Abstract
Purpose:
We aimed to investigate the influence of different methods of region-of-interest (ROI) placement on apparent diffusion coefficient (ADC) values in breast tumours and their accuracy in differentiating benign versus malignant tumors in mass and nonmass lesions.
Methods and Materials:
In this prospective study, 79 patients with 98 breast lesions, from 2015 until 2017, were investigated by 1.5-T breast MRI. Histopathology evaluation were done for all malignant lesions and most of the benign ones. ADC values were measured in normal breast tissue and by two ways of ROI placement in the breast lesions (mass and non-mass): 1- ROI covering the whole lesion, 2- ROI in the highest part (most restricted area) of the lesion in DWI images. The accuracy of these two approaches were compared.
Results:
The age range was 17-68 years with mean age 43.3 ± 9.9 years. 49% of the lesions were benign and 51% of tumors were malignant. Our results revealed that the measured ADC values in normal breast tissue were higher than breast lesions (P≤0.01). Appropriate cut off determination in non-mass was not valid by both methods, but in mass in the first way was 1.45×10-3mm2/s and in the most restricted part was 1.16×10-3 mm2/s. ADC values differed significantly between the two ways of ROI placement in mass lesions (P<.001). Most restricted part ADC showed the best diagnostic performance in mass lesions with area under curve 0.88 versus 0.82.
Conclusion:
ROI placement has significant impact on the meseaured ADC values of breast lesions and ROIs in most restricted parts were more accurate than whole-lesion ROIs. Cut-off values differed significantly based on the methods of measurement.
Keywords: Breast cancer, magnetic resonance imaging, diffusion magnetic resonance imaging
Introduction
Dynamic contrast-enhanced magnetic resonance imaging (DCE-MRI) has an essential role in breast lesion characterization and local staging. It is very sensitive to identify cancer by lesion morphologic and kinetic features (Kinkel et al., 2000;Warren et al., 2005).
Diffusion Weighted Imaging (DWI) is another method improves value of MRI in lesion characterization without using contrast material (Hirano et al., 2012) and is a useful sequence in differentiation of benign and malignant breast lesions (Hatakenaka et al., 2008; Patridge et al., 2010). This method evaluates the random movement of water molecules, shows the diffusivity of the different tissues and depicts cellular density and tissue microstructure. The apparent diffusion coefficient (ADC) can quantify this diffusivity (Woodhams et al., 2005; Hirano et al., 2012). Studies about this issue represented that ADC number is significantly lower in malignant breast lesions compared to benign breast lesions, because of higher cellular density in malignant lesions (Hirano et al., 2012; Hatakenaka et al., 2008).
ADC is usually measured by placing regions-of-interest (ROIs) inside the lesion. The employed b- value has influence on ADCs for example lower b-values result in higher ADCs. Also different strategies exist in the literature for placing ROI, but there is no agreement (Orponent et al., 2015; Bicket et al., 2016). A range of different sizes and places of ROI (in most restricted region, average of multiple ROIs in different regions or covering the whole lesion) and as a result different cut points have been proposed (Partridge et al., 2009; Park et al., 2007; Hirano et al., 2012). This study focuses on the investigation of ADC values by two different methods of ROI placement (in the whole lesion and its most restricted part) in mass and non-mass lesions to evaluate their efficacy in differentiation of benign and malignant breast lesions.
Materials and Methods
Study population
Between 2015 and 2017, 79 patients with 98 breast lesions were investigated in this prospective study, by 1.5-T breast MRI. The MRI Center is a major referral center for breast MRI. These patients referred either with clinical indications or due to request of their surgeon for example for evaluation of palpable breast lesions, bloody nipple discharge, abnormalities in their mammogram or for screening of high risk state. Our institutional ethics board approved this prospective study. Informed consent was obtained from all individual participants after explaining all aspects and aims of study.
