Abstract
Background
The role of noninvasive liver fibrosis markers which were developed to evaluate the severity of chronic liver disease remains unclear in cirrhosis.
Aims
To evaluate the correlation between noninvasive markers and hemodynamic parameters and their prognostic performance in cirrhotic patients.
Methods
A total of 242 cirrhotic patients undergoing hemodynamic study were analyzed. The correlations between noninvasive models, including FIB-4, aspartate aminotransferase to platelet ratio index, cirrhosis discriminant score, Lok index, Goteborg University Cirrhosis Index, and albumin-bilirubin (ALBI) score and hemodynamic parameters were investigated, along with their predictive accuracy for short- and long-term survival.
Results
There was a significant correlation between all noninvasive markers and hepatic venous pressure gradient (HVPG), and ALBI score had the best correlation (r = 0.307, p<0.001). For the prediction of 3-month and 6-month mortality, serum sodium (sNa) levels had the highest area under curve (AUC; 0.799 and 0.818, respectively) among all parameters, and ALBI score showed the best performance (AUC = 0.691 and 0.740, respectively) compared with other 5 noninvasive models. Of 159 patients with low MELD scores (<14), high ALBI score (>-1.4) and low sNa (<135 mmol/L) predicted early mortality. In the Cox multivariate model, ALBI, MELD, HVPG and sNa were independent predictors of long-term survival.
Conclusions
Among noninvasive markers, ALBI score is best correlated with HVPG and associated with short-term outcome in cirrhotic patients. A high ALBI score and low sNa identify high-risk patients with low MELD scores. High MELD, HVPG, ALBI and low sNa levels are independent predictors of survival. Independent studies are required to confirm our findings.
Introduction
Portal hypertension (PH) is responsible for complications of liver cirrhosis, such as variceal bleeding, ascites, hepatic encephalopathy and hepatorenal syndrome [1]. Because of these complications, PH represents the main cause of mortality in patients with advanced cirrhosis. Increased intrahepatic vascular resistance and hyperdynamic circulatory alteration both contribute to PH [2].Several studies showed that a reduction in portal pressure determined as the hepatic venous pressure gradient (HVPG) provided effective protection of variceal bleeding [3–5]. Subsequent studies further demonstrated that HVPG predicted the occurrence of decompensation and mortality in cirrhotic patients while a decrease in HVPG improved long-term survival [6,7]. In addition, hemodynamic changes in advanced cirrhosis were found to associate with the development of hepatorenal syndrome [8]. Thus, it is postulated that hemodynamic parameters may provide unique information on the prognosis of cirrhotic patients.
In the past decade, several noninvasive fibrosis markers involving routine laboratory parameters have been developed as an alternative to liver biopsy for evaluating the severity of chronic liver disease, including the FIB-4 index [9], aspartate aminotransferase (AST) to platelet ratio index (APRI) [10], cirrhosis discriminant index (CDS) [11],Lok index [12] and Goteborg University Cirrhosis Index (GUCI) [13].Recently, the albumin-bilirubin (ALBI) score, an alternative measure of liver dysfunction that was initially proposed for use in patients with hepatocellular carcinoma (HCC) [14], has also been found to correlatesignificantly with histological staging in patients with primary biliary cirrhosis [15].As hepatic fibrosis contributes to elevated intrahepatic vascular resistance, various noninvasive markers have been evaluated for their correlation with HVPG in cirrhotic patients. Both the APRI [16] and Lok index [17,18] have shown reliable performance for predicting PH. However, there has been no study evaluating the relationship between portal pressure and ALBI score, and the correlation between noninvasive models and hemodynamic parameters other than HVPG remains unknown. Although noninvasive fibrosis markers have been evaluated for their predictive value with respect to survival in chronic liver disease [17,19,20], the results were inconsistent.The prognostic ability of these models in comparison with the established risk factors such as the model of end stage liver disease (MELD) score, Child-Turcotte-Pugh (CTP) score, serum sodium (sNa) level and HVPG for outcome prediction in cirrhotic patients is still unclear.
This study aimed to investigate the correlation between various noninvasive markers of liver fibrosis and hemodynamic parameters and to assess their prognostic impact on short-term and long-term survival in cirrhotic patients.
Materials and methods
Patients
This retrospective cohort study included 242 consecutive adult patients with cirrhosis who had been admitted to Taipei Veterans General Hospital from May 1992 to March 2005for the evaluation of the severity of liver disease and degree of PH. These patients underwent HVPG measurement because it is considered a unique prognostic marker in cirrhosis. The diagnosis of liver cirrhosis was based on characteristic findings including physical stigmata of cirrhosis, biochemical data and image findings [21,22]. Patients with the following conditions were excluded from the study: (1) previous transjugular intra-hepatic porto-systemic shunt placement or portal vein thrombosis; (2) active variceal hemorrhage; (3) hepatic encephalopathy; (4) active infection; (5) HCC; and (6) use of β-blockers or vasoactive drugs upon enrollment. None of the patients with hepatitis B or C had received specific anti-viral treatment (interferon, nucleoside or nucleotide analogues) during the study period.
Medical records were reviewed for data collection including the etiology of liver disease, the presence of esophageal varicesandascites, and transplant-free survival. The severity of ascites was classified into 3 categories: no ascites, response to diuretics, and diuretic-resistant ascites.The presence of ascites was defined by ultrasonography, and the lack of response to diuretics was defined as a mean weight loss less than 0.8 kg over four days under maximal dose of diuretics ora reappearance of grade 2 or 3 ascites within 4 weeks of paracentesis [23].A written informed consent was obtained from each patient before the hemodynamic study was performed.This study complies with current ethical guidelines according to the Declaration of Helsinkiand was approved by theInstitutional Review Board, Taipei Veterans General Hospital, Taipei, Taiwan(No.2017-06-006AC).
