Abstract
Background:
This study aimed to assess the effects of Gluten free diet (GFD) on components of metabolic syndrome (MES).
Materials and Methods:
In this randomized clinical trial, 50 subjects diagnosed with MES were randomly divided into two groups (n=25). The first group received a GFD and the second group continued their regular diet. Biochemical markers of MES and blood pressure were measured before and after 8-week intervention.
Results:
Forty five subjects completed the study. A post-hoc comparison of the groups showed no effects of the GFD and control diet on LDL cholesterol, total cholesterol, fasting insulin, HOMA-IR, systolic and diastolic blood pressure levels. The GFD reduced fasting blood glucose, waist circumference (WC) and serum triglyceride concentration significantly compared with the control diet (p<0.05).
Conclusion:
Short-term GFD reduced WC and improved glycemic control and Triglyceride level in subjects with the metabolic syndrome.
Keywords: Gluten free diet, metabolic syndrome, obesity
Introduction
Metabolic syndrome is a cluster of conditions related to cardiovascular disorders risk factors such as blood pressure, dyslipidemia, hyperglycemia, excess body fat around the waist and insulin resistance. According to the National Cholesterol Education Program (NECP) and Adult Treatment Panel III, characteristics of MES are abdominal obesity (wait circumference ≥ 95 cm), high fasting serum glucose (>100 mg/dL), elevated triglyceride (>150 mg/dL), high blood pressure (systolic blood pressure ≥130 mmHg and diastolic blood pressure ≥ 85 mmHg) and low HDL cholesterol (<40 mg/dL). Having three or more of these criteria is essential for diagnosis of MES (Expert Panel on Detection 2001).
According to the National Health and Nutrition Examination Survey (NHANES), the prevalence of MES was 33% during 2003-2012. In Iran, the prevalence of MES was 32% based on the International Diabetes Federation definition and 33% based on the ATPIII definition which is higher than the prevalence of MES in most developing countries (Aguilar et al., 2015; Zabetian et al., 2007). MES is defined by a combination of interconnected physiological, biochemical, clinical, metabolic and environmental factors (Noori et al., 2007). In this regard, the current treatment for MES is modification in life-style including diet, physical activity and limited medications (Statins, Metformin and antihypertensive medicines), which have specific side effects (Lebovitz, 2011).
According to available evidence, the fundamental strategy for management of obesity is reduction in energy intake (Fung et al., 2001; Thomas et al., 2006). It seems that some nutrients can also play a role in management of weight. Gluten is a complex protein of Glutenin and Prolamin, which exist in wheat, oat and barley. Although the gluten free diet (GFD) has been considered as the main treatment for celiac disease (CD), it could be useful in preventing and treating of several other disorders such as Rheumatoid arthritis, type I diabetes, obesity and insulin resistance (Thomas et al., 2006).
The mechanism of action of the GFD for prevention of weight gain is explained by reduction of peripheral adipose tissue and the size of adipocytes. Adipose tissue hypertrophy is one of the most important factors of adipose tissue inflammation and insulin resistance. Thus, dietary approaches limiting adipose tissue extension are assumed to improve insulin resistance (Lammers et al., 2008).
It has been shown that individuals without CD following a GFD compared to those consuming usual diet had a significant decrease in weight over 1 year, lower waist circumference, and higher HDL cholesterol levels (Kim et al., 2017; Jönsson et al., 2015; DiGiacomo et al., 2013). In contrast, studies on CD patients have shown that GFD increase risk of metabolic syndrome because of improvement in intestinal absorption, and being high in sugar, fat, and calories (Tortora et al., 2015).
To our knowledge, no study have evaluated the effects of GFD on components of metabolic syndrome, and results of cross-sectional studies are contraversial (Lebwohl et al., 2017; Ibrügger et al., 2014; Ukkola et al., 2012; Marcason, 2011). Hence, we designed the current study to assess the effects of GFD on components of metabolic syndrome in patients diagnosed with metabolic syndrome.