Patients with suspicious findings based on DCE MRI underwent biopsy. The pathologic diagnosis was based on core needle biopsy, excisional biopsy, or examination of lumpectomy or mastectomy specimens. The studied patients fulfilled our inclusion criteria: no diagnostic or therapeutic intervention before MRI investigation; adequate size for visibility in DWI and ADC; histopathological proof of all assessed BIRADS 4 and 5 lesions but some benign masses based on BIRADS 2 MRI features were also included like as stability during 2 consecutive years (The BIRADS 3 lesions which followed for 2 years by MRI and were stable and then categorized as BIRADS 2 lesions).
Breast MRI Protocol
MRI examinations were performed in a standard prone position by a 1.5 T Signa system (General Electric Medical Systems, USA) using a bilateral phased-array 4 channel breast coil. MRI was done during the second week of the menstrual cycle in premenopausal patients. The applied MRI examination included an axial T1-weighted and axial short inversion time inversion-recovery (STIR) images, followed by six series of axial dynamic T1-weighted 3D (after bolus injection of 0.2 mmol/kg of gadolinium-DTPA (Dotarem, Guerbet), followed by 15 mL normal saline), fat-suppressed spoiled gradient-echo images and DWI sequence after 10 min of injection of gadolinium.
These sequences parameters are as follows
1) The axial T1-weighted sequences: repetition time (TR)/echo time (TE): 400/10; bandwidth (BW): 31.25 Hz/pixel; field of view (FOV): usually 32 mm; Slice thickness: 5.0 mm; Matrix size: 384 × 256; number of excitations (NEX): 1
2) Axial STIR: TR/TE: 4500/63; bandwidth: 62.50; FOV: usually 32; Slice thickness: 5.0 mm; Matrix size: 320 x 256; NEX: 1.
3) The dynamic T1-weighted 3D, fat-suppressed spoiled gradient-echo sequence: TR/TE: 9/4; BW: 31.25; FOV: 32; Slice thickness: 4.0 mm with no intersection gap; Matrix size: 352 × 288; NEX: 1; flip angle (FA): 300.
4) DWI echo planer image: TR/TE: 7700/89, FOV: 38, Flip angle: 90, NEX: 4, matrix: 192×192 pixels, Slice thickness: 5 mm with spatial fat suppression and by two respective b factors (0 and 800 s/mm2). The ADC maps were automatically calculated by MRI system software.
Image analysis
One radiologist with more than ten-year experience reviewed all MR images and was blinded to the pathology results. Each lesion was evaluated by the American College of Radiology BIRADS breast MRI lexicon based on its morphologic and kinetic findings. Lesion size (the largest diameter) was measured on the DCE MRI images.
First, one ROI was drawn freehand to cover the entire lesion (mass or non mass) on the slice with the largest tumor dimensions. Then one small ROI about 10±2 mm2 were placed on the most restricted region inside the solid part on the ADC map. The ROIs were selected as being clearly high-signal on the DWI, also we avoided the region of high signal intensity on the T2 weighted sequence (to exclude the T2 shine-through effect). Care was taken to avoid cystic or necrotic, fatty regions and hematoma inside the mass. Two ADC values (whole lesion ADC and most restricted part ADC) were calculated for all selected lesions. In addition, two large round ROIs were placed in healthy fibroglandular tissue of contralateral breast and its average was calculated as an ADC value of normal breast tissue.
Statistical analysis
The collected data was assessed by SPSS version 22. In data description, frequency distribution, mean, standard deviation, maximum and minimum are used. Mean± standard deviation (SD) was used for continuous variables and absolute values and percentages were used for categorical variables. One way Anova in data analysis and Duncan is used to identify the relation among quantities variables. To evaluate the potentiality of ADC value in differentiation of benign and malignant lesions receiver operating characteristics (ROC) test is used. After cutoff point determination, ADC identification coefficients including, sensitivity, PPV, NPV and accuracy were determined.