Noninvasive markers of liver fibrosis
Results of laboratory tests performed on the same day before hemodynamic measurement were used to determine of noninvasive markers. These markers, including the FIB-4, APRI, CDS, Lok index, GUCI and ALBI score, were calculated based on the following formulas:
FIB-4 index [9] = age (years) x AST (U/L)/ (platelets (109/L) x alanine aminotransferase (ALT) (U/L)1/2)
APRI [10] = AST (/upper limit of normal AST)/platelets (109/L) × 100
CDS is determined as the sum of the following variables and ranges from 0 to 11 [11]: (1) platelet count (109/L): >340 = 0; 280–339 = 1; 220–279 = 2; 160–219 = 3; 100–159 = 4; 40–99 = 5; <40 = 6, (2) the ALT/AST ratio: >1.7 = 0; 1.2–1.7 = 1; 0.6–1.19 = 2; <0.6 = 3, and (3) the international normalized ratio of prothrombin time (INR): <1.1 = 0; 1.1–1.4 = 1; >1.4 = 2. Different points are given and added together according to the values of these parameters.
Lok index [12] = e(LogOddsLok) / (1 + e(LogOddsLok)); LogOddsLok = - 5.56 - (0.0089 x platelets(109/L)) + (1.26x AST /ALT ratio) + (5.27 x INR)
GUCI [13] = AST/upper limit of normal AST (U/L) x INR x 100/platelets (109 /L). Upper limit of normal AST equals 45 U/L.
ALBI score [14] = log(bilirubin[mmol/L]) x 0.66)—(albumin[g/L] x 0.085).Patients were stratified into three groups according to previously described cut-offs resulting in three grades: ALBI grade 1 (≤−2.60), grade 2 (>−2.60 to −1.39) and grade 3 (>−1.39).
Hemodynamic measurement
Patients underwent hemodynamic measurement after an overnight fast. Under local anesthesia, hepatic vein catheterization was performed using a 7F Swan-Ganzthermodilution catheter (Viggo-Spectramed, Oxnard, CA, USA) as previously described [24].Briefly, the catheter was inserted percutaneously using the Seldinger technique into the right internal jugular vein and was then advanced into the right hepatic vein [25], where the free hepatic venous pressure (FHVP) and wedge hepatic venous pressure (WHVP) were recorded with a multi-channel recorder (model 78534C, Hewlett Packard, Palo Alto, CA, USA). The zero reference point was set precisely at 5 cm below the sternum. Under continuous monitoring, confirmation of the wedged position was obtained after injecting a small amount of contrast medium, which demonstrated retention of the contrast medium in the occluded hepatic vein.
The HVPG was obtained by subtracting the FHVP from the WHVP. The catheter was then advanced into the right side of the heart and the pulmonary artery for systemic hemodynamic measurements, which included right atrial pressure (RAP), mean pulmonary arterial pressure, pulmonary capillary wedge pressure, and cardiac output (CO). CO was measured by the thermodilution method [26].The mean arterial pressure (MAP) and heart rate were recorded with an external vital sign monitor. Systemic vascular resistance (SVR) (dyne/s/cm5) was calculated as follows: ([MAP–RAP]×80)/CO.An excellent correlation between portal vein pressure and the WHVP has been established in previous studies [27,28].
Statistical analyses
Chi-squared test or Fisher’s exact test (two-tailed) was used to analyze categorical data, and Mann-Whitney ranked sum test was used for continuous data.Pearson’s correlation analysis was used to estimate the correlation between the MELD, sNa, CTP score, noninvasive markers and hemodynamic parameters. The differences in noninvasive markers with respect to the severity of ascites were assessed using the Kruskal-Wallis test. To assess the abilities of the MELD scores, sNa,HVPG and noninvasive markers to predict the risk of death at 3and 6months, the concordance (c-statistic) equivalent to the area under the receiver operating characteristic curve (AUC) was measured and compared by using the method of Hanley and McNeil [29].
To assess the prognostic predictors of long-term survival, all relevant clinical variables, including age, sex, etiology of cirrhosis, ascites,sNa, HVPG, CTP score, MELD score, and noninvasive markers were entered intounivariate survival analysis and Cox proportional hazards model to determine the adjusted relative risk. Independent prognostic predictors were also evaluated as dichotomous or continuous variables in the Cox model. All statistical analyses were conducted using SPSS for Windows version 12 (SPSS Inc., Chicago, IL) and MedCalc for Windows version 4.2 (MedCalc Software, Mariakerke, Belgium). A p value less than 0.05 was considered statistically significant.
Results
Patient demographics
Baseline clinicalcharacteristics and laboratory data are shown in Table 1. Patients were predominantly (86%) elderly (mean age: 62±11 years) male. The most common etiology of cirrhosis was viral hepatitis (76%).There were 101 (42%), 86 (36%) and 55(23%) patients belonging to CTP class A, B and C, respectively. Ascites was found in 103 (43%) patients and most patients had esophageal varices (79%). The follow-up period of these patients was up to 13 years (mean: 41 months). During the follow-up period, none underwent liver transplantation due to severe organ shortage in this area.
Table 1. Patient demographics.
| Demographics | |
|---|---|
| Age (years) | 62 ± 11 |
| Male, n (%) | 208 (86) |
| Etiology of cirrhosis, n (%) | |
| Hepatitis B | 142 (59) |
| Hepatitis C | 41 (17) |
| Hepatitis B and C | 11 (5) |
| Alcohol | 19(8) |
| NASH and cryptogenic | 29 (12) |
| Ascites, n (%) | |
| No ascites | 139 (57) |
| Response to diuretics | 39 (16) |
| Diuretic-resistant ascites | 64 (27) |
| CTP score | 7±2 |
| Class A/B/C, n (%) | 101(42)/86(36)/55(23) |
| Albumin (g/dl) | 3.3±0.6 |
| MELD score | 13 ±5 |
| Bilirubin (mg/dL) | 2.2±2 |
| INR of prothrombin time | 1.4±0.4 |
| Creatinine | 1.1±0.6 |
| Serum sodium (mmol/L) | 138±4 |
| Platelet (k/cumm) | 83±49 |
| ALT (U/L) | 50±50 |
| AST (U/L) | 67±44 |
| Esophageal varices, n (%) | 192 (79) |
| Hemodynamic parameters | |
| HVPG (mmHg) | 16±5 |
| CO (L/min) | 6.70±1.84 |
| SVR (dyne/s/cm5) | 1142±365 |
| MAP (mmHg) | 93±13 |
| Noninvasive markers of liver fibrosis | |
| FIB-4 | 9.8±6.9 |
| APRI | 2.7±2.2 |
| CDS | 8±1 |
| Lok index | 0.86±0.17 |
| GUCI | 3.8±3.5 |
| ALBI score | -1.8±0.6 |
| Grade 1/2/3, n (%) | 34 (14)/132 (55)/76 (31) |
| Follow-up duration (months) | 41±39 |
Values are presented as mean ± SD or numbers and percentages.