Materials and Methods
Study design and Participants
In this randomized clinical trial, fifty subjects diagnosed with MES according to the Adult Treatment Panel III (ATP III) criteria were selected via convenience sampling method. Participants were recruited from nutrition counseling clinics and health houses of Tehran municipality, Tehran, Iran. Study inclusion criteria were: having at least three components of MES, age range of 25-70 years, BMI 25-35 kg/m2, not using insulin or other diabetes medications for controlling blood sugar, non-pregnant, non-lactating, non-athletic, not having thyroid gland disorders, not using fat burning and weight loss drugs, multivitamin/mineral, omega-3 and green tea supplements in the past month, not using oral contraceptives, not being on a weight loss diet in the past month, not using antibiotics, corticosteroids, immune-suppressants and non-smoking. Exclusion criteria were: any dietary pattern changes through the study and not willing to participate in the study.
Ethical considerations
Qualified individuals according to the inclusion and exclusion criteria were informed about the study objectives and methods. Afterwards, written informed consent was obtained from all the participants. The study protocol was approved by the Ethics Committee of National Nutritional and Food Technology Research Institute (NNFTRI) Shahid Beheshti University of Medical Sciences and was registered in the Iranian Registry of Clinical Trials website (IRCT20170723035244N1).
Intervention
Subjects were block randomizedbygender into control and GFD groups. Blinding was not possible due to the nature of intervention. All participantswere asked not to change their lifestylehabitsand medications during the intervention.
The first group received GFD diet and the second group were informed to continue their regular diet for eight weeks. In the GFD diet, patients were assumed to receive less than 2-gram gluten per day. Through the intervention process, individuals received gluten free breads. Additionally, participants were informed to avoid other cereals containing gluten such as cakes, biscuits, macaroni, spaghetti, and all other foods containing wheat, or barley. The amount of bread consumed for one week (1,250 g) was provided to patients. Because there was a possibility that the rest of the family members may want to consume gluten free breads as well, we provided extra bread for patients who had this issue. Patients were implied to refer to clinics and health houses at appointed dates for receiving the gluten free products. A recommendation sheet containing information regarding controlling overweight and obesity was provided to all participants in both groups. To assess the procedure of the intervention, and participants’compliance, individual meetings were held for each patient every week.
Dietary intake, anthropometric and biochemical assessments
All participants were asked tocomplete 24 –h food recall for 3 days (2 weekdays and 1 weekend day) before and after intervention. They were provided with information for how to record their daily intake. All 24 h food records data were reviewed by a dietician, and analyzed using Nutritionist IV (The Hearst Corporation, San Bruno, CA).
Anthropometric characteristics(height, weight, waist circumference) were measured for all participants at the beginning and end of trial. weight was measured without shoes by Seca scales with a precision of 100 g (Seca, Homburg, Germany) and Height was determined using Seca stadiometer with an accuracy 0.1 cm.An anthropometric tape was utilized to measure the waist circumference on the midway waistline between rib margin and iliac bone. Body mass index (BMI) was calculated as body weight (kg) / height squared (m2). Systolic and diastolic blood pressures were measured on the right arm after at least 10 min of sitting.
Blood samples were collected after 12-14 hours fasting from a vein in the right elbow using disposable syringes and poured slowly in acid-washed pipes by a specialized technician. After centrifugation at 3,000 rpm for 10 minutes, the serum samples were stored in aliquots at -80°C. Blood glucose was measured by glucose oxidase technique, insulin by Immunoradiometric assay (Zelbio GMBH, Germany), total cholesterol, HDL and Triglyceride by Enzymatic methods, while low-density lipoprotein (LDL) cholesterol levels were calculated using the formula of friedwald equation.Insulin resistance was quantified using the homeostasis model of assessment insulin resistance (HOMA-IR) by the following equation (Pisprasert et al., 2013):
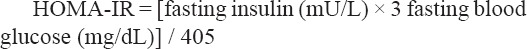
Sample size
Sample size estimations were calculated based on type I error (α) of 0.05 and type II error (β) of 0.20 (power = 80%) todetect a difference of 1kg/m2 in BMI with a standard deviation of 1.2 kg/m2 (Capristo et al. 2009). It was estimated that 44subjects in total were needed;however, considering 15 % drop-outrate, a total of 50patientswere recruited for the study.