Results
In this study, 79 patients with 98 breast lesions were studied. Their age range was 17-68 years with mean age 43.3 ± 9.9 years. Most of the patients were between 28-49 years old (70.9%). 49% (48) of the lesions were benign and 51% (50) of tumors were malignant. 71.4% (70) of lesions were mass and 28.6% (28) were lesions with non-mass like enhancement. The mean tumor size of benign and malignant lesions are measured 26.0± 20.2 mm, 34.3± 20.4 mm respectively and in mass and non-mass lesions are 23.5±10.6 mm and 46.4± 28.5 mm respectively.
Comparison of ADCs in two methods of ROI-placement
ADC values in the method of whole ROI placement of benign and malignant lesions are 1.49×10-3 mm2/s and 1.23×10-3 mm2/s respectively and in the most restricted part of the masses are 1.36×10-3 mm2/s and 1.06 ×10-3 mm2/s respectively (Table 1, Figure 1 and Figure 2).
Table 1.
Mean, Standard Deviation (SD), Maximum, Minimum of ADC Values for Benign and Malignant Lesions
| Variable | Mean (× 10-3 mm2/s) | SD (× 10-3 mm2/s) | Maximum (× 10-3 mm2/s) | Minimum (× 10-3 mm2/s) |
|---|---|---|---|---|
| whole ROI drawn method | ||||
| Benign | 1.49 | 0.29 | 2.37 | 0.85 |
| Malignant | 1.23 | 0.26 | 2.10 | 0.80 |
| Small ROI in most restricted part | ||||
| benign | 1.36 | 0.30 | 2.32 | 0.68 |
| Malignant | 1.06 | 0.26 | 2.10 | 0.66 |
Figure 1.
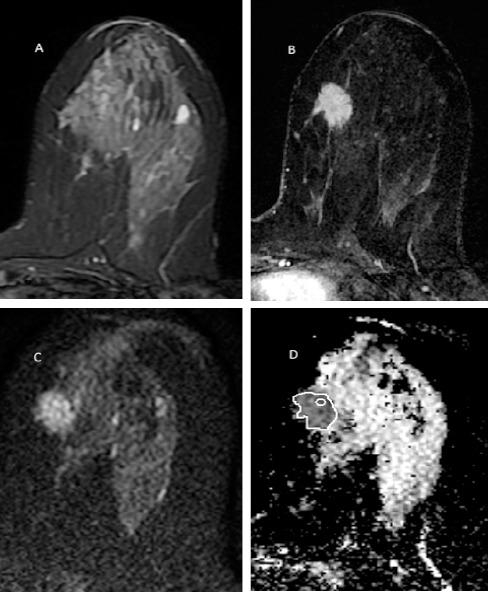
A 35 Years Old Women with Palpable Left Breast Lower Inner Quadrant Spiculated Mass. The mass has low T2 signal (A), homogenous enhancement with spiculated border in post contrast image (B) and is restricted in DWI with ADC value 1.02 × 10-3 mm2/s (C). Two methods of ROI placement is depicted in ADC image (D). The pathology was invasive lobular carcinoma.
Figure 2.
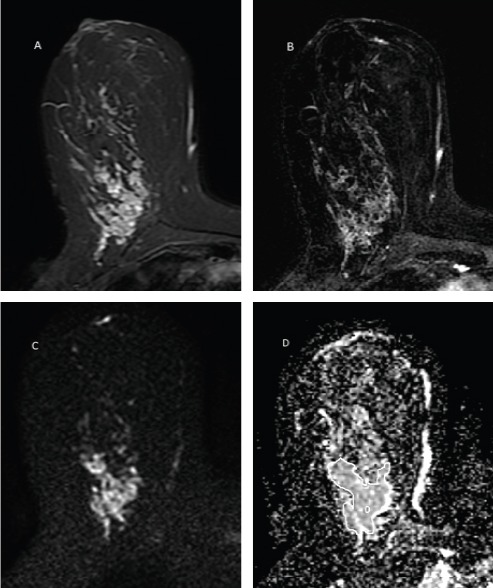
A 35 Years Old Women with Palpable Large Right Breast Lower Inner Quadrant Non-mass Lesion. The lesion has high T2signal (A), clumped pattern enhancement in post contrast image (B) and is mildly restricted in DWI with ADC value 1.60 × 10-3 mm2/s (C). Two methods of ROI placement is depicted in ADC image (D). The pathology was ductal carcinoma in situ.