Abbreviations: CTP, Child-Turcotte-Pugh score; MELD, model for end-stage liver disease; INR: international normalized ratio; ALT, alanine aminotransferase; AST, aspartate aminotransferase; HVPG, hepatic venous pressure gradient; CO, cardiac output; SVR, systemic vascular resistance; MAP, mean arterial pressure; APRI, AST to platelet ratio index; CDS, cirrhosis discriminant score; GUCI, Goteborg University Cirrhosis Index; ALBI, albumin-bilirubin.
Correlation between the MELD score, CTP score, noninvasive fibrosis markers, and hemodynamic parameters
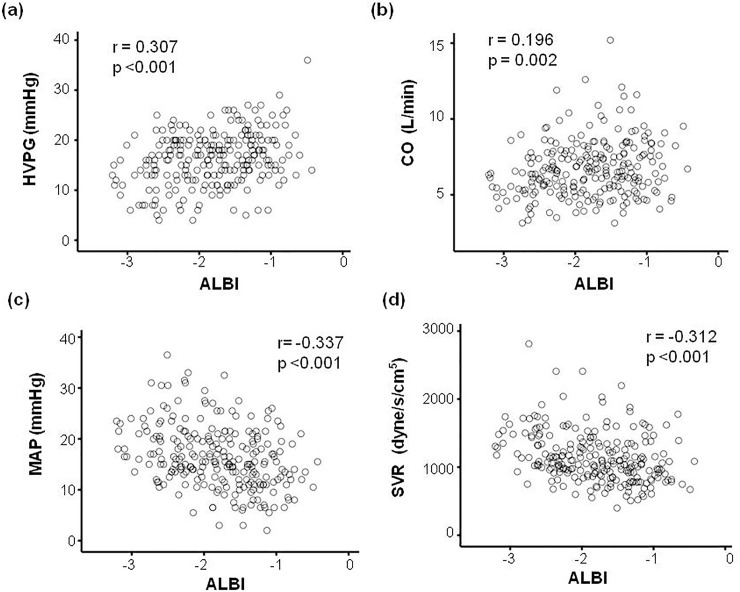
There was a positive and significant correlation between the MELD score, CTP score, all noninvasive markers and HVPG (Table 2). sNa had a significant inverse correlation with HVPG.The strongest correlation was observedbetween HVPG and the ALBI score (r = 0.307, p<0.001; Fig 1).The AUC for ALBI scores to predict clinically significant PH (HVPG≥10 mmHg) and severe PH (HVPG≥12 mmHg) were 0.721 and 0.671, respectively (p<0.001).
Table 2. Correlation coefficient (r) amongnoninvasiveliver fibrosismarkers and hemodynamic parameters in cirrhotic patients.
| MELD | CTP score | sNa | ALBI | FIB-4 | APRI | Lok index | CDS | GUCI | |
|---|---|---|---|---|---|---|---|---|---|
| HVPG (mmHg) | 0.205** | 0.260** | -0.187* | 0.307** | 0.270** | 0.238** | 0.302** | 0.261** | 0.212** |
| CO (L/min) | 0.140* | 0.111 | 0.002 | 0.196* | -0.057 | 0.036 | 0.143* | 0.148* | 0.061 |
| SVR (dyne/s/cm) | -0.192* | -0.217** | 0.093 | -0.312** | -0.066 | -0.158* | -0.248** | -0.265** | -0.194* |
| MAP (mmHg) | -0.196* | -0.279** | 0.312** | -0.332** | -0.187* | -0.203* | -0.238** | -0.276** | -0.237** |
MELD, model for end-stage liver disease; CTP score, Child-Turcotte-Pugh score; HVPG, hepatic venous pressure gradient; CO, cardiac output; SVR, systemic vascular resistance; MAP, mean arterial pressure; ALBI, albumin-bilirubinscore;FIB-4, fibrosis-4 score;APRI,aspartate transaminase-to-platelet ratio; CDS, cirrhosis discriminant index; GUCI, Göteborg University Cirrhosis Index
*p<0.05
** p<0.001
Fig 1. Correlation between albumin-bilirubin (ALBI) scores and hemodynamic parameters in cirrhotic patients.
The correlation between ALBI scores and hepatic venous pressure gradient (HVPG, panel A), cardiac output (CO, panel B), mean arterial pressure (MAP, panel C), and systemic vascular resistance (panel D).
The correlation between the MELD score, CTP score, non-invasive markers and individual hemodynamic parameters were also examined(Table 2). There was a significant and inverse correlation between MAP and the MELD score, CTP score, and noninvasive markers. CO positively correlated with the MELD and ALBI score, whereas SVR was inversely correlated with the MELD score, CTP score, and all noninvasive markers except FIB-4 index. Among these markers, ALBI scores had the best correlation with CO (r = 0.196, p = 0.002), SVR (r = -0.312, p<0.001) and MAP (r = -0.332, p<0.001) (Fig 1).
Association between noninvasive markers and severity of ascites
The comparison of noninvasive markers of liver fibrosis with respect to the severity of ascites is shown in Table 3. The ALBI score significantly increased with worsening ascites (p<0.001). Although the levels of noninvasive markers, except ALBI score, were higher in patients with ascites compared to those in patients without ascites, the difference between patients with ascites that was responsive or resistant to diuretics was not significant.
Table 3. Association between the severity of ascites and noninvasive markers of liver fibrosis.
| Severity of ascites | ||||
|---|---|---|---|---|
| No ascites | Respond to diuretics | Diuretics-resistant ascites | p value | |
| FIB-4 | 8.6±5.2 | 11.5±6.8 | 11.3±9.3 | 0.031 |
| APRI | 2.4±1.8 | 3.4±2.4 | 2.97±2.54 | 0.057 |
| CDS | 7±1 | 8±1 | 8±2 | 0.006 |
| Lok index | 0.83±0.17 | 0.9±0.13 | 0.9±0.17 | <0.001 |
| GUCI | 3.2±2.7 | 4.8±3.9 | 4.7±4.4 | 0.010 |
| ALBI | -2±0.6 | -1.6±0.5 | -1.4±0.5 | <0.001 |
Values are presented as mean ± SD
Abbreviations: APRI, AST to platelet ratio index; CDS, cirrhosis discriminant score; GUCI, Goteborg University Cirrhosis Index; ALBI, albumin-bilirubin.