Statistical Analysis
Data was analyzed using Statistical Package for the Social Sciences (SPSS ver.18) (SPSS Inc, Chicago, IL, USA) software. Descriptive analysis was used for measuring frequency, mean and standard deviation. Data normality was evaluated by Kolmogorov-Smirnov test and expressed by mean and standard deviation in Tables. Categorical variables were tested using the Chi-square test. For data with normal distribution, Paired t-test and independent t-test were used for inter and intra comparison of quantitative variables, respectively.Analysis of covariance was used toadjust for the baseline value of variables and mean differences in outcome variables between the two groups after trial. The statistical tests were two-sided, and a P value less than 0.05 were considered statistically significant.
Results
Forty-five subjects completed the study protocol: 22 subjects (6 M/16F) in the control group and 23 (6 M/17 F) in the GFD group. Five subjects dropped outfrom the study because of personal reasons. The study consort flow chart is presented in Figure 1.
Figure 1.
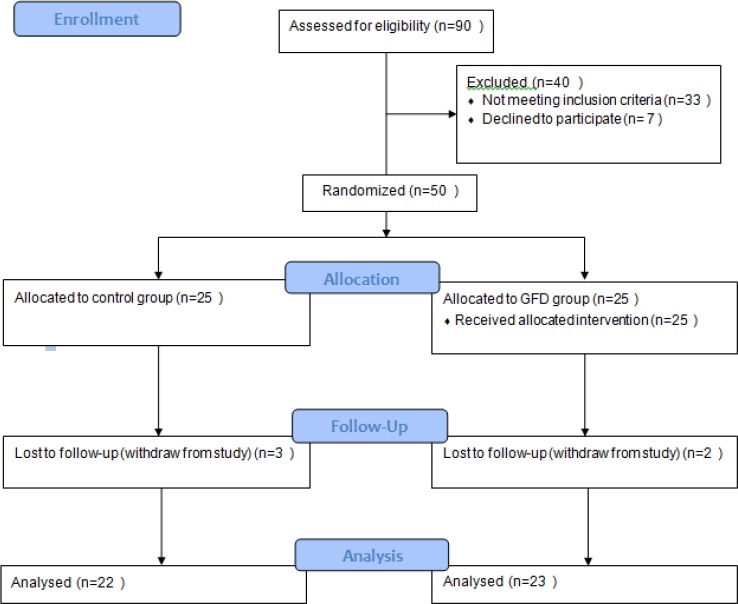
Flow Chart of the Study
The baseline clinical and anthropometric characteristics of the study population are reported in Table 1. Mean ± SD of participants’ age were 59.57±8.84 years in the intervention groups and 57.11±8.23 years in control group, and mean ± SD of body mass index was 32.60±4.70kg/m2, and 30.62±3.61kg/m2 in the intervention, and control groups respectively. There was no significant difference between intervention and control group at the baseline.
Table 1.