The results of one way Anova and Duncan test in ADC value investigation and in two ROI placement methods in benign and malignant masses and its comparison with ADC value in the normal breast tissue revealed that there is a significant difference in measured ADC values (p≤0.001). ADC value in the normal tissue (1.79 ×10-3 mm2/s) was significantly higher than ADC value in benign and malignant masses in both ROI placement techniques (Table 2). Also there was a significant difference between ADC value between two methods of ROI placement (p≤0.05) and its quantity in the whole ROI drown method was higher than its quantity by the second technique.
Table 2.
ADC Value Comparison in Normal Breast Tissue, Benign and Malignant Masses by Two ROI Methods
| Method | Number | Mean ± SD (× 10-3 mm2/s) | P value | |
|---|---|---|---|---|
| The normal breast tissue | Average of 2 large ROIs | 98 | 1.79±0.26 | <0.001 |
| Benign | In the whole mass | 48 | 1. 49±0.29 | <0.001 |
| In the most restricted part | 48 | 1.36±0.30 | ||
| Malignant | In the whole mass | 50 | 1.23± 0.26 | <0.001 |
| In the most restricted part | 50 | 1.06±0.26 |
Area under curve (AUC) values were 0.82 and 0.52 for whole ROIs and 0.88 and 0.50 for small ROIs for mass and non-mass lesions respectively. AUC was not significant (p≥0.05) for non-mass lesions and had no role in the characterization of these type of lesions by both methods. Also the AUC was higher in Small ROI technique in most restricted part and shows its more efficacy (Table 3 and Figure 3).
Table 3.
Area under Curve (AUC) Level and Appropriate Cutoff Points in Two ROI Methods in Mass and Non-mass
| ROI method | Cutoff point (× 10-3 mm2/s) | AUC | P value | |
|---|---|---|---|---|
| In the whole lesion | Mass | 1.45 | 0.825 | 0.000 |
| Non-mass | 1.39 | 0.529 | 0.796 | |
| In the most restricted part of lesion | Mass | 1.16 | 0.886 | 0.000 |
| Non-mass | 1.23 | 0.508 | 0.508 |
Figure 3.
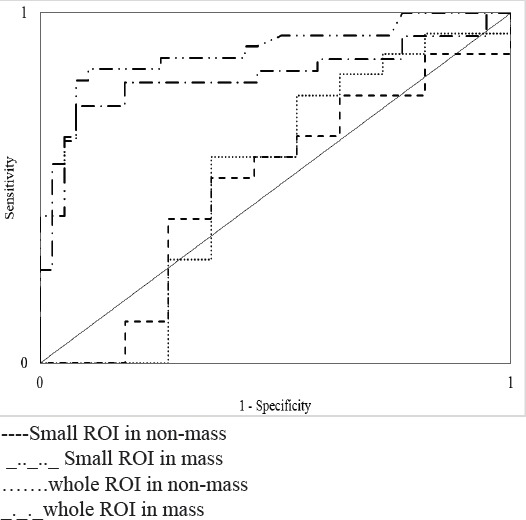
ROC Curve Related to ADC Value in Two ROI Methods in Mass and Non-mass.
Using whole ROI and applying a cut-off value of 1.45× 10-3 mm2s achieved 92.3% sensitivity, 74.1% specificity,81.8% PPV, 88.4% NPV, and 84.2% overall accuracy for differentiating benign and malignant masses. Small ROI with a cut-off value of 1.16× 10-3 mm2s for differentiating masses, had 89.7% sensitivity, 83.8% specificity, 87.5% PPV, 86.6% NPV, and 87.1 % overall accuracy.