Comparison of the predictive accuracy for mortality between the MELD score, HVPG, sNa and noninvasive markers of liver fibrosis at 3 and 6 months
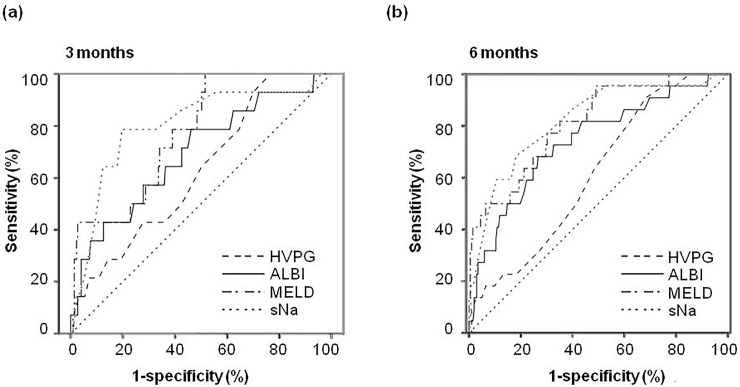
The prognostic performances of noninvasive markers, MELD, HVPG and sNa for predicting 3-month and 6-month mortality are shown in Table 4. Using 3-month mortality as the end point, the AUC was 0.773 for MELD score (p = 0.001), 0.626 for HVPG (p = 0.115) and 0.799 for sNa (p<0.001), respectively. Among the noninvasive markers, only ALBI score significantly predicted 3-month mortality according to the ROC curve analysis (AUC = 0.691, p = 0.016).The differences were not statistically significant between the MELD, HVPG, sNa and ALBI score (Table 4 and Fig 2A).At 6 months, the AUC was 0.813 for MELD score (p<0.001), 0.615 for HVPG (p = 0.081) and 0.818 for sNa (p<0.001). The FIB-4 index, CDS, Lok index, GUCI and ALBI score were significantly associated with 6-month mortality by the ROC analysis. Among the noninvasive models, the highest AUC was also observed for ALBI scores (0.740, p<0.001).The differences were not statistically significant between the MELD score,sNaand ALBI score.However, both the MELD score and sNa had a significantly higher AUC compared to HVPG (p = 0.003 and 0.004, respectively) (Table 4 and Fig 2B). The cut-off values that had the best predictive accuracies for the MELD, sNa and ALBI were determined from the ROC curve. In patients with low (< 14) MELD scores, those with sNa≤135 mmol or ALBI score >-1.4 had significantly higher mortality rates at both 3 and 6 months (Table 5).The AUC for ALBI score in predicting 3-month and 6-month mortality in patients with low MELD scores were 0.738 (p = 0.048) and 0.726 (p = 0.044), respectively. In patients without hyponatremia (sNa>135 mmol/L), those with ALBI scores >-1.4 had higher mortality rate at 3 months (6.8% vs. 1.4%, p = 0.079) and 6 months (12.8% vs 3.6%, p = 0.044).
Table 4. Comparison of AUC among MELD, HVPG, sNa and noninvasive fibrosis markers to predict survival at 3 months and 6 months.
| AUC at 3-month | 95% CI | p | AUC at 6-month | 95% CI | p | |
|---|---|---|---|---|---|---|
| MELD | 0.773 | 0.663–0.882 | 0.001 | 0.813 | 0.720–0.906 | <0.001 |
| HVPG (mmHg) | 0.626 | 0.489–0.762 | 0.115 | 0.615 | 0.506–0.724 | 0.081 |
| sNa (mmol/L) | 0.799 | 0.668–0.930 | <0.001 | 0.818 | 0.722–0.914 | <0.001 |
| FIB-4 | 0.546 | 0.370–0.723 | 0.560 | 0.643 | 0.511–0.775 | 0.028 |
| APRI | 0.549 | 0.370–0.727 | 0.541 | 0.593 | 0.461–0.726 | 0.151 |
| CDS | 0.547 | 0.396–0.697 | 0.559 | 0.695 | 0.572–0.817 | 0.003 |
| Lok index | 0.625 | 0.516–0.375 | 0.116 | 0.732 | 0.639–0.825 | <0.001 |
| GUCI | 0.574 | 0.397–0.750 | 0.355 | 0.640 | 0.505–0.774 | 0.032 |
| ALBI | 0.691 | 0.541–0.842 | 0.016 | 0.740 | 0.625–0.854 | <0.001 |
Abbreviations:AUC, area under the curves; CI, confidence interval; MELD, model for end-stage liver disease; HVPG, hepatic venous pressure gradient;sNa, serum sodium levels; APRI, AST to platelet ratio index; CDS, cirrhosis discriminant score; GUCI, Goteborg University Cirrhosis Index; ALBI, albumin-bilirubin
Fig 2. Comparison of the area under curve to predict short-term outcomes.
Area under curve of 3-month (panel A) and 6-month (panel B) mortality for model of end-stage liver disease (MELD) score, hepatic venous pressure gradient (HVPG), serum sodium (sNa) and albumin-bilirubin (ALBI) score.