Baseline Characteristics of the Study Participants
| Characterstrics | Total (n=45) | Control Diet (n=22) | GFD Diet (n=23) | P-value |
|---|---|---|---|---|
| Age (years) | 58.55±8.76a | 59.57±8.84 | 57.11±8.23 | 0.101 |
| Sex (female/male) | 31/11 | 16/6 | 17/6 | 0.647 |
| Height (cm) | 159±8.77 | 159.78±8.74 | 159.63±9.04 | 0.957 |
| Weight (kg) | 80.82±13.41 | 82.21±13.61 | 77.73±8.74 | 0.206 |
| BMI (kg/m2) | 32.06±8.68 | 32.60±4.70 | 30.62±3.61 | 0.242 |
| WC (cm) | 103.26±8.68 | 104.65±10.13 | 101.57±6.28 | 0.832 |
| FBS (mg/dl) | 123.47±33.14 | 127.04±29.23 | 119.15±38.11 | 0.562 |
| Insulin serum (mU/L) | 12.05±5.75 | 11.57±5.79 | 12.63±5.80 | 0.56 |
| HOMA-IR | 3.62±1.65 | 3.56±1.76 | 3.70±1.86 | 0.814 |
| Serum Lipids | ||||
| Total cholestrol (mg/dl) | 170.47±36.43 | 170.82±38.91 | 170.05±34.24 | 0.946 |
| HDL (mg/dl) | 46.33±11.86 | 46.64±7.83 | 45.56±15.56 | 0.724 |
| Triglycerides (mg/dl) | 159.5±60.70 | 161.73±72.57 | 156.78±44.06 | 0.796 |
| LDL (mg/dl) | 98.62±33.85 | 97.99±34.47 | 99.4±34.07 | 0.894 |
| Blood pressure | ||||
| Systolic (mmHg) | 14.06±1.09 | 14.10±1.04 | 14.02±1.17 | 0.806 |
| Diastolic (mmHg) | 8.81±.53 | 8.89±.56 | 8.72±.49 | 0.315 |
WC, waist circumference; BMI, body mass index; FBS, fasting blood sugar; LDL, low density lipoprotein; HDL, high density lipoprotein; HOMA-IR, homeostasis model assessment of insulin resistance;
Mean ± SD (all such values)
Dietary data of two groups at baseline, and after 8 weeks of intervention are shown in Table 2. There were no significant differences in energy intake and nutrient composition of the diets between two groups at baseline. At the end of the intervention, energy intakes decreased in both GFD and control groups. However, compared with the end of intervention, total fiber intake was lower in GFD. In Table 3, the intergroup differences of metabolic variables are shown. Weight loss was not significantly different between GFD and control diet groups. The mean values of waist circumference (WC) (P < 0.03) decreased significantly in the GFD group compared with the control group.
Table 2.
Energy and Nutrient Composition in the GFD and Control Groups at the Baseline and after 8 Weeks
| Baseline | 8 weeks | |||||
|---|---|---|---|---|---|---|
| GFD | Control | Pa | GFD | Control | Pb | |
| Energy (kcal/day) | 2,282.01±280.34 | 2,144.58±214 | 0.435 | 1,895.60±239.2 | 1,821.89±271.0 | 0.824 |
| Total carbohydrates (g/day) | 319.48±26.07 | 310.55±35.26 | 0.435 | 251.97±61.47 | 261.98±39.87 | 0.845 |
| Energy from carbohydrates | 56.30±4.57 | 57.94±6.58 | 0.745 | 53.17±12.97 | 57.55±8.76 | 0.746 |
| Total protein (g/day) | 86.88±10.15 | 78.65±8.73 | 0.435 | 76.29±9.99 | 70.82±8.38 | 0.689 |
| Energy from protein | 15.23±1.78 | 14.67±1.63 | 0.623 | 16.10±2.11 | 15.55±1.84 | 0.643 |
| Total fat (g/day) | 72.18±19.39 | 64.45±16.37 | 0.347 | 64.72±13.12 | 54.45±10.56 | 0.576 |
| Energy from fat | 28.47±7.65 | 27.05±6.87 | 0.215 | 30.73±6.23 | 26.90±5.22 | 0.435 |
| Fiber (g/day) | 16.89±2.65 | 15.71±2.12 | 0.541 | 13.71±1.76 | 15.61±2.34 | 0.134 |
Values are means ±SD;
p-values indicate differences between the control and GFD groups at baseline and 8 weeks (ANOVA).
Table 3.
