochowska E, Nowak G, et al. Chemotherapy and plasma adipokines level in patients with colorectal cancer. Postepy Hig Med Dosw (Online) 2017;71:281–90. doi: 10.5604/01.3001.0010.3813. [DOI] [PubMed] [Google Scholar]
- 38.Steppan CM, Bailey ST, Bhat S, et al. The hormone resistin links obesity to diabetes. Nature. 2001;409:307–12. doi: 10.1038/35053000. [DOI] [PubMed] [Google Scholar]
- 39.Wagsater D, Mumtaz M, Lofgren S, et al. Resistin in human colorectal cancer: increased expression independently of resistin promoter -420C >G genotype. Cancer Invest. 2008;26:1008–14. doi: 10.1080/07357900802087267. [DOI] [PubMed] [Google Scholar]
- 40.Zeidan B, Manousopoulou A, Garay-Baquero DJ, et al. Increased circulating resistin levels in early-onset breast cancer patients of normal body mass index correlate with lymph node negative involvement and longer disease free survival: a multi-center POSH cohort serum proteomics study. Breast Cancer Res. 2018;20:19. doi: 10.1186/s13058-018-0938-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang HP, Zou J, Xu ZQ, et al. Association of leptin, visfatin, apelin, resistin and adiponectin with clear cell renal cell carcinoma. Oncol Lett. 2017;13:463–8. doi: 10.3892/ol.2016.5408. [DOI] [PMC free article] [PubMed] [Google Scholar]