Table 5. Prognostic significance of serum sodium and albumin-bilirubin scores for short-term mortality in patients with low (<14) MELD scores.
| 3-month, n (%) | 6-month, n (%) | |||||
|---|---|---|---|---|---|---|
| Death | Alive | p | Death | Alive | p | |
| sNa≤135 mmol/L | 4 (21%) | 15 (79%) | 0.002 | 4 (21%) | 15 (79%) | 0.004 |
| sNa>135 mmol/L | 2 (1%) | 138 (99%) | 3 (2%) | 137 (98%) | ||
| ALBI>-1.4 | 3 (14%) | 18 (86%) | 0.031 | 3 (14%) | 18 (86%) | 0.048 |
| ALBI≤-1.4 | 3 (2%) | 135 (98%) | 4 (3%) | 134 (97%) | ||
Abbreviations:MELD, model for end-stage liver disease; sNa, serum sodium levels; ALBI, albumin-bilirubin
Survival analysis
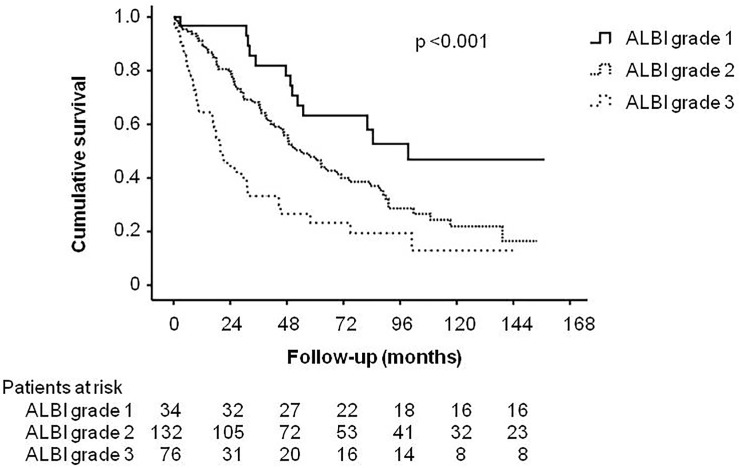
One hundred and thirty-one (54%) patients died during a mean follow-up period of 41±39 (range: 0.4–157.2) months. The most common causes of mortality were attributable to liver-related diseases(including liver failure, variceal bleeding, spontaneous bacterial peritonitis, hepatic encephalopathy, and hepatorenal syndrome; n = 124, 95%)(Table 6). In univariate survival analysis, male (p = 0.007), HVPG >16 mmHg (p = 0.001), CTP score >8 (p<0.001), MELD score >14 (p< 0.001), sNa≤135 mmol/L (p = 0.002) and the presence of ascites (p<0.001) were factors significantly associated with decreased survival. Among the noninvasive markers, higher ALBI grade (p<0.0001), FIB-4 index >8.4 (p = 0.005), CDS>7 (p = 0.001), Lok index>0.9 (p<0.0001), and GUCI>2.7 (p = 0.019)were associated with decreased survival. In the Cox multivariate analysis, sNa≤135 mmol/L (hazard ratio [HR]: 1.724, p = 0.035), MELD scores >14 (HR:1.829, p = 0.005), HVPG >16 mmHg (HR: 1.603, p = 0.012) and higher ALBI grade(grade 2 vs grade 1: HR: 1.647, p = 0.032; grade 3 vs grade 1: HR: 2.717, p = 0.001)wereindependent risk factors predictingpoor long-term survival (Table 7). Patients with ALBI grade 1 had a mean survival of 75.5±46.7 months and patients with ALBI grade 2 and 3 had mean survival of 42.9±37.1 and 22.4±27.5 months, respectively. The survival difference between patients with high and low ALBI grades is shown in Fig 3. When these parameters were treated as continuous variables in the Cox model, there was an additional risk of mortality of 6.9% (p = 0.001), 5.6% (p = 0.001), and 39.7% (p = 0.049) per unit increasein the MELD, HVPG, and ALBI score, respectively. Moreover, there was an additional risk of mortality of 7.5% per unit decrease in sNa levels (p = 0.002).
Table 6. Causes of death.
| Causes | N (%) |
|---|---|
| Liver related disease | 124 (95) |
| Liver failure | 32 (24) |
| Variceal bleeding | 29 (22) |
| Spontaneous bacterial peritonitis | 27 (21) |
| Hepatorenal syndrome | 22 (17) |
| Hepatic encephalopathy | 14 (11) |
| Pneumonia | 3 (2.2) |
| Soft tissue infection | 1 (0.7) |
| Meningitis | 1 (0.7) |
| Pulmonary hemorrhage | 1 (0.7) |
| Perforated peptic ulcer | 1 (0.7) |
Table 7. Prognostic factors associated with long-term survival in univariate and multivariate analysis.
| Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|
| N | Death (%) | p value | HR | 95% CI | P value | |
| Age (>65 /≤ 65year-old) | 116/126 | 70/61 | 0.068 | |||
| Gender (male/female) | 208/34 | 121/10 | 0.007 | |||
| sNa (≤135/>135 mmol/L) | 51/191 | 31/99 | 0.002 | 1.959 | 1.090–2.726 | 0.004 |
| Ascites (yes/no) | 103/139 | 66/65 | <0.001 | |||
| HVPG (>16/≤16 mmHg) | 122/120 | 76/55 | <0.001 | 1.549 | 1.072–2.238 | 0.020 |
| MELD scores(>14/≤14) | 83/159 | 50/81 | <0.001 | 1.844 | 1.212–2.807 | 0.004 |
| CTP score (>8/≤ 8) | 78/164 | 49/78 | <0.001 | |||
| ALBI grade | <0.001 | 0.008 | ||||
| Grade 2 vs Grade | 132/34 | 72/13 | 1.647 | 1.089–3.032 | ||
| Grade 3 vs Grade 1 | 76/34 | 46/13 | 2.717 | 1.728–5.335 | ||
| FIB-4 index (>8.4/≤ 8.4) | 121/121 | 72/59 | 0.005 | |||
| APRI (>2/≤ 2) | 125/117 | 72/59 | 0.053 | |||
| CDS (>7/≤ 7) | 177/65 | 105/26 | 0.001 | |||
| Lok index (>0.9/≤ 0.9) | 131/111 | 85/46 | <0.001 | |||
| GUCI (>2.7/≤2.7) | 122/120 | 73/58 | 0.019 | |||
Abbreviations: HR, hazard ratio; CI, confidence interval; sNa, serum sodium levels; HVPG, hepatic venous pressure gradient;MELD, model for end-stage liver disease; CTP, Child-Turcotte-Pugh score; ALBI, albumin-bilirubin; APRI, AST to platelet ratio index; CDS, cirrhosis discriminant score; GUCI, Goteborg University Cirrhosis Index.
Fig 3. Comparison of long-term survival according to differentALBI grades.
ALBI grade 1 patients had a significantly better long-term survival compared to other patient groups.