Anthropometric and Metabolic Parameters in the Two Study Groups after the Trial
| characterstrics | Control Diet (n=22) | GFD Diet (n=23) | P-valuea |
|---|---|---|---|
| Weight (kg) | 80.45±15.09b | 77.54± 8.98 | 0.171 |
| BMI (kg/m2) | 30.8± 4.1 | 30.1± ± 3.64 | 0.058 |
| WC (cm) | 101.23±5.97 | 101.73±9.13 | 0.001 |
| FBS (mg/dl) | 120.15±37.28 | 110.86±26.45 | 0.003 |
| Insulin serum (mU/L) | 14.45±7.26 | 12.38±6.37 | 0.619 |
| HOMA score | 12.36±6.85 | 10.19±6.37 | 0.153 |
| Serum Lipids | |||
| Total cholestrol (mg/dl) | 163.75±38.28 | 167.65±30.95 | 0.465 |
| HDL (mg/dl) | 45.89±14.80 | 48.26±8.41 | 0.745 |
| Triglycerides (mg/dl) | 154.75±41.31 | 138.52±42.13 | 0.032 |
| LDL (mg/dl) | 93.91±36.57 | 97.21±9.13 | 0.185 |
| Blood pressure | |||
| Systolic (mmHg) | 13.87±1.01 | 13.67±1.06 | 0.539 |
| Diastolic (mmHg) | 8.32±.50 | 8.67±.59 | 0.328 |
WC, waist circumference; FBS, fasting blood sugar; HOMA-IR, homeostasis model assessment of insulin resistance; LDL-C, low density lipoprotein; HDL-C, high-density lipoprotein cholesterol; CI, confidence interval; ANOVA, analysis of variance;
Based on an ANOVA model that regressed changes from baseline on treatment group, baseline value of the outcome;
Mean ± SD (all such values).
In both GFD and control groups, changes were observed in levels of total cholesterol[-3.17(-16.04,8.09) vs.-2.31(-6.29,1.24)], LDL cholesterol[-.75(-12.34, 8.83) vs. -3.12(-7.28,.90)], fasting insulin [.80(-1.04, 2.87) vs.2.02(-.41,4.75)mU/L] and HOMA-IR[-.16(-.71, .44) vs..50 (-.15, .35)] were not significantly different. Similar results were seen for systolic and diastolic blood pressure levels. Compared with the control diet, plasma triglyceride decreased significantly after the GFD [-23.21(-45.42,-3.58) vs.-2.05 (-6.29,1.94) mg/dL, P< 0.03)]. We observed that gluten exclusion improved glycemic control. Serum fasting glucose decreased significantly at the end of the study in GFD group compared with control group [-16.17 (-25.05, -9.15) vs. 1 (-3.65,-2.26), p< 0.05)].
Discussion
GFD is the primary treatment for individuals with gluten related disorders such as CD and gluten sensitivity none-celiac disease; however, some indirect evidence support the benefits of GFD for chronic diseases such as obesity, anddiabetes. Thus, GFD becomes a popular diet in the general population despite lack of evidence on its beneficial or harmful effects (Emilsson and Semrad, 2017). To our knowledge, the present study is the first trial examining the effects of GFD on obesity and metabolic variables in subjects with MES.
Our results showed that GFD induces significant reduction in WC in comparison to control diet. Reduction in WC without significant reduction in body weight may indicatepreferential loss of abdominal fat.Our results are in line with Soares et al findings, which have reported the effectiveness of gluten exclusion for weight loss as a healthy option for weight management (Soares et al., 2013). Furthermore, Kim et al., (2017) have shown that people who are on GFD, are at lower risk of obesity, metabolic syndrome and cardiovascular disorders; however, this study can not indicate that these effects are related with GFD because it might be possible that people following GFD might adhere a totally healthy diet, and these effects are due to it.
Although GFD is effective for weight loss in non-Celiac individuals, it increases weight in CD patients GFD (Dickey and Kearney, 2006; Tortora et al., 2015) because GFD induces intestinal healing of intestinal mucosal and subsequently promoting intestinal absorption efficiency (Cheng et al., 2010; Sima et al., 2010).
Our results showed that GFD improved glucose tolerance. These effects are probably associated with lower accumulation of visceral adiposity (Soares et al., 2013). The present results are consistent with a previous study showing that pigs fed a cereal-free diet had lower concentrations of C-reactive protein (CRP) and insulin resistance (Soares et al., 2013; Jönsson et al., 2005).