ADC values based on histopathology of breast lesions
In Table 4 the results of histopathology are presented. The majority of pathology results were invasive ductal carcinoma in 37.5% of the patients (27 persons). Nine patients with benign mass had no pathology and were considered benign according to their morphology or stability during 2-year MRI follow up. Generally the lower ADC values were related to malignant lesions, however the highest ADC value in the method of whole ROI placement and in most restricted part were respectively 1.76×10-3 mm2/s and 1.66×10-3 mm2/s and was related to ductal carcinoma in situ (DCIS). On the other hand the lowest ADC value by two methods was 0.90×10-3 mm2/s and was correspond to fibrocystic change with adenosis in histopathology results. Among malignant invasive lesions papillary carcinoma had the highest ADC quantity (1.38 ×10-3 mm2/s).
Table 4.
Histopathology Results and ADC Values in Two Methods of ROI Placement
| Histopathology results | Number | The whole ROI (× 10–3 mm2/s) | Small ROI (× 10–3 mm2/s) |
|---|---|---|---|
| Invasive ductal carcinoma | 37 | 1.19 | 1.04 |
| Mixed invasive and lobular carcinoma | 2 | 1.19 | 0.90 |
| Invasive lobular carcinoma | 5 | 1.13 | 1.04 |
| Absolute inflammatory | 7 | 1.19 | 0.97 |
| Fibrocystic change | 7 | 1.36 | 1.22 |
| Fibrocystic change with adenosis | 1 | 0.90 | 0.90 |
| Adenosis with ductal hyperplasia | 2 | 1.57 | 1.37 |
| Fibroadenoma | 7 | 1.57 | 1.46 |
| Ductal carcinoma in situ | 4 | 1.76 | 1.66 |
| Fat necrosis | 1 | 1.38 | 1.20 |
| Papillary carcinoma | 1 | 1.38 | 0.96 |
| Hamartoma | 2 | 1.60 | 1.46 |
| Fibrosis | 4 | 1.47 | 1.38 |
| Papillomatosis | 4 | 1.66 | 1.44 |
| Adenosis | 5 | 1.36 | 1.24 |
| Benign by follow up or morphology | 9 | 1.29 | 1.16 |
| Total | 98 |
Discussion
Distinguishing between benign and malignant lesions can be possible by ADC measurements, although there is some overlap in ADC values. Results of this study revealed than malignant lesions has lower ADC value compared to benign ones and normal breast tissue (1.79×10-3 mm2/s) like as many other previous essays. (Partridge et al., 2010; Hatakenaka et al., 2008; Woodhams et al., 2005; Park et al., 2007).
Based on previous literature, ADC values for malignant and benign lesions vary considerably (0.79–1.45 ×10-3 mm2/s and 1.20–1.71 ×10-3 mm2/s, respectively), because there is no standard method regarding ADC measurement. It can be influenced by way of ROI placement and the amount of b values. However the order of collecting DWI (before or after DCE-MRI) has been shown that has no significant effects on the ADC values (Arponent et al., 2015). Many different techniques of ROI placement exist: Woodham et al., (2005) used the mean of multiple ROI; Partrige et al., (2012) used the whole lesion ROI; Hirano et al., (2012) drown multiple ROIs and used minimum, average and maximum ADC; Hatakenka et al., (2008) used a ROI of at least 100 mm2.
Our study confirmed that the ADC values in breast lesions are significantly influenced by method of ROI placement and ROI in most restricted part of lesions perform better for the differentiation of benign and malignant breast lesions compared to whole drown ROI. ADC estimates of smaller ROIs were generally lower. This reflects the natural heterogeneity of breast lesions, which results in substantial differences in ADC estimate. Another likely explanation is that small ROIs in most restricted part include only the most viable and cellular portion of the lesions and as a result the estimated ADC may be more accurate for lesion differentiation. Also, by small ROIs it is easier to exclude necrotic and hemorrhagic areas. In recent investigations, also it has been shown that the small ROIs generally performing better than the whole method ones (Bickel et al., 2016; Nogueira et al., 2015; Arponent et al., 2015). A long the higher accuracy of small ROI, this technique has another advantage: it is much less time consuming compared to whole ROI placement (Nogueira et al., 2015).