Discussion
The present study showed that among the currently used noninvasive liver reserve models, ALBI score had the best correlation with HVPG and other hemodynamic parameters in patients with cirrhosis. Regarding their prognostic value for short-term outcomes, the ALBI score had the highest AUC for predicting 3-month and 6-month mortality compared to those for the other noninvasive markers. In patients with low MELD scores, those with low sNa or high ALBI score had greater short-term mortality. Furthermore, the ALBI as well as MELD score, HVPG and sNa were independently associated with long-term survival in cirrhotic patients.
Noninvasive markers validated in staging liver fibrosis have the advantage of availability and noninvasiveness. Nevertheless, very few of them have been evaluated with respect to their correlation with hemodynamic parameters including HVPG in cirrhotic patients. A previously proposed Fibrotest showed a moderate correlation with HVPG in patients with chronic liver disease with a correlation coefficient of 0.58, but only a weak correlation with HVPG in cirrhotic patients (r = 0.24) [30]. Another study showed that there was a significant correlation between the APRI and HVPG in patients with cirrhosis (r = 0.365) [16]. In our study, we found that all noninvasive markers were significantly correlated with HVPG but the correlation is weak in most models. PH depends not only on the hepatic fibrosis component but also on hemodynamic components which are often associated with splanchnic and portal flow [2]. Although fibrosis markers are related to the increase in intrahepatic vascularresistance consequent to tissue fibrosis, they may not reflect the complex hemodynamic changes characteristicof late PH; thusa fibrosis marker alone may be insufficient to correlate strongly with HVPG.
The ALBI score, a newly prognostic tool involving only two common laboratory parameters of albumin and bilirubin, was initially applied in patients with HCC for assessing the severity of liver dysfunction [14]. The ALBI score was reported to have a positive correlation with HVPG in patients with HCC in a small data set [31]. Our study, which is the first to evaluate the relationship between the ALBI score and hemodynamic parameters in cirrhotic patients, shows that the ALBI score was significantly correlated with HVPG and other hemodynamic parameters, with a higher correlation coefficient compared to that for other fibrosis markers. In addition, the development of ascites is a complication of PH that typically occurs above a HVPG of 12mmHg [32]. The severity of sodium retention increases throughout the natural history of cirrhosis due to the progression of systemic and portal hemodynamic abnormalities and the associated activation of neurohumoral vasoactive systems leading to diuretics-resistant ascites [33].In the present study, although all noninvasive markers were higher in patients with ascites compared to those without ascites, only the ALBI score showed a significant difference between patients with ascites responsive or resistant to diuretics. These findings indicate that the ALBI score is a more unique and specific factor linked to the severity of cirrhosis.
Liver functional reserve is considered as a crucial predictor of mortality in cirrhotic patients. In regard to short-term mortality, the ALBI score was used to predict in-hospital mortality in cirrhotic patients with acute upper gastrointestinal bleeding [34], but its prognostic values compared to the established models is unclear. We investigated the predictive value of the 6 currently used noninvasive models for short-term outcomes, and found that only the ALBI score was significantly associated with 3-month mortality and ALBI score also had the highest AUC for predicting 6-month mortality. There were no significant differences in AUC between the ALBI score, MELD and sNa, suggesting that they have similar predictive accuracies. In addition, the risk of mortality clearly increases with increasing MELD scores, but a substantial portion of patients with initially low MELD scores might have shortened survival [35].Hyponatremia has been proposed as an additional marker to identify patients with a high mortality risk among those with low MELD scores [35]. We demonstrated that a higher ALBI score (>-1.4) was able to predict early mortality in patients with low MELD scores and in patients without hyponatremia. ALBI score was also significantly associated with 3-month and 6-month mortality in patients with low MELD scores. Although the differences were not statistically significant between the MELD score, sNa and ALBI score in predicting short-term mortality, ALBI score can serve as a complimentary prognostic marker. Importantly, ALBI score could help identify high risk subjects in cirrhotic patients with low MELD score or normal serum sodium levels.These results suggest that the ALBI score is not only a prognostic factor of short-term mortality but also a complementary test to predict early mortality among the “low-risk” group.
Regarding the long-term survival, the APRI and FIB-4 have been reported as useful prognostic indicators in patients with chronic hepatitis C [20]. In addition, Lok index was found to predict survival in patients with alcoholic cirrhosis [17].Weevaluated the predictive values of noninvasive markers and the analysis was adjusted by the widely adopted predictorfor prognosis in cirrhosis including MELD, HVPG, sNa and CTP score with a follow-up period up to 13 years. In the univariate analysis, sex, sNa, presence of ascites, HVPG, the MELD score, CTP score, ALBI grade, FIB-4 index, CDS, Lok index and GUCI were significantly associated with survival. In the Cox multivariate model, the MELD, sNa, HVPG and ALBI grade were consistently identified as independent prognostic indicators. When treated as continuous variables, the MELD, sNa, HVPG and ALBI scores were still associated with long-term survival. In a systemic review of prognostic indicators in cirrhosis, serum albumin and bilirubin are the two most prominent individual prognostic variables [36].However, there are limited data on the prognostic information of ALBI score in cirrhotic patients. Chen et al reported that ALBI grades were predictors of long-term survival among patients with hepatitis B-related cirrhosis [19]during a 3-year follow-up; however, portal pressure and sNa were not analyzed in the multivariate model. In our study, sNa, MELD score, HVPG and ALBI score are all independent predictors of survival, suggesting that the ALBI score has enhanced prognostic information compared to that for other established indicators.
Our study has some limitations. First, we only included cirrhotic patients who underwent hemodynamic measurement and this may have led to certain selection bias. For example, none of our patients had hepatic encephalopathy at the time of enrollment which has been identified as an important prognostic predictor in cirrhosis.Nearly half (42%) of patients were in CTP class A, suggesting the number of patients with decompensated cirrhosis is relatively low. Thus, our findings may not be readily applicable in the population predominantly with advanced cirrhosis.Second, most patients in the present study had chronic hepatitis B infection as the etiology of cirrhosis and none of them received antiviral treatment at enrollment. The natural course of cirrhosis may vary in patients with different etiologies and specific treatment. Therefore, our results may not be readily applicable in those areas where alcoholism, non-alcoholic fatty liver disease or chronic hepatitis C are major causes of cirrhosis. Third, ALBI scores may be a potential useful predictor of hemodynamic parameters including HVPG in cirrhotic patients, but the correlation between ALBI scores and HVPG in patients without severe portal hypertension needs further studies to evaluate. Last, given the relatively low number of investigated patients, independent cohorts are needed to validate the obtained results.