In present study, no significant difference on systolic or diastolic blood pressure was seen between GFD and control groups. Several studies have demonstrated lower prevalence of hypertension in none-CD individuals after following GFD; however, some studies suggest some mechanism for anti-hypertensive action of gliadin in experimental models (Tavakkoli et al., 2014; Thewissen et al., 2011). Thus, further studies are needed to be able to conclude about the effects of gluten and its components on blood pressure.
Our findings did not show any significant difference in total cholesterol, HDL, and LDL cholesterol. In contrast to our results, several studies reported increased serum levels of HDL cholesterol in CD patients after the introduction of a GFD (Kim et al., 2017; Tortora et al., 2015), which should be due to mucosal healing and consequently improvement in intestinal absorption of HDL and apo-A1, the main apo-protein of circulating HDL cholesterol (Capristo et al., 2009).
We found favorable changes in serum triglyceride in GFD group as compared with control group. This lowering effect of GFD on triglyceride can lead to reduction of cardiovascular disease risks. Elimination of gluten-containing flour and cereals, processed foods and refined wheat products such as white bread from their diet in those who adhered to GFD, may have contributed to these results. While some previous studies have suggested that people on GFD consume more carbohydrates and sugars (Wild et al., 2010), other studies suggested that changes in lipid profile in GFD followers may be related to GFD imposed dietary modifications such as high fat consumption due to replacement of gluten-containing flour and grains with fats (García-Manzanares et al., 2011). Our study participants reported similar consumption of macronutrients in both groups, which shows that our results are not affected by other dietary components.
The main strengths of our study included assessment of dietary intervention compliance in study participants by taking frequent dietary recalls, and analyzing dietary data; these analyzes ensured us that other dietary components are similar in both groups. Due to high price of GFD, we provided a selection of gluten-free products for participants in GFD group. All provided gluten-free products were similar to their gluten-containing counterparts due to utilization of new formulation made up of bean and nut flours, and innovative processing techniques which improved rheological characteristics, texture, and nutritional content (such as calorie, macronutrient,..), which ensured us that participants can afford the study protocol.
A number of important limitations need to be acknowledged regarding the present study. First of all, participants were not screened for gluten sensitivity disorders (coeliac disease, wheat allergy, non-coeliac gluten sensitivity (NCGS)). Although there are no definite biomarkers to diagnose NCGS, and CD, some evidence suggested HLA-DQ assessment for screening of NCGS and CD patients (Ontiveros et al., 2015). Secondly, subjects adherence to GFD could only be assessed via interview by an expert dietian at each visit, however there are no clear guidelines to evaluate GFD compliance, more recently evidences have been proposed use of plasma alkylresorcinols (Lind et al., 2016) detection of immunodominant gluten peptides (GIP) in feces and urine, as novel methods to assess adherence to GFD. Thirdly, the present study evaluated only short term metabolic effects of GFD in metabolic syndrome subjects. Long term follow up of health outcome of GFD in metabolic syndrome subjects could provide valuable information about the potential benefits or hazards of GFD. Another limitation of current study, as with most of clinical trials on dietary interventions, is the absence of blinding after randomization. However, we tried to minimize this limitation by advising the participants that the study is going to compare the metabolic effects of two diets in metabolic syndrome subjects.
In Conclusion, our study indicates that GFD significantly improved some key features of MetS including blood glucose and serum TGs. This is a preliminary short-term GFD intervention study. Intervention trials of longer duration with higher sample size will provide new insights on the effects of Gluten restriction on metabolic variables and obesity in non-celiac individuals. In parallel, further studies on possible mechanisms for the effects of GFD are recommended.