Our study revealed that both technique had deficiency in differentiation of benign and malignant non-mass lesions (AUC: 0.5 in whole ROI, 0.52 in small ROI method, P value>0.05). This result could be partly related to their un-sharp boundary and their tendency to replace not displace normal surrounding tissue and partly due to the histopathology nature of non- mass malignant lesions (most of non-mass malignant lesions were DCIS, not invasive) especially when looking at the small ROI technique failure in their differentiation. Partridge et al found that ADC measurement may be more useful in discriminating masses compared to non-mass lesions (AUC 0.8 vs 0.66) (Partridge et al.,2010). Also Imamura et al showed no significant difference between ADC value of malignant and benign non-mass lesions (0.96×10-3 mm2/s vs 1.20×10-3 mm2/s respectively) but they proposed adding ADC results to DCE MRI pattern improve its accuracy (Imamura et al., 2010).
Among our malignant invasive lesions papillary carcinoma had the highest ADC value (1.38 ×10-3 mm2/s). In Woodham et al. study the highest ADC value was also related to intracystic papillary carcinoma and then mucinous carcinoma and malignant Phyllodes (2.6×10-3 mm2/s, I.75×10-3 mm2/s and 1.67×10-3 mm2/s respectively) (Woodhams et al., 2005). We have no case of mucinous carcinoma or malignant Phyllodes. Mucin pool and higher extracellular water content in mucinous carcinoma may be a reason for higher ADC values in this type of tumors. In contrast to previous literatures, ADCs in our DCIS lesions were very high (1.76×10-3 mm2/s and 1.66×10-3 mm2/s in whole ROI placement and in small ROI method respectively). The ADC values of this type of tumors range 0.90 -1.36×10-3mm2/s in previous studies (Woodham et al., 2005; Hirano et al., 2011; Park et al., 2007 and Partridge et al., 2009). May be this discrepancy was related to the limited number (4 lesions) of our DCIS cases or the pattern of their presentation. All 4 DCIS cases presented as non mass enhancement. Another limitation of our study apart from the relatively small number of different lesion subtypes was using only one small ROI in most restricted part. Because in some masses and especially non mass lesions more than one restricted regions could be identified and in the future the diagnostic ability of one small ROI versus average of 2 to 3 small ROIs in most restricted parts of lesions can be evaluated.
In conclusion, small ROIs in most restricted part proved to be more accurate than whole ROIs method and its drawing is less time consuming. Both techniques have less performance in differential diagnosis of non-mass lesions. The ADC cut-off points differed significantly depending on measurement technique and the standardization of ADC ROI placement is suggested based on our findings.
References
- 1.Abdulghaffar W, Tag–Aldeen MM. Role of diffusion –weighted imaging (ADC) and apparent diffusion coefficient (DWI) in differentiating between benign and malignant breast lesions. Egypt J Radiol Nucl Med. 2013;44:945–51. [Google Scholar]
- 2.Arantes pareira FP, Martins G, Figneired F, et al. Assessment of breast lesion with diffusion weighted MRI : comparing the use of different b values. AJR Am J Roentgenol. 2009;193:1030–35. doi: 10.2214/AJR.09.2522. [DOI] [PubMed] [Google Scholar]
- 3.Hatakenaka M, Soeda H, Yabnnehi H, et al. Apparent diffusion coefficients of breast tumors : clinical application. Magn Reson Med Sci. 2008;7:23–9. doi: 10.2463/mrms.7.23. [DOI] [PubMed] [Google Scholar]