In conclusion, the ALBI score has the best correlation with hemodynamic parameters and is a feasible marker for short-term outcome prediction in cirrhotic patients. A high ALBI score may identify high-risk patients with low MELD scores and can serve as a complimentary prognostic marker to predict early mortality among this population.Increased ALBI scores, MELD, HVPG, and low sNa are prognostic predictors of decreased long-term survival.Our studydemonstrates the predictive values of ALBI scores in cirrhosis and future prospective cohort with large sample size are needed to validate the findings in the present study and explore the applicability of ALBI score in patients with cirrhosis.
Supporting information
(XLSX)
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
This study was supported by grants (V107C-003, VN107-04) from Taipei Veterans General Hospital, Taiwan.
References
- 1.Bosch J, Garcia-Pagan JC. Complications of cirrhosis. I. Portal hypertension. J Hepatol. 2000;32:141–156. [DOI] [PubMed] [Google Scholar]
- 2.Iwakiri Y. Pathophysiology of portal hypertension. Clin Liver Dis. 2014;18:281–291. 10.1016/j.cld.2013.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Feu F, Garcia-Pagan JC, Bosch J, Luca A, Terés J, Escorsell A, et al. Relation between portal pressure response to pharmacotherapy and risk of recurrent variceal haemorrhage in patients with cirrhosis. Lancet 1995;346:1056–1059. [DOI] [PubMed] [Google Scholar]
- 4.Escorsell A, Bordas JM, Castaneda B, Llach J, García-Pagán JC, Rodés J, et al. Predictive value of the variceal pressure response to continued pharmacological therapy in patients with cirrhosis and portal hypertension. Hepatology 2000;31:1061–1067. 10.1053/he.2000.6779 [DOI] [PubMed] [Google Scholar]
- 5.Merkel C, Bolognesi M, Sacerdoti D, Bombonato G, Bellini B, Bighin R, et al. The hemodynamic response to medical treatment of portal hypertension as a predictor of clinical effectiveness in the primary prophylaxis of variceal bleeding in cirrhosis. Hepatology 2000;32:930–934. [DOI] [PubMed] [Google Scholar]
- 6.Groszmann RJ, Bosch J, Grace ND, Conn HO, Garcia-Tsao G, Navasa M, et al. Hemodynamic events in a prospective randomized trial of propranolol versus placebo in the prevention of a first variceal hemorrhage. Gastroenterology 1990;99:1401–1407. [DOI] [PubMed] [Google Scholar]
- 7.Abraldes JG, Tarantino I, Turnes J, Garcia-Pagan JC, Rodés J, Bosch J.Hemodynamic response to pharmacological treatment of portal hypertension and long-term prognosis of cirrhosis. Hepatology 2003;37:902–908. 10.1053/jhep.2003.50133 [DOI] [PubMed] [Google Scholar]
- 8.Moller S, Henriksen JH. Cirrhotic cardiomyopathy. J Hepatol. 2010;53:179–190. 10.1016/j.jhep.2010.02.023 [DOI] [PubMed] [Google Scholar]
- 9.Vallet-Pichard A, Mallet V, Nalpas B, Verkarre V, Nalpas A, Dhalluin-VenierV,et al. FIB-4: an inexpensive and accurate marker of fibrosis in HCV infection. comparison with liver biopsy and fibrotest. Hepatology 2007;46:32–36. 10.1002/hep.21669 [DOI] [PubMed] [Google Scholar]
- 10.Wai CT, Greenson JK, Fontana RJ,Kalbfleisch JD, Marrero JA, Conjeevaram HS,et al. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology 2003;38:518–526. 10.1053/jhep.2003.50346 [DOI] [PubMed] [Google Scholar]
- 11.Bonacini M, Hadi G, Govindarajan S, Lindsay KL. Utility of a discriminant score for diagnosing advanced fibrosis or cirrhosis in patients with chronic hepatitis C virus infection. Am J Gastroenterol. 1997;92:1302–1304. [PubMed] [Google Scholar]
- 12.Lok AS, Ghany MG, Goodman ZD, Wright EC, Everson GT, Sterling RK,et al. Predicting cirrhosis in patients with hepatitis C based on standard laboratory tests: results of the HALT-C cohort. Hepatology 2005;42:282–292. [DOI] [PubMed] [Google Scholar]
- 13.Islam S, Antonsson L, Westin J, Lagging M. Cirrhosis in hepatitis C virus-infected patients can be excluded using an index of standard biochemical serum markers. Scand J Gastroenterol. 2005;40:867–872. 10.1080/00365520510015674 [DOI] [PubMed] [Google Scholar]
- 14.Johnson PJ, Berhane S, Kagebayashi C, Satomura S, Teng M, Reeves HL,et al. Assessment of liver function in patients with hepatocellular carcinoma: a new evidence-based approach-the ALBI grade. J Clin Oncol. 2015;33:550–558. 10.1200/JCO.2014.57.9151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chan AW, Chan RC, Wong GL,Wong VW, Choi PC, Chan HL,et al. New simple prognostic score for primary biliary cirrhosis: Albumin-bilirubin score. J Gastroenterol Hepatol. 2015;30:1391–1396. 10.1111/jgh.12938 [DOI] [PubMed] [Google Scholar]
- 16.Verma V, Sarin SK, Sharma P, Kumar A. Correlation of aspartate aminotransferase/platelet ratio index with hepatic venous pressure gradient in cirrhosis. United European Gastroenterol J. 2014;2:226–231. 10.1177/2050640614527084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cho EJ, Kim MY, Lee JH, Lee IY, Lim YL, Choi DH,et al. Diagnostic and Prognostic Values of Noninvasive Predictors of Portal Hypertension in Patients with Alcoholic Cirrhosis. PLoS One 2015;10:e0133935 10.1371/journal.pone.0133935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lisotti A, Azzaroli F, Buonfiglioli F, Montagnani M, Cecinato P, Turco L,et al. Indocyanine green retention test as a noninvasive marker of portal hypertension and esophageal varices in compensated liver cirrhosis. Hepatology 2014;59:643–650. 10.1002/hep.26700 [DOI] [PubMed] [Google Scholar]