References
- 1.Aguilar M, Taft B, Sharon T, Benny L, Robert JW. Prevalence of the metabolic syndrome in the United States 2003-2012'. JAMA. 2015;313:1973–74. doi: 10.1001/jama.2015.4260. [DOI] [PubMed] [Google Scholar]
- 2.Capristo E, Noemi M, Sara F, et al. Increased serum high-density lipoprotein-cholesterol concentration in celiac disease after gluten-free diet treatment correlates with body fat stores. J Clin Gastroenterol. 2009;43:946–49. doi: 10.1097/MCG.0b013e3181978e4d. [DOI] [PubMed] [Google Scholar]
- 3.Cheng J, Pardeep SB, Anne RL, Peter HRG. Body mass index in celiac disease: beneficial effect of a gluten-free diet. J Clin Gastroenterol. 2010;44:267–71. doi: 10.1097/MCG.0b013e3181b7ed58. [DOI] [PubMed] [Google Scholar]
- 4.Dickey W, Natalie K. Overweight in celiac disease: prevalence, clinical characteristics, and effect of a gluten-free diet. Am J Gastroenterol. 2006;101:2356–59. doi: 10.1111/j.1572-0241.2006.00750.x. [DOI] [PubMed] [Google Scholar]
- 5.DiGiacomo DV, Christina AT, Peter HG, Ryan TD. Prevalence of gluten-free diet adherence among individuals without celiac disease in the USA: results from the Continuous National Health and Nutrition Examination Survey 2009–2010. Scand J Gastroenterol. 2013;48:921–5. doi: 10.3109/00365521.2013.809598. [DOI] [PubMed] [Google Scholar]
- 6.Emilsson L, Semrad CE. Obesity, metabolic syndrome, and cardiac risk factors: going gluten-free, for better or worse? Dig Dis Sci. 2017;62:2215–6. doi: 10.1007/s10620-017-4649-0. [DOI] [PubMed] [Google Scholar]
- 7.Expert Panel on Detection, Evaluation. Executive summary of the third report of the national cholesterol education program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III) JAMA. 2001;285:2486. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 8.Fung TT, Eric BR, Donna S, et al. Association between dietary patterns and plasma biomarkers of obesity and cardiovascular disease risk. Am J Clin Nutr. 2001;73:61–7. doi: 10.1093/ajcn/73.1.61. [DOI] [PubMed] [Google Scholar]
- 9.García-Manzanares Á, Alfredo JL, Sonia GC, Jesús MF. Resolution of metabolic syndrome after following a gluten free diet in an adult woman diagnosed with celiac disease. World J Gastrointestinal pathophysiol. 2011;2:49. doi: 10.4291/wjgp.v2.i3.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ibrügger S, Rikke JG, Henrik V, et al. Two randomized cross-over trials assessing the impact of dietary gluten or wholegrain on the gut microbiome and host metabolic health. J Clin Trials. 2014;4 2167-0870.1000178. [Google Scholar]
- 11.Jönsson T, Ashfaque AM, Kristina S, et al. Digested wheat gluten inhibits binding between leptin and its receptor. BMC Biochem. 2015;16:3. doi: 10.1186/s12858-015-0032-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jönsson T, Stefan O, Bo A, et al. Agrarian diet and diseases of affluence–Do evolutionary novel dietary lectins cause leptin resistance? BMC Endocr Disord. 2005;5:10. doi: 10.1186/1472-6823-5-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim H, Michael FD, Justin M, et al. Obesity, metabolic syndrome, and cardiovascular risk in gluten-free followers without celiac disease in the United States: Results from the National Health and Nutrition Examination Survey 2009–2014. Dig Dis Sci. 2017;10:1–9. doi: 10.1007/s10620-017-4583-1. [DOI] [PubMed] [Google Scholar]
- 14.Lammers KM, Ruliang Lu, Julie B, et al. Gliadin induces an increase in intestinal permeability and zonulin release by binding to the chemokine receptor CXCR3. Gastroenterology. 2008;135:194–204. doi: 10.1053/j.gastro.2008.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lebovitz HE. Type 2 diabetes mellitus-current therapies and the emergence of surgical options. Nat Rev Endocrinol. 2011;7:408–19. doi: 10.1038/nrendo.2011.10. [DOI] [PubMed] [Google Scholar]