- 4.Hirano M, Satake H, Ishiguki S, et al. Diffusion –weighted imaging of breast masses: comparison of diagnostic performance using various apparent diffusion coefficient parameters. AJR Am J Roentgenol. 2012;198:717–22. doi: 10.2214/AJR.11.7093. [DOI] [PubMed] [Google Scholar]
- 5.Immure T, Isomoto I, Sueyoshi E, et al. Diagnostic performance of ADC for non- mass like breast lesions on, MR imaging. Magn Reson Med Sci. 2010;9:217–25. doi: 10.2463/mrms.9.217. [DOI] [PubMed] [Google Scholar]
- 6.Kamitani T, Matsue Y, Yabuuchi H, et al. Correlations between apparent diffusion coefficient values and prognostic factors of breast cancer. Magn Reson Med Sci. 2013;12:193–9. doi: 10.2463/mrms.2012-0095. [DOI] [PubMed] [Google Scholar]
- 7.Kul S, Cansu A, Alhan E, et al. Contribution of diffusion –weighted imaging to dynamic contrast enhanced MRI in the characterization of breast tumors. AJR Am J Roentgenol. 2011;196:210–7. doi: 10.2214/AJR.10.4258. [DOI] [PubMed] [Google Scholar]
- 8.Kul S, Oguz S, Eyuboglu I, komureuglu R. Can unenhanced breast MRI be used to decrease negative biopsy rates. DIR. 2015;21:287–92. doi: 10.5152/dir.2014.14333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nogueira L, Brandaos F, Matos E, et al. Diffusion –weighted, imaging : determination of the pair of b-values to discriminate breast lesions. Br J Radiol. 2014;2014:87. doi: 10.1259/bjr.20130807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Orguc S, Basara I, Coskun T. diffusion weighted MR imaging of the breast : comparison of apparent diffusion coefficient values of normal breast tissue with benign and malignant breast lesions. Singapore Med J. 2012;53:737–43. [PubMed] [Google Scholar]
- 11.Partridge Sc, Demartini WB, Kurland BF, et al. Quantitative diffusion –weighted imaging as an adjunct to conventional breast MRI for improved position predictive value. AJR Am J Roentgenol. 2009;193:1719–22. doi: 10.2214/AJR.08.2139. [DOI] [PubMed] [Google Scholar]
- 12.Partridge SC, Mullins CD, Kurland BF, et al. Apparent diffusion coefficient values for discriminating benign and malignant breast MRI lesions : effects of lesion type and size. AJR Am J Roentgenol. 2010;194:1664–73. doi: 10.2214/AJR.09.3534. [DOI] [PubMed] [Google Scholar]
- 13.Partridge SC, Rahbar H, Muthy R, et al. Improved diagnostic accuracy of breast MRI through combined apparent diffusion coefficients and dynamic contrast- enhanced kinetics. Magn Reson Med. 2011;65:1759–67. doi: 10.1002/mrm.22762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Parsian S, Rahbar H, Allison KH, et al. Nonmalignant breast lesions: ADCs of benign and high risk subtypes assessed as fals positive at dynamic enhanced MR imaging. Radiology. 2012;2012:256. doi: 10.1148/radiol.12112672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ramirez –Galvan YA, Cardona –Huerta S, Ibarra –fombona E, Elizondo Riojas G. Apparent diffusion coefficient (ADC) value to evaluate B1-RADs4 breast lesions: correlation with pathological findings. J Clin Imaging Sci. 2014;39:51–5. doi: 10.1016/j.clinimag.2014.09.015. [DOI] [PubMed] [Google Scholar]
- 16.Satake H, Nishio A, Ikeda M, et al. Predictive value for malignancy of suspicious breast masses of BIRADS categories 4 and 5 using ultrasound elastography and MR diffusion –weighted imaging. AJR Am J Roentgenol. 2011;196:202–9. doi: 10.2214/AJR.09.4108. [DOI] [PubMed] [Google Scholar]
- 17.Woodhams R, Matnnga R, kan S, et al. ADC mapping of benign and malignant breast tumors. Magn Reson Med Sci. 2005;4:35–42. doi: 10.2463/mrms.4.35. [DOI] [PubMed] [Google Scholar]
- 18.Woodhams R, Ramadar S, Stanwell P, et al. Diffusion –weighted imaging of the breast : principles and clinjcal applications. Radiographics. 2011;31:1059–84. doi: 10.1148/rg.314105160. [DOI] [PubMed] [Google Scholar]