- 19.Chen RC, Cai YJ, Wu JM, Wang XD, Song M, Wang YQ, et al. Usefulness of albumin-bilirubin grade for evaluation of long-term prognosis for hepatitis B-related cirrhosis. J Viral Hepat. 2017;24:238–245. 10.1111/jvh.12638 [DOI] [PubMed] [Google Scholar]
- 20.Nunes D, Fleming C, Offner G, Craven D, Fix O, Heeren T,et al. Noninvasive markers of liver fibrosis are highly predictive of liver-related death in a cohort of HCV-infected individuals with and without HIV infection. Am J Gastroenterol. 2010;105:1346–1353. 10.1038/ajg.2009.746 [DOI] [PubMed] [Google Scholar]
- 21.Huo TI, Wu JC, Lin HC, Lee FY, Hou MC, Lee PC, et al. Evaluation of the increase in model for end-stage liver disease (DeltaMELD) score over time as a prognostic predictor in patients with advanced cirrhosis: risk factor analysis and comparison with initial MELD and Child-Turcotte-Pugh score. J Hepatol. 2005;42:826–832. 10.1016/j.jhep.2005.01.019 [DOI] [PubMed] [Google Scholar]
- 22.Huo TI, Lin HC, Wu JC, Lee FY, Hou MC, Lee PC,et al. Proposal of a modified Child-Turcotte-Pugh scoring system and comparison with the model for end-stage liver disease for outcome prediction in patients with cirrhosis. Liver Transpl. 2006;12:65–71. 10.1002/lt.20560 [DOI] [PubMed] [Google Scholar]
- 23.EASL clinical practice guidelines on the management of ascites, spontaneous bacterial peritonitis, and hepatorenal syndrome in cirrhosis. J Hepatol. 2010;53:397–417. 10.1016/j.jhep.2010.05.004 [DOI] [PubMed] [Google Scholar]
- 24.Tsai YT, Lee FY, Lin HC, Chang TT, Lay CS, Wang SS,et al. Lack of effects of isosorbide-5-mononitrate on hepatic hemodynamics in HBsAg-positive cirrhosis. Hepatology 1989;10:283–287. [DOI] [PubMed] [Google Scholar]
- 25.Lebrec D, Degott C, Rueff B, Benhamou JP. Transvenous (transjugular) liver biopsy. An experience based on 100 biopsies. Am J Dig Dis. 1978;23:302–304. [DOI] [PubMed] [Google Scholar]
- 26.Forrester JS, Ganz W, Diamond G, McHugh T, Chonette DW, Swan HJ. Thermodilution cardiac output determination with a single flow-directed catheter. Am Heart J. 1972;83:306–311. [DOI] [PubMed] [Google Scholar]
- 27.Lin HC, Tsai YT, Lee FY, Chang TT, Wang SS, Lay CS,et al. Comparison between portal vein pressure and wedged hepatic vein pressure in hepatitis B-related cirrhosis. J Hepatol. 1989;9:326–330. [DOI] [PubMed] [Google Scholar]
- 28.Garcia-Tsao G, Groszmann RJ, Fisher RL, Conn HO, Atterbury CE, Glickman M. Portal pressure, presence of gastroesophageal varices and variceal bleeding. Hepatology 1985;5:419–424. [DOI] [PubMed] [Google Scholar]
- 29.Hanley JA, McNeil BJ. A method of comparing the areas under receiver operating characteristic curves derived from the same cases. Radiology 1983;148:839–843. 10.1148/radiology.148.3.6878708 [DOI] [PubMed] [Google Scholar]
- 30.Thabut D, Imbert-Bismut F, Cazals-Hatem D, Messous D, Muntenau M, Valla DC,et al. Relationship between the Fibrotest and portal hypertension in patients with liver disease. Aliment Pharmacol Ther. 2007;26:359–368. 10.1111/j.1365-2036.2007.03378.x [DOI] [PubMed] [Google Scholar]
- 31.Toyoda H, Lai PB, O'Beirne J, Chong CC, Berhane S, Reeves H, et al. Long-term impact of liver function on curative therapy for hepatocellular carcinoma: application of the ALBI grade. Br J Cancer. 2016;114:744–750. 10.1038/bjc.2016.33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Morali GA, Sniderman KW, Deitel KM, Tobe S, Witt-Sullivan H, Simon M,et al. Is sinusoidal portal hypertension a necessary factor for the development of hepatic ascites? J Hepatol. 1992;16:249–250. [DOI] [PubMed] [Google Scholar]
- 33.Salerno F, Guevara M, Bernardi M, Moreau R, Wong F, Angeli P,et al. Refractory ascites: pathogenesis, definition and therapy of a severe complication in patients with cirrhosis. Liver Int.2010;30:937–947. 10.1111/j.1478-3231.2010.02272.x [DOI] [PubMed] [Google Scholar]
- 34.Zou D, Qi X, Zhu C, Ning Z, Hou F, Zhao J,et al. Albumin-bilirubin score for predicting the in-hospital mortality of acute upper gastrointestinal bleeding in liver cirrhosis: A retrospective study. Turk J Gastroenterol. 2016;27:180–186. 10.5152/tjg.2016.15502 [DOI] [PubMed] [Google Scholar]
- 35.Peng Y, Qi X, Tang S, Deng H, Li J, Ning Z,et al. Child-Pugh, MELD, and ALBI scores for predicting the in-hospital mortality in cirrhotic patients with acute-on-chronic liver failure. Expert Rev Gastroenterol Hepatol. 2016;10:971–980. 10.1080/17474124.2016.1177788 [DOI] [PubMed] [Google Scholar]
- 36.D'Amico G, Garcia-Tsao G, Pagliaro L. Natural history and prognostic indicators of survival in cirrhosis: a systematic review of 118 studies. J Hepatol. 2006;44:217–231. 10.1016/j.jhep.2005.10.013 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLSX)
Data Availability Statement
All relevant data are within the manuscript and its Supporting Information files.