- 16.Lebwohl B, Yin C, Geng Z, et al. Long term gluten consumption in adults without celiac disease and risk of coronary heart disease: prospective cohort study. BMJ. 2017;357:1892. doi: 10.1136/bmj.j1892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lind MV, Mia LM, Jüri JR, et al. Plasma alkylresorcinols reflect gluten intake and distinguish between gluten-rich and gluten-poor diets in a population at risk of metabolic syndrome. J Nutr. 2016;146:1991–8. doi: 10.3945/jn.116.236398. [DOI] [PubMed] [Google Scholar]
- 18.Marcason W. Is there evidence to support the claim that a gluten-free diet should be used for weight loss? J Am Diet Assoc. 2011;111:1786. doi: 10.1016/j.jada.2011.09.030. [DOI] [PubMed] [Google Scholar]
- 19.Noori NPM, Asgari S, Azizi F. Calcium and vitamin D intake and metabolic syndrome prevalence in Tehranian adults: Tehran glucose and lipid study. Iran J Endocrinol Metab. 2007;9:191–200. [Google Scholar]
- 20.Ontiveros N, Hardy MY, Cabrera-Chavez F. Assessing of celiac disease and nonceliac gluten sensitivity. Gastroenterol Res Pract. 2015;2015:723954. doi: 10.1155/2015/723954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pisprasert V, Katherine HI, Maria FL, Munoz A, Garvey WT. Limitations in the use of indices using glucose and insulin levels to predict insulin sensitivity: impact of race and gender and superiority of the indices derived from oral glucose tolerance test in African Americans. Diabetes Care. 2013;36:845–53. doi: 10.2337/dc12-0840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sima H, Hekmatdoost A, Ghaziani T, et al. The prevalence of celiac autoantibodies in hepatitis patients. Iran J Allergy Asthma Immunol. 2010;9:157. [PubMed] [Google Scholar]
- 23.Soares FLP, Rafael dOM, Lílian GT, et al. Gluten-free diet reduces adiposity, inflammation and insulin resistance associated with the induction of PPAR-alpha and PPAR-gamma expression. J Nutrit Biochem. 2013;24:1105–11. doi: 10.1016/j.jnutbio.2012.08.009. [DOI] [PubMed] [Google Scholar]
- 24.Tavakkoli A, Suzanne KL, Christina AT, et al. Characteristics of patients who avoid wheat and/or gluten in the absence of celiac disease. Dig Dis Sci. 2014;59:1255–61. doi: 10.1007/s10620-013-2981-6. [DOI] [PubMed] [Google Scholar]
- 25.Thewissen BG, Anneleen P, Inge C, Kristof B, Jan AD. Inhibition of angiotensin I-converting enzyme by wheat gliadin hydrolysates. Food Chem. 2011;127:1653–58. [Google Scholar]
- 26.Thomas KE, Anna S, Alessio F, Stefanie NV. Gliadin stimulation of murine macrophage inflammatory gene expression and intestinal permeability are MyD88-dependent: role of the innate immune response in Celiac disease. J Immunol. 2006;176:2512–21. doi: 10.4049/jimmunol.176.4.2512. [DOI] [PubMed] [Google Scholar]
- 27.Tortora RP, Capone GDS, Imperatore N, et al. Metabolic syndrome in patients with coeliac disease on a gluten-free diet. Aliment Pharmacol Ther. 2015;41:352–59. doi: 10.1111/apt.13062. [DOI] [PubMed] [Google Scholar]
- 28.Ukkola A, Markku M, Kalle K, et al. Changes in body mass index on a gluten-free diet in coeliac disease: a nationwide study. Eur J Int Med. 2012;23:384–88. doi: 10.1016/j.ejim.2011.12.012. [DOI] [PubMed] [Google Scholar]
- 29.Wild D, Robins GG, Burley VJ, Howdle PD. Evidence of high sugar intake, and low fibre and mineral intake, in the gluten-free diet. Aliment Pharmacol Ther. 2010;32:573–81. doi: 10.1111/j.1365-2036.2010.04386.x. [DOI] [PubMed] [Google Scholar]
- 30.Zabetian A, Hadaegh F, Azizi F. Prevalence of metabolic syndrome in Iranian adult population, concordance between the IDF with the ATPIII and the WHO definitions. Diabetes Res Clin Pract. 2007;77:251–7. doi: 10.1016/j.diabres.2006.12.001. [DOI] [PubMed] [Google Scholar]


